Abstract
Hospital-acquired pneumonia (HAP) and community-acquired pneumonia (CAP) are among the most common infections treated in the hospital setting, and together they place a significant burden on healthcare systems. Successful management of HAP and CAP depends on rapid initiation of empirical antibiotic therapy with broad-spectrum antibiotics. Ceftobiprole is a new-generation, broad-spectrum cephalosporin antibiotic for the treatment of HAP (excluding ventilator-associated pneumonia) and CAP. It displays potent in vitro activity against a broad range of pathogens important in pneumonia. This review summarizes the pharmacokinetic profile of ceftobiprole, and considers the pharmacokinetic parameters and pharmacodynamics underlying the choice of dosing regimen. Ceftobiprole shows linear pharmacokinetics after single and multiple doses and is eliminated predominantly through the kidneys. Ceftobiprole is administered as a 500 mg intravenous infusion over 2 h every 8 h, and steady-state concentrations are reached on the first day of dosing. Dose adjustment is recommended for patients with moderate or severe renal impairment and for those with end-stage renal disease. Extending the infusion time of ceftobiprole to 4 h is recommended to optimize drug exposure in critically ill patients with augmented renal clearance. However, there is no need for dose adjustments based on age, sex or ethnicity, or for patients with severe obesity. Population pharmacokinetic modelling and Monte Carlo simulations were used to determine the optimal dosing regimen for ceftobiprole in special patient populations, including paediatric patients. Future studies of ceftobiprole in patients with HAP and CAP would be of interest.
Key Points
| Ceftobiprole is a new-generation cephalosporin antibiotic for the treatment of hospital-acquired pneumonia (excluding ventilator-associated pneumonia) and community-acquired pneumonia. |
| Ceftobiprole is administered as a 500 mg infusion over 2 h every 8 h. No dose adjustments are required based on sex, ethnicity or age, or for patients with hepatic impairment or severe obesity. |
| Ceftobiprole is eliminated predominantly through the kidneys. Dose adjustment is recommended for patients with moderate or severe renal impairment and for patients with end-stage renal disease; as for all β-lactams, for patients with augmented renal clearance, extending the infusion time up to 4 h may help to optimize ceftobiprole exposure. |
Introduction
The burden of illness associated with pneumonia is considerable, both in terms of patient morbidity/mortality and the use of healthcare resources. Hospital-acquired pneumonia (HAP) occurs in approximately 3–10 of every 1000 hospital admissions, making it one of the most common nosocomial infections [1, 2]. HAP is associated with increases in the length of hospital stay and additional healthcare costs, and therefore has a marked impact on healthcare resource use [3, 4].
Community-acquired pneumonia (CAP; pneumonia acquired outside a hospital setting or an extended-care facility) is a considerable cause of morbidity and mortality [5]. Annual incidences of 5–11 cases per 1000 population have been reported in studies in Europe and the US, and up to 60 % of these individuals can require hospital treatment [6, 7]. Among patients hospitalized with CAP, mortality is significant, with rates of up to 14 % [5, 7]. The impact of CAP on healthcare resources is also considerable; the costs of hospitalization are a high proportion of the overall costs of care, with length of hospital stay being a major factor [6].
In HAP, Gram-negative pathogens are the most frequent cause of infection, with Pseudomonas aeruginosa, Acinetobacter baumannii (although not in Northern Europe), Escherichia coli, and Klebsiella and Enterobacter species all commonly isolated from patients [8, 9]. Gram-positive pathogens such as Staphylococcus aureus (including methicillin-resistant S. aureus [MRSA]) and Streptococcus pneumoniae are also detected, albeit less frequently [8, 9]. S. pneumoniae is the most commonly identified cause of CAP; other commonly isolated pathogens include Haemophilus influenzae and S. aureus [5–7].
The successful management of HAP and CAP depends on rapid and adequate empirical antibiotic therapy. A range of factors are taken into consideration when choosing a treatment, including suspected pathogens, patterns of local antibiotic resistance, illness severity and patient risk factors [7, 9, 10]. Effective treatment relies on broad-spectrum antibiotics that generate rapid effects.
Ceftobiprole is a new-generation, broad-spectrum cephalosporin approved in 13 European countries and in Canada for the treatment of HAP (excluding ventilator-associated pneumonia [VAP]) and CAP [11–13]. The aims of this review were to summarize the pharmacokinetic profile of ceftobiprole in healthy individuals and patients, examine the pharmacokinetic parameters and pharmacodynamics underlying the choice of dosing regimen, and consider the pharmacokinetics and dosing of ceftobiprole in special patient populations, including critically ill patients treated in an intensive care unit (ICU), and those with renal impairment. Papers reporting the pharmacokinetics of ceftobiprole were identified from PubMed searches, using terms including BAL5788, ceftobiprole/ceftobiprole medocaril, pneumonia and pharmacokinetic/pharmacokinetics. References were screened by title and abstract to identify potentially relevant articles; the bibliographies of appropriate papers were also screened to identify further studies for inclusion. In addition, relevant abstracts presented at the European Congresses of Clinical Microbiology and Infectious Diseases (ECCMID) for 2004–2016 and Annual Interscience Conferences on Antimicrobial Agents and Chemotherapy (ICAAC) for 2004–2015 were identified.
Ceftobiprole Overview
Ceftobiprole is the active moiety of the prodrug ceftobiprole medocaril. The recommended dose in adults with normal renal function is ceftobiprole 500 mg, administered as a 2-h intravenous infusion every 8 h [14, 15]. Dose adjustments are recommended according to renal function, as described in detail below (see Sect. 5 for further details) [14].
Ceftobiprole has shown antimicrobial activity in vitro against a broad range of pathogens important in pneumonia, including Gram-positive pathogens, such as MRSA, S. pneumoniae and Enterococcus faecalis, and Gram-negative pathogens, such as H. influenzae and P. aeruginosa [11, 16–18]. Findings from two randomized, double-blind, phase III clinical studies have demonstrated the efficacy of ceftobiprole in patients with HAP (excluding VAP) or CAP [19, 20]. In 781 patients with HAP, ceftobiprole (500 mg every 8 h) demonstrated non-inferiority to ceftazidime (2 g every 8 h) plus linezolid (600 mg every 12 h) for the primary efficacy measure of clinical cure rate at the test-of-cure (TOC) visit [19]. In addition, ceftobiprole showed non-inferiority to the combination for clinical cure in patients with HAP (excluding VAP), but not in patients with VAP alone [19]. Ceftobiprole was efficacious for the treatment of HAP associated with Gram-positive pathogens, such as S. aureus and MRSA, and with Gram-negative pathogens, such as P. aeruginosa [19]. In 706 patients hospitalized with CAP, ceftobiprole (administered as above) was non-inferior to ceftriaxone (2 g every 24 h) with or without linezolid (administered as above) for the same primary efficacy measure [20]. Ceftobiprole treatment was generally well tolerated in both phase III studies [19, 20]. The efficacy of ceftobiprole for the treatment of HAP has been reviewed in detail by Scheeren [13].
Overall Pharmacokinetic Profile of Ceftobiprole in Healthy Individuals
The pharmacokinetics of ceftobiprole following single- and multiple-dose administration have been assessed in several studies in healthy individuals [15, 21, 22]. The prodrug ceftobiprole medocaril was rapidly converted, probably by type A esterases, to the active substance ceftobiprole following 30-min intravenous infusion [23, 24]. Peak plasma concentrations were reached at the end of the infusion, while the following biphasic decrease reflected the rapid distribution [half-life (t ½) in the distribution phase, 0.57 ± 0.22 min] of ceftobiprole into other body compartments. Ceftobiprole exhibited linear pharmacokinetics after single- or multiple-dose administration over the dose range 125–1000 mg [23–25]. In addition, ceftobiprole pharmacokinetics were shown to be time-independent [15]. Steady-state concentrations were attained rapidly within the first day of dosing [14]; accumulation with multiple dosing every 8 h was minimal in healthy individuals with normal renal function [15].
