Abstract
The importance of renal ammonia metabolism in acid-base homeostasis is well known. However, the effects of renal ammonia metabolism other than in acid-base homeostasis are not as widely recognized. First, ammonia differs from almost all other solutes in the urine in that it does not result from arterial delivery. Instead, ammonia is produced by the kidney and only a portion of the ammonia produced is excreted in the urine. The remainder is returned to the systemic circulation through the renal veins. In normal individuals, systemic ammonia addition is metabolized efficiently by the liver, but in patients with either acute or chronic liver disease, conditions that increase renal ammonia addition to the systemic circulation can cause precipitation and/or worsening of hyperammonemia. Second, ammonia appears to serve as an intra-renal paracrine signaling molecule. Hypokalemia increases proximal tubule ammonia production and secretion and it increases reabsorption in the thick ascending limb of the loop of Henle, thereby increasing delivery to the renal interstitium and the collecting duct. In the collecting duct, ammonia decreases potassium secretion and stimulates potassium reabsorption, thereby decreasing urinary potassium excretion and enabling feedback correction of the initiating hypokalemia. Finally, hypokalemia’s stimulation of renal ammonia metabolism and hypokalemia contributes to development of metabolic alkalosis, which can stimulate NaCl reabsorption and thereby contribute to the intravascular volume expansion, increased blood pressure and diuretic resistance that can develop with hypokalemia. In this review, we discuss the evidence supporting these novel non-acid-base roles of renal ammonia metabolism.
Introduction
Renal ammonia1 metabolism has a critical role in mammalian biology. Ammonia’s role as a critical component of both basal net acid excretion and the response to acid-base disorders, such as metabolic acidosis, is well known. Less well recognized is that ammonia produced in the kidney can be added to the systemic circulation and increase systemic ammonia. In patients with impaired hepatic ammonia metabolism, this may lead to either precipitation or worsening of hyperammonemic (hepatic) encephalopathy. Also less well recognized is that intrarenal ammonia may function as a paracrine signaling molecule. It is produced in the proximal tubule through mechanisms regulated by extracellular potassium and then acts in the collecting duct to alter collecting duct potassium secretion in a manner that allows feedback regulation of the initiating potassium disorder. In this review, we discuss the processes regarding these effects of ammonia that are independent of its traditional view in systemic acid-base homeostasis.
Brief review of ammonia’s role in acid-base homeostasis
Overview of acid-base homeostasis
Acid-base homeostasis is necessary for the normal maintenance of health. Disordered acid-base homeostasis leads to a wide variety of complications, including failure to thrive, skeletal osteopenia and/or osteoporosis, recurrent nephrolithiasis, impaired thyroid hormone action and impaired glucose tolerance [1, 2]. The kidney’s contribution to acid-base homeostasis involves two fundamental processes: reabsorption of filtered bicarbonate, and generation of new bicarbonate [3, 4]. Filtered bicarbonate reabsorption is necessary for acid-base homeostasis, but it is not sufficient. This is because systemic bicarbonate is used to buffer endogenous acid production, resulting in depletion of bicarbonate stores. Thus, even with 100 % reabsorption of filtered bicarbonate, the kidneys need to generate “new” bicarbonate to maintain acid-base homeostasis. New bicarbonate generation is equivalent to net acid excretion, and involves the separate processes of titratable acid excretion and ammonia excretion. Under basal conditions, ammonia excretion is the quantitatively larger component of new bicarbonate generation, and in response to acid-base disorders, almost all of the change in new bicarbonate generation is attributable to variations in ammonia metabolism [5, 6]. Organic anion excretion can also contribute to acid-base homeostasis, but in humans it is quantitatively a substantially lesser component [3, 7, 8].
Renal ammonia metabolism
The renal handling of ammonia differs fundamentally from that of almost every other urinary solute. Other solutes are delivered to the kidney via the renal artery, and undergo variable components of glomerular filtration and renal epithelial cell transport that result in urinary excretion. This results in a renal vein content less than renal arterial content. Moreover, urinary excretion is only a minor component of filtered load (Table I). Renal ammonia metabolism uses a different paradigm. Total filtered ammonia, assuming an arterial ammonia of 25 µmol/L [9] and a normal adult glomerular filtration rate (GFR), 120 mL/minute, is 4.32 mmol/24 hours; this contrasts with the normal urinary ammonia excretion of ~30 mmol/24 hours [10]. Thus, the majority of urinary ammonia excretion does not result from glomerular filtration.
Table I.
Common urine solutes*
| Solute | Units | Filtered per day | Urinary excretion, per day |
Percent of filtered load excreted in urine |
|---|---|---|---|---|
| Water | L | 180 | ~1 | ~0.6 % |
| Na+ | mmol | 25,200 | 100–200 | 0.4 – 0.8 % |
| K+ | mmol | 720 | 60 | 8.3 % |
| HCO3− | mmol | 4,320 | 0 | 0 % |
| Ammonia | mmol | 4.3 | 30 | ~700 % |
Calculations are based upon normal adult glomerular filtration rate (GFR), 120 mL/min, serum Na+ 140 mmol/liter, serum K+ 4.0 mmol/L, serum bicarbonate 24 mmol/L. Urinary solute excretion is variable, and typical norms are shown.
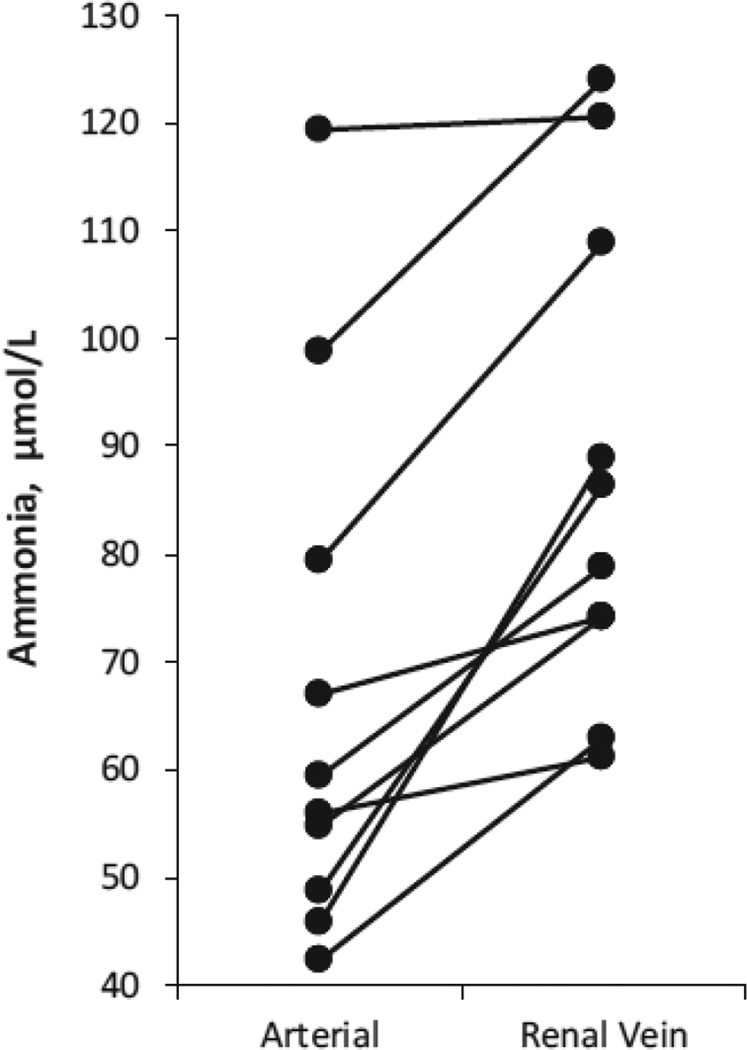
Kidneys actually generate ammonia from the metabolism of specific amino acids, predominantly glutamine, with a lesser component from alanine metabolism [4, 6, 11]. The proximal tubule is the primary site of ammonia generation, and is the site of changes in ammonia generation in response to physiologic stimuli [12–16]. Ammonia generation from glutamine, when carried to its completion, results in generation of two NH4+ and two bicarbonate molecules [6]. Several recent reviews of the mechanisms of renal ammonia generation are available for the interested reader [3, 4, 6, 11]. The bicarbonate generated is added to the systemic circulation through the renal vein, thereby serving as a source of “new bicarbonate”. Ammonia generated in the kidney is either excreted into the urine or enters peritubular capillaries and is added to the systemic circulation via the renal vein [3, 6, 17, 18]. As a result, the kidneys, even though they excrete ammonia, also act as a source of systemic ammonia generation (Figure 1).
Figure 1. Renal vein ammonia concentration is higher than renal arterial ammonia, indicating systemic ammonia addition of renal origin.
Measurements of arterial and renal vein ammonia concentrations were made in ten patients. Data calculated from results presented in [19]. All patients had impaired liver function, likely contributing to mild elevation in arterial ammonia levels.
