Abstract
Background
Metal debris and ion release has raised concerns in joint arthroplasty. The purpose of this study was to characterize the sources of metallic ions and particulate debris released from long-term (in vivo > 15y) TKA femoral components.
Methods
A total of 52 CoCr femoral condyles were identified as having been implanted for more than 15 years. The femoral components were examined for incidence of five types of damage (metal-on-metal wear due to historical polyethylene insert failure, MACC at taper interfaces, cement interface corrosion, third-body abrasive wear, and ICIC). Third-body abrasive wear was evaluated using the Hood method for polyethylene components and a similar method quantifying surface damage of the femoral condyle was used. The total area damaged by ICIC was quantified using digital photogrammetry.
Results
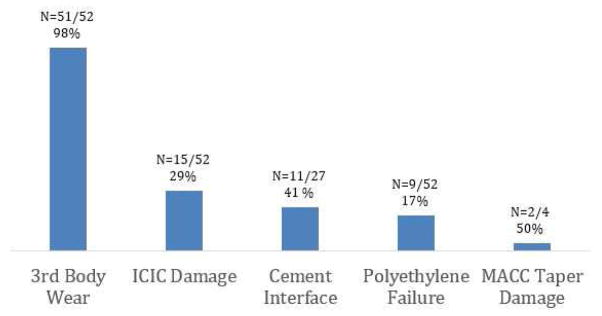
Surface damage associated with corrosion and/or CoCr debris release was identified in 98% (n=51) of the CoCr femoral components. Five types of damage were identified: 98% of femoral components exhibited 3rd body abrasive wear (mostly observed as scratching, n=51/52), 29% of femoral components exhibited ICIC damage (n=15/52), 41% exhibited cement interface damage (n=11/27), 17% exhibited metal-on-metal wear following wear-through of the polyethylene insert (n=9/52), and 50% of the modular femoral components exhibited MACC taper damage (n=2/4). The total ICIC damaged area was an average of 0.11 ± 0.12 mm2 (Range: 0.01–0.46mm2).
Conclusion
Although implant damage in TKA is typically reported with regard to the polyethylene insert, the results of this study demonstrate that abrasive and corrosive damage occurs on the CoCr femoral condyle in vivo.
Keywords: CoCr Femoral Components TKA, Fretting Corrosion, Third Body Wear, ICIC Damage, Revision TKA
Introduction
The release of metal debris from modular and bearing surfaces in total hip arthroplasty (THA) has become a concern due to the potential for adverse biological reactions in the surrounding tissue [1]. The mechanisms of metal release in THA are primarily due to wear, electrochemical dissolution, or a combination of the two processes [1]. The CoCr alloys used in orthopaedic devices rely on the formation of a passive film to prevent degradation of the alloy [2, 3] and the passive film is one of the key kinetic barriers preventing implant corrosion [1]. These films form and reform spontaneously on the metal surface, however they are only effective if they can withstand fracture or abrasion caused by fretting, micro motion, applied stress, or if the films are exposed to conditions in which they can rapidly reform [1]. Although there is little in the literature describing metal release in total knee arthroplasty TKA, the metallic alloys used in TKA are largely the same as those used in THA [4].
Similar to THA, the CoCr femoral component in TKA can undergo material loss due to wear and/or corrosion, with the release of metal debris and metal ions. The mechanisms of metal debris generation in TKA include: mechanically assisted crevice corrosion (MACC) of modular tapers [5], direct inflammatory cell induced corrosion (ICIC) [6], degradation at the backside interface with the bone cement layer [1, 2], scratching due to third body debris (e.g. bone cement particles or metallic debris) [7, 8], or complete wear-through of the polyethylene tibial insert and subsequent metal-on-metal wear between the femoral condyle and tibial baseplate. Although many of these mechanisms have been studied in depth in THA, inflammatory cell induced corrosion (ICIC) is a newly appreciated phenomenon. ICIC has recently been investigated in both THA and TKA by Gilbert et al. [6] and is described as a corrosive attack by inflammatory cells.
