Abstract
In order to summarize the principal findings on risk and protective factors for childhood asthma, we retrieved systematic reviews on these topics in children (ages 1 to 18 years), up to January 2016, through MEDLINE, EMBASE, CINAHL, SCOPUS and CDSR. Two hundred twenty seven studies were searched from databases. Among those, 41 systematic reviews (SRs) were included: 9 focused on prenatal factors, 5 on perinatal factors, and 27 on postnatal factors. Of these 41 SRs, 83% had good methodological quality, as determined by the AMSTAR tool. After reviewing all evidence, parental asthma, prenatal environmental tobacco smoke and prematurity (particularly very preterm birth) are well-established risk factors for childhood asthma. Current findings do suggest mild to moderate causal effects of certain modifiable behaviors or exposures during pregnancy (maternal weight gain or obesity, maternal use of antibiotics or paracetamol, and maternal stress), the perinatal period (birth by Caesarean delivery), or postnatal life (severe RSV infection, overweight or obesity, indoor exposure to mold or fungi, and outdoor air pollution) on childhood asthma, but this suggestive evidence must be confirmed in interventional studies or (if interventions are not feasible) well-designed prospective studies.
Keywords: asthma, wheeze, children, meta-analysis, protective factors, risk factors
INTRODUCTION
Over the last three decades, childhood asthma became a major public health problem worldwide, particularly in industrialized nations. This rapid rise in asthma burden can only be explained by changes in environment or lifestyle.1
A justifiable interest in understanding the etiology of the “asthma epidemic” has led to publication of hundreds of articles on environmental risk factors for childhood asthma in recent years. Attempts to synthesize the results of these studies have in turn led to systematic reviews, which can be defined as scientific investigations with pre-planned methods and a collection of primary studies as their “subjects”, using strategies to identify bias and random error.2 However, reading around 50 published systematic reviews of risk factors for childhood asthma would be both challenging and time-consuming. Thus, a summary and interpretation of these systematic reviews could be helpful to researchers, clinicians and public health practitioners interested in asthma in children.
The objective of this article is to synthesize and critically examine the main findings of systematic reviews of risk or protective factors for childhood asthma, organized according to their presence or predominance in the prenatal, perinatal and postnatal periods of life.
We identified studies published in the MEDLINE, EMBASE, CINAHL, and SCOPUS databases up to January 2016, using the terms: “((risk factors OR protective factors) AND (asthma or wheeze*) AND (child*) AND (meta-analysis)).” No language restriction was employed; if a study was published in more than one language, the latest version was chosen. To be included in this review, all studies also had to: 1) be systematic reviews with a meta-analysis of observational or interventional studies of risk or protective factors for childhood asthma, and 2) focus on children (ages 1 to 18 years) or (if children and adults were included) report a separate analysis in children. Studies were excluded if they: 1) were published solely in abstract form , 2) focused on risk factors specific to a country, geographic region, or particular study group (e.g. ISAAC, ENRIECO, GA2LEN), or 3) focused on genetics or pharmacologic treatments for asthma.
Data Extraction and Assessment of Risk of Bias
Titles, abstracts, and citations for studies meeting the inclusion criteria outlined above were independently analyzed by two authors (J.C.R. and E.F.). Full texts of all studies were then evaluated for eligibility, and the methodological quality of the eligible systematic reviews was assessed using the AMSTAR (Assess Systematic Reviews) tool.3 The AMSTAR tool has a maximum of 11 points; a systematic review assigned 8 or more points is deemed of good quality. Disagreements between the two reviewers were discussed and resolved by consensus.
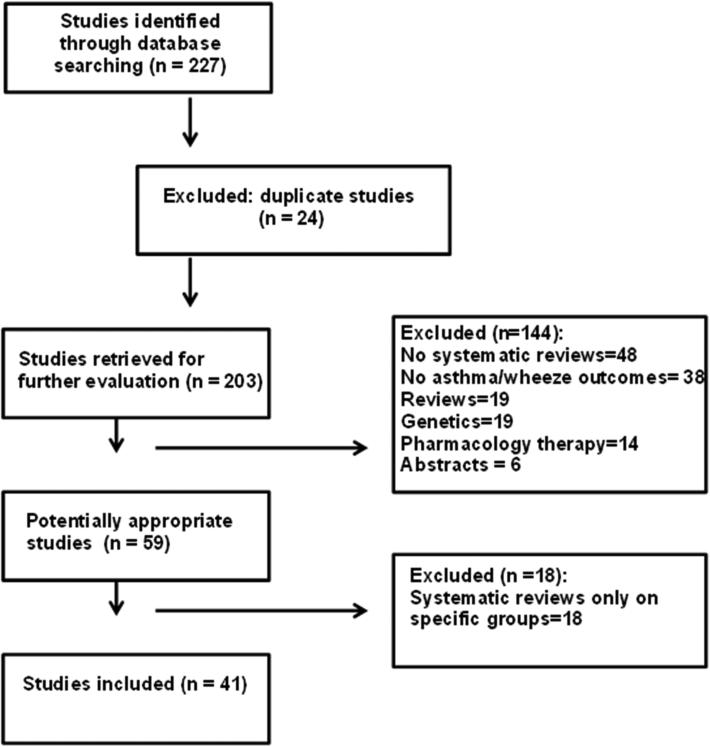
Of the two hundred and twenty seven studies retrieved from the databases (Figure 1), 41 studies were included in this review. Of these 41 studies, 9 examined familial or prenatal factors; 5 examined perinatal factors; and 27 examined post-natal factors. The quality of their methodology (assessed using the AMSTAR tool) is shown in Table 1 (83% were of good quality, receiving ≥8/11 points).
Figure 1.
Study selection flowchart.
Table 1.
Evaluation of the quality (using the AMSTAR tool3) of the 41 systematic reviews included.
| Ref./AMSTAR's Questions | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lim et al.[4] | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y |
| Forno et al. [5] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Crider et al. [6] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Nurmatov et al. [7] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Beckhaus et al. [8] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Van de Loo et al. [9] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Zhao et al. [10] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Eyers et al. [11] | Y | Y | Y | Y | N | Y | CA | CA | Y | Y | N |
| Cheelo et al. [12] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Burke et al. [13] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | CA |
| Thavagnanam et al. [14] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N |
| Jaakkola et al. [15] | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y |
| Been et al. [16] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Mu et al. [17] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Das & Naik [18] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| El-Zein et al. [19] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Gdalevich et al. [20] | Y | Y | N | Y | N | Y | Y | Y | Y | N | N |
| Brew et al. [21] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Dogaru et al. [22] | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y |
| Lodge et al. [23] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Muley et al. [24] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Yang et al. [25] | Y | Y | Y | CA | Y | Y | CA | CA | Y | Y | Y |
| Garcia-Marcos et al. [26] | Y | Y | Y | Y | Y | Y | N | CA | Y | N | Y |
| Seyedrezazadeh et al. [27] | Y | Y | Y | CA | Y | Y | Y | Y | CA | N | Y |
| Elazad et al. [28] | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y |
| Zuccotti et al. [29] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Thakkar et al. [30] | Y | N | Y | Y | N | Y | Y | Y | N | N | Y |
| Marra et al. [31] | Y | Y | Y | CA | Y | Y | Y | Y | Y | Y | N |
| Murk et al. [32] | Y | Y | N | Y | N | Y | Y | Y | Y | Y | Y |
| Regnier et al. [33] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Maas et al. [34] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Fisk et al. [35] | Y | N | N | Y | N | N | Y | Y | N | Y | Y |
| Tischer et al. [36] | Y | N | N | Y | N | Y | Y | Y | Y | Y | Y |
| Mendy et al. [37] | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y |
| Genuneit [38] | Y | CA | Y | Y | Y | Y | Y | CA | Y | Y | N |
| Gasana et al. [39] | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y |
| Jaakkola & Knight [40] | Y | CA | CA | CA | Y | Y | N | CA | Y | NA | Y |
| Vork et al. [41] | Y | CA | Y | Y | N | Y | CA | CA | Y | N | CA |
| Lieberoth et al. [42] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Flaherman et al. [43] | Y | N | N | Y | Y | Y | N | N | Y | Y | Y |
| Egan et al. [44] | Y | N | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| Chen et al. [45] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
AMSTAR= Asses Systematic Reviews, Ref. =reference, Y= yes, N=no, CA=cannot answer, NA= not applicable.
