Abstract
Purpose
Poor adherence to recombinant human growth hormone (r-hGH) therapy is associated with reduced growth velocity in children with growth hormone deficiency (GHD). This twelve-month observational study was to assess adherence in r-hGH patients treated with the easypod™, an electronic, fully automated injection device designed to track the time, date and dose administered.
Methods
Ninety-seven prepubertal patients receiving r-hGH therapy were included in the study from ten Italian clinical sites and 88 completed the study. To avoid possible confounding effects, only GHD patients (79/88; 89.7 % of the overall study population) were considered in the final analysis. The primary endpoint—adherence to treatment—was calculated as the proportion of injections correctly administered during the observational period out of the expected total number of injections. The relevant information, tracked by the easypod™, was collected at months 6 (V1) and 12 (V2) after baseline (V0). At study termination, adherence data were partially available from 16 patients and fully available from 53 patients. As secondary endpoints, serum IGF-1 levels, fasting serum glucose and insulin levels and key anthropometric characteristics (height, waist circumference and BMI) were also determined.
Results
The easypod™ data showed that 56.7 % of the patients were considered to be fully (≥92 %) adherent to their treatment throughout the period V0–V2. Treatment improved stature, significantly increased IGF-1 and produced a non-significant increase in blood glucose and insulin levels.
Conclusions
The injection-recording system and other characteristics of easypod™ could enhance the ability of physicians to monitor adherence to r-hGH treatment.
Keywords: Growth disorders (GD), IGF-1, Easypod™, Adherence
Introduction
Growth hormone (GH) has been used as an elective treatment for severely GH-deficient children and adolescents since the 1960s [1]. Due to the limited availability of human pituitary-derived hormone, GH use for other conditions related to short stature could not be seriously considered until the mid-1980s, when recombinant human GH (r-hGH) became available, thus opening up access to GH treatment to children and adolescents with causes of short stature other than GH deficiency (GHD) [2]. The indications for r-hGH in Italy are limited to the following conditions [3]: GHD, growth failure in girls with gonadal dysgenesis (Turner Syndrome), growth failure in prepubertal children due to chronic renal failure (CRF) and failure of growth in short children born small for gestational age (SGA) [4]. The marketing authorisation (MA) of some marketed products has been extended to additional indications (e.g. Prader–Willi syndrome and short stature associated with altered function of the SHOX gene) [4].
The need for frequent injections over a long period of time has stimulated research into easier methods of administration, to improve patients’ adherence to their therapy. Non-adherence, as well as low adherence, is unavoidably associated with both individual and social treatment failures, such as less favourable clinical outcomes, lower quality of life and higher healthcare costs [5].
Several devices for r-hGH administration have been developed over time. So far, five broad categories of GH injection device are available, including syringes with needle, injection pens, self-injection pens, needle-free devices and electronic devices. As reported in a recent survey by patients, parents, physicians and nurses who had experience with administration of r-hGH, an optimal r-hGH device should fulfil the following characteristics: reliability; ease of use; lack of pain during injection; safety in use and storage and minimum number of steps before injection preparation. In addition, a good tracking system, allowing effective and objective monitoring of treatment adherence, was considered extremely important by the physicians [6–10].
Materials and methods
This was an observational, prospective study with the primary objective of monitoring adherence to r-hGH treatment for 1 year in prepubertal patients with growth disorders. The patients enrolled all received r-hGH therapy with the easypod™ Clinical Kit, a system comprising an electronic, automated injection device (easypod™) with a docking station for recording r-hGH administration data to enable objective monitoring of actual drug usage. The secondary objectives were to monitor the effect of r-hGH treatment on serum IGF-1 concentrations, fasting serum glucose and insulin and on anthropometric characteristics (height, waist circumference and BMI).
The first patient was enrolled on the 19th of March 2010; the last study visit was performed on the 28th of January 2013. The study was approved by the ad hoc local ethics committees, and informed consent was obtained by patient’s parents or legal guardians. Eligibility for the study was based on the following inclusion criteria:
Prepubertal patients with short stature (under 14 years of age) with growth disorders receiving r-hGH therapy (according to the local SmPC), who were either naïve or unsatisfied with their current device and were candidates to continue r-hGH therapy with easypod™ according to clinical practice;
Receiving r-hGH prescribed according to the local SmPC;
Written informed consent obtained from the parent(s)/legal guardian(s) at the beginning of the study.
Exclusion criteria included acquired GHD due to CNS tumour, infection, pituitary infiltration, history of cranial or spinal irradiation or cranial surgery; previous treatment with corticosteroids, except for topical or inhaled administration for atopic disease and/or for hormonal substitution at a stable dosage for at least 3 months and concomitant significant diseases.