The main pharmacokinetic parameters for ceftobiprole in healthy individuals following a single 500 mg dose administered by 2-h intravenous infusion are shown in Table 1 [26], together with the pharmacokinetic data for other cephalosporin antibiotics commonly used in HAP and CAP [14, 26–35]. The pharmacokinetics observed after multiple-dose administration of ceftobiprole 500 mg every 8 h were similar to those seen with a single dose [26]. Systemic exposure [area under the plasma concentration–time curve from time zero to 8 h (AUC8)] and maximum plasma concentration (C max) on day 5 were similar to those observed on day 1 of dosing (AUC8, 102 ± 11.9 and 90.0 ± 12.4 mg h/L, respectively; C max, 33.0 ± 4.83 and 29.2 ± 5.52 mg/L, respectively), while values for renal clearance (CLR), total systemic clearance (CLT) and elimination half-life (t ½) were virtually unchanged on days 1 and 5 (CLR, 4.28 ± 0.57 and 4.08 ± 0.72 L/h, respectively; CLT, 4.98 ± 0.58 and 4.89 ± 0.69 L/h, respectively; t ½, 3.3 ± 0.3 and 3.1 ± 0.3 h, respectively) [15, 26]. The mean volume of distribution at steady state (V SS) with ceftobiprole was 29 % lower on day 5 compared with day 1 (15.5 ± 2.33 vs. 21.7 ± 3.3 L). The V SS was similar to the volume of the extracellular fluid compartment in adults, consistent with findings seen with other β-lactam agents [26]. Protein binding with ceftobiprole (16 %) [14] was consistent with the values reported for ceftaroline (~20 %) [32], ceftazidime (10–23 %) [33, 36, 37] and cefepime (~20 %) [34], whereas higher values have been reported for cefotaxime (37 %) [35] (Table 1). High concentration-dependent plasma protein binding has been shown for ceftriaxone (41–99 %) [38, 39]. The half-life of ceftobiprole was approximately 3 h, which was comparable to that for ceftaroline. Ceftazidime, cefepime and cefotaxime had shorter half-lives (1.95, 2.00 and 1.04 h, respectively), whereas ceftriaxone had a longer half-life (6.30 h) [28, 40] (Table 1).
Table 1.
Single-dose pharmacokinetic parameters and EUCAST clinical breakpoints for ceftobiprole and other cephalosporins
| Parameter | Ceftobiprole 500 mg [15, 24, 26] | Ceftazidime 1000 mg [27, 33, 67] | Ceftriaxone 500 mg [28, 40] | Ceftaroline 600 mg [30, 32] | Cefotaxime 500 mg [31, 35, 74] | Cefepime 500 mg [29, 34] |
|---|---|---|---|---|---|---|
| Pharmacokinetic parameters following single-dose administration in healthy individuals | ||||||
| Number of patients | 28 | 15a | 12 | 6b | 9 | |
| Infusion time (min) | 120 | 30 | 30 | 60 | 5 | 30 |
| C max (mg/L) | 29.2 ± 5.52 | 86.29 ± 13.06 | 82.0 ± 10.4 | 28.4 ± 7.0 | 37.9 ± 2.1 | 31.9 ± 6.0 |
| t max (h) | – | – | 0.5 | 1.0 | – | – |
| AUC∞ (mg·h/L) | 104 ± 13.9 | 150.30 ± 19.84 | 551 ± 91 | 75.6 ± 9.7 | 30.6 ± 2.2 | 56.6 ± 11.4 |
| t ½ for the distribution phase (h) | – | – | 0.21c | – | 0.19 ± 0.03 | – |
| t ½ for the post-distribution phase (h) | 3.1 ± 0.3 | 1.95 + 0.25 | 6.30c | 2.9 ± 0.4 | 1.04 ± 0.07 | 2.00 ± 0.64 |
| V d | 21.7 ± 3.3 L | 0.21 ± 0.03 L/kg | 8.46 ± 1.11 L | 29.3 ± 5.2 Ld | 19.1 ± 1.2 L/1.73 m2 | 18.3 ± 1.9 L |
| CLT | 4.89 ± 0.69 L/h | 0.095 ± 0.014 L/h/kg | 0.929 ± 0.15 L/h | 7.11 ± 0.89 L/he | 14.72 ± 1.17 L/h/1.73 m2 | 9.12 ± 1.68 L/h |
| CLR | 4.08 ± 0.72 L/h | 0.084 ± 0.014 L/h/kg | 0.373 ± 0.60 L/h | 3.36 ± 0.83 L/h | 8.81 ± 1.12 L/h/1.73 m2 | 8.28 ± 1.98 L/h |
| Urinary excretion (%)f | 83.1 ± 9.06 | 88.26 ± 5.50 | 38 ± 7g [28] (~60 %) [42] |
46.8 ± 6.1 | 58.8 | 91.0 ± 15.2 |
| Protein binding (%) | 16 | 10–23 | 41–99 | ~20 | 37 | ~20 |
| EUCAST MIC breakpoints (S≤/R>) [75] | ||||||
| Staphylococcus aureus | 2/2 | ND | NDh | 1/1 | NDh | NDh |
| Streptococcus pneumoniae | 0.5/0.5 | ND | 0.5/2 | 0.25/0.25 | 0.5/2 | 1/2 |
| Enterobacteriaceae | 0.25/0.25 | 1/4 | 1/2 | 0.5/0.5 | 1/2 | 1/4 |
| Pseudomonas aeruginosa | IE | 8 | ND | ND | ND | 8 |
Data are expressed as mean ± standard deviation or mean ± standard error (cefotaxime), except for t max, which is expressed as median
AUC ∞ area under the plasma concentration–time curve from time zero extrapolated to infinity, C max maximum plasma concentration, CL CR creatinine clearance, CL R renal clearance, CL T total systemic clearance, EUCAST European Committee on Antimicrobial Susceptibility Testing, F m fraction of the dose metabolized, IE insufficient evidence, MIC minimum inhibitory concentration, ND breakpoint not defined (susceptibility testing not recommended), R resistant, S susceptible, t ½ half-life, t max time to C max, V d volume of distribution, V z volume of distribution based on the terminal phase
aMean weight 72.0 kg
bIndividuals with normal renal function (CLCR > 80 mL/min)
cHarmonic mean
d V z/F m, volume of distribution based on the terminal phase/fraction of the dose metabolized
eCL/Fm, plasma clearance/fraction of the dose metabolized
fUnchanged drug over 24 h
g n = 11
hSusceptibility can be inferred from cefoxitin testing (S ≤/R>, >4 mg/L)
Penetration of ceftobiprole into lung tissue, as measured by concentrations in the epithelial lining fluid (ELF), was assessed in healthy individuals at a presumed steady state (following the fourth dose of ceftobiprole) [41]. This showed that mean ceftobiprole concentrations in the ELF were lower than in the plasma over the 8 h following the start of infusion. Population pharmacokinetic modelling based on these data showed that mean penetration into the ELF was 25.5 % (median 15.3 %; interquartile range 7.9–30.4 %; calculated from the ratio of ELF/total ceftobiprole) [41]. In the murine model of pneumonia, lung penetration of ceftobiprole, based on AUC values in the ELF and plasma, was higher (median 68.8 %) than in healthy individuals [41]. However, the measured ELF levels of antibiotics may not accurately predict β-lactam concentrations at the site of infection [42], especially in studies in healthy volunteers.
A review of clinical studies comparing the ELF concentrations of ceftobiprole with those of other cephalosporins showed that lung penetration with the majority of agents was similar to that observed with ceftobiprole [43]. For most of the oral or parenteral cephalosporins studied, the percentage lung penetration, calculated from the ratio of ELF to total plasma concentrations, ranged from 10 to 38 % [43]. Similarly, the lung penetration of ceftazidime (4 g/day) was 21 % in a study in 15 adults with HAP who were receiving mechanical ventilation [44]. However, higher values were reported with cefepime during continuous intravenous infusion (4 g/day) in patients with severe nosocomial pneumonia [45]. After 2 days of therapy, cefepime showed similar concentrations in the ELF and plasma, giving a mean penetration of approximately 100 % [46]. It is currently unclear what impact an infection may have on ceftobiprole lung penetration in humans [41].