The lack of information on the prevalence and clinical relevance of metal release in TKA prompted us to search our multi-institutional orthopedic implant retrieval program for femoral components from TKA that were implanted for more than 15 years. We elected to investigate long-term TKA femoral components because we hypothesized that these femoral components would be the most likely to have evidence of degradation.
In this study, we investigated the mechanisms of metal release from CoCr femoral components in TKA by analyzing a series of retrieved long-term retrievals. We asked: (1) what are the sources of metallic ions and particulate debris released from long-term (in vivo > 15y) TKA femoral components; (2) what is the prevalence and extent of affected surface area by ICIC; and (3) what is the extent of corrosion damage at the implant-cement interface?
Materials and Methods
Clinical Demographics and Implant Characterization
Between 2000 and 2014, more than 2,700 total knee replacement systems (consisting of all or some components, depending on availability, including femoral, tibial, and patellar components) were retrieved during revision surgeries as part of an IRB-approved, multi-institutional orthopedic implant retrieval program. Seventy-two systems were identified as being implanted for greater than 15 years. Of these 72, twenty of the femoral components were retained in the patient during revision surgery and therefore removed from this study. Thus, 52 CoCr femoral components were examined for damage mechanisms that could lead to release of metal debris or particles (Table 1). The femoral components were implanted for 18 ± 3 years (range: 15 to 33 years). The patient age at the time of implantation was 57 ± 11 years (range: 21 to 78 years). Fifty-four percent of the patients were female (n=28/52). Seventy seven percent of the femoral components were from a primary surgery (n=40/52). The systems were predominantly revised for loosening (n=19/52: 37%), polyethylene wear (n=15/52: 29%), instability (n=5/52: 10%), and pain (n=4/52: 8%). Medical records were examined for evidence of metal-release and adverse reactions to metal debris. Six patients (12%) had evidence of metallosis or reported metal-on-metal articulation resulting from wear-through of the polyethylene tibial insert.
Table 1.
Clinical Information Corresponding to 52 Retrieved Long-Term (in-vivo >15y) CoCr Femoral Condyles.
| Clinical Information | |
|---|---|
| Patients | |
| Male (n) | 24 |
| Female (n) | 28 |
| Mean Age at Implantation | 57 ± 11 (21–78) years |
| Mean Time in situ | 18 ± 3 (15–33) years |
| Primary surgery (n) | 40 |
| Reason for revision (n) | |
| Loosening | 19 |
| Polyethylene Wear | 15 |
| Instability | 5 |
| Pain | 4 |
| Other | 9 |
| Documented Adverse Reactions | 6 |
| Metallosis | 5 |
Data are presented as mean ± standard deviation (range), or as a count (n).
The femoral condyles were all CoCr alloy and were from 5 manufacturers (in alphabetical order): Biomet (Warsaw, IN; n = 4/52), DePuy Synthes (Warsaw, IN; n = 13/52), Smith and Nephew (Memphis, TN; n = 4/52), Stryker (Mahwah, NJ; n = 13/52) and Zimmer (Warsaw, IN; n = 18/52). Eight of the fifty-two femoral condyles were fabricated with a porous coating (fiber mesh, n=3 and beads, n=5) and were not cemented (Table 2). In 22 cases, porous coatings were used in conjunction with cement fixation including, beads (n = 14/52), plasma spray (n = 3/52) and fiber metal mesh (n = 5/52). The tibial trays were fabricated from historical gamma air-sterilized ultra high molecular weight polyethylene (UHMWPE, n = 38), conventional gamma inert sterilized UHMWPE (n = 10), carbon fiber reinforced UHMWPE (Poly II, Zimmer, Warsaw, IN, [n = 3]), and 1 high pressure crystallized UHMPWE (Hylamer-M, DePuy Synthesis, Warsaw, IN). None of the polyethylene components in this study were fabricated from highly cross-linked polyethylene.
Table 2.