Differential handling of the ammonia generated in the kidneys, urinary excretion versus systemic addition, has a critical effect on the acid-base aspects of ammonia metabolism. Ammonia added to the systemic circulation undergoes, in the individual with normal hepatic function, rapid hepatic metabolism; this is necessary to maintain low arterial ammonia levels and prevent hyperammonemic encephalopathy. There are two pathways of hepatic ammonia metabolism, one produces urea and the other glutamine [20–24]. The ureagenic pathway uses equal amounts of bicarbonate and NH4+. As a result, the bicarbonate produced during renal ammonia generation is used in the hepatic metabolism of ammonia to urea. Ammonia can also be metabolized by glutamine synthetase, in an enzymatic process that regenerates glutamine. However, this reaction generates one H+ for each NH4+ metabolized [25], and the H+ is then buffered by bicarbonate. Consequently, hepatic ammonia metabolism, whether resulting in urea or glutamine production, consumes an equal amount of bicarbonate as was made during the renal generation of ammonia. Thus, only the ammonia generated in the kidneys and excreted in the urine is associated with a net addition of bicarbonate to the extracellular body fluids.
Clinical implications of renal ammonia addition to the systemic circulation
Renal ammonia addition to the systemic circulation has important clinical implications. The acid-base effect has been described above. The second effect is related to changes in arterial ammonia concentrations. In individuals with intact hepatic function, rapid hepatic metabolism prevents significant changes in arterial ammonia [26–28]. However, in patients with acute or chronic liver disease, who have an impaired ability to metabolize ammonia, increased renal ammonia addition may lead to hyperammonemia. The renal contribution to hyperammonemia probably occurs in a wide variety of conditions, including hypokalemia, gastrointestinal bleeding, high protein diet and valproic acid-induced hyperammonemia.
Hypokalemia
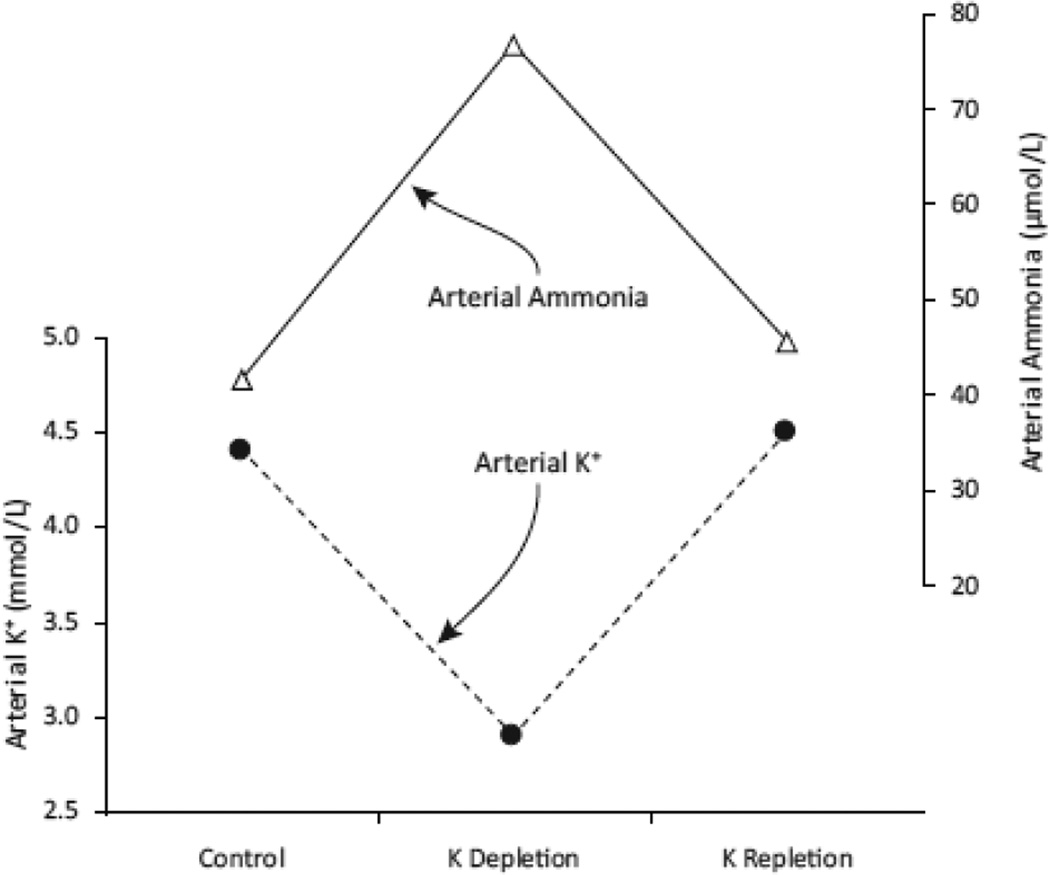
Hypokalemia is one of the more common electrolyte disorders observed in clinical medicine. It occurs in 3–8 % of hospitalized patients, and the incidence is increased in patients treated with diuretics, whether for hypertension, heart failure or other volume overload conditions [29–31]. Not as well-recognized, however, is that hypokalemia also can increase arterial ammonia levels in patients with liver disease (Figure 2). In one study, K+ depletion increased arterial ammonia levels from ~45 to ~80 µmol/L, and these effects were reversible with K+ supplementation [34]. This appears to occur because of changes in renal ammonia metabolism. Specifically, hypokalemia increases renal ammonia generation, urinary ammonia excretion, and, importantly, systemic ammonia addition from the kidneys [32, 33]. Studies in animal models show that hypokalemia stimulates all major components of ammonia metabolism, renal glutamine uptake, ammonia generation and multiple components of renal epithelial cell ammonia transport [35–38]. The mechanism through which hypokalemia stimulates these components has not been definitively determined. Some [39, 40], but not other [41], studies have shown that hypokalemia causes intracellular acidosis in renal cortical, presumably proximal tubule, cells, and this might be the proximate signal initiating increased renal ammoniagenesis.
Figure 2. Hypokalemia increases arterial ammonia levels in patients with cirrhosis.
Data showing effects of diuresis-induced potassium depletion and then correction by either diet or diet with supplemental potassium salts. All changes are statistically significant. Data from [32, 33]. All patients had impaired liver function, likely contributing to mild elevation in arterial ammonia levels.
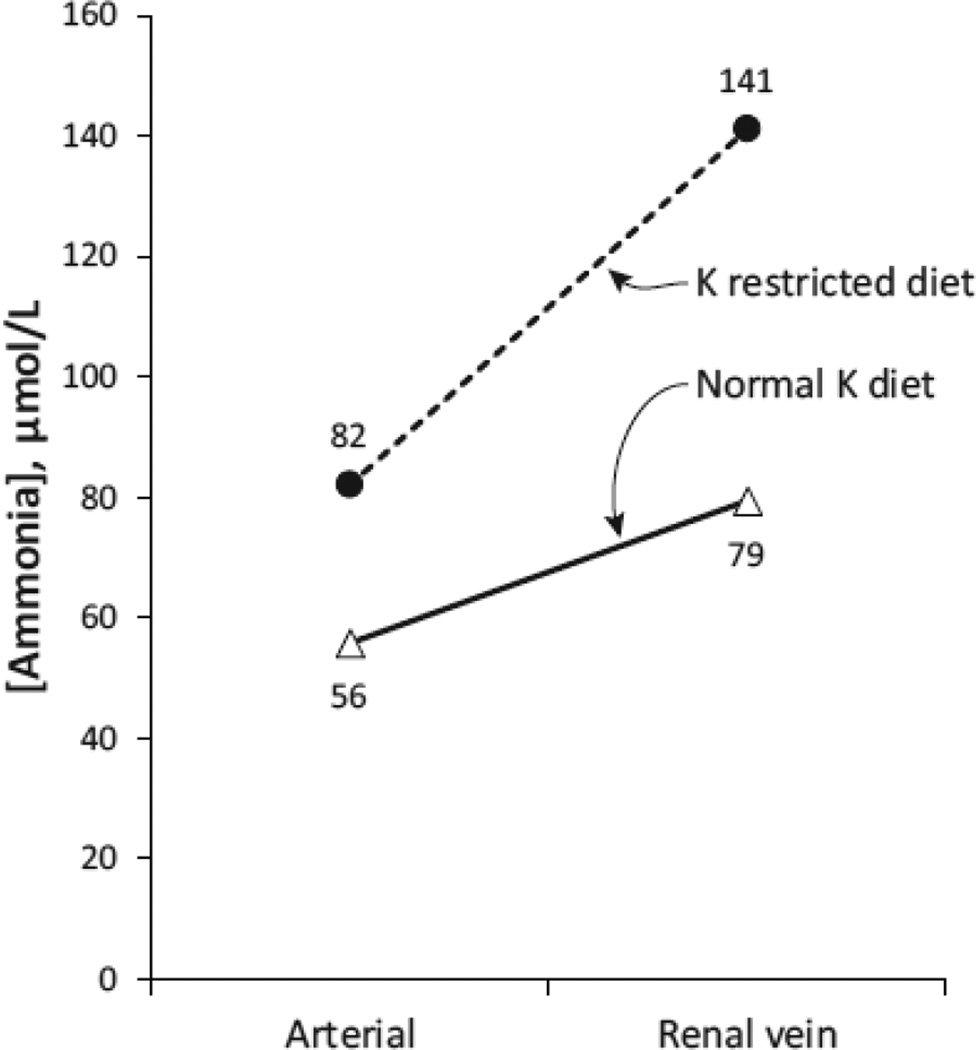
This stimulation of renal ammonia metabolism increases systemic ammonia addition of renal origin. In one set of studies, patients were studied on a normal potassium diet and following induction of a potassium restricted diet [32]. Arterial ammonia levels and renal venous ammonia levels were measured to assess renal ammonia addition to the systemic circulation (Figure 3). While on a normal potassium diet, renal ammonia generation and systemic addition increased renal vein ammonia concentration above renal arterial ammonia concentration by an average of 23 µmol/L. When hypokalemia was induced by a potassium restricted diet, the systemic ammonia addition of renal origin, measured as the increase in the concentration of ammonia in the renal venous fluid, increased ~2.5 fold. Although renal plasma flow was not determined, it is highly unlikely that hypokalemia decreased renal plasma flow by more than 70 %, which would be necessary to account for this change in renal vein ammonia. These human studies have been extended by animal studies showing that dialysis-induced hypokalemia substantially increases systemic ammonia addition of renal origin [32].