The penetration of ceftobiprole into soft tissue and bone has also been examined. Following a single 2-h infusion of ceftobiprole 500 mg in healthy volunteers, ceftobiprole systemic exposure and peak concentrations (measured using in vivo microdialysis) were lower in both skeletal muscle and subcutaneous adipose tissue than in plasma [mean penetration: 69 % for skeletal muscle and 49 % for adipose tissue (calculated as tissue-to-plasma AUC ratios for free drug)] [47], which is in line with values reported for other cephalosporins. In adults receiving ceftobiprole 500 mg as a 2-h intravenous infusion before undergoing total hip replacement surgery, ceftobiprole exposure in cortical bone was almost 3.5-fold higher than that in cancellous bone [48]. Bone penetration of ceftobiprole (the ratio of AUC in bone relative to that in unbound plasma) was 0.22 for cortical bone and 0.06 for cancellous bone (0.15–0.3) [49]. In a rabbit model of tibial osteomyelitis, after ceftobiprole treatment for 4 weeks, bacterial titres in the infected tibia were below the limit of detection in all of the evaluable animals, compared with 73 % of those animals treated with either vancomycin or linezolid [50].
Elimination of ceftobiprole occurs predominantly through renal excretion [15, 26]. With multiple dosing, approximately 80–90 % of the administered dose is recovered in the urine as unchanged ceftobiprole [15, 23]. Elimination occurs mainly by glomerular filtration, and does not involve active tubular secretion [15]. Renal drug−drug interactions are therefore not expected with ceftobiprole [11]. The linear relationship between systemic and renal clearance observed for ceftobiprole allows the accurate prediction of dose modification for patients with renal impairment (as discussed in Sect. 5.1 below) [51].
Role of Pharmacokinetic Characteristics in the Tolerability of Ceftobiprole
The predominantly renal elimination observed with ceftobiprole is typical of most cephalosporins. For example, ceftazidime [27], cefepime [29] and ceftaroline [32] are eliminated mainly via the kidneys. By contrast, ceftriaxone shows substantial elimination via non-renal pathways, through biliary excretion into the gut [28]. In regard to drug tolerability, one potential benefit of renal elimination is that it may limit antibiotic exposure in the gut, although to date there are no studies that specifically address this. Ceftobiprole has shown no significant effect on the intestinal microflora of healthy individuals [52] and was generally well tolerated in clinical studies [19, 20]. Over a 3-week study period, no measurable concentrations of ceftobiprole were found in the faeces of healthy individuals who received intravenous infusions of ceftobiprole 500 mg every 8 h for the first 7 days. The lack of ceftobiprole in the intestine is thought to account for the minor effects on intestinal microflora. In addition, no new colonizing ceftobiprole-resistant aerobic or anaerobic bacteria were detected among the intestinal microflora, and no Clostridium difficile strains or toxins were found [52]. Furthermore, a study in mice showed that ceftobiprole did not promote the growth of C. difficile in caecal contents, whereas ceftazidime, cefoxitin, ceftriaxone, cefotaxime and ertapenem all promoted significant C. difficile growth when compared with saline controls, and were associated with toxin production [53].
Finally, ceftobiprole treatment was generally well tolerated in the phase III clinical studies in patients with HAP or CAP [19, 20]. The incidence of treatment-related adverse events was similar to that with other cephalosporins, with the most frequent being hyponatraemia, diarrhoea, nausea and phlebitis [19].
Pharmacokinetic/Pharmacodynamic Relationships and Dosing Considerations for Ceftobiprole
For β-lactam antibacterial agents, duration of exposure is the key factor in determining therapeutic efficacy. The main pharmacokinetic/pharmacodynamic index shown to correlate with therapeutic efficacy is the length of time that the unbound drug concentration exceeds the minimum inhibitory concentration (fT > MIC), typically expressed as a proportion of the dosing interval (%fT > MIC) [54]. An appropriate dosing regimen can be determined based on a suitable %fT > MIC target once the dose−effect relationships have been established for a particular drug [55, 56].
Non-clinical studies in animals have shown a strong correlation between %fT > MIC and efficacy for ceftobiprole. The %fT > MIC values required for the static doses were 36–45 % for Enterobacteriaceae, 14–28 % for S. aureus and 15–22 % for S. pneumoniae [57]. Based on findings from the in vivo murine pneumonia and thigh infection models, the most appropriate pharmacodynamic targets chosen for ceftobiprole dose selection analyses were a %fT > MIC of 30 % for documented Gram-positive infections and 50 % for broad-spectrum coverage of both Gram-positive and Gram-negative pathogens; a %fT > MIC of 50 % was used to determine the European Committee on Antimicrobial Susceptibility Testing (EUCAST) non-species-specific breakpoint (4 mg/L) [15, 22, 58] as this is the exposure correlated with a 1- to 2-log10 kill.
The knowledge gained from pharmacokinetic studies in healthy volunteers and animal models was used to determine the optimal dosing regimen for ceftobiprole in patients with HAP or CAP [22, 59]. However, individual variations in pharmacokinetics may complicate calculation of the optimal dosing regimen. Monte Carlo simulations are therefore considered a valuable method for assessing the probability of achieving defined pharmacodynamic target values with different dosing regimens based on population pharmacokinetic modelling data [60, 61]. In an initial simulation, pharmacokinetic data from individuals involved in the multiple ascending dose, phase I clinical study were used for the population pharmacokinetic modelling [22], with ceftobiprole concentrations in plasma predicted using a two-compartment model. The analyses examined the effects of different dosing regimens on target attainment for %fT > MIC of 30–60 % across a range of MIC values (1–16 mg/L). Ceftobiprole 500 mg every 8 h showed a probability of target attainment (PTA) of 100 % for %fT > MIC of 30 and 40, and 99 % for %fT > MIC of 50 % at an MIC of 4 mg/L, and a PTA of 100 % for %fT > MIC of 50 and 60 % at an MIC of 2 mg/L [22], although it should be acknowledged that this simulation was based on data from a relatively small number of healthy individuals (n = 12).
A subsequent modelling analysis examined the effect of lung penetration of ceftobiprole (as measured by ELF concentrations) on the exposure targets required for valid antimicrobial activity in a murine model of S. aureus pneumonia [41]. In this model, the %fT > MIC for ceftobiprole in ELF to kill 1 log10 and 2 log10 colony-forming units (CFU)/g of lung tissue were 13 and 24 %, respectively [41]. This is noteworthy, given that β-lactam agents may differ in their penetration into lung tissue [43], and drug concentration at the site of infection may be an important factor in determining treatment outcome; this mixed analysis could therefore provide additional data for the optimal ceftobiprole dose to be used for the treatment of pneumonia in humans [41]. On the basis of the cell kill targets identified in the pneumonia model, as well as data from population pharmacokinetic modelling of ceftobiprole in healthy volunteers, Monte Carlo simulations were used to determine the target attainment rate [calculated as the cumulative fraction of predicted response (CFR)] for a 2-h infusion of ceftobiprole 500 mg every 8 h using the MIC distribution for ceftobiprole for over 4950 MRSA isolates. The findings showed that the CFR for ELF against MRSA in humans was 85.6 % for a 1 log10 CFU/g kill, and 79.7 % for a 2 log10 CFU/g kill [41].
A further simulation study used pharmacokinetic data from 150 individuals included in phase I and phase II trials [59]. The main parameters for ceftobiprole were established using population pharmacokinetic modelling, with Monte Carlo simulations applied to determine the PTA with ceftobiprole 500 mg every 8 h, administered as 30-min, 1- or 2-h infusions, for %fT > MIC values of 30–60 % at different MICs (0.25–8 mg/L). Target attainment was determined for different rates of creatinine clearance (CLCR). At normal CLCR values (80–120 mL/min), the PTA with ceftobiprole 500 mg every 8 h, administered as a 2-h infusion, was at least 96 % for a %fT > MIC of 30 %, and at least 80 % for %fT > MIC of 50 % at an MIC of ≤4 mg/L (Table 2) [59].