Device Information Corresponding to 52 Retrieved Long-Term (in-vivo >15y) CoCr Femoral Condyles.
| Device Information (Count) | |
|---|---|
| Number of CoCr Femoral Components | 52 |
| Porous Coatings | 22 |
| Beads | 14 |
| Plasma Spray | 3 |
| Fiber Mesh | 5 |
| Polyethylene Inserts | |
| Historical | 38 |
| Gamma Inert Sterilized | 10 |
| Carbon-Fiber Reinforced | 3 |
| Hylamer-M | 1 |
| Manufacturers | |
| Biomet (Warsaw, IN) | 4 |
| Depuy Synthes (Warsaw, IN) | 13 |
| Smith and Nephew (Memphis, TN) | 4 |
| Stryker (Mahwah, NJ) | 13 |
| Zimmer (Warsaw, IN) | 18 |
Data are presented as counts.
Identification of damage mechanisms
Prior to examination, the femoral components were first cleaned and disinfected in two consecutive soaks (20 minutes) with 1:10 ratio of detergent (Discide®; AliMed, Dedham, Massachusetts) to water solution. The femoral components were then placed in a sonicator for 20 minutes in water to remove any loose debris. Visual inspection (with the naked eye and/or up to 10X magnification with a stereomicroscope) of the femoral components revealed five predominant damage mechanisms that could result in the production of metal debris or the release of metal ions. Abrasive wear mechanisms included scratching (due to 3rd body wear debris particles) and complete wear-through of the polyethylene component resulting in metal-on-metal articulation between the femoral condyle and the tibial baseplate. Corrosive damage mechanisms that were noted included ICIC, MACC in modular tapers, and discoloration of the backside of the femoral component at the interface with the cement layer.
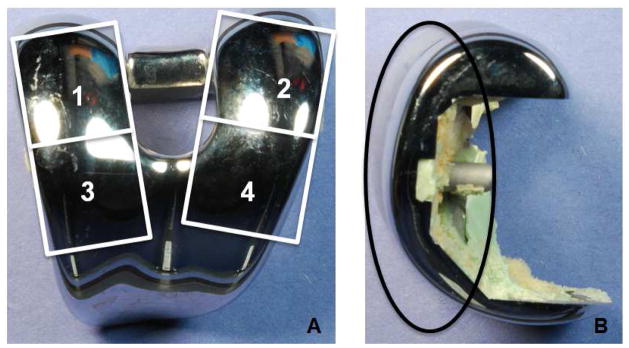
Third body abrasive wear was semi-quantitatively assessed by inspecting the articulating surfaces (the polyethylene insert and the CoCr femoral condyle) using a modified Hood method only inspecting for third body wear [9]. For the polyethylene insert, three independent observers inspected eight zones (four quadrants on each condyle) of the bearing surface for seven damage modes: burnishing, pitting, delamination, abrasion, embedded debris, scratching and surface deformation [9]. Any discrepancies between observers were resolved in a meeting among the investigators. For each zone and each type of damage, a score ranging from 0 to 3 was assigned, depending on the severity of damage of each damage mode. A score of 0 indicated no damage, a score of 1 indicated damage that covered less than 10% of available area, a score of 2 was given when a damage mode covered between 10 and 50% of the surface, and a score of 3 was given when a damage mode covered more than 50% of the surface [9]. Polyethylene failure was defined as full wear-through of the polyethylene insert allowing for metal-on-metal articulation. Similarly, the bearing surface of the CoCr femoral component was divided into quadrants and scored for damage by the same three investigators (figure 1). We assessed each quadrant of the bearing surface femoral components for 3 damage modes: scratching, indentations or lacerations, and pitting. A score of 1 described damage that covered less than 10% of the surface, a score of 2 described damage that was present over 10% – 30% of the surface, a score of 3 described damage that covered 30% – 50% of the surface and a score of 4 described damage that covered more than half of the surface.
Figure 1.
Each CoCr femoral component was evaluated using a semiquantitative scoring method for scratching and pitting. A) The bearing surface was split into posterior (numbers 1 and 2) and anterior (numbers 3 and 4) regions. The medial and lateral regions of the condyles were scored separately resulting in a total of 4 quadrants to describe the condition of the bearing surface. B) A side view of the primary bearing regions (enclosed within the black oval) that were evaluated.