Figure 3. Hypokalemia increases systemic ammonia addition of renal origin.
Representative example of the effect of spontaneous hypokalemia induced by dietary potassium restriction on systemic ammonia addition of renal origin. Results from patient with chronic liver disease on normal potassium diet (65 mEq per day) and low potassium diet (30 mEq per day). Serum potassium decreased from 3.5 to 2.7 mEq per liter. There was no significant change in arterial pH. Data from [32]. All patients had impaired liver function, likely contributing to mild elevation in arterial ammonia levels.
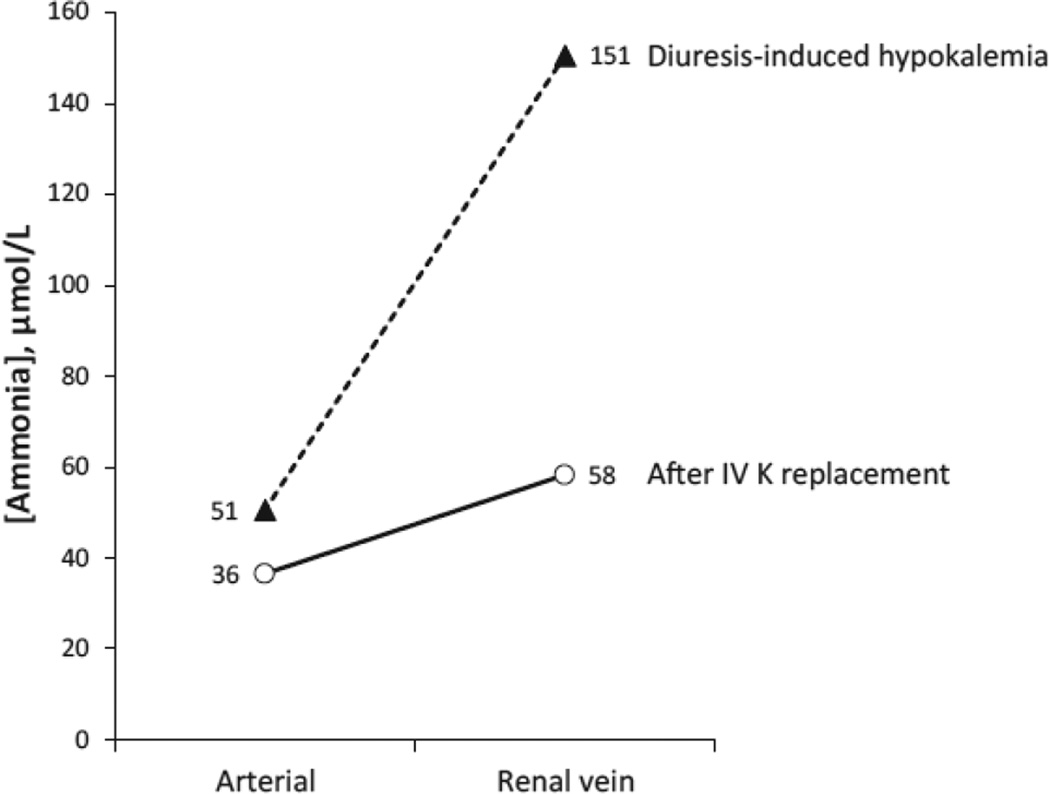
Another experimental model tested whether the effects of diuresis-induced hypokalemia and systemic ammonia addition of renal origin were reversible with acute intravenous potassium replacement [32]. During the diuresis-induced hypokalemia, ammonia concentration in the renal vein exceeded that in the renal artery by an average of 100 µmol/L (Figure 4). Following intravenous potassium replacement, the increment decreased substantially, to an amount similar to that observed in the patients on a normal potassium diet in the previous study, 22 µmol/L. Hypokalemia’s stimulation of renal-to-systemic ammonia addition appears to be rapidly reversible with correction of the hypokalemia.
Figure 4. Effect of intravenous K+ replacement on renal ammonia addition to systemic circulation.
Representative example of effect of correcting hypokalemia with intravenous K+ on systemic ammonia addition of renal origin. Acute intravenous potassium replacement decreases arterial ammonia concentration and decreases systemic ammonia addition of renal origin, as indicated by the decrease in the gradient between arterial and renal vein ammonia concentration. Data from [32]. All patients had impaired liver function, likely contributing to mild elevation in arterial ammonia levels.
Gastrointestinal bleeding
Another clinical condition associated with precipitation and/or worsening of hepatic (hyperammonemic) encephalopathy is gastrointestinal bleeding. Traditionally, the increase in arterial ammonia levels during gastrointestinal bleed is thought to result from increased colonic ammonia production. However, increased systemic ammonia addition of renal origin likely also contributes substantially.
In one study, intestinal and renal ammonia balance was measured in patients with cirrhosis and active variceal bleeding [42]. Intestinal tract net ammonia consumption averaged 121 nmol/kilogram/minute. When extrapolated to a 70 kg individual, this is an average rate of ammonia consumption of 12 mmol/24 hours. Thus, worsening hyperammonemia could not be attributed in this circumstance to net intestinal ammonia production. Unfortunately, the measurements of “net intestinal ammonia production” in this study resulted from measurement of arterial ammonia, hepatic vein ammonia and hepatic venous blood flow rates.
However, these authors did assess renal ammonia production by measuring arterial ammonia, renal vein ammonia and renal plasma flow [42]. They documented net ammonia production of 572 nmol/kilogram/minute. When extrapolated over 24 hours in a 70 kg individual, this is equivalent to systemic ammonia addition of renal origin of 58 mmol/24 hours. Because similar measurements were not made in similarly ill patients without active bleeding, one cannot conclude definitively that renal ammonia addition was increased. However, this rate of renal ammonia addition, ~60 mmol/d, is ~2-fold higher than the average of 30 mmol/d typically seen.
In the same study, a more detailed analysis of the site of ammonia generation during a GI bleed was assessed during more controlled circumstances, i.e., not during an acute GI bleed [42]. These authors studied patients with biopsy-proven cirrhosis undergoing assessment of transjugular intrahepatic portosystemic shunt (TIPSS) patency. They measured arterial ammonia, intestinal tract-specific (i.e., not involving the liver) ammonia production and renal ammonia production, and did so before and after a simulated GI bleed. They mimicked the GI bleed by providing an intestinal load of 46 g of an amino acid solution designed to mimic the amino acid composition of hemoglobin, administered via a nasogastric tube. Intestinal tract ammonia production increased over the four hour time course of the experiment, from ~600 nmol/kilogram/minute to ~1000 nmol/kilogram/minute, i.e., a 400 nmol/kilogram/minute increase. In addition, renal ammonia addition to the systemic circulation increased from ~100 nmol/kilogram/minute to ~600 nmol/kilogram/minute. These findings indicate that nasogastric administration of an amino acid load, designed to mimic hemoglobin, stimulates renal ammonia generation and addition to the systemic circulation that is at least as much, if not greater, than the increase in intestinal ammonia production.
Whether these changes in ammonia addition also occur in response to changes in dietary protein has been addressed indirectly in studies assessing the renal response to dietary protein changes. High dietary protein increases multiple components of renal ammonia production and metabolism, and low-protein diets decreases renal ammonia metabolism [43–45]. These effects on renal ammonia metabolism are paralleled by changes in urinary ammonia excretion, and appear to reflect a renal response designed to maintain acid-base homeostasis in the face of varying levels endogenous acid production that result from changes in dietary protein metabolism. The finding, discussed above, that nasogastric amino acid administration increases systemic ammonia addition of renal origin suggests that changes in dietary protein intake will cause parallel changes, but this has not been determined specifically.