Approximately 100 patients were estimated as a suitable sample size for the assessment of their adherence to treatment. Due to a slow recruitment rate, enrolment was stopped at the attainment of 97 participants. After one screening failure, a total of 96 patients were actually recruited. Eight patients did not complete the observational period for various reasons, so 88 patients completed the study.
Due to the small number of patients with conditions other than GHD, and in order to avoid possible confounding effects, only the 79 patients with GHD were included in the analysis data set. Patients could elect to discontinue their participation at any time. The disposition of the patients is summarised in Table 1.
Table 1.
Patient disposition
| Status | Frequency | Percentage |
|---|---|---|
| Screened | 97 | 100 |
| Screening failure | 1 | 1 |
| Enrolled | 96 | 99 |
| Early termination | 8 | 8 |
| Completed | 88 | 92 |
| Analysis data set (only GHD patients | 79 | 82 |
The study design is summarised in Fig. 1:
Fig. 1.
Study design and plan
The study duration for each recruited patient was 1 year, unless they discontinued prematurely.
Each patient was enrolled in the study at baseline visit, after the assessment of eligibility criteria. Easypod™ devices were supplied by Merck Serono SpA, sponsor of the study. A support service, provided by the sponsor, was guaranteed to the enrolled patients in order to train them in correct device usage and replacement procedures, should malfunction of the device occur during the study.
Pursuant to the observational nature of the study, the r-hGH treatment was administered according to routine clinical practice, independent of the patient’s participation in the study. The outcome measurements for both the primary and the secondary endpoints were assessed at visits V0, V1 and V2, respectively. The adherence to the treatment for each patient was estimated as the proportion of injections correctly administered during the observational period out of the expected total number of injections. The target rate for full adherence was defined as ≥92 % at the start of the study.
Adherence was calculated only for patients reporting at least 150 injections every 6 months (at least 300 injections throughout the overall 12-month observational period). The adherence rate was calculated as follows:
The statistical analysis was performed with SAS® software, version 9.2 (SAS Institute, Cary, NC, USA). As this was an observational study, no distinction between intention to treat and per protocol data sets was made, no subsets were identified and only descriptive statistical analysis was performed. IGF-1 concentrations were measured by local laboratories using standard assays. IGF-1 SDS was calculated using the normative data for the method [11].
Results
Within an overall study population composed of 88 enrolled subjects, 79 patients, all with GHD, were included in the final analysis. Of these, 52 (66 %) were male and 27 (34 %) were female. Median age at enrolment was 10 years (interquartile range 9–12).
Adherence to treatment
Adherence data were available from 53/79 (67.09 %) participants for the whole 12-month study period and from 16/79 (20.25 %) patients for a 6-month follow-up (either from V0–V1 or from V1–V2). Only 16 patients reported ≥150 injections over 6 months between V0–V1 and V1–V2. Overall, 30/53 patients reported a total number of injections ≥300 across the whole observation period. Easypod™ data showed that 17/30 (56.67 %) patients administering at least 300 injections across the 1-year follow-up period completed the study with the preset target adherence rate 92 %. With respect to the length of the follow-up period, administration data collected through the easypod™ were available from 28/53 (52.83 %) adherent patients. Relevant results are summarised in Tables 2 and 3.
Table 2.
Adherence by number of injections
| Adherence by number of injections (V0–V2) | ||||
|---|---|---|---|---|
| Adherence rate | Number of injections | |||
| <300 | ≥300 | Total | ||
| Frequency (%) | <92 % | 12 (22.64 %) | 13 (24.53 %) | 25 (47.17 %) |
| Frequency (%) | ≥92 % | 11 (20.7 %) | 17 (32.1 %) | 28 (52.8 %) |
| Total (%) | 23 (43.4 %) | 30 (56.6 %) | 53 (100 %) | |
Table 3.
Adherence by follow-up period
| Adherence | Number of subjects (%) | |
|---|---|---|
| Between V0 and V1 | Not adherent | 29 (45.3) |
| Adherent | 35 (54.7) | |
| Between V1 and V2 | Not adherent | 24 (41.4) |
| Adherent | 34 (58.6) | |
| Between V0 and V2 (whole study period) | Not adherent | 25 (47.2) |
| Adherent | 28 (52.8) |
Changes from baseline of height SDS were evaluated, showing a significant increase in height across the 12 months of follow-up (Table 4).
Table 4.