To identify regions of ICIC damage, components were initially screened via visual inspection at low magnification by two investigators. Regions of interest were identified as having a frosted or discolored appearance. ICIC in these regions was subsequently confirmed using optical microscopy and scanning electron microscopy (SEM, Environmental Scanning Electron Microscope, XL30, FEI, Hillsboro, Oregon) by looking for characteristic features of ICIC. The affected area includes features that are interconnected and give the impression of a cell moving on the surface. These features can include a combination of circular crater-like morphologies, and irregular crater-like morphologies. These features were 10 – 100 microns in length, which is consistent with the size of a cell [6]. The extent of ICIC damage was estimated using digital photogrammetry [10]. The area affected by ICIC was confirmed using optical microscopy and outlined using a permanent marker. Images were taken using a digital SLR camera with a calibrated ruler in the same focal plane as the affected surface. Using commercial computer software (Adobe Photoshop, Adobe Systems Inc., San Jose, CA and GIMP 2.8.14), the affected area was digitally isolated. Using the known pixel dimensions, the affected area was calculated in mm2. If there were more than one region with ICIC on the implant, the regions were summed to obtain a cumulative affected area.
Adhered cement on nonporous devices was removed to evaluate the damage between the cement mantle and backside of the femoral components. To remove the cement, the femoral components were boiled in toluene for two to three hours depending on the cement thickness and quantity. After boiling in toluene, the femoral components were sonicated for 10 minutes to remove any residual cement. To ensure that this process did not cause damage to the current study’s implants, retrieved components without cement that were unrelated to this study, were subjected to the toluene boiling procedure. The implants were examined before and after boiling. There was no evidence of damage caused by the boiling procedure on these components. After boiling and sonication, the components were visually inspected for signs of corrosion, staining and discoloration [1, 2].
A fretting corrosion evaluation was conducted on CoCr femoral condyles that had a modular junction (n = 4). The junctions were visually inspected for evidence of fretting corrosion damage that was characterized using a modified semiquantitative score adapted from the Higgs-Goldberg method [3, 11]. Within this scoring system, a score of 1 was assigned when the damage was considered minimal which indicated fretting on less than 10% of the surface. A score of 2 was utilized to describe mild damage indicating fretting that occurred on more than 10% of the surface. The score of 3 was used to describe moderate damage. A score of 3 was given when fretting occurred on more than 30% of the implant surface with an aggressive local corrosion attack. Lastly, a score of 4 was used to describe severe describing fretting on more than 50% of the surface and a severe corrosion attack with abundant corrosion debris. The femoral components were independently evaluated by three experienced investigators. Any differences among these investigator’s damage scores were resolved in a conference, resulting in a final damage score for each component.
Statistical Analysis
Depending on the analysis, the data in this study both continuous and ordinal. For continuous variables, we determined that the data was not normal using the Shapiro-Wilk test. Therefore, we used nonparametric descriptive statistics for both ordinal and non-normal data. As such, we report the median and interquartile range throughout this study.
Results
Surface damage indicative of corrosion and/or wear of the CoCr femoral condyles was identified in 98% (n=51/52) of the femoral components. The three most prevalent damage modes were third-body wear, ICIC damage, and discoloration at the cement interface. Fifty-one of the 52 femoral components (98%; Figure 2) exhibited some third body wear, 15 femoral components (29%; Figure 2) had ICIC damage, and 9 of the femoral components (17%; Figure 2) exhibited wear-through of the polyethylene component resulting in metal-on-metal articulation. Eleven of the 27 (40%; Figure 2) nonporous-coated femoral condyles had evidence of cement interface degradation in the form of discoloration and 2 of the 4 (50%, Figure 2) modular femoral components had moderate to severe MACC taper damage (score ≥3). Third-body wear (primarily in the form of scratching on the CoCr femoral components) was the most prevalent damage mechanism (Fig 3). Mild damage was observed in 38% (n=20/52) of the femoral components (score of 1), moderate damage was observed in 37% (n=19/52) of the femoral components (score of 2) and severe damage was observed in 22% (n=12/52) of the femoral components (score of 3). The corresponding polyethylene damage scores for each quadrant of the bearing surface were predominantly a score of 3 out of a maximum score of 3 (n=34/38; 89%), indicating severe damage.