Valproic acid encephalopathy
Valproic acid is an effective anticonvulsant used in many types of epilepsy and is usually well tolerated, but occasionally causes hyperammonemic encephalopathy in the absence of overt liver disease. As many as 10–15 % of pediatric patients treated with valproic acid develop hyperammonemia [46]. At least in part this appears to be due to renal ammonia generation. Valproic acid, and several of its metabolites, increase renal glutamine uptake and renal ammonia production quite dramatically [47–49]. The increased ammonia production results in both an increase in urinary ammonia excretion and a simultaneous quantitatively similar increase in systemic ammonia addition arising from the kidneys [50–55]. Another study demonstrated the critical role of renal ammonia addition by showing that valproic acid-induced hyperammonemia did not occur in animals with a previous bilateral nephrectomy [55]. There is also likely to be a hepatic contribution, resulting from impaired hepatic ammonia metabolism, that contributes to the hyperammonemia that develops.
Intrarenal roles of ammonia other than acid-base homeostasis
A variety of studies over many years have investigated the observation of a correlation between potassium disorders and abnormal ammonia metabolism. As discussed previously, hypokalemia stimulates renal ammonia metabolism and increases urinary ammonia excretion. Simultaneous with the changes in urinary ammonia excretion are changes in potassium excretion [56, 57].
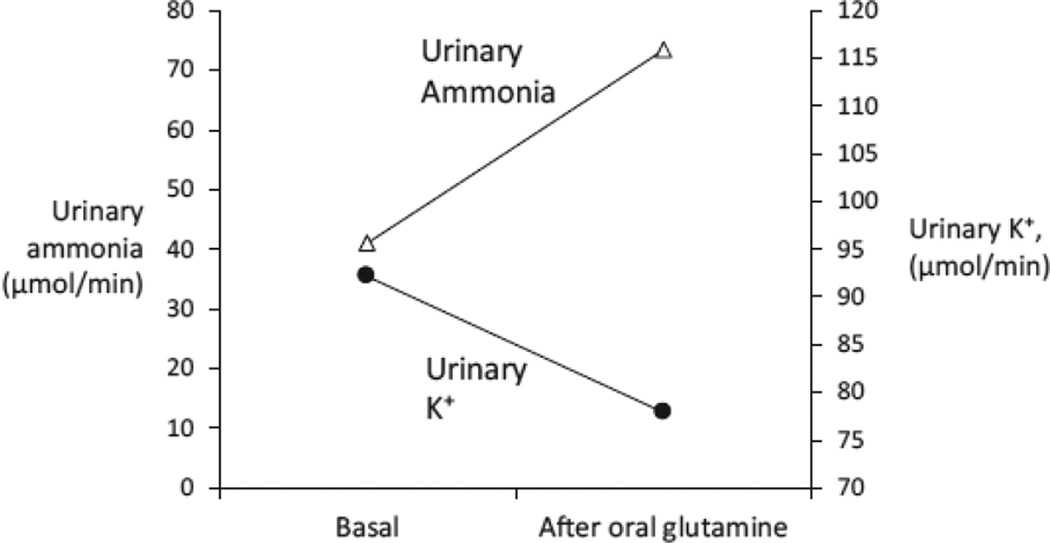
Tannen and colleagues investigated whether these were causally related by stimulating renal ammonia metabolism independent of changes in systemic acid-base status or serum potassium [58]. They took advantage of the finding that glutamine is limiting for renal ammonia metabolism, under both basal and acidotic conditions, and stimulated renal ammonia generation by administering glutamine. They found that glutamine administration increased urinary ammonia excretion rapidly, and simultaneously decreased urinary potassium excretion (Figure 5). The potassium conservation could not be accounted for by changes in urinary sodium excretion or in plasma potassium or acid-base parameters. Quantitatively, the change in urinary ammonia excretion, an increase of ~32 µmole/minute, was significantly greater than the change in urinary potassium excretion, a decrease of ~14 µmol/minute. Thus, the change in K+ excretion could not be simply attributed to a renal K+ for NH4+ exchange process. These results suggested that there might be a correlation between effects of renal ammonia metabolism and renal potassium handling.
Figure 5. Effect of stimulating renal ammonia metabolism with glutamine on urinary ammonia and K+ excretion.
After ingesting a constant-formula diet of normal electrolyte content for 3 days, normal men ingested glutamine, 4.3 mmol/kg. Glutamine ingestion resulted in an increase in urinary ammonia excretion and a concomitant decrease in potassium excretion. Data from [58].
Further studies in animal models sought to determine where in the kidney changes in ammonia metabolism altered potassium transport. In these studies, intravenous glutamine administration to rats had the exact same effect on urinary ammonia and potassium excretion as observed in humans [59]. Free-flow micropuncture was used to assess the site along the nephron/collecting duct where potassium transport was altered. Glutamine administration did not significantly alter the fractional delivery of potassium to the micropuncturable distal tubule, indicating no change in combined proximal tubule and loop of Henle K+ reabsorption [59]. Instead, all of the regulation of potassium handling occurred distal to the “micropuncturable distal tubule,” a region which includes portions of the distal convoluted tubule (DCT) and connecting segment and the entire collecting duct. In the absence of glutamine stimulation, there was net potassium secretion. However, following glutamine treatment, there was net potassium reabsorption. Thus, stimulating ammonia metabolism with glutamine decreased renal potassium excretion and all of the effects on potassium transport occurred in the “distal nephron,” i.e., somewhere in the DCT, connecting segment and collecting duct.
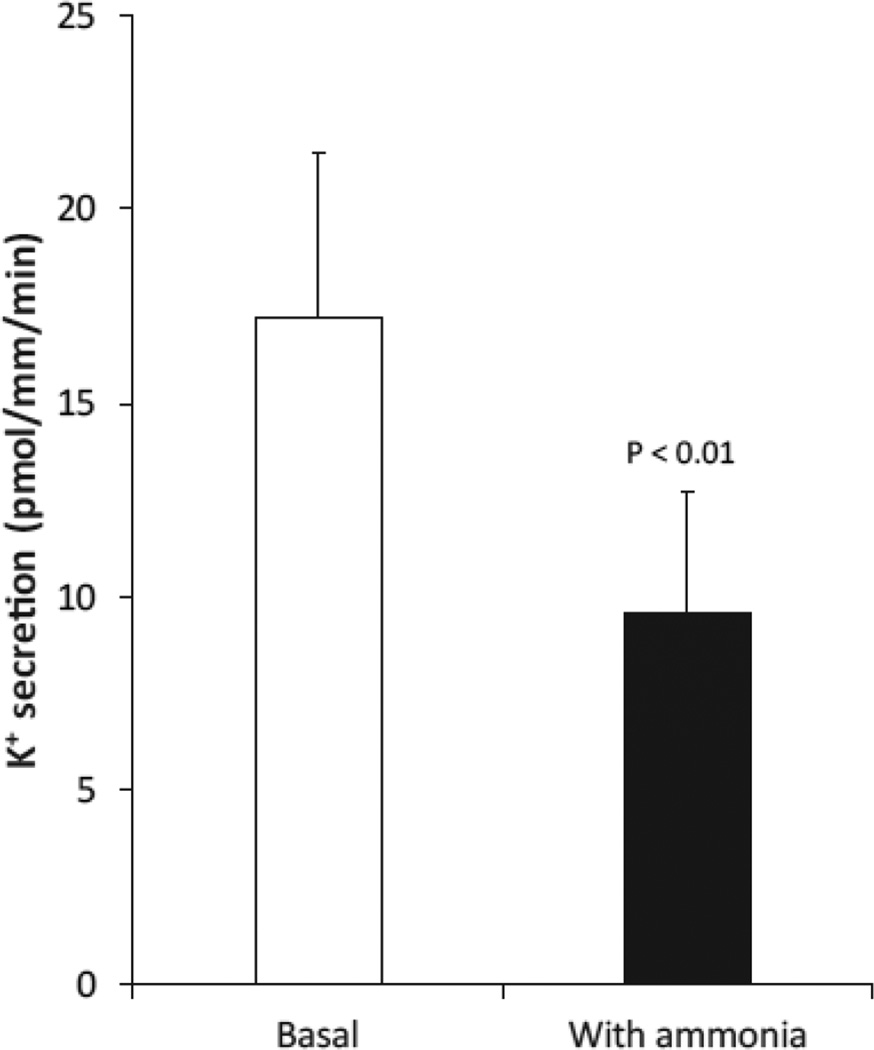
The next set of studies examining this issue, from the laboratory of Hamm et al., used in vitro microperfusion of cortical collecting duct segments [60]. This technique allows one to examine the effects of specific stimuli on transport without having to worry about systemic and/or non-specific effects. They showed that ammonia rapidly and significantly decreased cortical collecting duct potassium secretion by ~40 % (Figure 6). Thus, the effects of glutamine administration increased renal ammonia metabolism and altered “distal tubule” potassium handling, and are attributable, at least in part, to the ability of ammonia to significantly and substantially inhibit net potassium secretion in the cortical collecting duct.