AMSTAR's Questions: 1. Was an ‘a priori” design provided? 2. Was there duplicate study selection and data extraction? 3. Was a comprehensive literature search performed? 4. Was the status of publication (i.e., grey literature) used as an inclusion criterion? 5. Was a list of studies (included and excluded) provided? 6. Were the characteristics of the included studies provided? 7. Was the scientific quality of the included studies assessed and documented? 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? 9. Were the methods used to combine the findings of studies appropriate? 10. Was the likelihood of publication bias assessed? 11. Was the conflict of interest included?
Table 2 summarizes the main findings of systematic reviews of risk or protective factors for asthma or wheeze. For each meta-analysis, Table 2 shows the pooled odds ratio (OR) and its 95% confidence interval (CI), and the I2 statistic or Cochran chi-square heterogeneity test Q for heterogeneity across studies. A two-sided P <0.01 for the Cochran chi-square indicates significant heterogeneity; for the I2 statistic, cut-off values of 0%, 25%, 50% and 75% were used for no, low, moderate, and high heterogeneity, respectively.
| Authors | # studies | study design | total # subjects | OR or RR [95%CI] for meta-analysis | heterogeneity |
|---|---|---|---|---|---|
| Familial or prenatal factors | |||||
| Lim4 | 33 | 25 PBC, 8 CC | 254820 | OR=3.04 [2.59-3.56] for maternal asthma and asthma in children; OR= 2.44 [2.14-2.79] for paternal asthma and asthma in children | Q stat p=0.13; p=0.06 |
| Forno5 | 14 | 14 O | 108321 | OR= 1.21 [1.07-1.37], p=0.003 for maternal overweight/obesity in pregnancy and current asthma/wheeze in children | I2=69.1 % |
| Crider6 | 5 | 3 C, 2 NCC | 45642 | RR=1.01 [0.78-1.30], p=0.95 for prenatal folate exposure and childhood asthma | I2=0% |
| Nurmatov7 | 62 | 21 C, 15 CC, 26 CS | NR | OR=0.25 [0.10-0.40], p=0.002 for high vitamin A level and childhood asthma; OR=0.68 [0.52-0.88], p=0.004 for high maternal vitamin E intake during pregnancy and childhood asthma; OR=0.56 [0.42-0.73], p<0.001 for high maternal vitamin D intake during pregnancy and childhood asthma; OR=0.75 [0.60-0.94], p=0.01 for high fruit intake and childhood asthma | I2=0%; I2=0%; I2=15.8%; I2=66%; |
| Beckhaus8 | 32 | 29 PC, 3 RC | 123032 | OR=0.58 [0.38-0.88], p=<0.01 for higher maternal vit D and childhood wheeze; OR=0.54 [0.41-0.71], p=<0.0001 for higher maternal vit E and childhood wheeze; OR=0.57 [0.41-0.81] p=0.002 for higher maternal zinc intake and childhood wheeze | I2=59%; I2=0%; I2=0% |
| Van de Loo9 | 10 | 8 C, 1 CC, 1 CS | 3210204 | OR=1.45 [1.25-1.68] for maternal stress and childhood asthma; OR=1.87 [1.42-2.45] for maternal stress and childhood wheeze | I2=13%; I2=76% |
| Zhao10 | 10 | 7 C, 3 CC | 16610 | OR=1.20 [1.13-1.27] for maternal antibiotics exposure and childhood asthma/wheeze | I2=83% |
| Eyers11 | 6 | 5 PC, 1 CS | 28038 | OR=1.21 [1.02-1.44] for exposure to paracetamol during pregnancy and wheezing | I2=76% |
| Cheelo12 | 11 | 9 PC, 1 RC | 921529 | OR=1.39 [1.01-1.91]; OR=1.17 [1.04-1.31]; and 1.49 [1.37-1.63] for exposure to paracetamol during 1st; 3rd and 2nd-3th trimester, respectively; and childhood asthma | I2=64%; I2=0%; I2=0% |
| Burke13 | 79 | 79 PC | NR | OR=1.41 [1.20-1.67]; OR=1.28 [1.14-1.44]; and OR= 1.52 [1.23-1.87] for prenatal maternal smoking and wheeze in children age ≤ 2 years; 3-4 years; and 5-18 years old, respectively | I2=85.2%; I2=65.6%; I2=21.1% |
| OR= 1.70 [1.24-2.35]; OR= 1.65 [1.20-2.28]; and OR= 1.18 [0.99-1.40] for postnatal maternal smoking and wheeze in children age ≤ 2 years; 3-4 years; and 5-18 years, respectively | I2=0%; I2=48.5%; I2=19.4% | ||||
| OR=1.35 [1.10-1.66]; OR=1.06[0.88-1.27]; and OR=1.32 [1.12-1.55] for household ETS exposure and wheeze in children age ≤ 2 years; 3-4 years; and 5-18 years, respectively | I2=64.5%; I2=54.4%; I2=0% | ||||
| Perinatal factors | |||||
| Thavagnanam14 | 23 | 22 C, 1 CC | 1206679 | OR=1.22 [1.14-1.29], p<0.001 for birth by Caesarean section and childhood asthma | I2=46% |
| Jaakkola15 | 19 | 13 C, 5 CS, 1 CC | 456651 | OR=1.074 [1.07-1.08], p<0.001 for preterm delivery and childhood asthma | Q stat (p=0.001) |
| Been16 | 17 | 7 RC, 5 PC, 5 CS | 874710 | aOR=1.46 [1.29-1.65], p<0.001 for preterm (<37 wk) delivery and childhood wheeze; aOR=2.81 [2.55-3.12], p<0.001 for very preterm (<32 wk) delivery and childhood wheeze | I2=80%; and I2=0% |
| Mu 17 | 17 | 15 C, 2 CS | 38115 | OR=1.28 [1.09-1.50], p<0.003 for low birth weight as compared with birth weight >2,500g and childhood asthma; OR=1.34 [1.13-1.60], p<0.001 for low birth weight as compared to birth weight 2500-4000 g and childhood asthma | I2=51%; I2=62% |
| Das & Naik18 | 7 | 6 R, 1 C | 101499 | OR=4.26 [4.04-4.5], p<0.0001 for neonatal hyperbilirubinemia and childhood asthma; OR=3.81 [3.53-4.11], p<0.0001 for neonatal phototherapy (bilirubin >15 mg/dl) and childhood asthma | I2=0%; I2=26% |
| Postnatal factors | |||||
| El-Zein19 | 16 | 7 RC, 5 CS, 3 CC, 1 PC | 67179 | OR=0.86 [0.79-0.93] for BCG vaccination and childhood asthma | I2=0% |
| Gdalevich20 | 12 | 12 PC | 8183 | OR=0.70 [0.60-0.81] for exclusive breast-feeding during first 3 months and childhood asthma | I2=11.3% |
| Brew21 | 31 | 15 LBC, 10 CS, 6 CC | 417880 | OR=0.92 [0.86-0.98], p=0.008 for any breast-feeding and current wheeze in children; OR=1.10 [1.00-1.22], p=0.05 for any breast-feeding and childhood asthma | I2= 40%; I2=4% |