Table 2.
Probabilities of target attainment with ceftobiprole 500 mg administered as a 2-h infusion every 8 h [59]
| Creatinine clearance (mL/min) | Probability of target attainment (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 % fT > MIC for MIC (mg/L) of: | 40 % fT > MIC for MIC (mg/L) of: | 50 % fT > MIC for MIC (mg/L) of: | ||||||||||
| 0.5 | 1 | 2 | 4 | 0.5 | 1 | 2 | 4 | 0.5 | 1 | 2 | 4 | |
| 80 | 99 | 99 | 99 | 98 | 99 | 99 | 99 | 94 | 99 | 98 | 97 | 89 |
| 100 | 99 | 99 | 99 | 97 | 99 | 99 | 98 | 92 | 99 | 98 | 95 | 85 |
| 120 | 99 | 99 | 99 | 96 | 99 | 99 | 97 | 89 | 99 | 98 | 94 | 80 |
MIC minimum inhibitory concentration, fT > MIC time free (unbound) drug concentration is above MIC
Additional analyses examined the target attainment for specific organisms, based on ceftobiprole MIC data obtained from either surveillance programmes or ceftobiprole clinical studies [59]. As the authors state, these showed that a 2-h infusion of ceftobiprole 500 mg every 8 h had an estimated target attainment of >90 % for %fT > MIC of 50 % across the whole range of MRSA and methicillin-susceptible S. aureus (MSSA) isolates at a MIC of 0.5 and 1 mg/L, respectively. The same ceftobiprole regimen also showed a good estimated target attainment against Gram-negative susceptible pathogens [59]. However, it is not entirely clear whether the authors calculated a PTA based on an epidemiological cut-off (ECOFF) value for a specific population, or whether they calculated the CFR.
Taken together, data from Monte Carlo analyses carried out among healthy volunteers demonstrate that ceftobiprole 500 mg infused over a 2-h period every 8 h is the optimal regimen to provide adequate coverage against pathogens with an MIC of ≤4 mg/L.
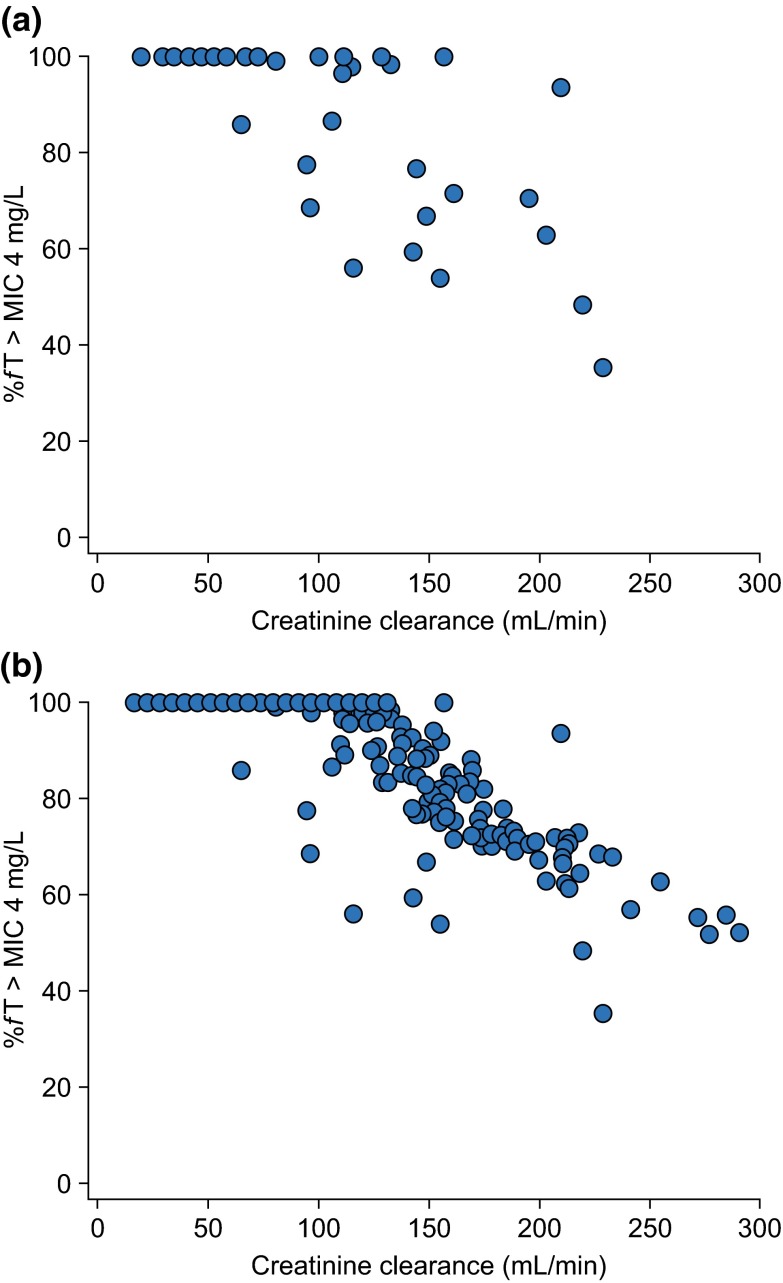
Modelling analyses were also used to determine the actual observed target attainment (OTA) with ceftobiprole 500 mg administered over a 2-h period every 8 h among patients with HAP enrolled in a phase III study [62]. A population model was used to calculate individual exposures to ceftobiprole for study populations based on either covariates or patient samples. The OTA in both groups was then determined for different %fT > MIC targets at a range of MIC values. The analysis showed an attainment of higher than 90 % for %fT > MIC of up to 70 % in patients with HAP for MIC values up to 4 mg/L. Interestingly, it is notable that Monte Carlo simulations based on data from healthy individuals might be adequate in predicting actual exposure to ceftobiprole among this study population [62]. A further analysis was performed using these data to determine the potential effects of augmented clearance on ceftobiprole concentrations among the study population. Pharmacokinetic parameter estimates for each patient were used to determine the %fT > MIC at 4 mg/L for patients with known ceftobiprole plasma concentrations (n = 52), as well as for the overall patient group, based on CLCR values (n = 391) and the population model [62]. Figure 1 shows the %fT > MIC at an MIC of 4 mg/L as a function of CLCR for both groups. The majority of patients had a %fT > MIC above 50 % (stasis target) or 60 % (1-log kill target), although high clearance rates suggested that some patients may have been underexposed.
Fig. 1.
Percentage of time the plasma drug concentration is above the MIC (%fT > MIC) at 4 mg/L: Monte Carlo simulations for patients in the HAP study. a %fT > MIC determined based on the population model and actual ceftobiprole plasma concentrations (n = 52); b %fT > MIC calculated based on creatinine clearance as a covariate in the overall patient population (n = 391). MIC minimum inhibitory concentration, HAP hospital-acquired pneumonia
In a further Monte Carlo simulation based on the population of patients with HAP from the phase III study, the antimicrobial activity of ceftobiprole 500 mg (administered over a 2-h period every 8 h) against MRSA isolates from ICUs was compared with that for other anti-MRSA antimicrobial agents used at standard doses (dalbavancin 1000 mg, daptomycin 4 or 6 mg/kg/day, tigecycline 50 mg every 12 h, linezolid 600 mg every 12 h, and vancomycin 1 or 1.5 g every 12 h) [63]. Ceftobiprole, together with dalbavancin, was found to have the highest CFR for the pharmacokinetic/pharmacodynamic target against MRSA, and provides optimal coverage up to the EUCAST non-species-specific breakpoint MIC of 4 mg/L.