Figure 6. Effects of ammonia on cortical collecting duct (CCD) K+ secretion.
Net K+ secretion was determined in isolated perfused rabbit CCD segments. Ammonia addition to extracellular solutions significantly inhibited net K+ secretion. Data from [60].
Collecting duct potassium handling involves separate components of potassium secretion and potassium reabsorption [29, 61, 62]. Potassium secretion is primarily linked in the principal cell to a mechanism of sodium reabsorption occurring through the sodium channel, ENaC, coupled to potassium secretion. Hamm et al., using the isolated-perfused collecting duct approach, also showed that ammonia significantly and rapidly impaired sodium reabsorption with a similar time course and magnitude as the effects on potassium secretion [60]. Whether this was due to changes in intracellular pH was not examined specifically. However, chronic ammonia exposure decreases intracellular pH in collecting duct intercalated cells [63]. If similar intracellular pH changes occurred in Na+-transporting principal cells, this could explain, at least in part, the inhibition of Na+ reabsorption because decreased intracellular pH is known to inhibit ENaC [64, 65].
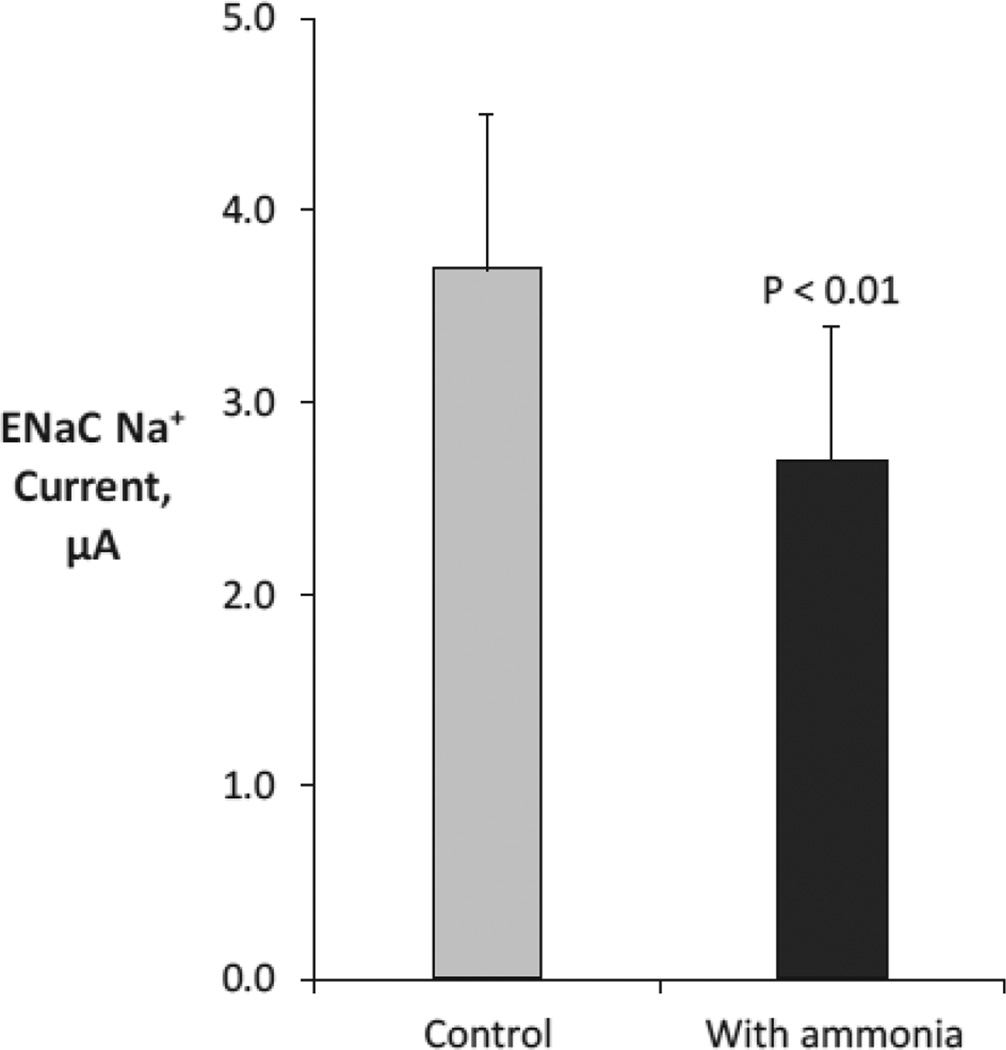
Further studies, using heterologous expression of ENaC in the Xenopus oocyte and detailed electrophysiologic studies, showed that ammonia had direct effects to inhibit ENaC-mediated sodium transport (Figure 7) [66]. This effect was independent of effects of ammonia on intracellular pH. Thus, at least a component of the effects of ammonia on collecting duct potassium secretion can be linked to ammonia acting as a “potassium sparing diuretic” that blunts collecting duct potassium secretion.
Figure 7. Effect of ammonia on ENaC-mediated Na+ transport.
ENaC was expressed in Xenopus oocytes, and effects of ammonia on ENaC-mediated Na+ transport, measured as current, were measured. Data from [66].
Collecting duct potassium handling also involves potassium reabsorption, which occurs primarily via H-K-ATPase [29, 67, 68]. Two sets of studies have addressed the effects of ammonia on this component of potassium handling. Studies in the isolated, perfused collecting duct showed that ammonia stimulated reabsorption of the potassium analog, rubidium [60]. Other studies in the isolated, perfused cortical collecting duct showed that ammonia stimulated proton secretion [63]. There are two mechanisms of apical proton secretion in the cortical collecting duct, H-ATPase and H-K-ATPase. Inhibiting apical H-K-ATPase completely blocked the effects of ammonia on proton secretion, whereas inhibiting H-ATPase did not [63]. Although continuous ammonia exposure caused intracellular acidification in H+-transporting intercalated cells [63], this was unlikely to explain the activation of H-K-ATPase. Specifically, similar changes in intracellular pH through alternative mechanisms not involving changes in either extracellular or intracellular pH did not alter H+ secretion [63]. These observations indicate that ammonia stimulates H-K-ATPase, directly contributing to the increased H+ secretion observed and likely stimulating unidirectional K+ reabsorption.
This effect of ammonia and H-K-ATPase appears to be a specific defect involving specific intracellular signaling pathways. Further studies have shown that ammonia activates H-K-ATPase through mechanisms involving intracellular calcium, microtubules, SNARE proteins and activation of MAPK ([69] and unpublished observations). Although ammonia altered intracellular pH, equivalent intracellular pH changes induced by an alternative, non-specific mechanism did not alter H-K-ATPase activity [63].
Overall, these observations of a correlation between systemic potassium, renal ammonia metabolism and renal potassium transport suggest that ammonia can be considered an intrarenal, paracrine signaling molecule. A paracrine signaling molecule is produced by one set of cells in a tissue in response to a stimuli, and acts on other cells in the same tissue in order to enable correction of the initiating stimuli. Hypokalemia directly stimulates proximal tubule ammonia generation and luminal secretion [38]. Because ammonia is reabsorbed in the thick ascending limb by transport of NH4+ at the K+-binding site of NKCC2 [70], hypokalemia increases ammonia reabsorption, leading to increased interstitial ammonia concentrations, particularly in the cortex and outer medulla [71], the major sites of collecting duct K+ transport. This ammonia can then act in the collecting duct to regulate net K+ transport, inhibiting unidirectional potassium secretion and stimulating unidirectional potassium reabsorption [60, 63, 66, 69, 72]. Thus, ammonia meets all criteria necessary to be identified as a renal paracrine signaling molecule. However, the specific receptor protein(s) through which ammonia initiates these effects in collecting duct intercalated and principal cells has not been identified.
Indirect effects of ammonia metabolism on sodium chloride transport
A third effect of ammonia metabolism other than in acid-base homeostasis has to do with indirect effects of ammonia on renal sodium chloride transport. For this consideration, we’ll return to a non-acid-base-mediated stimulation of ammonia metabolism, hypokalemia. Hypokalemia and/or potassium deficiency is well known to cause sodium chloride retention, mild intravascular volume expansion, increases in blood pressure and diuretic resistance [73–75]. In addition, hypokalemia often results in mild metabolic alkalosis [76, 77]; this results in large part from the increased ammonia metabolism and urinary ammonia excretion, discussed above in detail.
