Abstract
Background
This study evaluated the social environment of bariatric surgery patients in the preoperative period.
Methods
Forty bariatric surgery patients (mean = 46.2 ± 11.2 years), 35 adult cohabitating family members (mean = 45.2 ± 12.7 years), and 15 cohabitating children (mean = 11.5 ± 3.6 years) were recruited from a large rural medical center. Adult participants (patients and family members) completed height, weight, body composition, blood draws, and physical activity assessments (accelerometry), as well as eating behavior and social support inventories before the patient underwent bariatric surgery. Child participants completed demographic, height, and weight assessment only.
Results
Over 90 % of adult family members were overweight or obese (body mass index (BMI) ≥25 kg/m2, as were 50 % of children (BMI percentile ≥85 %). More than one third (37.1 %) of family members met the criteria for moderate to severe insulin resistance. Physical activity measured by accelerometry was moderately correlated between the patient and adult family members (r = 0.46, p = 0.023). Bariatric surgery patients reported high levels of social support from their family members on multiple social support measures.
Conclusions
Many family members of bariatric surgery patients also lived with obesity and related comorbidities, and demonstrate high sedentary behavior. However, patients reported high levels of support from family members, including support in following a healthy diet and engaging in physical activity. Engaging families in behavior change may help bariatric surgery patients and their families to become healthier.
Keywords: Bariatric surgery, Families, Obesity
Introduction/Purpose
Individuals with obesity (body mass index (BMI) ≥30 kg/m2) tend to have family members that also live with obesity [1–4]. Couples display similar BMIs [5] that trend together over time [6]. When one spouse participates in behavioral [7] or surgical [8, 9] weight loss treatment, the other may also experience reductions in weight. Children with obese parents are more likely to live with obesity [1], which may relate to modeling, shared environments, and genetics [2–4]. In one study [10], an individual’s risk of obesity increased by 57 % if a friend developed obesity, suggesting a “contagion of obesity” within social networks. While some studies found similar social network effects [11], others were less conclusive [12].
The Main Effect Model [13] proposes that social support influences health through positive effect, self-worth, and modeling of behavior. Bariatric surgery patients are required to implement numerous behavioral and dietary changes [14]. Adherence to this regimen is paramount to maximize weight loss. Under this model [13], behavior change following surgery may be influenced by social support.
The social environment of bariatric surgery patients and the role of family in postoperative outcomes are not yet well understood. The studies or reviews evaluating social support suggest that previous research is limited by non-validated questionnaires [15, 16] or small samples [17, 18], or studied gastric banding only [19]. Findings are inconsistent, with some reporting an association between support and postoperative outcomes [20, 21], and others reporting no such connection [17, 18, 22–24]. Three studies [8, 9, 25] indicated that patients’ family members also live with overweight or obesity. However, how family dietary and physical activity behaviors support or hinder behavior change in bariatric surgery patients remains largely unexplored.
Given the shared home environment, patients’ cohabitating family members could benefit from the positive behavioral and environmental changes associated with bariatric surgery. One study [26] found that postoperative mothers modeled healthy eating more frequently for their children than preoperative mothers. Conversely, families could negate positive behavior change in patients if they engage in unhealthful behaviors or are unsupportive. A better understanding of the family environment of bariatric surgery patients could provide insight into how this environment influences outcomes. The aim of this pilot, observational study was to obtain a comprehensive assessment of the family environment of bariatric surgery patients, including the health and health behaviors of adult family members residing with patients, the weight of their children, and the amount and type of social support perceived by patients before surgery.
Materials and Methods
We recruited 40 adult bariatric surgery patients and their cohabitating adult family members (n = 35) and/or children (n = 15) from the bariatric surgery program of a rural medical center from March 2013 to January 2015. Research staff introduced the study to patients during their preoperative behavior classes. All adult participants provided written informed consent. Children provided written consent and parental consent was provided as well. Surgical candidacy criteria served as eligibility criteria for patients. Non-surgical adults were included if they lived with a participating bariatric surgery patient and were ≥18 years old. Children were eligible if they lived with a bariatric surgery patient and were 5–16 years old. We excluded participants who reported pregnancy, planned pregnancy, or lactation, or had embedded electronic medical devices.