The relationship between ceftobiprole exposure and microbiological eradication at the end of therapy (EOT) was also evaluated using data from the phase III study of patients with HAP [64]. Of note, pharmacokinetic/pharmacodynamic analysis showed that %fT > MIC (based on the highest observed MIC of any pathogen cultured at baseline and/or the EOT) was the most significant predictor of microbiological eradication at the EOT (p < 0.0001) in multiple logistic regression analysis. Moreover, univariate analysis showed a significant correlation between %fT > MIC and microbiological eradication at the EOT. Furthermore, %fT > MIC was also a significant predictor of clinical cure at the TOC visit (p = 0.0062) [64].
Pharmacokinetics and Dosing of Ceftobiprole in Special Patient Populations
As noted above, the approved dose of ceftobiprole in adults with normal renal function is 500 mg, administered as a 2-h intravenous infusion every 8 h [14]. Ceftobiprole has been shown to be generally well tolerated at doses of up to 1000 mg every 8 h [25]. However, there is a need for dose adjustment in patients with moderate or severe renal impairment or end-stage renal disease (ESRD) [14], and additional patient-related factors should also be considered when selecting an appropriate regimen.
Patients with Renal Impairment
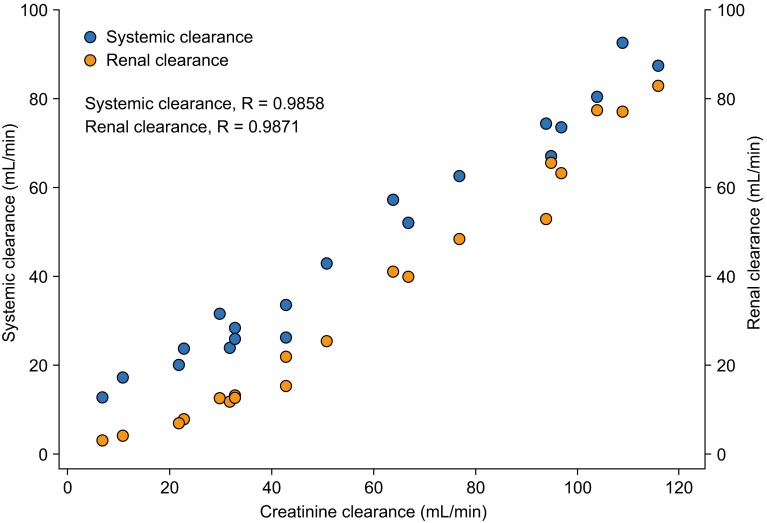
The pharmacokinetics of ceftobiprole are affected by renal impairment, consistent with its predominantly renal excretion through glomerular filtration [13]. A study compared the pharmacokinetic parameters following a single 30-min intravenous infusion of ceftobiprole 250 mg in healthy individuals with normal renal function and those with differing degrees of renal impairment (Table 3) [51, 65]. Systemic exposure increased linearly with the severity of renal impairment; t ½ was also increased in individuals with reduced renal function, particularly in those with severe impairment. Total CLR decreased with decreasing renal function, and marked reductions were observed with moderate (80 %) and severe (91 %) impairment, when compared with normal renal function, although it should be noted that there were only small numbers of individuals in each group (Table 3) [51]. Systemic and renal clearance showed a good linear correlation with CLCR (correlation coefficient = 0.9858 and 0.9871, respectively) (Fig. 2), confirming that dose modification of ceftobiprole in patients with renal impairment can be accurately predicted on the basis of CLCR.
Table 3.
Main pharmacokinetic parameters following a single intravenous infusion of ceftobiprole in healthy individuals and those with renal impairment or end-stage renal disease requiring dialysis [51, 65]
| Parameter | Renal impairment studya | ESRD studyb | |||||
|---|---|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | Healthy | Pre-dialysis | Post-dialysis | |
| CLCR > 80 mL/min | CLCR 50–80 mL/min | CLCR 30 to < 50 mL/min | CLCR < 30 mL/min | ||||
| [n = 5] | [n = 5] | [n = 5] | [n = 5] | [n = 6] | [n = 5] | [n = 5] | |
| C max (mg/L) | 20.6 ± 2.06 | 20.1 ± 1.45 | 24.4 ± 1.65 | 22.8 ± 3.48 | 11.1 ± 1.77 | 13.3 ± 2.33 | 21.1 ± 14.7 |
| AUClast (mg·h/L) | 52.4 ± 6.95 | 72.7 ± 13.9 | 139 ± 15.7 | 174 ± 44.5 | 44.3 ± 7.12 | 118 ± 8.73 | 249 ± 49.0 |
| AUC∞ (mg·h/L) | 52.8 ± 6.91 | 74.8 ± 15.6 | 151 ± 21.6 | 222 ± 71.0 | 45.2 ± 6.84 | 143 ± 8.53 | 311 ± 75.1 |
| t ½ (h) | 3.45 ± 0.37 | 4.75 ± 0.81 | 6.87 ± 1.12 | 11.1 ± 1.96 | 3.0 ± 0.4 | 20.7 ± 1.83 | 20.5 ± 5.33 |
| V SS (L) | 15.8 ± 1.81 | 18.0 ± 0.76 | 14.2 ± 0.80 | 16.9 ± 2.39 | 24.4 ± 3.68 | 52.5 ± 5.23 | 23.9 ± 5.14 |
| CLT (L/h) | 4.80 ± 0.61 | 3.46 ± 0.71 | 1.68 ± 0.25 | 1.21 ± 0.36 | 5.62 ± 0.73 | 1.76 ± 0.10 | 0.845 ± 0.21 |
| CLR (L/h) | 4.38 ± 0.51 | 2.48 ± 0.63 | 0.88 ± 0.25 | 0.41 ± 0.24 | 5.11 ± 0.81 | NC | NC |
| Urinary recovery (%) | 91.6 ± 6.55 | 71.1 ± 7.32 | 51.9 ± 9.93 | 31.5 ± 9.65 | 88.6 ± 4.06 | NC | NC |
Data are expressed as mean ± standard deviation
AUC area under the plasma concentration–time curve, AUC last AUC from time zero to the last measurable concentration, AUC ∞ AUC from time zero extrapolated to infinity, C max maximum plasma concentration, CL CR creatinine clearance, CL R renal clearance, CL T total systemic clearance, ESRD end-stage renal disease, NC not calculated, t ½ elimination half-life, V SS volume of distribution at steady state
aCeftobiprole 250 mg administered as a 30-min infusion
bCeftobiprole 250 mg administered as a 120-min infusion
Fig. 2.
Correlation between creatinine clearance and systemic and renal clearance following a single intravenous infusion of ceftobiprole 250 mg over 30 min in individuals with normal renal function and those with renal impairment [51]. Data are shown for individuals with normal renal function (CLCR >80 mL/min; n = 5) and those with mild (CLCR 50–80 mL/min; n = 5), moderate (CLCR 30 to <50 mL/min; n = 5) or severe (CLCR <30 mL/min; n = 5) renal impairment. CL CR creatinine clearance
A study in patients with ESRD requiring dialysis showed that systemic exposure to ceftobiprole [in terms of AUC from time zero to infinity (AUC∞)] was 3.2-fold higher than in healthy individuals when ceftobiprole was administered pre-dialysis, and approximately sevenfold higher when it was administered post-dialysis (Table 3) [65]. In addition, systemic clearance was markedly reduced in patients with ESRD compared with healthy individuals, while t ½ was approximately sevenfold longer, although, as with the renal impairment study, the number of participants was small (Table 3). The estimated mean extraction ratio of ceftobiprole during a 4-h dialysis was 0.685, and mean dialysis clearance was 7.91 L/h [65].
Consistent with these findings, dose adjustment is recommended for ceftobiprole in patients with moderate or severe renal impairment [14]. In those with moderate impairment (CLCR 30 to <50 mL/min), the recommended dose is 500 mg, administered as a 2-h intravenous infusion every 12 h, whereas for those with severe impairment (CLCR <30 mL/min) the recommended dose is 250 mg, administered as a 2-h intravenous infusion every 12 h. For patients with ESRD, the recommended dose is 250 mg once every 24 h, regardless of haemodialysis application [14]. Similar to ceftobiprole, other cephalosporins used in the treatment of HAP or CAP have been shown to require dose adjustments in patients with renal impairment. For ceftaroline, dose reductions are recommended for patients with moderate−severe impairment and those with ESRD [30]. Similar adjustments are also recommended for cefepime [66] and ceftazidime [67].