Figure 2.
Third-body wear (typically in the form of scratching, n=51/52), ICIC damage (n=15/52), and damage at the cement mantle-implant interface (n=11/27) were the most prevalent of the damage modes. Polyethylene wear through (n=9/52) and MACC taper damage (n=2/4) were also observed.
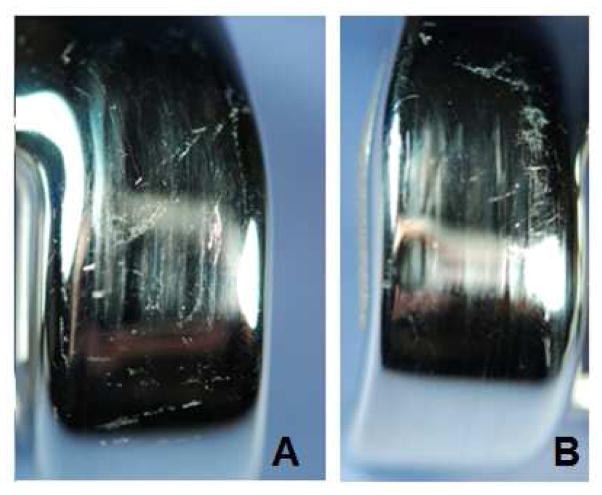
Figure 3.
Examples of severe third body damage and scratching on the bearing surface. A). Vertical plowing with erratic scratching throughout the left bearing surface. B). Aggressive plowing with erratic scratching toward the left of the bearing surface.
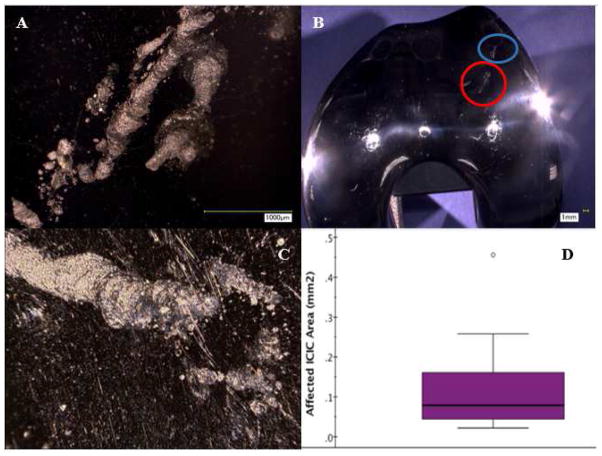
ICIC was identified on the bearing surface of 29% (n=15/52, Figure 4) of the CoCr femoral components. Suspected affected areas (Figure 4B) were observed up to 4000X magnification using digital optical microscopy (DOM) (figure 4A). The confirmed ICIC damage consisted of circular pits and indentations that were interconnected with a spiraling or trailing morphology consistent with cell movement on the surface (Fig. 4C). The median cumulative area with ICIC damage was 0.07 mm2 (interquartile range: 0.12 mm2, Fig. 4D).
Figure 4.
(A) High magnification digital photograph of the region circled in red in (B) revealing the circular damage scars that are associated with ICIC [6]. (B) Digital photography of a femoral condyle that illustrates the macro appearance of the ICIC affected area (circled in red and blue), which have a frosted appearance. (C) Three-dimensional stacked image created using digital photograph of ICIC damage in circled blue region. (D) The ICIC damage area varied among the femoral components. The median area that was affected by ICIC was 0.07 mm2 (interquartile range: 0.12 mm2).
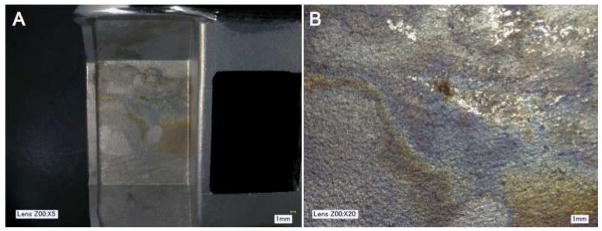
Twenty-seven of the femoral components did not have a porous backside, which allowed for optical observations of corrosion at the interface. Discoloration, staining, fretting scars, or blackened debris were observed in 11/27 femoral components (41%, Figure 5A and B).