Study procedures were administered preoperatively. Adults were compensated $100 and completed questionnaires separately. Children were not compensated and participated in height and weight measurements only. This study was approved by the health system’s Institutional Review Board.
Anthropometric Measures
Weight was measured to the nearest 0.1 kg and height to the nearest 0.1 cm.
Demographics and Medical History
Baseline demographic and medical information were collected through the electronic medical record (EMR) and a questionnaire.
Physical Activity (Adults)
Patients and adult family members were provided instructions and asked to wear an accelerometer (ActiGraph® GTX3, ActiGraph Corp., Pensacola, FL) for seven consecutive days. Accelerometers provide valid and reliable quantification of physical activity and sedentary behaviors in normal weight to obese adults [27]. Data were downloaded using the low-frequency extension and analyzed using Actilife Version 6.12.0 (ActiGraph Corp., Pensacola, FL). Prior to analysis, data were screened for wear time using the Choi algorithm [28]. A minimum of 10 h of valid wear time was required to count as a valid wear day. Participants were included in the analyses if they had four or more valid wear days [29]. There were 28 dyads that had accelerometer files and three dyads were excluded due to insufficient valid wear days. We used the axis-1 counts per minute (CPM) to differentiate time spent in sedentary (<100 CPMs), light-intensity (100–2019 CPMs), and moderate to vigorous (≥2020 CPMs) physical activities (sedentary, LPA, moderate to vigorous physical activity (MVPA)) and are presented as minutes per day of total accumulation [30]. We also present the MVPA data as minutes per week spent in bouts lasting 10 min or greater.
Eating Behavior (Adults)
Patients and adult family members completed the Eating Inventory (EI) [31], a 51-item questionnaire assessing cognitive control of eating (α= 0.92), disinhibition (α= 0.91), and hunger (α= 0.85).
Social Support (Adults)
Perceived social support in bariatric surgery patients was assessed using the Social Support for Healthy Behaviors scale [32], a 48-item questionnaire assessing social support for diet and exercise (Cronbach’s α = 0.69–0.80) validated in individuals seeking to make healthful behavior change. Answers are on a Likert scale ranging from “almost never” to “almost always,” with eight sub-scales (support/sabotage) (α = 0.61–0.91) [32]. Patients completed the Medical Outcomes Study (MOS) social support survey [33], a questionnaire assessing types of social support: emotional/informational, tangible, affectionate, and positive social interactions. Answers were provided in a Likert scale ranging from “none of the time” to “all of the time.” The MOS was created for use in individuals with chronic health conditions [33].
Lipids, Insulin, and Glucose (Adults)
Blood samples were obtained after an overnight fast (12 h) from patients and their adult family members. Lipid panels were conducted on Roche “P” modular instruments using Roche reagent. Total and HDL cholesterol and triglycerides were measured enzymatically and LDL cholesterol calculated by Freidwald calculation. Seven milliliters of blood were drawn. Fasting plasma glucose was measured using a spectrophotometric (colorimetric) method, and fasting insulin measured using the electrochemiluminescence method. Diabetes was a fasting glucose >125 mg/dL, fasting serum >17 ml/U/L, or as diagnosed in the medical record. Impaired fasting glucose was defined as ≥100 mg/dL or as diagnosed in the medical record. Homeostasis Model Assessment (HOMA) was calculated to assess insulin resistance [34] and values greater than three used as markers of insulin resistance. Dyslipidemia was an LDL >150 mg/dL, HDL <40 mg/dL, cholesterol >200 mg/dL, triglycerides >150 mg/dL, or as a diagnosis in the medical record.
Body Composition (Adults)
Body composition was assessed using air displacement plethysmography [35, 36] by BodPod® (COSMED, Chicago, IL).