Treatment in Critically Ill Patients
Pathophysiological changes are common in critically ill patients, which could affect the pharmacokinetics of antibiotics [68, 69]. A systematic review of pharmacokinetic studies assessing intravenous β-lactam antibiotics in infected patients treated in the ICU reported significant changes in the pharmacokinetics of all six of the agents studied [70]. Increased capillary permeability as a result of infection can lead to the movement of fluid to the interstitial space, thus increasing the volume of distribution of hydrophilic drugs such as β-lactam antibiotics, and decreasing their plasma concentration. In addition, increases in renal perfusion are often observed in these patients, leading to higher CLCR and increased elimination of hydrophilic drugs [68, 69]. Patients with augmented CLR may therefore require higher maintenance doses of hydrophilic antibiotics through more frequent administrations and/or longer or even continuous infusions in order to maintain adequate therapeutic exposure to the antimicrobial agent [69].
A recent open-label study carried out in 33 adults treated in the ICU examined the pharmacokinetics of high-dose ceftobiprole administered over a longer infusion period (1000 mg over 4 h) [71]. The frequency of administration was chosen on the basis of CLCR measurements (every 8 h in those with CLCR >80 mL/min and every 12 h in those with CLCR 50–79 mL/min). Blood samples for pharmacokinetic analysis were collected during the morning dose of day 2 [71]. Systemic clearance of ceftobiprole was higher for patients with high CLCR (>150 mL/min) than for those with normal (80–100 mL/min) or low CLCR (50–79 mL/min), with a twofold difference between the high and low clearance groups (Table 4) [71]. However, the analysis included only small numbers of patients with high and low CLCR (six and five patients, respectively), which represents a potential limitation of the study. Further analysis of these patients showed that mechanical ventilation had no consistent effects on the pharmacokinetics of ceftobiprole, although the number of ventilated patients in each group was low. Pharmacodynamic analysis demonstrated that prolongation of the infusion time to 4 h was sufficient to provide plasma levels of ceftobiprole above the EUCAST non-species-specific breakpoint MIC of 4 mg/L for the entire dosing interval, even among patients in the ICU with CLCR >150 mL/min [71]. In patients with high CLCR, fT > MIC for ceftobiprole 1000 mg was reduced compared with patients with normal or low CLCR (Table 5). However, ceftobiprole 1000 mg every 8 h allowed maintenance of plasma free concentrations above the MIC for the entire dosing interval (%fT > MIC > 100). By extrapolating these findings to a 500 mg dose, the %fT > MIC for ceftobiprole among patients with high CLCR was 91 % (Table 5) [71]. Therefore, extending the infusion time to 4 h may help optimize drug exposure when standard doses of ceftobiprole 500 mg every 8 h are administered to patients with augmented CLR (CLCR >150 mL/min) [14]. The findings of this study are consistent with the Monte Carlo simulations assessing the effects of augmented clearance on ceftobiprole concentrations (as discussed in Sect. 4). High CLR (e.g. among the critically ill) might contribute to a lower %fT > MIC in some patients. However, a previous ceftobiprole Monte Carlo simulation has demonstrated that, while high CLR contributes to low exposure in the subpopulation, not all patients with high CLCR have low exposure to ceftobiprole, reflecting the inaccuracy of CLCR estimates as a surrogate for CLR [62]. Moreover, data for target attainment derived from healthy individuals predicted exposure in a patient population, including those who are severely ill [62].
Table 4.
Main pharmacokinetic parameters for high-dose ceftobiprole administration in intensive care unit patients, according to creatinine clearance [71]
| Parameter | Lowa | Normalb | Highb |
|---|---|---|---|
| CLCR 50–79 mL/min | CLCR 80–150 mL/min | CLCR >150 mL/min | |
| [n = 5] | [n = 20] | [n = 6] | |
| C max (mg/L) | 51.6 ± 11.2 | 37.8 ± 7.3 | 27.6 ± 7.3 |
| t max (h) | 4.7 (3.4–6.0) | 4.0 (3.5–4.5) | 3.9 (3.5–4.0) |
| AUClast (mg·h/L) | 405 ± 93.2 | 269 ± 116 | 180 ± 75.3 |
| t ½ (h) | 4.5 ± 1.0 | 3.8 ± 1.6 | 3.8 ± 1.2 |
| V SS (L) | 23.7 ± 6.6 | 23.1 ± 6.3 | 29.4 ± 7.5 |
| CLT (L/h) | 3.8 ± 0.6 | 5.2 ± 1.2 | 7.4 ± 1.5 |
| Protein binding (%) | 19.1 ± 4.4 | 20.5 ± 7.3 | 21.6 ± 3.5 |
Data are expressed as mean ± standard deviation, except for t max, which is expressed as median (range)
AUC last area under the plasma concentration–time curve from time zero to the last measurable concentration, C max maximum plasma concentration, CL CR creatinine clearance, CL T total systemic clearance, t ½ elimination half-life, t max time to C max, V SS volume of distribution at steady state
aCeftobiprole 1000 mg administered as a 4-h infusion every 12 h
bCeftobiprole 1000 mg administered as a 4-h infusion every 8 h
Table 5.
Pharmacodynamic analysis of ceftobiprole treatment in patients treated in the intensive care unit [71]
| Ceftobiprole 1000 mg (observed) | Ceftobiprole 500 mg (extrapolated) | ||||
|---|---|---|---|---|---|
| CLCR
50–79 mL/min |
CLCR
80–150 mL/min |
CLCR >150 mL/min | CLCR
80–150 mL/min |
CRCR >150 mL/min | |
| T > MICa (h) | 19.6 ± 4.1 | 14.5 ± 6.0 | 12.0 ± 5.0 | – | – |
| fT > MICa (h) | 18.2 ± 4.0 | 13.2 ± 5.5 | 10.8 ± 5.2 | 9.5 ± 4.3 | 7.3 ± 3.9 |
| %fT > MICa (q8 h) | >100 | >100 | >100 | >100 | 91 |
Data are expressed as mean ± standard deviation, unless otherwise stated
CL CR creatinine clearance, MIC minimum inhibitory concentration, T > MIC time the plasma drug concentration is above the MIC, fT > MIC time the free (unbound) drug concentration is above the MIC, q8 h every 8 h
aMIC = 4 mg/L
Paediatric Patients
A recent open-label study evaluated the pharmacokinetics of a single dose of ceftobiprole in 55 paediatric patients (aged 3 months to <18 years) requiring systemic antibiotics [72]. Ceftobiprole was administered as a 2-h infusion, with doses adjusted to achieve exposures equivalent to those in adults following standard dosing (15 mg/kg for patients aged 3 months to <2 and 2 to <6 years; 10 mg/kg for those aged 6 to <12 years; and 7 mg/kg for individuals aged 12 to <18 years). Ceftobiprole pharmacokinetic parameters in paediatric patients were broadly within the range of those reported for adults [15, 26]. Ceftobiprole exposure was up to 20 % lower in patients aged <12 years (mean ± SD: C max 24.4 ± 9.1 to 28.7 ± 7.0 µg/mL; AUC∞ 79.5 ± 16.2 to 87.7 ± 28.2 µg·h/mL) than in adults (C max 29.2 ± 5.5 µg/mL; AUC∞104 ± 13.9 µg·h/mL), and approximately 40 % lower in patients aged 12 to <18 years (C max 17.4 ± 3.2 µg/mL; AUC∞ 63.5 ± 14.3 µg·h/mL) than in adults [72]. When adjusted for body weight, volume of distribution and total clearance decreased with increasing age in paediatric patients, whereas CLR and t ½ (not adjusted for body weight) were similar across the age groups. In paediatric patients, ceftobiprole concentrations remained above the target MIC of 4 mg/L for 66.5 to 75.3 % of an 8-h time period (%T > MIC), and ceftobiprole treatment was generally well tolerated [72]. The lower ceftobiprole exposure observed in patients aged 12 years to <18 years compared with adults has implications for the design of future studies in paediatric patient populations. Ceftobiprole is currently not approved for use in paediatric patients.