Figure 5.
(A) Macro photograph of corrosion between the cement mantle and backside of a femoral component. (B) Digital micrograph of the damaged region, showing discoloration.
Discussion
The release of metal ions and debris has been identified in THA as a concern due to its association with adverse local tissue reactions in a subset of patients [1]. Although TKAs are fabricated from the same materials as THA, there is little in the literature describing damage to the CoCr femoral components in TKA. The purpose of this retrieval study was to investigate the prevalence of metal release in long-term implanted (> 15 years in vivo) TKAs. We found evidence of metal release or corrosive damage in 98% (n=51/52) of the femoral components in this study. The results of this study indicate that the most prevalant forms of ion or metalic debris release were of third body wear (present in 98% of the components, n=51/52), ICIC damage (present in 29% of the components, n=15/52), and damage at the cement mantle interface in non porous implants (present in 41% of the components, n=11/27). Additionally, polyethylene wear-through was observed (present in 17% of the components, n=9/52), and MACC taper damage in 50% of the modular implants (n=2/4).
There were limitations in this study. Only CoCr femoral components that were implanted for greater than 15 years were included in this study, and therefore, the incidence of these damage mechanisms in short-term components remains unclear. We chose implants that were in vivo for an extended period of time because we reasoned that these implants would be most likely to have evidence of damage and/or corrosion. However, due to the long-term duration of these TKA systems, all but 14 of the tibial inserts were fabricated from historical gamma air sterilized polyethylene (with the remaining 3 components being fabricated from carbon fiber reinforced polyethylene, 10 components being fabricated from gamma inert sterilized polyethylene and 1 being fabricated from Hylamer-M polyethylene). Given the improved wear and oxidative properties of newer polyethylene formulations, polyethylene wear-through is less likely to appear with contemporary gamma inert sterilized and highly crosslinked polyethylenes. Additionally, the damage scoring methods used in this study were semi-quantitative in nature. Although these methods do not help elucidate the volume of material lost, they can be effective in describing the extent of the damage and can be useful for qualitative comparisons to previous studies that used similar techniques. Lastly, all of these femoral components were retrieved components, restricting the cohort to only represent a population of implants that were revised or removed. However, in the absence of availability of implants recovered at autopsy, analysis of retrieved components remains the primary method by which to gain insight into the in vivo performance joint replacements.
In this study, the most prevalent damage mechanism was scratching on articulating surface possibly caused by third-body debris (ie, bone chips, bone cement, metallic debris, or carbides). A simulator study suggested that scratching induces a rougher surface that leads to faster wear of the polyethylene components [12]. In addition, Kretzer et al. [13] performed a simulator study using all polymer bearings within the simulator allowing the resultant metallic wear to be solely from the CoCr implants. This study was performed in the absence of third body debris. The study reported polyethylene (7.28±0.27 mg/106 cycles), and metallic (1.63±0.28 mg for cobalt, 0.47±0.06 mg for chromium, 0.42±0.06 mg for molybdenum and 1.28±0.14 mg for titanium) wear, reporting 12% of the wear weight as metallic debris [13]. The absence of third body debris made the authors speculate that this wear could be due to carbides removed from the bulk alloy[13]. Additionally, retrieval studies have shown that a relationship exists between third body particles, increased polyethylene wear, and increased roughness of the CoCr femoral condyle [7, 8]. In the current study, we observed 9 cases of complete wear-through of the polyethylene insert. However, the polyethylenes utilized in these explants were either gamma air sterilized unfilled polyethylene or carbon fiber reinforced polyethylene. Both of these materials are susceptible to oxidative degradation [14–16], which can accelerate the wear processes of polyethylene. Thus, it is unclear if the polyethylene failures were due to oxidative degradation, third body wear of the condyles, or a combination of the two processes. Future research investigating the effect of articulating CoCr femoral component roughness against wear and oxidative resistant materials (e.g. gamma inert sterilized UHMWPE or highly cross-linked polyethylene) should further elucidate the impact of scratching on the wear performance of polyethylene tibial inserts.