Statistical Analyses
Descriptive statistics characterized the sample and surveys. Independent samples t tests compared eating scale means between patients and family members. Physical activity and body composition differences between patients and family members were analyzed using paired t tests. Significance levels were p < 0.05.
Results
For adult family members, all but one participant (adult child) was a spouse or partner of a bariatric surgery patient. Three of the 15 children were from the same family. Sample characteristics are in Table 1. Of the 55 families approached to participate, 40 agreed (72.7 %).
Table 1.
Sample characteristics
| Bariatric surgery patients (n = 40) | Adult family members (n = 35) | Children (n = 15) | |
|---|---|---|---|
| Age (years), M (SD) | 46.2 (11.2) | 45.2 (12.7) | 11.5 (3.6) |
| Sex (%) | |||
| Female | 77.5 % | 28.6 % | 46.7 % |
| Male | 22.5 % | 71.4 % | 53.3 % |
| Race/ethnicity (%) | |||
| White | 95.0 % | 97.1 % | 93.3 % |
| Black | 2.5 % | 2.9 % | 6.7 % |
| Other/not reported | 2.5 % | – | – |
| Ethnicity (%) | |||
| Hispanic | 2.5 % | 2.9 % | 13.3 % |
| Marital status | |||
| Married | 80.0 % | 77.1 % | N/A |
| Single | 5.0 % | 11.4 % | |
| Divorced/separated | 7.5 % | 8.6 % | |
| Living as married/other | 7.5 % | 2.9 % | |
| Education | |||
| High school/GED | 57.5 % | 60.0 % | N/A |
| 1–2 years college | 20.0 % | 20.0 % | |
| 4-year college | 15.0 % | 11.4 % | |
| Postgraduate | 7.5 % | 8.6 % | |
| Height (cm), M (SD) | 167.5 (9.9) | 172.0 (7.6) | 151.7 (19.6) |
| Weight (kg), M (SD) | 132.8 (26.0) | 102.9 (31.6) | 56.8 (22.2) |
| BMI (kg/m2), M (SD) | 47.4 (9.2) | 34.8 (11.4) | N/A |
| BMI z score, M (SD) | N/A | N/A | 1.3 (0.7) |
| BMI percentile, M (SD) | N/A | N/A | 85.7 (13.1) |
| Weight category (%)a | |||
| Normal | – | 8.7 % | 50.0 % |
| Overweight | – | 31.3 % | 12.5 % |
| Obese | 100 % | 60.0 % | 37.5 % |
| Fasting glucose (mg/dL), M (SD) | 101.1 (25.1) | 102.5 (26.8) | N/A |
| Fasting insulin (mg/dL), M (SD) | 19.3 (12.9) | 13.5 (10.4) | |
| HOMAb (mass units) | 5.0 (4.6) | 3.7 (3.4) | |
| Cholesterol (mg/dL) | 183.8 (31.0) | 181.3 (32.1) | |
| HDL | 45.2 (12.0) | 53.8 (22.6) | |
| LDL | 109.8 (25.5) | 103.8 (32.6) | |
| Triglycerides (mg/dL) | 144.3 (55.6) | 118.2 (72.9) | |
For adults: normal = BMI < 25 kg/m2, overweight = BMI 25–29.9 kg/m2, obese = 30+ kg/m2 . For children, BMI percentile for overweight = 85–94.9 %, obese = 95 % or higher
HOMA calculation = (glucose × insulin)/405. Glucose values were missing for n = 2 patients and lipids for n = 3 patients
Weight
In adult family members, 91.3 % (Table 1) lived with overweight or obese (body mass index ≥25 kg/m2), as were 50 % of children (BMI percentile ≥85 %) [37].
Comorbidity
In adult family members, 31 % had elevated fasting glucose (≥100 mg/dL, Table 1), 37.1 % had moderate to severe insulin resistance based on the HOMA calculation (>3 mass units), 23 % had elevated triglyceride levels (≥150 mg/dL), and 43 % had low HDL levels (<40 mg/dL).