Changes from baseline in height SDS
| Changes from baseline | n | Mean | Standard deviation | Median | Interquartile range | p value |
|---|---|---|---|---|---|---|
| Height SDS V0 | 77 | −2.2 | 0.8 | −2.2 | −2.67 to −1.79 | |
| Height SDS V2 | 76 | −1.7 | 0.7 | −1.7 | −2.33 to −1.27 | |
| Height SDS V2–V0 | 75 | 0.5 | 0.3 | 0.5 | 0.25–0.64 | <0.0001 |
No correlation was found, using a linear regression model, between the change in height SDS among fully adherent patients (300 injections in the whole period) and the adherence rate (coefficient β = 0.01241, p = 0.123).
Serum glucose and insulin concentrations increased slightly, but not significantly, from baseline to V1 and V2, while IGF-1 significantly increased, as expected (Table 5).
Table 5.
Changes from baseline in IGF-1 blood glucose and insulin levels
| Changes from baseline and between visits | n | Mean | Standard deviation | Median | Interquartile range | p value |
|---|---|---|---|---|---|---|
| IGF-1 V0 (ng/mL) | 67 | 204 | 129 | 183 | 116–256.3 | |
| IGF-1 V1 (ng/mL) | 52 | 278 | 136 | 275 | 185.5–356.75 | |
| IGF-1 V1–V0 | 50 | 89 | 131 | 77 | 19–154 | <0.0001 |
| IGF-1 V2 (ng/mL) | 63 | 290 | 143 | 292 | 162–374 | |
| IGF-1 V2–V0 | 54 | 97 | 148 | 88 | 42 – 158 | <0.0001 |
| Blood glucose V0 (mg/dL) | 40 | 82 | 9 | 81 | 77–87 | |
| Blood glucose V1 (mg/dL) | 56 | 86 | 9 | 87 | 81–92 | |
| Blood glucose V1–V0 | 39 | 2 | 9 | 1 | −4 to 8 | 0.2262 |
| Blood glucose V2 (mg/dL) | 63 | 89 | 42 | 87 | 80–91 | |
| Blood glucose V2–V0 | 38 | 3 | 11 | 2 | −5 to 9 | 0.2043 |
| Insulin V0 (μU/mol) | 37 | 6 | 5 | 5 | 3–8.25 | |
| Insulin V1 (μU/mol) | 45 | 7 | 4 | 7 | 3.7–9.5 | |
| Insulin V1–V0 | 29 | 1 | 4 | 1 | −0.9 to 4 | 0.1459 |
| Insulin V2 (μU/mol) | 51 | 8 | 6 | 7 | 4.99–10 | |
| Insulin V2–V0 | 28 | 1 | 4 | 1 | −0.76 to 3.8 | 0.1628 |
Assessment of anthropometric characteristics revealed a statistically significant increase in height both at visit V1 and V2 (Table 6).
Table 6.
Changes from baseline in height parameters
| Changes from baseline and between visits | n | Mean | Standard deviation | Median | Interquartile range | p value |
|---|---|---|---|---|---|---|
| Height V0 (cm) | 79 | 126 | 16 | 129 | 118.7–136.2 | |
| Height V1 (cm) | 79 | 130 | 16 | 133 | 122.3–141 | |
| Height V1–V0 | 79 | 4 | 2 | 4 | 3.3–5.5 | <0.0001 |
| Height V2 (cm) | 79 | 134 | 15 | 137 | 126.7–144.6 | |
| Height V2–V0 | 79 | 8 | 2 | 8 | 6.7–9.5 | <0.0001 |
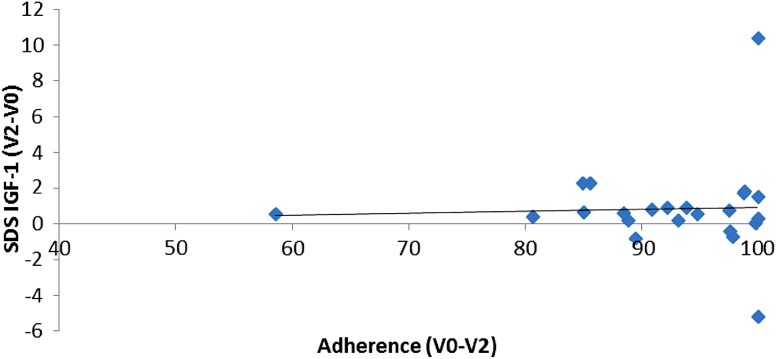
A linear regression model showed no relationship between the changes observed in IGF-1 standard deviation score (IGF-1 SDS) and the adherence rate of patients with at least 300 injections in the whole period (coefficient β = 0.01122, p = 0.8517). Detailed results are summarised in Fig. 2.