ICIC is a phenomenon that, up until recently [6], has not been reported in the literature. In this study, we observed evidence of ICIC on 29% (n=15/52) of the long-term TKA femoral components. In a study of metal-on-polyethylene THAs, metal-on-metal hips, and knee components, Gilbert et al. [6] observed ICIC on 74% (n=51/69) of the components studied. However, they did not break down the prevalence of ICIC by implant type so it is difficult to compare directly to the current study. Additionally, 11 of the components in that study were revised for ALTRs, whereas we had no documented instances of ALTR in our study. This may explain some of the differences in the prevalence of ICIC observed between the two studies. Although evidence of ICIC was observed in nearly a third of the long-term knees in our collection, the damage did not cover a large area. We estimated that ICIC damage covered 0.11 ± 0.12 mm2 (Range: 0.01–0.46mm2) of surface area. This is a small percentage of the surface area of a femoral condyle that is typically on the order of thousands of square millimeters. Gilbert et al. [6] did not report the total affected area, but they did report that the size of the cells attacking the surface as between 20 to 300 μm, with potentially larger giant cells. At this time, it is unknown how ICIC affects clinical outcomes or whether devices with larger amounts of ICIC fare poorer. However, given the prevalence observed in long-term implants, more studies should be conducted to fully understand the complex interaction between metallic bearing surfaces and inflammatory cells. In addition to ICIC, we observed MACC in 50% (2 of 4) of the CoCr femoral components in this series. Due to the limited sample size of components with modular stems in the study, it is difficult to draw conclusions from this finding.
One other source of degradation identified in this study was damage at the backside interface of the femoral component and the cement layer. This was observed in 41% (n=11/27) of the retrievals in this study. Although there is little in the literature on this damage in TKA, several studies have looked at damage at the cement mantle interface in THA. Recently Bryant et al. [2] conducted a study on retrieved THA stems to characterize failure mechanisms, one being damage between the cement mantle and femoral stem. Initial spot energy-dispersive spectroscopy (EDS) analysis revealed debris collected on the cement particles with a Cr2O3 composition. EDS mapping also identified areas rich in Cr, O, and N, compared with clean bulk material [2]. The observation of Cr rich oxides are products of fretting corrosion and are indicative of fretting corrosion of CoCr alloys. In a study of twenty-five cobalt-alloy femoral THA femoral components Gilbert et al. [1] reported migrated foreign body particles with the composition of CrPO4. The particles elicited a foreign-body tissue response through fibrosis, necrosis and giant cells [1]. Due to the non-destructive nature of the current study, EDS measurements were not available as the femoral components were too large to fit into the experimental chamber of our institution’s SEM. However, we did observe discoloration that is indicative of corrosion (Figure 4B). More research is required to understand the composition of these oxide films. However, the clinical consequences of this damage are not clear, as it is unknown if the corrosion products from the cement-implant interface will be liberated and cause a foreign body tissue reaction in TKA.
In summary, this study tracked the prevalence of five major damage mechanisms in long-term (in vivo >15 years) TKA femoral components that may lead to release of metal ions or debris. We observed the presence of both abrasive (scratching and third body wear) and/or corrosive degradation processes (fretting corrosion indicated by discoloration, inflammatory cell induced corrosion, and mechanically assisted crevice corrosion) in 98% of the explants in this retrieval cohort, n=51/52. The clinical implications of these findings are as yet unclear as the devices were not revised for adverse local tissue reactions or biological reactions to CoCr. However, 6 of the 52 patients had reported observations of metallosis that was characterized as dark staining of the tissue surrounding the implant. Additionally, some cases of loosening or pain may have been due to debris but was not recognized as such. Therefore, surgeons should be aware of these damage mechanisms that may affect the performance of TKA systems.