Body Composition
Table 2 presents the results of air displacement plethysmography assessment of body composition in a subset of bariatric surgery patients and their adult family member (n = 32 dyads). Three dyads did not complete body composition assessments (8.5 %). Patients had significantly higher body weights, percent body fat, and total fat than their adult family members (all p < 0.05) but most adult family members were also obese by percent body fat standards [38].
Table 2.
Height, weight, and air displacement plethysmography body-derived measures of body composition
| Bariatric surgery patient (n = 32) | Adult family member (n = 32) | p value | |
|---|---|---|---|
| Age | 48.9 (11.2) | 45.6 (12.5) | 0.34 |
| Female (%) | 75 | 25 | – |
| Height (cm) | 168.8 (9.3) | 172.3 (7.7) | <0.0001 |
| Weight (kg) | 130.5 (22.7) | 97.0 (22.7) | <0.0001 |
| Body fat (%) | 50.8 (7.2) | 35.8 (11.1) | <0.0001 |
| Body fat (kg) | 66.8 (17.2) | 35.9 (16.4) | <0.0001 |
| Fat free mass (kg) | 67.8 (12.2) | 61.1 (16.4) | 0.48 |
Data are presented as mean (SD)
Physical Activity
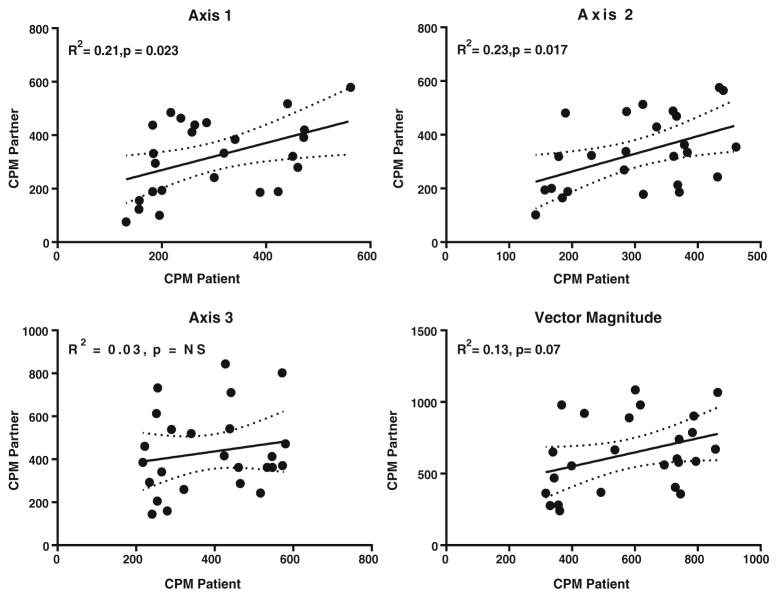
Table 3 presents the quantitative measurements of physical activity and sedentary behavior in a subset of bariatric surgery patients and their adult family members (n = 25 dyads; n = 28 dyads completed accelerometry but three dyads were excluded from analyses due to insufficient wear time, or 10.7 %). There were no differences between patients and family members for sedentary time, low-intensity physical activity, or moderate to vigorous physical activity (MVPA) (all p > 0.05). Overall, patients and their family members spent ~60 % of their time sedentary, with minimal time spent in MVPA. Finally, the axis-1 activity counts per minute of the patients demonstrated a moderate positive correlation (r = 0.46, p = −0.023) with their family members’ axis-1 activity counts per min (Fig. 1). Axis-2 counts were significant, though axis-3 and vector magnitude were not significantly associated between patients and family members.
Table 3.