| Dogaru22 | 113 | 57 C, 47 CS, 13 CC | 832013 | OR= 0.76 [0.67-0.86] for breastfeeding (more vs. less) and current asthma in children; OR=0.81 [0.76-0.87] for breastfeeding (more vs. less) and current wheezing in children | I2=91.6%; I2=86.6% |
| Lodge23 | 42 | 23 C, 17 CS, 2 CC | 391238 | OR=0.90 [0.84-0.97] for breastfeeding (more vs. less) and childhood asthma | I2=63% |
| Muley24 | 5 | 5 RCT | 1932 | OR= 0.97 [0.65-1.47], p=0.9 for dietary omega-3-fatty acid supplementation on childhood asthma incidence | I2=52.2% |
| Yang25 | 4 | 4 C | 12481 | OR=0.76 [0.61-0.94] for fish intake in infancy and childhood asthma incidence; OR=0.71 [0.42-0.96] for children whose mothers had high levels of n3PUFA in breast milk and childhood asthma incidence | I2=11.5%; I2=0% |
| Garcia-Marcos26 | 8 | 8 CS | 39804 | OR= 0.85 [0.75-0.98], p=0.02 for Mediterranean diet adherence in children and current wheezing; OR=0.86 [0.78-0.95], p=0.004 for Mediterranean diet adherence in children and asthma ever | Q stat p=0.24; p=0.98 |
| Seyedrezazadeh27 | 14 | 13 CS, 1 C | 346615 | RR= 0.81 [0.74–0.88]; for high intake of fruits and childhood wheeze; RR= 0.88 [0.79-0.97] for high intake of vegetables and childhood wheeze | I2=83.1%; I2=83.7% |
| Elazad28 | 5 | 5 RCT | 3257 | RR=0.99 [0.81-1.21], p=0.92 for probiotics supplementation during pregnancy and childhood asthma | I2=0% |
| Zuccotti29 | 8 | 8 RCT | 1890 | RR=0.99 [0.77-1.27], p=0.95 for probiotics infants supplementation and childhood asthma; RR=0.91 [0.67-1.23], p=0.76 for probiotics infants supplementation and wheezing | NR |
| Thakkar30 | 5 | 4 PC, 1 PB | 2824 | OR= 5.6 [4.3-6.9] for gastroesophageal reflux prevalence and childhood asthma | NR |
| Marra31 | 8 | 5 RC, 3 PC | 12082 | OR= 2.05 [1.41-2.99] for antibiotic exposure during first year of life in all studies and incident of childhood asthma; OR=1.12 [0.88-1.42] for antibiotic exposure during first year of life in prospective studies and incident of childhood asthma | Q stat p<0.01 |
| Murk32 | 22 | 8 RC, 8 DB, 6 PC | 685820 | OR=1.52 [1.30-1.77], p<0.001 for antibiotic exposure during pregnancy or in the first year of life and childhood asthma | I2=95% |
| Regnier33 | 15 | 6 L | 82008 | OR=3.84 [3.23-4.58], p=0.03 for RSV hospitalization in early life and incidence of asthma/wheeze | I2=45% |
| Mass34 | 9 | 9 LBC | 3271 | OR=0.72 [0.54-0.96], p=0.02 for multifaceted intervention reducing inhalant & food allergens before 5 years of age and childhood asthma; OR=0.52 [0.32-0.85], p=0.009 for multifaceted intervention reducing inhalant & food allergens after 5 years of age and childhood asthma | I2=0%; I2=0% |
| Fisk35 | 17 | NR | NR | OR=1.53 [1.39-1.68] for presence of dampness & mold in home and wheezing; OR= 1.56 [1.30–1.86] for presence of dampness & mold in home and current asthma | NR |
| Tischer36 | 61 | 29 CS, 16 CC, 16 C | 203798 | OR=1.49 [1.28-1.72] for visible mold exposure and childhood asthma; OR=1.68 [1.48–1.90] for visible mold exposure and wheeze | NR |
| Mendy37 | 19 | 10 C, 8 CS, 1 CC | 13793 | OR=1.48 [1.10-1.98] for early endotoxin exposure and wheeze at infants/toddlers; OR=0.82 [0.69-0.97] for early endotoxin exposure and asthma at school-age | I2=46.2%; I2=0% |
| Genuneit38 | 29 | 29 LBC | 130428 | OR=0.77 [0.60-0.99] for living in a farm residence and childhood asthma | I2= 68% |
| Gasana39 | 19 | 10 CS, 9 C | 163597 | OR=1.05 [1.0-1.11]; 1.02 [1.0-1.04] and 1.06 [1.01-1.12] for NO2, N2O and CO exposure and childhood asthma prevalence, respectively. OR=1.04 [1.01-1.07] for SO2 exposure and prevalence of wheezing in children, and OR=1.05 (1.04-1.07) for PMs exposure in incidence of wheezing in children | I2=0%; I2=0%; I2=37.7%. I2=16.5% I2=0% |
| Jaakola&Knight 40 | 5 | 3 CS, 2 CC | 20899 | OR=1.55 [1.18-2.05] for PVC surface materials at home and childhood asthma | Q stat p=0.64 |
| Vork41 | 8 | 8 C | 25167 | aRR=1.33 [1.41-1.36] for exposure to secondhand tobacco and childhood asthma incidence | NR |
| Lieberoth42 | 7 | 4 RC, 3 PC | 22859 | OR=1.37 [1.15-1.64], p=0.0005 for early menarche (<12 years) and childhood asthma | I2=55% |
| Flaherman43 | 12 | 8 PC, 4 RC | 121386 | RR=1.50 [1.2-1.8] for BMI ≥ 85 percentile and childhood asthma | NR |
| Egan44 | 6 | 6 PC | 25374 | RR=1.35 [1.15-1.58], p=0.0002 for overweight (BMI ≥ 85 percentile) and childhood asthma; RR=1.50 [1.22-1.83], p<0.001 for obese (BMI >95 percentile) and childhood asthma | I2=2%; I2=44% |
| Chen45 | 6 | 6 PC | 18760 | RR=1.19 [1.03-1.37], p=0.02 for overweight and childhood asthma incidence; and RR=2.02 [1.16-3.50], p=0.01 for obesity and childhood asthma incidence | NR |
BMI= body mass index; C=case control; C=cohort; CS= cross-sectional; DB=data-base; ETS= environmental tobacco smoke; L=longitudinal; LBC= longitudinal birth-cohort; NCC= nasted case-control;; NR=not reported; O=observational; PMs= particulate matters; PB= population based; PBC= population-based cohort; PC= prospective cohort; PVC= polyvinyl chloride; PUFA= polyunsaturated fatty acids; RCT= randomized clinical trial; RC= retrospective cohort; RSV= respiratory syncytial virus; TS= time-series
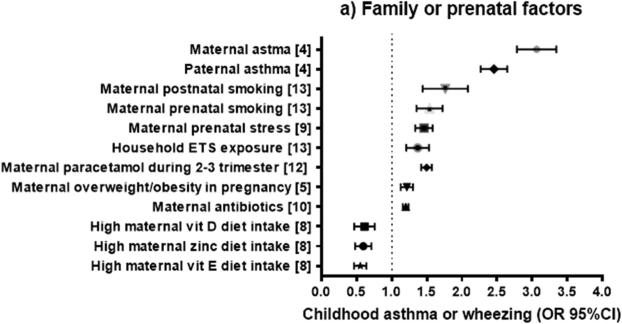
FAMILIAL OR PRENATAL FACTORS (Figure 2a)
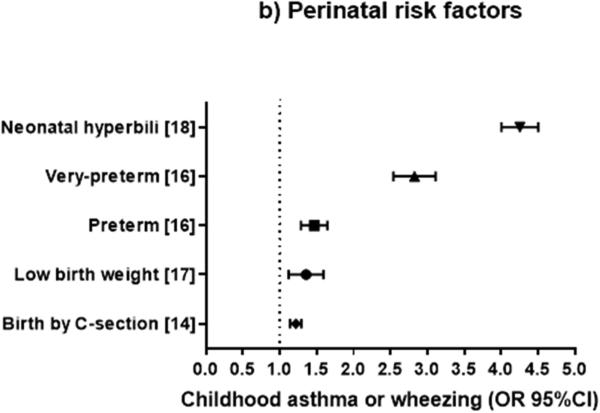
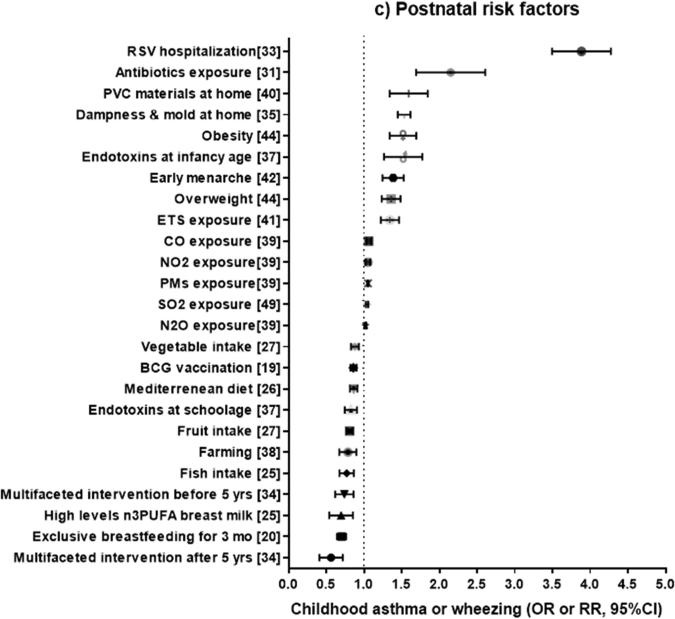
Figure 2.