Patients with Severe Obesity
Patients who are obese may have physiological alterations that influence the pharmacokinetics of drugs. An open-label, single-centre study compared the pharmacokinetics of a single intravenous infusion of ceftobiprole 500 mg over 2 h in adults who were severely obese [body mass index (BMI)] >40 kg/m2) and those who were not obese (BMI 18–30 kg/m2) [73]. Mean BMI was 45.5 kg/m2 in the severely obese group (n = 12) compared with 24.0 kg/m2 in the non-obese group (n = 13); other baseline characteristics were similar in the two groups. Volume of distribution and total clearance of ceftobiprole were 25.9 and 19.1 % higher, respectively, in severely obese than non-obese individuals (Table 6) [73]. Ceftobiprole exposure was lower in severely obese adults than in those who were not obese. Plasma concentrations of unbound ceftobiprole remained above the target MIC of 4 mg/L (fT > MIC) for 76.6 and 79.7 % of an 8-h dosing interval in severely obese and non-obese individuals, respectively [73]. Although volume of distribution and total clearance were higher, and exposure was lower, in severely obese adults compared with non-obese individuals (Table 6) following a single ceftobiprole infusion, %fT > MIC was similar in the two groups, indicating that there is no need for dose adjustment of ceftobiprole in severely obese patients.
Table 6.
Main pharmacokinetic parameters following a single intravenous infusion of ceftobiprole in patients who were severely obese and those who were not obese [73]
| Severely obese [n = 11]a | Non-obese [n = 13] | |
|---|---|---|
| C max (µg/mL) | 21.4 ± 3.0 | 30.2 ± 4.3 |
| AUC∞ (µg·h/mL) | 91.0 ± 11.7 | 110 ± 20.1b |
| t ½ (h) | 3.4 ± 0.3 | 3.2 ± 0.5b |
| V dz (L) | 27.2 ± 3.9 | 21.6 ± 5.1b |
| CL (L/h) | 5.6 ± 0.7 | 4.7 ± 0.7b |
| %fT > MIC (4 mg/L)c | 76.6 ± 9.2c | 79.7 ± 7.3 |
Data are expressed as mean ± standard deviation
AUC ∞ AUC from time zero extrapolated to infinity, C max maximum plasma concentration, CL clearance, MIC minimum inhibitory concentration, T > MIC time the plasma drug concentration is above the MIC, t ½ elimination half-life, V dz volume of distribution
aOne subject who received treatment was excluded from the pharmacokinetic analysis
b n = 12
c8-h dosing interval
Patients with Ventilator-Associated Pneumonia
In the phase III HAP study, ceftobiprole 500 mg, administered as a 2-h infusion every 8 h, did not demonstrate non-inferiority to ceftazidime 2 g every 8 h/linezolid 600 mg every 12 h in the subgroup of VAP patients [19]. The clinical cure rates at the TOC visit were lower with ceftobiprole than with ceftazidime/linezolid in the intent-to-treat (ITT; 23.1 vs. 36.8 %) and clinically effective (CE; 37.7 vs. 55.9 %) populations. A similar pattern of results was seen with microbiological eradication rates at TOC [19]. All-cause mortality at 30 days in patients with VAP was 26.9 % for ceftobiprole and 19.8 % for ceftazidime/linezolid; 30-day pneumonia-specific mortality was similar in the two groups (ceftobiprole 8.7 %; ceftazidime/linezolid 7.5 %) [19]. Furthermore, bacteriological eradication at the EOT was found to be similar in patients with or without VAP [64], and Monte Carlo simulations have demonstrated that the PTA in patients with VAP is no different from that in those without VAP [62].
Although the peculiar pathophysiological status might account for this, an analysis of the subgroup of mechanically ventilated patients with HAP who did not have VAP suggested that ventilation itself did not affect these outcomes. In these patients, clinical cure rates with ceftobiprole were similar to those with ceftazidime/linezolid in the ITT population (30.4 vs. 27.1 %), and were higher in the CE population (55.3 vs. 40.5 %) [19]. Multivariate analysis of the VAP subgroup did not identify any specific patient factor or combination of factors that could account for the differences in treatment outcomes between the two groups [19]. Furthermore, the OTA in patients with VAP is similar to that in those without VAP, and bacterial eradication at EOT is also similar between the two groups [62, 64]. Interestingly, augmented renal function (CLCR >150 mL/min) did not correlate with the observed findings, suggesting that the data observed in patients with VAP might reflect the heterogeneous nature of this population [19]. Ceftobiprole is not licensed for the treatment of VAP.
Other Patient Populations
There is no need for dose adjustment of ceftobiprole in patients with hepatic impairment as the drug undergoes minimal hepatic metabolism and its pharmacokinetics are unlikely to be affected by reduced hepatic function [14]. Likewise, there is no need for dose adjustment based on sex or ethnicity, or in elderly patients [14]. Regarding sex, one pharmacokinetic study in healthy individuals showed that after a single 30-min infusion of ceftobiprole 750 mg, the AUC∞ was higher (15 %) and both systemic clearance and volume of distribution were lower (12 and 29 %, respectively) in women compared with men [15]; however, the differences were no longer apparent after the parameters were adjusted for body weight or %T > MIC. For ethnicity, a study comparing the pharmacokinetics of single- and multiple-dose ceftobiprole regimens in healthy Japanese men demonstrated that no difference in ceftobiprole disposition existed compared with historical data from Caucasian individuals; this was also confirmed in a population analysis [65]. Finally, with regard to age, a population analysis used to assess the effect of age on the pharmacokinetics of ceftobiprole showed that CLT was typically lower in elderly individuals than in younger individuals [65]. However, age was not a statistically significant covariate in the final population pharmacokinetic model, in which lower CLCR in the elderly explained the lower systemic clearance of ceftobiprole [65]. Therefore, no specific ceftobiprole dose adjustments are required based on age alone, except in cases of moderate and severe renal impairment, as recommended for the general population [14].
Limitations
The studies reporting the pharmacokinetics of ceftobiprole do, however, have some limitations. Evaluation of ceftobiprole pharmacokinetics in individuals with renal impairment and those with ESRD involved only limited numbers of subjects in each assessment group. Similarly, although overall patient numbers were larger in the study of adults treated in the ICU, the number of patients with augmented CLR was small. The pharmacokinetic modelling data used in Monte Carlo simulations to determine the optimal ceftobiprole dosing regimen were based on data from healthy volunteers, an approach that it has been suggested may not accurately reflect the pharmacokinetics observed in patients. However, the value of this approach has been supported by a study comparing modelled pharmacokinetic data from healthy volunteers with actual ceftobiprole exposure data in patients with HAP; this analysis clearly demonstrated that the Monte Carlo simulations adequately predicted actual exposure to ceftobiprole in this patient population [62]. Future pharmacokinetic/pharmacodynamic studies in patients with HAP and CAP would prove valuable, given the limited pharmacokinetic data currently available from these patients, especially in patients with VAP.
Summary
Ceftobiprole is a new-generation, broad-spectrum cephalosporin that has recently been approved for the treatment of HAP (excluding VAP) and CAP [11]. It shows antimicrobial activity in vitro against a broad range of Gram-positive and Gram-negative pathogens important in pneumonia, including MRSA [11, 16]. The prodrug ceftobiprole medocaril is rapidly and almost completely converted to the active substance, ceftobiprole, following intravenous infusion [23, 24]. The efficacy of ceftobiprole 500 mg, infused over 2 h every 8 h, has been demonstrated in two randomized, double-blind, phase III clinical studies carried out among patients with HAP (excluding VAP) or CAP [19, 20].