This ammonia-induced effect to stimulate metabolic alkalosis may have significant effects on renal sodium chloride transport. Human clinical studies show that metabolic alkalosis decreases diuretic responsiveness [78]. This effect may occur, at least in part, through a 2-oxoglutarate-dependent pathway. Metabolic alkalosis increases renal excretion of the organic anion, 2-oxoglutarate [79–82], and 2-oxoglutarate stimulates sodium and chloride reabsorption [79]. Detailed studies have shown that luminal 2-oxoglutarate acts through a specific G-protein-coupled receptor, Oxgr1, expressed in the apical membrane of pendrin-positive collecting duct cells, i.e., non-A non-B cells and Type-B intercalated cells [79]. Activation of Oxgr1 by luminal 2-oxoglutarate then stimulates sodium and chloride reabsorption through activation of sodium transporting protein, NDCBE (sodium-dependent chloride bicarbonate exchanger) and the chloride reabsorbing protein, pendrin [79]. By increasing sodium chloride reabsorption, this can explain a component of the hypokalemia-induced sodium chloride retention, volume expansion, increased blood pressure and diuretic resistance.
Another important mechanism through which hypokalemia stimulates salt retention involves the DCT and does not involve ammonia. Hypokalemia increases phosphorylation of NCC [83], which increases its activity [84], leading to increased NaCl reabsorption. This likely occurs because hypokalemia causes hyperpolarization of DCT cells resulting from K+ transport through the basolateral K+ channel, KCNJ10 (Kir4.1) [85]. The hyperpolarization presumably increases basolateral Cl− exit, presumably via the basolateral Cl− channel, ClC-Kb in association with its accessory subunit, barttin [86], decreasing intracellular Cl−. Decreased intracellular Cl− activates the signaling protein WNK4, increasing SPAK phosphorylation, which then phosphorylates and activates the apical Na+-Cl− cotransporter, NCC [83, 87]. At this time, the relative roles of the ammonia-dependent pathway and ammonia-independent pathway in hypokalemia-dependent increases in NaCl reabsorption and intravascular volume expansion has not been studied specifically.
Summary
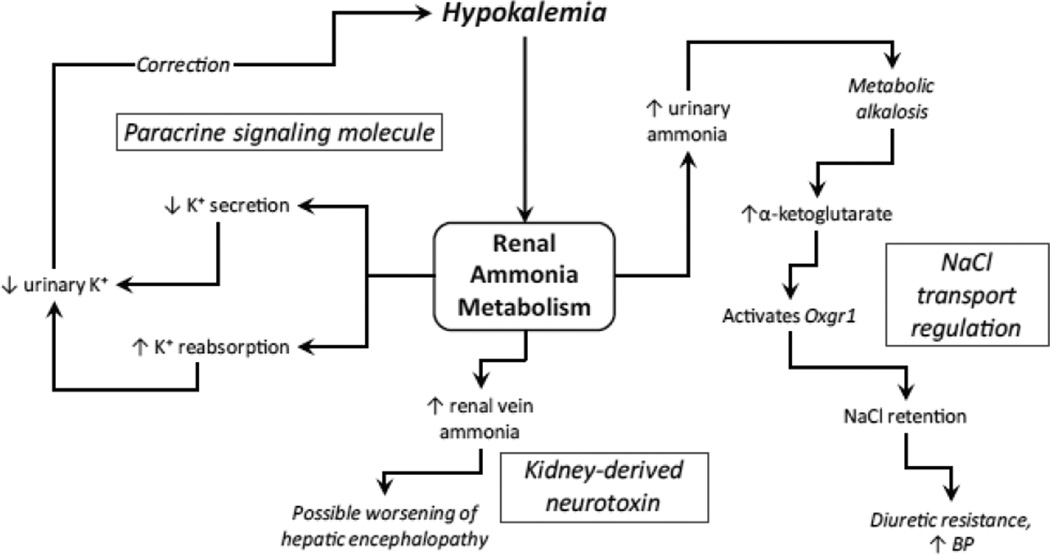
In this review, we have attempted to discuss roles of renal ammonia metabolism other than its traditionally considered role as a component of net acid excretion. We do not mean to diminish the importance of ammonia’s role in acid-base homeostasis. Instead, we wish to highlight additional roles of renal ammonia metabolism (Figure 8). First, the kidneys produce ammonia, and under normal circumstances excrete ~50 % of the produced ammonia into the urine. The remaining proportion of ammonia produced in the kidney leads to systemic ammonia addition of renal origin. In patients at risk of hyperammonemia, such as those with acute or chronic liver disease, this systemic ammonia addition of renal origin can lead to hyperammonemia and subsequent precipitation and/or exacerbation of hepatic encephalopathy. Second, ammonia is a paracrine signaling molecule, produced in response to changes in extracellular potassium, and then regulating collecting duct potassium transport through effects on both principal cell potassium secretion and intercalated cell potassium reabsorption. Finally, stimulation of renal ammonia metabolism, particularly in response to hypokalemia, can be, albeit indirectly, related to its effect in causing generation of metabolic alkalosis, by stimulating collecting duct sodium reabsorption through a 2-oxoglutarate-Oxgr1-NDCBE and pendrin-mediated mechanism.
Figure 8. Effects of renal ammonia metabolism that are in addition to its primary role in systemic acid-base homeostasis.
Acknowledgments
Generation and publication of this review was supported by funds from the NIH (R01–DK045788 and R01–DK107798).
Footnotes
Ammonia can exist in two molecular forms, NH3 and NH4+. When referring to the combination of both molecules, we use the term “ammonia” in this topic review. When referring specifically to molecular form of NH3, we state specifically “NH3.” When referring specifically to molecular form NH4+, we state specifically “NH4+.”
Conflict of interest:
The authors declare that they have no conflict of interest.
References
- 1.Gil-Pena H, Mejia N, Santos F. Renal Tubular Acidosis. J Pediatr. 2013;164:691–698. doi: 10.1016/j.jpeds.2013.10.085. [DOI] [PubMed] [Google Scholar]
- 2.Mitch WE. Metabolic and clinical consequences of metabolic acidosis. J Nephrol. 2006;19:S70–S75. [PubMed] [Google Scholar]
- 3.Weiner ID, Verlander JW. Renal acidification mechanisms. In: Tall MW, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM, Wm B, editors. Brenner and Rector's The Kidney. 10th. New York: Saunders Press; 2015. pp. 234–257. [Google Scholar]
- 4.Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol. 2015;10:2232–2242. doi: 10.2215/CJN.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiner ID, Mitch WE, Sands JM. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol. 2014;10:1444–1458. doi: 10.2215/CJN.10311013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner ID, Verlander JW. Renal Ammonia Metabolism and Transport. Compr Physiol. 2013;3:201–220. doi: 10.1002/cphy.c120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halperin ML, Dhadli SC, Kamel KS. Physiology of Acid-Base Balance: Links With Kidney Stone Prevention. Semin Nephrol. 2006;26:441–446. doi: 10.1016/j.semnephrol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Unwin RJ, Capasso G, Shirley DG. An overview of divalent cation and citrate handling by the kidney. Nephron Physiol. 2004;98:15–20. doi: 10.1159/000080259. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson LS, Broberg S, Bjorkman O, Wahren J. Ammonia metabolism during exercise in man. Clin Physiol. 1985;5:325–336. doi: 10.1111/j.1475-097x.1985.tb00753.x. [DOI] [PubMed] [Google Scholar]
- 10.Elkinton JR, Huth EJ, Webster GD, Jr, McCance RA. The renal excretion of hydrogen ion in renal tubular acidosis. Am J Med. 1960;36:554–575. doi: 10.1016/0002-9343(60)90090-5. [DOI] [PubMed] [Google Scholar]
- 11.Curthoys NP, Moe OW. Proximal Tubule Function and Response to Acidosis. Clin J Am Soc Nephrol. 2014;9:1627–1638. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright PA, Knepper MA. Phosphate-dependent glutaminase activity in rat renal cortical and medullary tubule segments. Am J Physiol. 1990;259:F961–F970. doi: 10.1152/ajprenal.1990.259.6.F961. [DOI] [PubMed] [Google Scholar]
- 13.Wright PA, Knepper MA. Glutamate dehydrogenase activities in microdissected rat nephron segments: effects of acid-base loading. Am J Physiol. 1990;259:F53–F59. doi: 10.1152/ajprenal.1990.259.1.F53. [DOI] [PubMed] [Google Scholar]
- 14.Nagami GT. Ammonia production and secretion by S3 proximal tubule segments from acidotic mice: role of ANG II. Am J Physiol Renal. 2004;287:F707–F712. doi: 10.1152/ajprenal.00189.2003. [DOI] [PubMed] [Google Scholar]
- 15.Nagami GT, Sonu CM, Kurokawa K. Ammonia production by isolated mouse proximal tubules perfused in vitro: effect of metabolic acidosis. J Clin Invest. 1986;78:124–129. doi: 10.1172/JCI112540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagami GT. Role of angiotensin II in the enhancement of ammonia production and secretion by the proximal tubule in metabolic acidosis. Am J Physiol Renal. 2008;294:F874–F880. doi: 10.1152/ajprenal.00286.2007. [DOI] [PubMed] [Google Scholar]