Accelerometer-derived measures of time spent in sedentary activity, low-intensity activity (LPA), and moderate to vigorous physical activity (MVPA)
| Physical activity | Bariatric surgery patient (n = 25) | Adult family member (n = 25) | p value |
|---|---|---|---|
| Sedentary (min/day) | 525 (104) | 520 (123) | 0.85 |
| LPA (min/day) | 317 (84) | 332 (108) | 0.55 |
| MVPA (min/day) | 20 (21) | 24 (17) | 0.34 |
| MVPA (min/week) 10-min bouts | 35 (70) | 42 (70) | 0.68 |
| Sedentary (%) | 61 (10) | 59 (13) | 0.56 |
| LPA (%) | 37 (9) | 38 (12) | 0.68 |
| MVPA (%) | 2 (2) | 3 (2) | 0.36 |
| Axis-1 counts per minute (CPM) | 289 (125) | 329 (140) | 0.16 |
Sedentary (<100 CPM), LPA (100–2019 CPM), MVPA (>2020 CPM); CPM were adjusted for wear time using the Troiano algorithm. Data are presented as mean (SD)
Fig. 1.
Association in physical activity measured as counts per minute between patients and adult family members (n = 25 dyads)
Social Support
Patients (n = 40) reported high social support (mean=85.5±17.4, Table 4). Affectionate support (i.e., show of love, hugs) was rated highest (mean= 96.9±58.1) followed by positive social interactions (i.e., someone to have fun/relax with, mean=85.6±20.7). Patients reported high social support from family members for healthy diet (mean =3.3 ±0.8) and physical activity (mean=3.1±0.8) behaviors, and endorsed lower levels of sabotage regarding healthy eating (mean = 2.0 ± 0.6) and physical activity (mean =2.0 ±0.5, Table 4).
Table 4.
Perceived social support reported by treatment-seeking populations [41]
| Bariatric surgery (n = 40, present study) mean (SD) (1–5) | Behavioral weight loss (n = 267) [41] mean (SD) (1–5) | Chronic health conditions (n = 2987) [33] | |
|---|---|---|---|
| Social support for healthy behaviors scales [32, 41] | – | ||
| Support from family for healthy eating | 3.3 (0.8) | 2.3 (0.8) | – |
| Sabotage from family for healthy eating | 2.0 (0.6) | 2.2 (0.8) | – |
| Support from family for physical activity | 3.1 (0.8) | 2.3 (0.8) | – |
| Sabotage from family for physical activity | 2.0 (0.5) | 2.3 (0.8) | – |
| Medical Outcomes Study (MOS) social support survey [33] | Scaled score* (SD) (0–100) | Scaled score* (SD) (0–100) | |
| Emotional/informational support | 81.5 (18.5) | – | 69.6 (25.5) |
| Tangible support | 85.2 (17.1) | – | 69.8 (28.5) |
| Affectionate support | 96.9 (58.1) | – | 73.7 (28.3) |
| Positive social interactions | 85.6 (20.7) | – | 69.8 (26.0) |
| Overall support index | 85.5 (17.4) | – | 70.1 (24.2) |
Scaled score = 100 × (observed mean − minimum possible score)/(maximum possible − minimum possible)
Eating Behaviors
On the EI, patients (n = 40) reported mean ± SD cognitive control of 14.6 ± 4.5, disinhibition 8.6 ± 3.5, and hunger 5.1 ± 4.5. Overall, adult family members (n = 35, all weight categories) reported mean ± SD cognitive control of 9.0 ± 4.8, disinhibition of 6.5 ± 4.0, and hunger of 3.2 ± 4.4. Patients reported significantly higher cognitive control (t = 5.21, p < 0.001) and greater disinhibition (t = 2.51, p = 0.01) compared to adult family members.
In adult family members with obesity only (n = 21) and patients, there were no differences (p > 0.05) in disinhibition (7.0 ± 4.0 versus 8.6 ± 3.5, respectively) or hunger ratings (3.0 ± 4.6 versus 5.1 ± 4.5).