Fig. 2.
Linear regression IGF-1 SDS/adherence
Discussion
In this study, we report the adherence rate measured by easypod in the 53 of 79 prepubertal patients with GHD (52 [66 %] boys, 27 [34 %] girls) for whom data were available, who had completed >300 injections over 12 months. Demographic characteristics of the patients included were consistent with the usual profile of easypod™ Clinical Kit users. The majority of patients showed good adherence to treatment, better than that reported in previous studies [12, 13]. However, it must be stated that our study was prospective, and we used an objective method to measure adherence. In addition, it must be pointed out that the patients (and their parents) were aware that adherence to treatment was being monitored and this fact may have influenced the outcome. We found no correlation between change in height SDS and the adherence rate. This may be due to the fact that the great majority of patients had a high adherence rate, and the number of patients was too small to find a correlation with a parameter which varies very little.
As expected, we found a slight but non-significant increase in serum insulin and glucose concentrations. A number of studies have shown that such an increase has no clinical significance [14, 15]. IGF-1 significantly increased but always remained within the normal range of concentrations.
The data collected by easypod™ Clinical Kit showed that 56.67 % of patients were fully adherent (adherence 92 %) to treatment for the whole period of observation (V0–V2). Our results did not show a relationship between the changes observed in IGF-1 SDS and the adherence rate of patients with at least 300 injections in the whole period. Notably, IGF-1 concentrations significantly increased in the whole studied population both at V1 and at V2. Consequently, IGF-1 concentrations were increased to therapeutic levels in the overall population. Further studies are needed, either to specifically assess the minimum number of injections necessary to achieve a therapeutic effect or to compare IGF-1 SDS levels between adherent and non-adherent patients.
Adherence to treatment has been demonstrated to be critical for the achievement of both medical and economic expected outcomes of GH therapy [16–18]. However, Fisher et al. [18] have shown that non-adherence to GH therapy in paediatric patients is affected by several factors, among which the adoption of a needle-free injection device in place of a multi-dose injection pen may not play a crucial role, according to Verrips et al. [19]. Nevertheless, Cutfield et al. [20] have pointed out how subjective (parent-reported) and objective (empty vial count) adherence rates may differ from one another, thus demonstrating unequivocally the usefulness of a device, like the easypod™, that is able to collect objective data.
Conclusions
The results of the present study suggest that easypod™ may represent a helpful option that could assist physicians in effective monitoring of adherence to r-hGH treatment. The treatment resulted in significant changes in height SDS and IGF-1 concentrations. Further studies are needed to compare growth and IGF-1 levels between adherent vs non-adherent patients. Nevertheless, our study demonstrates that, even when therapeutic adherence is not strictly observed, but IGF-1 levels are maintained at therapeutic levels, with modern, easy to use, recombinant hormone formulations, r-hGH supplementation is safe and efficacious.
Acknowledgments
The trial was sponsored by Merck Serono SpA, Rome, Italy. The authors would like to thank the patients and their families, co-investigators and the study teams at each of the participating centres. The authors also thank Francesca Vannini, Clinical Research Manager, Merck Serono SpA, Rome, Italy and Anna Mattia, Clinical Operations Manager, Fullcro s.r.l., Rome, Italy for their support in the study conduction. Medical writing assistance was provided by David Candlish, inScience Communications, Chester, UK, funded by Merck KGaA, Darmstadt, Germany.