Odds ratio (OR) or relative risk (RR) (and 95% confidence intervals) for childhood asthma or wheeze from systematic reviews of familial or prenatal factors (2a), perinatal factors (2b), and postnatal factors (2c). The OR or RR were chosen for asthma if available, or for current wheezing otherwise. When two ORs or RRs existed for the same outcome, we selected the highest estimate for risk factors and the lowest estimate for protective factors.
Parental asthma
A meta-analysis of 33 studies showed that children with maternal asthma (defined as self-reported physician-diagnosed asthma or as self-reported ever asthma) had approximately threefold greater odds of asthma than those without maternal asthma, with no significant heterogeneity4. Moreover, children with paternal asthma had 2.4 times higher odds of asthma than those without paternal asthma, with no significant heterogeneity. Compared with paternal asthma, maternal asthma was associated with significantly greater odds of childhood asthma (P= 0.04). However, there was no significant difference in the magnitude of the effect estimates between maternal (OR=2.85) and paternal (OR=2.48) asthma in an analysis restricted to 18 studies in which parental asthma was diagnosed by a physician (P=0.37). Similarly, there was no significant difference in effect estimates between maternal asthma (OR=3.15) and paternal asthma (OR=2.60) in an analysis restricted to children 5 years and older (P= 0.14).
Maternal weight gain and obesity during pregnancy
Forno et al.5 conducted a meta-analysis of 14 studies of gestational obesity (n=12) or weight gain (n=5) during pregnancy and childhood asthma, with an aggregate sample size of 108,321 mothers whose children were followed up to an age ranging from 14 months to 16 years. In this meta-analysis, maternal overweight or obesity during pregnancy was significantly associated with 1.21 times increased odds of current asthma or wheeze in participating children, with moderate heterogeneity across studies. Moreover, each 1-kg/m2 increment in maternal body mass index (BMI) was significantly associated with 2%-3% increased odds of childhood asthma. In that study the association between maternal obesity and childhood asthma was stronger in studies with lower prevalence of maternal asthma; other factors including maternal age or the child's age at follow up did not modify the effect size.
Maternal folate or vitamin D status during pregnancy
Crider et al.6 performed a systematic review of five studies (including 45,642 mother-child pairs) of prenatal folate or folic acid use and childhood asthma. In that meta-analysis, there was no significant association between maternal intake of folic acid supplements before the second trimester of pregnancy and asthma at school age (range: 5.5 to 7 years), with no heterogeneity across studies. Because of substantial heterogeneity in exposures and outcomes, no aggregate effect estimates could be generated for other indicators of folate status (e.g. serum folate). However, most primary effect estimates showed no significant association between folate status and asthma.
Maternal diet and maternal dietary intake of nutrients or vitamins during pregnancy
Nurmatov et al. 7 examined 62 studies of diet, vitamins or nutrients and asthma, wheeze or atopic disorders in children. Meta-analyses of 3-4 birth cohort studies showed that high maternal dietary intake of vitamin D and vitamin E during pregnancy was significantly associated with 44% and 32% reduced odds of wheezing outcomes, respectively, in early childhood (at ages 2 to 5 years). Maternal intake of vitamin D was not significantly associated with asthma at age 5 years. Results from other meta-analyses of a few birth cohorts were not supportive of prenatal effects of vitamin C, zinc or selenium on childhood wheeze.
More recently, Beckhaus and colleagues8 examined 32 studies of maternal diet (including diverse nutrients, food groups, and dietary patterns) during pregnancy and childhood asthma. In that meta-analysis, higher maternal intake of vitamin D, vitamin E and zinc during pregnancy were each associated with lower odds of wheeze -but not asthma- in childhood. No significant findings were reported for maternal intake of other vitamins (A, B, or C), copper, calcium, magnesium, manganese, selenium, or specific food groups such as vegetables, fruits, fish, meat, dairy, or fats.
Maternal stress
Van de Loo et al. 9 examined 10 observational studies of prenatal maternal stress and respiratory morbidity in childhood. The prevalence of wheeze, asthma and other respiratory symptoms was higher in children of mothers who were exposed to or experienced some form of psychological stress during pregnancy than in children of mothers who did not (OR= 1.56 [1.36–1.80], I2=18 Subgroup analysis demonstrated that maternal stress during pregnancy was significantly associated with asthma (4 studies) or wheeze (8 studies).
Maternal use of antibiotics
In a meta-analysis of 10 studies (7 cohort studies and 3 case-control studies)10, maternal use of antibiotics during pregnancy was associated with 1.2 times increased odds of childhood wheeze or asthma, but there was high heterogeneity across studies. After excluding case-control studies and prospective studies of inadequate quality, the association remained significant and of similar magnitude (pooled OR=1.18, 95% CI=1.11 to 1.26), but the heterogeneity across studies was markedly decreased (I2=46.7%). In that analysis, the association between prenatal use of antibiotics and childhood wheeze or asthma was stronger for antibiotic use in the third trimester of pregnancy than that in the first or second trimester of pregnancy.
Maternal use of paracetamol
Several meta-analyses have been published on maternal paracetamol (acetaminophen) use during pregnancy and childhood asthma. In a meta-analysis of six studies (five prospective cohorts and one cross-sectional study)11, paracetamol use during pregnancy was significantly associated with 1.2 times increased odds of wheeze at ages 2.5 years to 7 years. However, there was high heterogeneity across studies.