Conclusion
This is the first study to describe the family environment of bariatric surgery patients by assessing a combination of social support and weight-related health measures, eating behaviors, and physical activity in cohabitating family members. Over 90 % of adult family members were overweight or obese, over one third met criteria for moderate to severe insulin resistance, and almost one quarter had elevated triglycerides. Moreover, 50 % of patients’ children lived with overweight or obesity. Most male adult family members were obese by percent body fat standards, as were female adult family members. While patients had more body fat than family members, most patients were women, who typically have more body fat than men. Several studies [8, 9, 25, 39, 40] also evaluated the weight of patients’ family members and similarly found high rates of obesity. Collectively, our findings indicate that bariatric surgery patients’ family members also live with overweight and obesity, and in our study, many lived with obesity-related comorbidities.
Patients and their adult family members demonstrated a high degree of time spent in sedentary behaviors, with little time spent in 10-min bouts of MVPA (over 8.5 h per day in sedentary behaviors). Berglind and colleagues [39] recently reported that bariatric surgery patients spent over 7 h per day in sedentary behaviors. We observed that patients and their adult family members spent about 35–40 min per week in 10-min bouts of MVPA, which is slightly higher but remains consistent with the recent observations [39].
Patients reported higher cognitive control but greater disinhibition compared to their adult family members (all weights). Preoperative patients are in the process of learning about the postoperative diet and are asked to lose weight, which is likely reflected in their higher cognitive control scores. Patients’ higher disinhibition scores compared to family members suggests that they may still experience some loss of control, but eating behavior change can take time to adopt and sustain. Of note, adult family members living with obesity reported eating behaviors that were comparable to patients, including disinhibition and sensitivity to hunger. Future studies could evaluate the potential benefit of working with family members with obesity to better manage disinhibition and hunger.
Patients perceived high levels of social support, including support in making healthy diet choices and in being physically active. When compared to overweight and obese participants of a behavioral weight loss program [41], our patients’ ratings of family support for healthy eating and physical activity were 43 and 35 % higher, respectively. Patients in our study reported particularly high levels of affection in their social relationships. Compared to individuals with other health conditions [33], our patients reported 17 % higher average emotional/informational social support, 22 % higher tangible support, 31 % higher affectionate support, and 23 % higher positive social interactions. Patients may have over-reported levels of support to portray a positive image before surgery, but were informed that answers would not be reviewed by their providers. Finally, participating families may be more supportive of each other than families who declined. Future studies could explore differences between participating families versus those that decline, as well as the impact of families on bariatric surgery outcomes.
Our study has several limitations. Our sample was predominantly White and may not reflect families of other races. The sample size was small to assess the feasibility of recruiting families and our data were collected preoperatively only. Future studies could follow patients over time to evaluate the impact of social support and familial dietary and activity patterns on weight loss, as well as changes in social support and familial health behaviors. To date, three studies [8, 9, 40] have examined changes in weight in spouses of bariatric surgery patients, with mixed results. Two studies [9, 39] found that obese spouses experienced reductions in weight, while a third [40] found that many obese spouses gained weight and postulated that spouses may consume the patients’ leftover food.
A family-based intervention for bariatric surgery patients and their families could be a novel avenue for obesity treatment. Walters-Bugbee and colleagues [26] found that postoperative mothers modeled healthy eating for their children, suggesting that patients’ behavioral changes have the potential to impact their families. Most adult family members in our study were overweight or obese and lived a largely sedentary lifestyle, but appeared engaged in helping the patient adopt behavioral changes. Bariatric surgery patients may be more motivated to implement and sustain healthful changes if supported by family members also making healthy changes. Approaching this type of obesity intervention using a family-systems framework [42] extends the reach of surgical weight loss interventions beyond the patient, leverages the motivation of a patient seeking bariatric surgery, and targets the home environment to promote behavior change.
Acknowledgments
Funding Support for this project was provided by internal grants at Geisinger and NIH grants 1F32DK096756 and t P30 DK072488. These funding sources did not play any role in the study design, collection, analysis, interpretation of data, writing the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest The authors declare that they have no conflicts of interest.