Compliance with ethical standards
Conflict of interest
The authors take full responsibility for the content of the paper. All authors have read and approved the final version of the manuscript. Raffaella Perrone is an employee of Merck Serono SpA, Italy, and Salvatore Longobardi is an employee of Merck KGaA, Darmstadt, Germany. Mariacarolina Salerno, Piernicola Garofalo, Giuliana Cardinale, Maria Rosaria Licenziata, Guiseppe Citro, Manuella Caruso Nicoletti, Marco Cappa and Mohamad Maghnie declare no conflicts of interest. Sandro Loche has received fees as a consultant to Merck Serono.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Ayyar Vageesh S. History of growth hormone therapy. Indian J Endocrinol Metab. 2011;15(Suppl3):S162–S165. doi: 10.4103/2230-8210.84852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richmond E, Rogol AD. Current indications for growth hormone therapy for children and adolescents. Endocr Dev. 2010;18:92–108. doi: 10.1159/000316130. [DOI] [PubMed] [Google Scholar]
- 3.SAIZEN 8 mg/mL Italian SmPC, last revised July 2015. https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002392_026863_RCP.pdf&retry=0&sys=m0b1l3
- 4.Farber RS, Kerrigan JR. The multiple indications for growth hormone treatment of pediatric patients. Pediatr Ann. 2006;35(12):926–932. doi: 10.3928/0090-4481-20061201-06. [DOI] [PubMed] [Google Scholar]
- 5.Haverkamp F, Johansson L, Dumas H, et al. Observations of non-adherence to recombinant human growth hormone therapy in clinical practice. Clin Ther. 2008;30:307–316. doi: 10.1016/j.clinthera.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen JT. Improvement of patient convenience in treatment with growth hormone. J Pediatr Endocrinol. 1994;7:175–180. doi: 10.1515/JPEM.1994.7.4.283. [DOI] [PubMed] [Google Scholar]
- 7.Marchisotti FG, Carvalho LR, Berger K, et al. Growth hormone (GH) deficiency treatment in children: comparison between uses of pen versus bottles/syringes on GH administration. Arquiv Bras Endocrinol Metabol. 2007;51:1093–1096. doi: 10.1590/S0004-27302007000700011. [DOI] [PubMed] [Google Scholar]
- 8.Sjoblom K, Albertsson-Wikland K, Bengtsson BA, et al. Patient evaluation of a new injection pen for growth hormone treatment in children and adults. Acta Paediatr Suppl. 1995;411:63–65. doi: 10.1111/j.1651-2227.1995.tb13867.x. [DOI] [PubMed] [Google Scholar]
- 9.Dahlgren J, Veimo D, Johansson L, Bech I. Patient acceptance of a novel electronic auto-injector device to administer recombinant human growth hormone: results from an open-label, user survey of everyday use. Curr Med Res Opin. 2007;23:1649–1655. doi: 10.1185/030079907X210589. [DOI] [PubMed] [Google Scholar]
- 10.Dahlgren J. Easypod: a new electronic injection device for growth hormone. Expert Rev Med Device. 2008;5:297–304. doi: 10.1586/17434440.5.3.297. [DOI] [PubMed] [Google Scholar]
- 11.Elmlinger MW, Kühnel W, Weber MM, Ranke MB. Reference ranges for two automated chemiluminescent assays for serum insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) Clin Chem Lab Med. 2004;42:654–664. doi: 10.1515/CCLM.2004.112. [DOI] [PubMed] [Google Scholar]
- 12.Hindmarsh PC, Brook CG. Compliance with growth hormone treatment—is it a problem? Horm Res. 1999;51(Suppl 3):104–108. doi: 10.1159/000053170. [DOI] [PubMed] [Google Scholar]
- 13.Aydın BK, Aycan Z, Sıklar Z, et al. Adherence to growth hormone therapy: results of a multicenter study. Endocr Pract. 2014;20(1):46–51. doi: 10.4158/EP13194.OR. [DOI] [PubMed] [Google Scholar]
- 14.Balaž M, et al. Improved adipose tissue metabolism after 5-year growth hormone replacement therapy in growth hormone deficient adults: the role of zinc-α2-glycoprotein. Adipocyte. 2014;4(2):113–122. doi: 10.4161/21623945.2014.973772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stochholm K, et al. Reviewing the safety of GH replacement therapy in adults. Growth Horm IGF Res. 2015;25(4):149–157. doi: 10.1016/j.ghir.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Stanhope R, Moyle L, MacSwiney M. Patient knowledge and compliance with growth hormone treatment. Arch Dis Child. 1993;68:525–529. doi: 10.1136/adc.68.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tauber M, Payen A, Cartault A, et al. User trial of easypod, an electronic autoinjector for growth hormone. Ann Endocrinol. 2008;69:511–516. doi: 10.1016/j.ando.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Fisher BG, Acerini CL. Understanding the growth hormone therapy adherence paradigm: a systematic review. Horm Res Paediatr. 2013;79:189–196. doi: 10.1159/000350251. [DOI] [PubMed] [Google Scholar]
- 19.Verrips GH, Hirasing RA, Fekkes M, et al. Psychological responses to the needle-free Medi-Jector or the multidose Disetronic injection pen in human growth hormone therapy. Acta Paediatr. 1998;87:154–158. doi: 10.1080/08035259850157589. [DOI] [PubMed] [Google Scholar]
- 20.Cutfield WS, Derraik JG, Gunn AJ, et al. Noncompliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6:e16223. doi: 10.1371/journal.pone.0016223. [DOI] [PMC free article] [PubMed] [Google Scholar]