A meta-analysis of eleven birth cohort studies12 reported that maternal paracetamol use in the first, third, and second/third trimesters is significantly associated with 39%, 17% and 49% increased odds of childhood asthma, respectively. However, there was considerable heterogeneity across studies. In contrast to the findings for prenatal use of paracetamol, there was no significant association between paracetamol use during infancy and childhood asthma after adjustment for lower respiratory tract infections.12
Environmental tobacco smoke (ETS)
Burke et al.13 performed a meta-analysis of 79 studies of ETS, wheeze, and asthma. The studies were grouped by type of ETS (prenatal maternal, postnatal maternal, postnatal paternal, or in the household) and the age at which the outcome was assessed (≤2, 3-4, or 5-18 years). Prenatal maternal smoking was significantly associated with increased incidence of asthma or wheeze in all age groups, with ORs ranging from 1.28 in children ages 3 to 4 years to 1.52 in children ages 5 to 18 years. In that analysis, there was moderate to high heterogeneity across the two age groups below 5 years but not in the age groups 5 years and older. Post-natal maternal smoking was also significantly associated with wheeze in all age groups, but the magnitude of the association was higher in children younger than 5 years (ORs= 1.65 to 1.70) than in those 5 years and older (OR=1.18). Household ETS exposure was significantly associated with wheeze, with similar findings across all age groups (ORs ranging between 1.06 and 1.32).Paternal smoking was significantly associated with 1.39 times increased odds of wheeze in 5-18 year olds; limited data precluded a meta-analysis in younger children. A meta-analysis of prenatal maternal, paternal and household ETS exposure and asthma yielded findings that were similar to those for wheeze; although some results were not significant, they were in the same direction of association as in the analysis of wheeze.
PERINATAL FACTORS (Figure 2b)
Birth by Caesarean section
In a meta-analysis of 23 studies14, birth by Caesarean section was significantly associated with 22% excess odds of asthma (aged 1 to 28 years), with moderate heterogeneity across studies. Restricting the analysis to childhood asthma (ascertained before age 18 years) yielded similar results (OR=1.20), with reduced heterogeneity across studies.
Preterm delivery
In a meta-analysis of nineteen studies including predominantly children, Jaakkola et al.15 reported that prematurity (a gestational age <37 weeks) was associated with asthma at ages 1 to 31 years (fixed-effect OR=1.074, random-effect OR=1.37), with significant heterogeneity across studies. In a meta-regression adjusting for asthma definition, study design, population size, geographic location, language and year of publication, the estimated effect of preterm birth on asthma decreased as the age of the subjects increased.
In a meta-analysis of 17 studies including 874,710 children16, preterm birth was associated with 1.46 times increased odds of asthma or wheezing disorders, but there was high heterogeneity across studies. The strength of the association between preterm birth and wheezing disorders was similar between children aged <5 years and older children. Of note, children born very preterm (<32 weeks gestation) had a greater increase in the odds of asthma (OR=2.81) than those born moderately preterm (32-36 weeks gestation, OR=1.37). Findings were most pronounced for studies with low risk of bias and were consistent across sensitivity analyses. The population-attributable risk (PAR) of asthma for preterm birth was estimated as 3.1%, with 1.2% attributable to very preterm birth and 1.9% attributable to moderately preterm birth.
Birth weight
A meta-analysis of twelve studies including 38,115 children17 showed that low birth weight (<2,500 g) was associated with 1.28 to 1.34 times higher odds of asthma than a birth weight > 2,500 g or between 2,500 g and 4,000 g, with moderate heterogeneity across studies. In this analysis, a birth weight >4,000 g was not significantly associated with asthma.
Neonatal hyperbilirubinemia
A meta-analysis of seven studies (six retrospective studies and one cohort study) including 101,499 children18 showed that neonatal hyperbilirubinemia significantly increased the odds of childhood asthma (OR=4.26), without heterogeneity across studies. Neonatal phototherapy also increased the odds of asthma (OR=3.81) with low heterogeneity. As observational studies were included, the GRADE evidence generated was of low quality.
POST NATAL FACTORS (Figure 2c)
Bacillus Calmette-Guérin (BCG) vaccination
El-Zein et al.19 performed a meta-analysis of sixteen studies of BCG vaccination and asthma with a total of 67,179 participants. In this analysis, BCG vaccination was significantly associated with a 14% reduction in the odds of asthma, with no heterogeneity across studies. However, only one prospective study was included in this meta-analysis.
Breastfeeding
In a meta-analysis of 12 studies including 8,183 subjects20, exclusive breast-feeding during the first 3 months of life was associated with 30% reduced odds of childhood asthma (mean age 4.1 years), with minimal heterogeneity across studies. The estimated protective effect of breastfeeding on asthma was greater in studies of children with familial history of atopy (OR = 0.52 [0.35-0.79]) than in studies of the general population (OR = 0.73 [0.62-0.86]). After the analysis was restricted to studies that only included children without a family history of atopy, there was no significant association between breastfeeding and childhood asthma (OR=0.99 [0.48-2.03]).
In another meta-analysis, including 31 studies and 417,880 subjects21, there was no significant association between any or exclusive breast feeding (for 3 or 4 months) and asthma or current wheeze in children aged >5 years, though there was moderate heterogeneity across studies. However, a subgroup analysis revealed that any breast feeding is associated with a mild reduction (8%) in the odds of current wheeze (with moderate heterogeneity across studies), but also non-significantly associated with a 10% increment in the odds of asthma (P= 0.05, with minimal heterogeneity across studies).
A meta-analysis of 113 studies22 showed that breastfeeding for at least six months was associated with 24% reduced odds of “recent asthma”, as well as with 19% reduced odds of “recent wheeze”, with high heterogeneity across studies. After stratification by age, there was a strong inverse association between breastfeeding and asthma or wheeze up to age 2 years (without heterogeneity), but this association weakened as age increased.
In a recent meta-analysis of 42 studies 23, ever (vs. never) breastfeeding was associated with 12% reduced risk of asthma in children aged 5-18 years, with moderate heterogeneity across studies. There was a reduced risk of asthma for ever-breastfed children in high-income countries (OR=0.90 [0.83, 0.97], I2=18%), as well as in medium/low income countries (OR=0.78 [0.70, 0.88], I2=0%). This association was attenuated and became non-significant when the analysis was restricted to cohort studies.
Diet and nutrients
In meta-analyses of observational studies of children, Nurmatov et al7 showed that serum vitamin A level and high dietary intake of fruits are significantly associated with 75% reduced odds of asthma and 25% reduced odds of wheeze, respectively.
Muley et al.24 conducted a meta-analysis of five randomized clinical trials (RCTs) of omega-3 fatty acids (for up to 12 months) to prevent asthma, including 1,932 children who were randomized, had available outcomes, and were followed for an average of 3.5 years (range 0.5-8 years). In that analysis, there was no significant association between dietary omega-3 fatty acid supplementation and asthma. A meta-analysis of four cohort studies including 12,481 children25 reported a 24% reduced risk of asthma for fish intake and a 29% reduction in the risk of asthma among children whose mothers had high levels of long-chain n-3 PUFA in breast milk, with minimal heterogeneity across studies.
In a meta-analysis of eight cross-sectional studies.,26 a Mediterranean diet (generally low in saturated fatty acids and rich in fiber, antioxidants, and n-3 PUFA derived from olive or fish oil) was significantly associated with 15% lower odds of current wheeze and 14% lower odds of ever asthma. Of note, however, some results were driven mainly by studies performed in Mediterranean populations. Consistent with findings for a Mediterranean diet, Seyedrezazadeh et al.27 reported lower risk of wheeze among children with high intake of fruits and vegetables, with very similar results for asthma.