Compliance with Ethical Standards
Ethical Statements This study was approved by the Medical Center’s Institutional Review Board (IRB) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Reilly JJ, et al. Early life risk factors for obesity in childhood: cohort study. BMJ. 2005;330(7504):1357. doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams TD, et al. Familial aggregation of morbid obesity. Obes Res. 1993;1(4):261–70. doi: 10.1002/j.1550-8528.1993.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 3.Reed DR, Bradley EC, Price RA. Obesity in families of extremely obese women. Obes Res. 1993;1(3):167–72. doi: 10.1002/j.1550-8528.1993.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Reed DR, Price RA. Familial risk ratios for extreme obesity: implications for mapping human obesity genes. Int J Obes Relat Metab Disord. 1997;21(10):935–40. doi: 10.1038/sj.ijo.0800498. [DOI] [PubMed] [Google Scholar]
- 5.Katzmarzyk PT, Hebebrand J, Bouchard C. Spousal resemblance in the Canadian population: implications for the obesity epidemic. Int J Obes Relat Metab Disord. 2002;26(2):241–6. doi: 10.1038/sj.ijo.0801870. [DOI] [PubMed] [Google Scholar]
- 6.Jeffery RW, Rick AM. Cross-sectional and longitudinal associations between body mass index and marriage-related factors. Obes Res. 2002;10(8):809–15. doi: 10.1038/oby.2002.109. [DOI] [PubMed] [Google Scholar]
- 7.Gorin AA, et al. Weight loss treatment influences untreated spouses and the home environment: evidence of a ripple effect. Int J Obes (Lond) 2008;32(11):1678–84. doi: 10.1038/ijo.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodard GA, et al. Halo effect for bariatric surgery: collateral weight loss in patients’ family members. Arch Surg. 2011;146(10):1185–90. doi: 10.1001/archsurg.2011.244. [DOI] [PubMed] [Google Scholar]
- 9.Willmer M, et al. Changes in BMI and psychosocial functioning in partners of women who undergo gastric bypass surgery for obesity. Obes Surg. 2015;25(2):319–24. doi: 10.1007/s11695-014-1398-4. [DOI] [PubMed] [Google Scholar]
- 10.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370–9. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 11.Hruschka DJ, et al. Shared norms and their explanation for the social clustering of obesity. Am J Public Health. 2011;101(Suppl 1):S295–300. doi: 10.2105/AJPH.2010.300053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen-Cole E, Fletcher JM. Is obesity contagious? Social networks vs. environmental factors in the obesity epidemic. J Health Econ. 2008;27(5):1382–7. doi: 10.1016/j.jhealeco.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98(2):310–57. [PubMed] [Google Scholar]
- 14.Mechanick JI, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient©—2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Surg Obes Relat Dis. 2013;9(2):159–91. doi: 10.1016/j.soard.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Herpertz S, et al. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obes Res. 2004;12(10):1554–69. doi: 10.1038/oby.2004.195. [DOI] [PubMed] [Google Scholar]
- 16.Vallis MT, Ross MA. The role of psychological factors in bariatric surgery for morbid obesity: identification of psychological predictors of success. Obes Surg. 1993;3(4):346–59. doi: 10.1381/096089293765559025. [DOI] [PubMed] [Google Scholar]
- 17.Delin CR, Watts JM, Bassett DL. An exploration of the outcomes of gastric bypass surgery for morbid obesity: patient characteristics and indices of success. Obes Surg. 1995;5(2):159–70. doi: 10.1381/096089295765557962. [DOI] [PubMed] [Google Scholar]
- 18.Shiri S, et al. Positive psychological impact of bariatric surgery. Obes Surg. 2007;17(5):663–8. doi: 10.1007/s11695-007-9111-5. [DOI] [PubMed] [Google Scholar]
- 19.Larsen JK, et al. Psychosocial functioning before and after laparoscopic adjustable gastric banding: a cross-sectional study. Obes Surg. 2003;13(4):629–36. doi: 10.1381/096089203322190871. [DOI] [PubMed] [Google Scholar]