- 17.Weiner ID, Verlander JW. Ammonia transport in the kidney by Rhesus glycoproteins. Am J Physiol Renal. 2014;306:F1107–F1120. doi: 10.1152/ajprenal.00013.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal. 2011;300:F11–F23. doi: 10.1152/ajprenal.00554.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owen EE, Tyor MP, Flanagan JF, Berry JN. The kidney as a source of blood ammonia in patients with liver disease: the effect of acetazolamide. J Clin Invest. 1960;39:288–294. doi: 10.1172/JCI104039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haussinger D, Lamers WH, Moorman AF. Hepatocyte heterogeneity in the metabolism of amino acids and ammonia. Enzyme. 1992;46:72–93. doi: 10.1159/000468779. [DOI] [PubMed] [Google Scholar]
- 21.Haussinger D. Glutamine metabolism in the liver: overview and current concepts. Metabolism. 1989;38:14–17. doi: 10.1016/0026-0495(89)90133-9. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser S, Gerok W, Haussinger D. Ammonia and glutamine metabolism in human liver slices: new aspects on the pathogenesis of hyperammonaemia in chronic liver disease. Eur J Clin Invest. 1988;18:535–542. doi: 10.1111/j.1365-2362.1988.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 23.Haussinger D. Structural-functional organization of hepatic glutamine and ammonium metabolism. Biochem Soc Trans. 1987;15:369–372. doi: 10.1042/bst0150369. [DOI] [PubMed] [Google Scholar]
- 24.Haussinger D. Regulation of hepatic ammonia metabolism: the intercellular glutamine cycle. Adv Enzyme Regul. 1986;25:159–180. doi: 10.1016/0065-2571(86)90013-0. [DOI] [PubMed] [Google Scholar]
- 25.Verlander JW, Chu D, Lee HW, Handlogten ME, Weiner ID. Expression of glutamine synthetase in the mouse kidney: localization in multiple epithelial cell types and differential regulation by hypokalemia. Am J Physiol Renal. 2013;305:F701–F713. doi: 10.1152/ajprenal.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koen H, Okuda K, Musha H, Tateno Y, Fukuda N, Matsumoto T, Shisido F, Rikitake T, Iinuma T, Kurisu A, Arimizu N. A dynamic study of rectally absorbed ammonia in liver cirrhosis using ammonia and a positron camera. Dig Dis Sci. 1980;25:842–848. doi: 10.1007/BF01338526. [DOI] [PubMed] [Google Scholar]
- 27.Cooper AJ. Ammonia metabolism in normal and portacaval-shunted rats. Adv Exp Med Biol. 1990;272:23–46. doi: 10.1007/978-1-4684-5826-8_2. [DOI] [PubMed] [Google Scholar]
- 28.Conn HO. Studies of the source and significance of blood ammonia. IV. Early ammonia peaks after ingestion of ammonium salts. Yale J Biol Med. 1972;45:543–549. [PMC free article] [PubMed] [Google Scholar]
- 29.Gumz ML, Rabinowitz L, Wingo CS. An Integrated View of Potassium Homeostasis. N Engl J Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol. 2011;7:75–84. doi: 10.1038/nrneph.2010.175. [DOI] [PubMed] [Google Scholar]
- 31.Weiner ID, Wingo CS. Hypokalemia - consequences, causes and correction. J Am Soc Nephrol. 1997;8:1179–1188. doi: 10.1681/ASN.V871179. [DOI] [PubMed] [Google Scholar]
- 32.Gabuzda GJ, Hall II. Relation of potassium depletion to renal ammonium metabolism and hepatic coma. Medicine (Baltimore) 1966;45:481–489. doi: 10.1097/00005792-196645060-00011. [DOI] [PubMed] [Google Scholar]
- 33.Shear L, Gabuzda GJ. Potassium deficiency and endogenous ammonium overload from kidney. Am J Clin Nutr. 1970;23:614–618. doi: 10.1093/ajcn/23.5.614. [DOI] [PubMed] [Google Scholar]
- 34.Baertl JM, Sancetta SM, Gabuzda GJ. Relation of acute potassium depletion to renal ammonium metabolism in patients with cirrhosis. J Clin Invest. 1963;42:696–706. doi: 10.1172/JCI104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han KH, Lee HW, Handlogten ME, Bishop JM, Levi M, Kim J, Verlander JW, Weiner ID. Effect of Hypokalemia on Renal Expression of the Ammonia Transporter Family Members, Rh B Glycoprotein and Rh C Glycoprotein, in the Rat Kidney. Am J Physiol Renal. 2011;301:F823–F832. doi: 10.1152/ajprenal.00266.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busque SM, Wagner CA. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal. 2009;297:F440–F450. doi: 10.1152/ajprenal.90318.2008. [DOI] [PubMed] [Google Scholar]
- 37.Hossain SA, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal. 2011;301:F969–F978. doi: 10.1152/ajprenal.00010.2011. [DOI] [PubMed] [Google Scholar]
- 38.Nagami GT. Effect of bath and luminal potassium concentration on ammonia production and secretion by mouse proximal tubules perfused in vitro. J Clin Invest. 1990;86:32–39. doi: 10.1172/JCI114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adam WR, Koretsky AP, Weiner MW. 31P-NMR in vivo measurement of renal intracellular pH: effects of acidosis and K+ depletion in rats. Am J Physiol. 1986;251:F904–F910. doi: 10.1152/ajprenal.1986.251.5.F904. [DOI] [PubMed] [Google Scholar]
- 40.Jones B, Simpson DP. Influence of alterations in acid-base conditions on intracellular pH of intact renal cortex. Ren Physiol. 1983;6:28–35. doi: 10.1159/000172878. [DOI] [PubMed] [Google Scholar]
- 41.Schoolwerth AC, Culpepper RM. Measurement of intracellular pH in suspensions of renal tubules from potassium-depleted rats. Miner Electrolyte Metab. 1990;16:191–196. [PubMed] [Google Scholar]
- 42.Olde Damink SW, Jalan R, Deutz NE, Redhead DN, Dejong CH, Hynd P, Jalan RA, Hayes PC, Soeters PB. The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with cirrhosis. Hepatology. 2003;37:1277–1285. doi: 10.1053/jhep.2003.50221. [DOI] [PubMed] [Google Scholar]
- 43.Bounoure L, Ruffoni D, Muller R, Kuhn GA, Bourgeois S, Devuyst O, Wagner CA. The Role of the Renal Ammonia Transporter Rhcg in Metabolic Responses to Dietary Protein. J Am Soc Nephrol. 2014;25:2040–2052. doi: 10.1681/ASN.2013050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brosnan JT, McPhee P, Hall B, Parry DM. Renal glutamine metabolism in rats fed high-protein diets. Am J Physiol. 1978;235:E261–E265. doi: 10.1152/ajpendo.1978.235.3.E261. [DOI] [PubMed] [Google Scholar]
- 45.Lee HW, Osis G, Handlogten ME, Guo H, Verlander JW, Weiner ID. Effect of dietary protein restriction on renal ammonia metabolism. Am J Physiol Renal Physiol. 2015;308:F1463–F1473. doi: 10.1152/ajprenal.00077.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue K, Takahashi T, Yamamoto Y, Suzuki E, Takahashi Y, Imai K, Inoue Y, Hirai K, Tsuji D, Itoh K. Influence of glutamine synthetase gene polymorphisms on the development of hyperammonemia during valproic acid-based therapy. Seizure. 2015;33:76–80. doi: 10.1016/j.seizure.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Elhamri M, Ferrier B, Martin M, Baverel G. Effect of valproate, sodium 2-propyl-4-pentenoate and sodium 2-propyl-2-pentenoate on renal substrate uptake and ammoniagenesis in the rat. J Pharmacol Exp Ther. 1993;266:89–96. [PubMed] [Google Scholar]
- 48.Martin G, Durozard D, Besson J, Baverel G. Effect of the antiepileptic drug sodium valproate on glutamine and glutamate metabolism in isolated human kidney tubules. Biochim Biophys Acta. 1990;1033:261–266. doi: 10.1016/0304-4165(90)90130-o. [DOI] [PubMed] [Google Scholar]
- 49.Doval M, Culebras M, Lopez-Farre A, Rengel M, Gougoux A, Vinay P, Lopez-Novoa JM. Effect of valproate on lactate and glutamine metabolism by rat renal cortical tubules. Proc Soc Exp Biol Med. 1989;190:357–364. doi: 10.3181/00379727-190-42872. [DOI] [PubMed] [Google Scholar]
- 50.Marini AM, Zaret BS, Beckner RR. Hepatic and renal contributions to valproic acid-induced hyperammonemia. Neurology. 1988;38:365–371. doi: 10.1212/wnl.38.3.365. [DOI] [PubMed] [Google Scholar]
- 51.Rengel M, Doval M, Culebras M, Gougoux A, Vinay P, Lopez-Novoa JM. Ammoniagenesis and valproic acid in the rat in vivo: role of the kidney. Contrib Nephrol. 1988;63:132–135. doi: 10.1159/000415710. [DOI] [PubMed] [Google Scholar]