Probiotics
A meta-analysis of RCTs28 showed that probiotic supplementation during pregnancy or infancy was not significantly associated with physician-diagnosed asthma or incident wheeze. In a more recent meta-analysis of RCTs, Zuccotti et al.29 also reported no significant association between probiotic supplementation and asthma or wheeze.
Gastroesophageal reflux (GER)
Thakkar et al.30 examined five studies of GER that included children with and without asthma. In those studies, estimates of the prevalence of GER were 22% and 4.8% in children with and without asthma, respectively. Moreover, GER was significantly associated with 5.6 times increased odds of asthma (pooled OR=5.6 [4.3 to 6.9]). Because of methodologic limitations, paucity of population-based studies, and a lack of longitudinal studies, several aspects of this association are unclear.
Antibiotics
In a meta-analysis of eight studies by Marra et al31, including 12,082 subjects (of whom 1,817 had asthma), antibiotic use in the first year of life was significantly associated with twofold increased odds of childhood asthma, with significant heterogeneity across studies. However, this association was markedly weakened and became non-statistically significant when the analysis was restricted to prospective studies (OR=1.12, 95% CI= 0.88 to 1.42), which are less susceptible to “reverse causation” or recall bias.
Murk et al.32 recently showed that antibiotic exposure during pregnancy or in the first year of life (22 studies, n= 685,820 subjects) was significantly associated with 1.52 times increased odds of childhood asthma however, there was high heterogeneity across studies. Similar to the findings of Marra et al,31 there was no significant association between antibiotic use in the first year of life and asthma when the analysis was restricted to prospective studies (OR=1.07, 95% CI=0.89 to 1.28), with no heterogeneity across studies. In contrast, prenatal use of antibiotics was significantly associated with childhood asthma, even when the analysis was restricted to three prospective studies (OR=1.70, 95% CI=1.11– 2.60).
Viral infections
A meta-analysis of 15 studies including 82,008 children33 showed that hospitalization for respiratory syncytial virus (RSV) infection in early life was associated with 3.84 times increased odds of incident asthma or wheeze later in childhood, with low to moderate heterogeneity across studies. To date, there has been no meta-analysis of studies of rhinovirus infection and asthma.
Allergens
A meta-analysis34 of 9 studies including 3,271 children showed that multifaceted interventions to reduce both inhalant and food allergens were associated with 28% to 48% significantly reduced odds of asthma before and after age 5 years, with no heterogeneity across studies. In contrast, there was no significant association between mono-faceted interventions to reduce inhalant (but no food) allergens and asthma. Indirect comparisons between these treatments did not demonstrate a significant difference between multiple interventions and mono-interventions in reducing the odds of asthma in children under five years (OR=0.64 [0.40 - 1.04], p=0.07) or in those five years and older (OR=0.63 [0.35 - 1.13], p=0.12).
Mold and fungi
A meta-analysis of 17 studies found that household dampness and mold is associated with 53% increased odds of childhood wheeze, perhaps independently of allergy35. Moreover, a combined analysis of 33 studies of children and adults found that household dampness and mold is significantly associated with 56% increased odds of current asthma.
In another meta-analysis36 of 61 studies including over 200,000 subjects, signs of mold in the home were significantly associated with 49% and 68% increased odds of asthma and wheeze, respectively.
Endotoxin
A meta-analysis of 19 studies37 showed that endotoxin exposure in early life was significantly associated with 48% increased odds of wheeze in infants and toddlers, with moderate heterogeneity across studies. In contrast, endotoxin exposure in early life was significantly associated with 18% reduced odds of asthma at school age, with no heterogeneity across studies. All four studies in younger children and three out of five studies in school-aged children were prospective.
Farm environment
In a meta-analysis of birth cohort studies38, living in a farm residence was significantly associated with 23% reduced odds of asthma, with moderate heterogeneity across studies. This association was even stronger in children who lived in a farm and had parents who worked in a farm environment (OR=0.69 [0.64-0.76], I2=35%). The heterogeneity across studies was reduced when the analysis was restricted to geographic locations in the Alps.
Air pollution
A meta-analysis by Gasana et al.39 included 19 studies assessing different exposures: nitrogen oxides (nitric oxide [NO], nitrogen dioxide [NO2], nitrous oxide [N2O]), particulate matters (PMs), carbon monoxide (CO), sulfur dioxide (SO2), and ozone (O3). In that meta-analysis, N2O and NO2 were significantly associated with 2% and 5% increased odds of prevalent asthma, with no heterogeneity across studies; and CO was associated with 6% increased odds of prevalent asthma, with low heterogeneity across studies. Moreover, NO2 and SO2 were associated with increased incident asthma, with minimal heterogeneity across studies, and SO2 and PMs were each associated with increased odds of wheeze. Of note, no separate analysis was conducted for the 9 longitudinal studies.
Toxins
Jaakola & Knight40 conducted a meta-analysis of five (non-longitudinal) studies in children, which showed an association between polyvinyl chloride (PVC) surface materials in the home and 45% increased odds of asthma, without significant heterogeneity across studies.
ETS
In a meta-analysis of 38 studies41, ETS was significantly associated with 1.33 times increased risk of incident asthma among children ages 6-18 years, adjusting for atopy. There was no reported assessment of heterogeneity across studies.
Menarche
A meta-analysis of seven studies including 22,859 children42 showed that girls with early menarche (<12 years) had 1.37 times significantly increased odds of asthma, with moderate heterogeneity across studies. A sensitivity analysis showed that the risk estimate was not markedly changed when excluding any of the analyzed studies.
Overweight or obesity
In a meta-analysis of 12 studies including over 120,000 subjects43, a sub-group analysis of 4 studies showed that increased body weight during middle childhood was significantly associated with 50% increased risk of subsequent asthma, and a sub-group analysis of nine studies showed that high birth weight is associated with 20% increased risk of childhood asthma.
A meta-analysis of six studies including 25,734 children44 showed that obesity (defined as a body mass index [BMI] ≥95th percentile) was significantly associated with 50% increased risk of incident asthma in both boys and girls, with no heterogeneity across studies. The magnitude of this association was greater in girls (RR=1.53 [1.09 - 2.14], p = 0.01) than in boys (RR=1.40 [1.01 - 1.93], p=0.04). However, there was much more heterogeneity in studies of boys (I2= 81%) than in studies of girls (I2= 34%).
In another meta-analysis of six studies including 18,760 children45, overweight (defined as a BMI ≥=85th percentile) and obesity (BMI ≥95th percentile) were significantly associated with 1.19 times and 2.02 times increased risk of incident asthma, respectively. Moreover, there was a significant dose-response relationship between BMI and incident asthma. In this analysis, the estimated effect of obesity on asthma was greater in obese boys (RR = 2.47 [1.57–3.87], p < 0.001) than in obese girls (RR = 1.25 [0.51–3.03], p = 0.04).
A systematic review or meta-analysis is ultimately dependent on the underlying quality of the primary studies included in the analysis. Limitations include the design of the original studies (e.g. observational or cross-sectional), the covariates included in the original analyses (e.g. different studies may have adjusted for different confounders), and unknown sources of bias that may have been inadvertently included in the primary studies. The degree of heterogeneity between studies is also important and has been highlighted within each section. Moreover, results from such analysis have to be interpreted in the context of other supporting evidence.
Familial or prenatal factors
Current evidence supports strong causal effects (OR or RR ≥2) of parental (paternal or maternal) asthma (a non-modifiable risk factor) on childhood asthma. Systematic reviews of observational studies, together with findings from interventional studies and data on the impact of public health policy measures, strongly support mild to moderate causal effects (OR or RR ≥1.2 but <2) of prenatal ETS on childhood asthma.