Supplementary Material
Footnotes
This work was performed at the Implant Research Center at Drexel University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobs JJ, Gilbert JL, Urban RM. Current Concepts Review-Corrosion of Metal Orthopaedic Implants*. The Journal of Bone & Joint Surgery. 1998;80(2):268. doi: 10.2106/00004623-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Bryant M, Ward M, Farrar R, Freeman R, Brummitt K, Nolan J, Neville A. Characterisation of the surface topography, tomography and chemistry of fretting corrosion product found on retrieved polished femoral stems. Journal of the mechanical behavior of biomedical materials. 2014;32:321. doi: 10.1016/j.jmbbm.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clinical orthopaedics and related research. 2002;401:149. doi: 10.1097/00003086-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Black J. Requirements for successful total knee replacement. Material considerations. The Orthopedic clinics of North America. 1989;20(1):1. [PubMed] [Google Scholar]
- 5.Arnholt C, MacDonald DW, Tohfafarosh M, Gilbert JL, Rimnac CM, Kurtz SM, Klein G, Mont MA, Parvizi J, Cates HE. Mechanically assisted taper corrosion in modular TKA. The Journal of Arthroplasty. 2014 doi: 10.1016/j.arth.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert JL, Sivan S, Liu Y, Kocagöz SB, Arnholt CM, Kurtz SM. Direct in vivo inflammatory cell-induced corrosion of CoCrMo alloy orthopedic implant surfaces. Journal of Biomedical Materials Research Part A. 2014 doi: 10.1002/jbm.a.35165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clinical orthopaedics and related research. 1994;299:31. [PubMed] [Google Scholar]
- 8.Hirakawa K, Bauer TW, Yamaguchi M, Stulberg BN, Wilde AH. Relationship between wear debris particles and polyethylene surface damage in primary total knee arthroplasty. The Journal of arthroplasty. 1999;14(2):165. doi: 10.1016/s0883-5403(99)90120-1. [DOI] [PubMed] [Google Scholar]
- 9.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. Journal of biomedical materials research. 1983;17(5):829. doi: 10.1002/jbm.820170510. [DOI] [PubMed] [Google Scholar]
- 10.Grochowsky J, Alaways L, Siskey R, Most E, Kurtz S. Digital photogrammetry for quantitative wear analysis of retrieved TKA components. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;79(2):263. doi: 10.1002/jbm.b.30537. [DOI] [PubMed] [Google Scholar]
- 11.Higgs GB, Hanzlik JA, MacDonald DW, Gilbert JL, Rimnac CM, Kurtz SM. Is Increased Modularity Associated With Increased Fretting and Corrosion Damage in Metal-On-Metal Total Hip Arthroplasty Devices?: A Retrieval Study. The Journal of arthroplasty. 2013;28(8):2. doi: 10.1016/j.arth.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muratoglu OK, Burroughs BR, Bragdon CR, Christensen S, Lozynsky A, Harris WH. Knee simulator wear of polyethylene tibias articulating against explanted rough femoral components. Clinical orthopaedics and related research. 2004;428:108. doi: 10.1097/01.blo.0000143801.41885.8b. [DOI] [PubMed] [Google Scholar]
- 13.Kretzer JP, Reinders J, Sonntag R, Hagmann S, Streit M, Jeager S, Moradi B. Wear in total knee arthroplasty—just a question of polyethylene? International orthopaedics. 2014;38(2):335. doi: 10.1007/s00264-013-2162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medel F, Kurtz SM, Klein G, Levine H, Sharkey P, Austin M, Kraay M, Rimnac CM. Clinical, surface damage and oxidative performance of Poly II tibial inserts after long-term implantation. Journal of long-term effects of medical implants. 2008;18(2):151. doi: 10.1615/jlongtermeffmedimplants.v18.i2.40. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz SM, Rimnac CM, Hozack WJ, Turner J, Marcolongo M, Goldberg VM, Kraay MJ, Edidin AA. In vivo degradation of polyethylene liners after gamma sterilization in air. Journal of Bone and Joint Surgery-American Volume. 2005;87A(4):815. doi: 10.2106/JBJS.D.02111. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz SM, Hozack WJ, Purtill JJ, Marcolongo M, Kraay MJ, Goldberg VM, Sharkey PF, Parvizi J, Rimnac CM, Edidin AA. Otto Aufranc Award paper - Significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin Orthop Relat R. 2006;(453):47. doi: 10.1097/01.blo.0000246547.18187.0b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.