- 52.Imler M, Chabrier G, Marescaux C, Warter JM. Effects of 2,4-dinitrophenol on renal ammoniagenesis in the rat. Eur J Pharmacol. 1986;123:175–179. doi: 10.1016/0014-2999(86)90657-6. [DOI] [PubMed] [Google Scholar]
- 53.Warter JM, Brandt C, Marescaux C, Rumbach L, Micheletti G, Chabrier G, Krieger J, Imler M. The renal origin of sodium valproate-induced hyperammonemia in fasting humans. Neurology. 1983;33:1136–1140. doi: 10.1212/wnl.33.9.1136. [DOI] [PubMed] [Google Scholar]
- 54.Warter JM, Marescaux C, Brandt C, Rumbach L, Micheletti G, Chabrier G, Imler M, Kurtz D. Sodium valproate associated with phenobarbital: effects on ammonia metabolism in humans. Epilepsia. 1983;24:628–633. doi: 10.1111/j.1528-1157.1983.tb03428.x. [DOI] [PubMed] [Google Scholar]
- 55.Warter JM, Imler M, Marescaux C, Chabrier G, Rumbach L, Micheletti G, Krieger J. Sodium valproate-induced hyperammonemia in the rat: Role of the kidney. Eur J Pharmacol. 1983;87:177–182. doi: 10.1016/0014-2999(83)90327-8. [DOI] [PubMed] [Google Scholar]
- 56.Tannen RL. Relationship of renal ammonia production and potassium homeostasis. Kidney Int. 1977;11:453–465. doi: 10.1038/ki.1977.63. [DOI] [PubMed] [Google Scholar]
- 57.Tannen RL. The effect of uncomplicated potassium depletion on urine acidification. J Clin Invest. 1970;49:813–827. doi: 10.1172/JCI106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tannen RL, Terrien T. Potassium-sparing effect of enhanced renal ammonia production. Am J Physiol. 1975;228:699–705. doi: 10.1152/ajplegacy.1975.228.3.699. [DOI] [PubMed] [Google Scholar]
- 59.Jaeger P, Karlmark B, Giebisch G. Ammonium transport in rat cortical tubule: relationship to potassium metabolism. Am J Physiol. 1983;245:F593–F600. doi: 10.1152/ajprenal.1983.245.5.F593. [DOI] [PubMed] [Google Scholar]
- 60.Hamm LL, Gillespie C, Klahr S. NH4Cl inhibition of transport in the rabbit cortical collecting tubule. Am J Physiol. 1985;248:F631–F637. doi: 10.1152/ajprenal.1985.248.5.F631. [DOI] [PubMed] [Google Scholar]
- 61.Wang WH, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch. 2009;458:157–168. doi: 10.1007/s00424-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muto S. Potassium Transport in the Mammalian Collecting Duct. Physiol Rev. 2001;81:85–116. doi: 10.1152/physrev.2001.81.1.85. [DOI] [PubMed] [Google Scholar]
- 63.Frank AE, Wingo CS, Weiner ID. Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am J Physiol Renal. 2000;278:F219–F226. doi: 10.1152/ajprenal.2000.278.2.F219. [DOI] [PubMed] [Google Scholar]
- 64.Chalfant ML, Denton JS, Berdiev BK, Ismailov II, Benos DJ, Stanton BA. Intracellular H+ regulates the alpha-subunit of ENaC, the epithelial Na+ channel. Am J Physiol. 1999;276:C477–C486. doi: 10.1152/ajpcell.1999.276.2.C477. [DOI] [PubMed] [Google Scholar]
- 65.Konstas AA, Mavrelos D, Korbmacher C. Conservation of pH sensitivity in the epithelial sodium channel (ENaC) with Liddle's syndrome mutation. Pflugers Arch. 2000;441:341–350. doi: 10.1007/s004240000430. [DOI] [PubMed] [Google Scholar]
- 66.Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Ammonium interaction with the epithelial sodium channel. Am J Physiol Renal. 2001;281:F493–F502. doi: 10.1152/ajprenal.2001.281.3.F493. [DOI] [PubMed] [Google Scholar]
- 67.Greenlee MM, Lynch IJ, Gumz ML, Cain BD, Wingo CS. The renal H,K-ATPases. Curr Opin Nephrol Hypertens. 2010;19:478–482. doi: 10.1097/MNH.0b013e32833ce65f. [DOI] [PubMed] [Google Scholar]
- 68.Weiner ID, Linus S, Wingo CS. Disorders of potassium metabolism. In: Johnson RJ, Fluege J, Feehally J, editors. Comprehensive Clinical Nephrology. 4. Saunders, USA: 2010. pp. 118–129. [Google Scholar]
- 69.Frank AE, Wingo CS, Andrews PM, Ageloff S, Knepper MA, Weiner ID. Mechanisms through which ammonia regulates cortical collecting duct net proton secretion. Am J Physiol Renal. 2002;282:F1120–F1128. doi: 10.1152/ajprenal.00266.2001. [DOI] [PubMed] [Google Scholar]
- 70.Good DW. Ammonium transport by the loop of Henle. Miner Electrolyte Metab. 1990;16:291–298. [PubMed] [Google Scholar]
- 71.Wall SM, Davis BS, Hassell KA, Mehta P, Park SJ. In rat tIMCD, NH4+ uptake by the Na+, K+-ATPase is critical to net acid secretion during chronic hypokalemia. Am J Physiol. 1999;277:F866–F874. doi: 10.1152/ajprenal.1999.277.6.F866. [DOI] [PubMed] [Google Scholar]
- 72.Frank AE, Weiner ID. Effects of ammonia on acid-base transport by the B-type intercalated cell. J Am Soc Nephrol. 2001;12:1607–1614. doi: 10.1681/ASN.V1281607. [DOI] [PubMed] [Google Scholar]
- 73.Barri YM, Wingo CS. The effects of potassium depletion and supplementation on blood pressure: a clinical review. Am J Med Sci. 1997;314:37–40. doi: 10.1097/00000441-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Krishna GG, Kapoor SC. Potassium depletion exacerbates essential hypertension. Ann Intern Med. 1991;115:77–83. doi: 10.7326/0003-4819-115-2-77. [DOI] [PubMed] [Google Scholar]
- 75.Krishna GG. Effect of potassium intake on blood pressure. J Am Soc Nephrol. 1990;1:43–52. doi: 10.1681/ASN.V1143. [DOI] [PubMed] [Google Scholar]
- 76.Hernandez RE, Schambelan M, Cogan MG, Colman J, Morris RC, Sebastian A. Dietary NaCl determines severity of potassium depletion-induced metabolic alkalosis. Kidney Int. 1987;31:1356–1367. doi: 10.1038/ki.1987.150. [DOI] [PubMed] [Google Scholar]
- 77.Cremer W, Bock KD. Symptoms and course of chronic hypokalemic nephropathy in man. Clin Nephrol. 1977;7:112–119. [PubMed] [Google Scholar]
- 78.Loon NR, Wilcox CS. Mild metabolic alkalosis impairs the natriuretic response to bumetanide in normal human subjects. Clin Sci (Lond) 1998;94:287–292. doi: 10.1042/cs0940287. [DOI] [PubMed] [Google Scholar]
- 79.Tokonami N, Morla L, Centeno G, Mordasini D, Ramakrishnan SK, Nikolaeva S, Wagner CA, Bonny O, Houillier P, Doucet A, Firsov D. a-Ketoglutarate regulates acid-base balance through an intrarenal paracrine mechanism. J Clin Invest. 2013;123:3166–3171. doi: 10.1172/JCI67562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin M, Ferrier B, Baverel G. Transport and utilization of alpha-ketoglutarate by the rat kidney in vivo. Pflugers Arch. 1989;413:217–224. doi: 10.1007/BF00583533. [DOI] [PubMed] [Google Scholar]
- 81.Ferrier B, Martin M, Baverel G. Reabsorption and secretion of alpha-ketoglutarate along the rat nephron: a micropuncture study. Am J Physiol. 1985;248:F404–F412. doi: 10.1152/ajprenal.1985.248.3.F404. [DOI] [PubMed] [Google Scholar]
- 82.Balagura S, Pitts RF. Renal handling of a-ketoglutarate by the dog. Am J Physiol. 1964;207:483–494. doi: 10.1152/ajplegacy.1964.207.2.483. [DOI] [PubMed] [Google Scholar]
- 83.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int. 2016;89:127–134. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadchouel J, Ellison DH, Gamba G. Regulation of Renal Electrolyte Transport by WNK and SPAK-OSR1 Kinases. Annu Rev Physiol. 2016;78:367–389. doi: 10.1146/annurev-physiol-021115-105431. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1) Proc Natl Acad Sci U S A. 2014;111:11864–11869. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Subramanya AR, Ellison DH. Distal Convoluted Tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bazua-Valenti S, Chavez-Canales M, Rojas-Vega L, Gonzalez-Rodriguez X, Vazquez N, Rodriguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, Garcia-Valdes J, Hadchouel J, Gamba G. The Effect of WNK4 on the Na+-Cl−Cotransporter Is Modulated by Intracellular Chloride. J Am Soc Nephrol. 2015;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]