Current evidence suggest that maternal weight gain or obesity during pregnancy, maternal use of paracetamol, and maternal stress during pregnancy each have mild to moderate effects on increasing asthma risk in childhood, but interventional studies are needed to firmly establish causality. Similarly, maternal use of antibiotics during pregnancy may have mild to moderate causal effects on asthma, but this needs further assessment in longitudinal studies.
Maternal vitamin D insufficiency (a serum 25(OH)D <30 ng/ml) is not likely to have moderate or strong causal effects on asthma, but there is inconclusive evidence for modest (yet potentially clinically significant) effects from two RCTs46-47. In a RCT of high-dose prenatal vitamin D (4,400 IU/day) vs. low-dose prenatal vitamin D (400 IU/day) to prevent asthma or wheeze at age 3 years in 806 children in US46 , 218 children developed asthma or recurrent wheeze: 98 of 405 (24.3%; 95% CI, 18.7%-28.5%) in the intervention arm vs. 120 of 401 (30.4%, 95% CI, 25.7%-73.1%) in the control group (hazard ratio, 0.8 [0.6-1.0]; p=0.05). Future RCTs should have larger simple size and consider post-natal vitamin D supplementation.
Existing evidence does not support a causal role for maternal folic acid status on childhood asthma, and there is insufficient or conflicting evidence for a causal role of maternal diet or maternal nutrient intake during pregnancy on childhood asthma.
Perinatal factors
Moderate to high-quality evidence suggest that three correlated perinatal factors have mild to moderate effects on increasing the risk of childhood asthma (birth by Caesarean section, prematurity and low birth weight), and that very preterm birth is a strong risk factor for childhood asthma. Compared to vaginal delivery, Caesarean delivery before rupture of the membranes was recently shown to be more strongly associated with childhood asthma (incidence RR=1.20 [1.16-1.23] than Caesarean delivery after rupture of the membranes incidence RR=1.12 [1.09-1.16]).48 A RCT of vaginal vs. non-emergent caesarean delivery may be difficult to implement but could help to firmly establish causality. Whether neonatal hyperbilirubinemia predisposes to childhood asthma or wheeze cannot be determined from existing evidence, but warrants proper assessment in longitudinal studies.
Postnatal exposures
Severe RSV infection in early life (leading to hospitalization) is likely to be a strong risk factor for childhood asthma. Confirmation of a causal role of severe RSV infection (e.g. by vaccination) is pending, awaiting trials of RSV vaccination or treatment to prevent childhood asthma.
High quality evidence supports mild to moderate effects of both overweight/obesity and indoor mold/fungi on increasing the risk of childhood asthma. However, interventional studies are needed to both confirm causality and assess the relative contribution of these factors to disease pathogenesis. Studies of moderate quality also support mild to moderate effects of outdoor air pollution (particularly NO2 and SO2) on asthma risk, and warrant follow-up in well-designed longitudinal studies.
High quality evidence suggests that living in a farm residence or exposure to indoor endotoxin has mild to moderate protective effects against asthma, particularly at or after school age. Longitudinal studies are needed to identify specific exposures (e.g. microbial) underlying the putative protective effects of a farming environment against asthma. Although there is some evidence of potential beneficial effects of multifaceted interventions to reduce indoor allergen exposure, such interventions are generally cumbersome and likely to be prohibitively expensive.
There is limited, conflicting or insufficient evidence of a causal role of BCG vaccination, breastfeeding, diet or dietary patterns, early menarche and exposure to PVC in childhood asthma. Ongoing longitudinal studies of breastfeeding, accounting for introduction and parallel consumption of foodstuff, should help clarify its role, if any, on asthma. Similarly, longitudinal studies are needed to assess whether certain diets (e.g. Mediterranean) reduce asthma risk, as well as to better characterize the relation between PVC exposure and asthma. Current evidence does not support a causal role of postnatal use of antibiotics or probiotics in childhood asthma.
Summary
After reviewing all evidence, parental asthma, prenatal environmental tobacco smoke and prematurity (particularly very preterm birth) are well-established risk factors for childhood asthma. Current findings do suggest mild to moderate causal effects of certain modifiable behaviors or exposures during pregnancy (maternal weight gain or obesity, maternal use of antibiotics or paracetamol, and maternal stress), the perinatal period (birth by Caesarean delivery), or postnatal life (severe RSV infection, overweight or obesity, indoor exposure to mold or fungi, and outdoor air pollution) on childhood asthma, but this suggestive evidence must be confirmed in interventional studies or (if interventions are not feasible) well-designed prospective studies.
Acknowledgments
Funding Sources: This work was supported by grant #1141195 from Fondecyt-Chile, to Dr. Castro-Rodriguez. Dr. Celedón's contribution was supported by grants HL079966 and HL117191 from the U.S. National Institutes of Health (NIH), and by The Heinz Endowments. Dr. Forno's contribution was supported by grant HL125666 from the U.S. NIH.
Abbreviations
- AMSTAR
Assess Systematic Reviews
- BCG
Bacillus Calmette-Guérin
- BMI
body mass index
- CO
carbon monoxide
- CI
confidence intervals
- ETS
Environmental tobacco smoke
- GER
gastroesophageal reflux
- OR
odd ratio
- O3
ozone
- PAR
population-attributable risk
- PMs
particulate matters
- PUFA
polyunsaturated fatty acids
- PVC
polyvinyl chloride
- NO
nitric oxide
- NO2
nitrogen dioxide
- N2O
nitrous oxide
- SO2
sulfur dioxide
- RCT
randomized clinical trials
- RR
relative risk
- RSV
respiratory syncytial virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Dick S, Friend A, Dynes K, AlKandari F, Doust E, Cowie H, Ayres JG, Turner SW. A systematic review of associations between environmental exposures and development of asthma in children aged up to 9 years. BMJ Open. 2014;4:e006554. doi: 10.1136/bmjopen-2014-006554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–380. doi: 10.7326/0003-4819-126-5-199703010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim RH, Kobzik L, Dahl M. Risk for asthma in offspring of asthmatic mothers versus fathers: a meta-analysis. PLoS One. 2010;5:e10134. doi: 10.1371/journal.pone.0010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forno E, Young OM, Kumar R, Simhan H, Celedón JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics. 2014;134:e535–46. doi: 10.1542/peds.2014-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crider KS, Cordero AM, Qi YP, Mulinare J, Dowling NF, Berry RJ. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98:1272–81. doi: 10.3945/ajcn.113.065623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127:724–33. e1–30. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Beckhaus AA, Garcia-Marcos L, Forno E, Pacheco-Gonzalez RM, Celedón JC, Castro-Rodriguez JA. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: a systematic review and meta-analysis. Allergy. 2015;70:1588–604. doi: 10.1111/all.12729. [DOI] [PubMed] [Google Scholar]
- 9.van de Loo KF, van Gelder MM, Roukema J, Roeleveld N, Merkus PJ, Verhaak CM. Prenatal maternal psychological stress and childhood asthma and wheezing: a meta-analysis. Eur Respir J. 2016;47:133–46. doi: 10.1183/13993003.00299-2015. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D, Su H, Cheng J, Wang X, Xie M, Li K, Wen L, Yang H. Prenatal antibiotic use and risk of childhood wheeze/asthma: A meta-analysis. Pediatr Allergy Immunol. 2015;26:756–64. doi: 10.1111/pai.12436. [DOI] [PubMed] [Google Scholar]