- 20.Lanyon RI, Maxwell BM. Predictors of outcome after gastric bypass surgery. Obes Surg. 2007;17(3):321–8. doi: 10.1007/s11695-007-9059-5. [DOI] [PubMed] [Google Scholar]
- 21.Valley V, Grace DM. Psychosocial risk factors in gastric surgery for obesity: identifying guidelines for screening. Int J Obes. 1987;11(2):105–13. [PubMed] [Google Scholar]
- 22.Canetti L, Berry EM, Elizur Y. Psychosocial predictors of weight loss and psychological adjustment following bariatric surgery and a weight-loss program: the mediating role of emotional eating. Int J Eat Disord. 2009;42(2):109–17. doi: 10.1002/eat.20592. [DOI] [PubMed] [Google Scholar]
- 23.Ray EC, et al. Predicting success after gastric bypass: the role of psychosocial and behavioral factors. Surgery. 2003;134(4):555–63. doi: 10.1016/s0039-6060(03)00279-4. discussion 563–4. [DOI] [PubMed] [Google Scholar]
- 24.Livhits M, et al. Is social support associated with greater weight loss after bariatric surgery?: a systematic review. Obes Rev. 2011;12(2):142–8. doi: 10.1111/j.1467-789X.2010.00720.x. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch AG, et al. Collateral weight loss in children living with adult bariatric surgery patients: a case control study. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walters-Bugbee SE, et al. Maternal child feeding practices and eating behaviors of women with extreme obesity and those who have undergone bariatric surgery. Surg Obes Relat Dis. 2012;8(6):784–91. doi: 10.1016/j.soard.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Lopes VP, et al. Actigraph calibration in obese/overweight and type 2 diabetes mellitus middle-aged to old adult patients. J Phys Act Health. 2009;6(Suppl 1):S133–40. doi: 10.1123/jpah.6.s1.s133. [DOI] [PubMed] [Google Scholar]
- 28.Choi L, et al. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–64. doi: 10.1249/MSS.0b013e3181ed61a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee IM, Shiroma EJ. Using accelerometers to measure physical activity in large-scale epidemiological studies: issues and challenges. Br J Sports Med. 2014;48(3):197–201. doi: 10.1136/bjsports-2013-093154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troiano RP, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 31.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 32.Sallis JF, et al. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–36. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 33.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27(12):1692–7. [PubMed] [Google Scholar]
- 36.Ginde SR, et al. Air displacement plethysmography: validation in overweight and obese subjects. Obes Res. 2005;13(7):1232–7. doi: 10.1038/oby.2005.146. [DOI] [PubMed] [Google Scholar]
- 37.CDC. BMI percentile calculator for child and teen English version. [cited 2015 August 4] [Google Scholar]
- 38.Swain DP American College of Sports Medicine. ACSM’s resource manual for Guidelines for exercise testing and prescription. 7. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. p. 862. [Google Scholar]
- 39.Berglind D, et al. Women undergoing Rouxen-Y gastric bypass surgery: family resemblance in pre- to postsurgery physical activity and sedentary behavior in children and spouses. Surg Obes Relat Dis. 2015;11(3):690–6. doi: 10.1016/j.soard.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Madan AK, Turman KA, Tichansky DS. Weight changes in spouses of gastric bypass patients. Obes Surg. 2005;15(2):191–4. doi: 10.1381/0960892053268426. [DOI] [PubMed] [Google Scholar]
- 41.Kiernan M, et al. Social support for healthy behaviors: scale psychometrics and prediction of weight loss among women in a behavioral program. Obesity (Silver Spring) 2012;20(4):756–64. doi: 10.1038/oby.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitzman-Ulrich H, et al. The integration of a family systems approach for understanding youth obesity, physical activity, and dietary programs. Clin Child Fam Psychol Rev. 2010;13(3):231–53. doi: 10.1007/s10567-010-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]