- 11.Eyers S, Weatherall M, Jefferies S, Beasley R. Paracetamol in pregnancy and the risk of wheezing in offspring: a systematic review and meta-analysis. Clin Exp Allergy. 2011;41:482–9. doi: 10.1111/j.1365-2222.2010.03691.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheelo M, Lodge CJ, Dharmage SC, Simpson JA, Matheson M, Heinrich J, Lowe AJ. Paracetamol exposure in pregnancy and early childhood and development of childhood asthma: a systematic review and meta-analysis. Arch Dis Child. 2015;100:81–9. doi: 10.1136/archdischild-2012-303043. [DOI] [PubMed] [Google Scholar]
- 13.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 14.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38:629–33. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 15.Jaakkola JJ, Ahmed P, Ieromnimon A, Goepfert P, Laiou E, Quansah R, Jaakkola MS. Preterm delivery and asthma: a systematic review and meta-analysis. J Allergy Clin Immunol. 2006;118:823–30. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, Sheikh A. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu M, Ye S, Bai MJ, Liu GL, Tong Y, Wang SF, Sheng J. Birth weight and subsequent risk of asthma: a systematic review and meta-analysis. Heart Lung Circ. 2014;23:511–9. doi: 10.1016/j.hlc.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Das RR, Naik SS. Neonatal hyperbilirubinemia and childhood allergic diseases: a systematic review. Pediatr Allergy Immunol. 2015;26:2–11. doi: 10.1111/pai.12281. [DOI] [PubMed] [Google Scholar]
- 19.El-Zein M, Parent ME, Benedetti A, Rousseau MC. Does BCG vaccination protect against the development of childhood asthma? A systematic review and meta-analysis of epidemiological studies. Int J Epidemiol. 2010;39:469–86. doi: 10.1093/ije/dyp307. [DOI] [PubMed] [Google Scholar]
- 20.Gdalevich M, Mimouni D, Mimouni M. Breast-feeding and the risk of bronchial asthma in childhood: a systematic review with meta-analysis of prospective studies. J Pediatr. 2001;139:261–6. doi: 10.1067/mpd.2001.117006. [DOI] [PubMed] [Google Scholar]
- 21.Brew BK, Allen CW, Toelle BG, Marks GB. Systematic review and meta-analysis investigating breast feeding and childhood wheezing illness. Paediatr Perinat Epidemiol. 2011;25:507–18. doi: 10.1111/j.1365-3016.2011.01233.x. [DOI] [PubMed] [Google Scholar]
- 22.Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. 2014;179:1153–67. doi: 10.1093/aje/kwu072. [DOI] [PubMed] [Google Scholar]
- 23.Lodge CJ, Tan DJ, Lau M, Dai X, Tham R, Lowe AJ, Bowatte G, Allen KJ, Dharmage SC. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38–53. doi: 10.1111/apa.13132. [DOI] [PubMed] [Google Scholar]
- 24.Muley P, Shah M, Muley A. Omega-3 Fatty Acids Supplementation in Children to Prevent Asthma: Is it worthy? A Systematic Review and Meta-Analysis. J Allergy (Cairo) 2015;2015:312052. doi: 10.1155/2015/312052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Xun P, He K. Fish and fish oil intake in relation to risk of asthma: a systematic review and meta-analysis. PLoS One. 2013;8(11):e80048. doi: 10.1371/journal.pone.0080048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Marcos L, Castro-Rodriguez JA, Weinmayr G, Panagiotakos DB, Priftis KN, Nagel G. Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24:330–8. doi: 10.1111/pai.12071. [DOI] [PubMed] [Google Scholar]
- 27.Seyedrezazadeh E, Moghaddam MP, Ansarin K, Vafa MR, Sharma S, Kolahdooz F. Fruit and vegetable intake and risk of wheezing and asthma: a systematic review and meta-analysis. Nutr Rev. 2014;72:411–28. doi: 10.1111/nure.12121. [DOI] [PubMed] [Google Scholar]
- 28.Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132:e666–76. doi: 10.1542/peds.2013-0246. [DOI] [PubMed] [Google Scholar]
- 29.Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, Fantini MP, Gori D, Indrio F, Maggio L, Morelli L, Corvaglia L. Italian Society of Neonatology. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70:1356–71. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 30.Thakkar K, Boatright RO, Gilger MA, El-Serag HB. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125:e925–30. doi: 10.1542/peds.2009-2382. [DOI] [PubMed] [Google Scholar]
- 31.Marra F, Lynd L, Coombes M, Richardson K, Legal M, Fitzgerald JM, Marra CA. Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and metaanalysis. Chest. 2006;129:610–8. doi: 10.1378/chest.129.3.610. [DOI] [PubMed] [Google Scholar]
- 32.Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011;127:1125–38. doi: 10.1542/peds.2010-2092. [DOI] [PubMed] [Google Scholar]
- 33.Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–6. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 34.Maas T, Kaper J, Sheikh A, Knottnerus JA, Wesseling G, Dompeling E, Muris JW, van Schayck CP. Mono and multifaceted inhalant and/or food allergen reduction interventions for preventing asthma in children at high risk of developing asthma. Cochrane Database Syst Rev. 2009;(3):CD006480. doi: 10.1002/14651858.CD006480.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Fisk WJ, Lei-Gomez Q, Mendell MJ. Meta-analyses of the associations of respiratory health effects with dampness and mold in homes. Indoor Air. 2007;17:284–96. doi: 10.1111/j.1600-0668.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 36.Tischer C, Chen CM, Heinrich J. Association between domestic mould and mould components, and asthma and allergy in children: a systematic review. Eur Respir J. 2011;38:812–24. doi: 10.1183/09031936.00184010. [DOI] [PubMed] [Google Scholar]
- 37.Mendy A, Gasana J, Vieira ER, Forno E, Patel J, Kadam P, Ramirez G. Endotoxin exposure and childhood wheeze and asthma: a meta-analysis of observational studies. J Asthma. 2011;48:685–93. doi: 10.3109/02770903.2011.594140. [DOI] [PubMed] [Google Scholar]
- 38.Genuneit J. Exposure to farming environments in childhood and asthma and wheeze in rural populations: a systematic review with meta-analysis. Pediatr Allergy Immunol. 2012;23:509–18. doi: 10.1111/j.1399-3038.2012.01312.x. [DOI] [PubMed] [Google Scholar]
- 39.Gasana J, Dillikar D, Mendy A, Forno E, Ramos Vieira E. Motor vehicle air pollution and asthma in children: a meta-analysis. Environ Res. 2012;117:36–45. doi: 10.1016/j.envres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Jaakkola JJ, Knight TL. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ Health Perspect. 2008;116:845–53. doi: 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vork KL1, Broadwin RL, Blaisdell RJ. Developing asthma in childhood from exposure to secondhand tobacco smoke: insights from a meta-regression. Environ Health Perspect. 2007;115:1394–400. doi: 10.1289/ehp.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieberoth S, Gade EJ, Brok J, Backer V, Thomsen SF. Age at menarche and risk of asthma: systematic review and meta-analysis. J Asthma. 2014;51:559–65. doi: 10.3109/02770903.2014.903966. [DOI] [PubMed] [Google Scholar]
- 43.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch Dis Child. 2006;91:334–9. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egan KB, Ettinger AS, Bracken MB. Childhood body mass index and subsequent physician-diagnosed asthma: a systematic review and meta-analysis of prospective cohort studies. BMC Pediatr. 2013;13:121. doi: 10.1186/1471-2431-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen YC, Dong GH, Lin KC, Lee YL. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. 2013;14:222–31. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 46.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315:362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chawes BL, Bønnelykke K, Stokholm J, Vissing NH, Bjarnadóttir E, Schoos AM, et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA. 2016;315:353–61. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 48.Sevelsted A, Stokholm J, Bisgaard H. Risk of asthma from cesarean deliver depends on membrane rupture. J Pediatr. 2016 Jan 26;:S0022–3476(15)01656 X. doi: 10.1016/j.jpeds.2015.12.066. doi: 10.1016/j.jpeds.2015.12.066. [DOI] [PubMed] [Google Scholar]