Ceftobiprole exhibits linear pharmacokinetics over the dose range 125–1000 mg [23, 24], and steady-state concentrations are rapidly attained on the first day of dosing [14]. The elimination of ceftobiprole occurs predominantly through renal excretion. Therefore, prolongation of infusion time is recommended for patients with augmented renal clearance, whereas dose reductions are recommended for those with moderate or severe renal impairment and for patients with ESRD, irrespective of dialysis application [14]. A study in critically ill patients showed that the systemic clearance of ceftobiprole was increased in patients with augmented CLR; extending the infusion time of the standard 500 mg dose of ceftobiprole to 4 h may therefore help to optimize drug exposure in these patients. There is no need for dose adjustment based on sex, ethnicity, age or hepatic impairment [14].
Acknowledgments
The authors thank Oxford PharmaGenesis Ltd (Oxford, UK) who provided medical writing support. The authors take full responsibility for the content of the article.
Funding
Medical writing and editorial support for the development of this review was funded by Basilea Pharmaceutica International Ltd (Basel, Switzerland).
Compliance with Ethical Standards
Conflict of interest
Antonio Torres has participated in advisory boards for Basilea Pharmaceutica International Ltd, Bayer, Roche and AstraZeneca. Johan Willem Mouton has received research funding from Adenium, AstraZeneca, Basilea Pharmaceutica International Ltd, Cubist, Eumedica, Merck & Co, Pfizer, Polyphor, Roche, Shionogi and Wockhardt. Federico Pea has been on the speakers’ bureau of Angelini, AstraZeneca, Basilea Pharmaceutica International Ltd, Forest, Gilead, MSD, Novartis, Pfizer and Sanofi-Aventis; and has participated in advisory boards for Angelini, AstraZeneca, Basilea Pharmaceutica International Ltd, Gilead, MSD, Pfizer and Takeda. He is also a member of the Clinical Pharmacokinetics editorial board.
References
- 1.Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S48–S53. doi: 10.1086/653049. [DOI] [PubMed] [Google Scholar]
- 2.Kieninger AN, Lipsett PA. Hospital-acquired pneumonia: pathophysiology, diagnosis, and treatment. Surg Clin North Am. 2009;89(2):439–61, ix. [DOI] [PubMed]
- 3.American Thoracic Society Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 4.Leu HS, Kaiser DL, Mori M, Woolson RF, Wenzel RP. Hospital-acquired pneumonia. Attributable mortality and morbidity. Am J Epidemiol. 1989;129(6):1258–1267. doi: 10.1093/oxfordjournals.aje.a115245. [DOI] [PubMed] [Google Scholar]
- 5.Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371(17):1619–1628. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 6.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 7.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–55. [DOI] [PubMed]
- 8.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 9.Torres A, Ewig S, Lode H, Carlet J. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35(1):9–29. doi: 10.1007/s00134-008-1336-9. [DOI] [PubMed] [Google Scholar]
- 10.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, et al. Guidelines for the management of adult lower respiratory tract infections: summary. Clin Microbiol Infect. 2011;17(Suppl 6):1–24. doi: 10.1111/j.1469-0691.2011.03602.x. [DOI] [PubMed] [Google Scholar]
- 11.Syed YY. Ceftobiprole medocaril: a review of its use in patients with hospital- or community-acquired pneumonia. Drugs. 2014;74(13):1523–1542. doi: 10.1007/s40265-014-0273-x. [DOI] [PubMed] [Google Scholar]
- 12.Liapikou A, Cilloniz C, Torres A. Ceftobiprole for the treatment of pneumonia: a European perspective. Drug Des Devel Ther. 2015;9:4565–4572. doi: 10.2147/DDDT.S56616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheeren TW. Ceftobiprole medocaril in the treatment of hospital-acquired pneumonia. Futur Microbiol. 2015;10(12):1913–1928. doi: 10.2217/fmb.15.115. [DOI] [PubMed] [Google Scholar]
- 14.Summary of product characteristics—Zevtera. 20 Nov 2013. Available at: https://www.medicines.org.uk/emc/medicine/29764. Accessed 11 Jun 2015.
- 15.Murthy B, Schmitt-Hoffmann A. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin Pharmacokinet. 2008;47(1):21–33. doi: 10.2165/00003088-200847010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Farrell DJ, Flamm RK, Sader HS, Jones RN. Ceftobiprole activity against over 60,000 clinical bacterial pathogens isolated in Europe, Turkey, and Israel from 2005 to 2010. Antimicrob Agents Chemother. 2014;58(7):3882–3888. doi: 10.1128/AAC.02465-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell DJ, Flamm RK, Sader HS, Jones RN. Activity of ceftobiprole against methicillin-resistant Staphylococcus aureus strains with reduced susceptibility to daptomycin, linezolid or vancomycin, and strains with defined SCCmec types. Int J Antimicrob Agents. 2014;43(4):323–327. doi: 10.1016/j.ijantimicag.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Lascols C, Legrand P, Merens A, Leclercq R, Muller-Serieys C, Drugeon HB, et al. In vitro antibacterial activity of ceftobiprole against clinical isolates from French teaching hospitals: proposition of zone diameter breakpoints. Int J Antimicrob Agents. 2011;37(3):235–239. doi: 10.1016/j.ijantimicag.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Awad SS, Rodriguez AH, Chuang YC, Marjanek Z, Pareigis AJ, Reis G, et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin Infect Dis. 2014;59(1):51–61. doi: 10.1093/cid/ciu219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson SC, Welte T, File TM, Jr, Strauss RS, Michiels B, Kaul P, et al. A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int J Antimicrob Agents. 2012;39(3):240–246. doi: 10.1016/j.ijantimicag.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Dauner DG, Nelson RE, Taketa DC. Ceftobiprole: a novel, broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Am J Health Syst Pharm. 2010;67(12):983–993. doi: 10.2146/ajhp090285. [DOI] [PubMed] [Google Scholar]
- 22.Mouton JW, Schmitt-Hoffmann A, Shapiro S, Nashed N, Punt NC. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob Agents Chemother. 2004;48(5):1713–1718. doi: 10.1128/AAC.48.5.1713-1718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt-Hoffmann A, Nyman L, Roos B, Schleimer M, Sauer J, Nashed N, et al. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob Agents Chemother. 2004;48(7):2576–2580. doi: 10.1128/AAC.48.7.2576-2580.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt-Hoffmann A, Roos B, Schleimer M, Sauer J, Man A, Nashed N, et al. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob Agents Chemother. 2004;48(7):2570–2575. doi: 10.1128/AAC.48.7.2570-2575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt-Hoffmann A, Murthy B, Strauss RS, Pypstra R. Pharmacokinetics (PK) of multiple infusions of ceftobiprole (1000 mg every 8 hours) in healthy volunteers [abstract no. A-1943]. 46th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, American Society for Microbiology; 27–30 Sep 2006; San Francisco.
- 26.Murthy B, Skee D, Wexler D, et al. Pharmacokinetics of ceftobiprole following single and multiple intravenous infusions administered to healthy subjects [abstract P779]. Clin Microbiol Infect. 2007;13(Suppl s1):S194.
- 27.Paradis D, Vallee F, Allard S, Bisson C, Daviau N, Drapeau C, et al. Comparative study of pharmacokinetics and serum bactericidal activities of cefpirome, ceftazidime, ceftriaxone, imipenem, and ciprofloxacin. Antimicrob Agents Chemother. 1992;36(10):2085–2092. doi: 10.1128/AAC.36.10.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, et al. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981;20(5):634–641. doi: 10.1128/AAC.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992;36(3):552–557. doi: 10.1128/AAC.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riccobene T, Jakate A, Rank D. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J Clin Pharmacol. 2014;54(7):742–752. doi: 10.1002/jcph.265. [DOI] [PubMed] [Google Scholar]
- 31.Neu HC, Aswapokee P, Fu KP, Ho I, Matthijssen C. Cefotaxime kinetics after intravenous and intramuscular injection of single and multiple doses. Clin Pharmacol Ther. 1980;27(5):677–685. doi: 10.1038/clpt.1980.96. [DOI] [PubMed] [Google Scholar]
- 32.Kiang TK, Wilby KJ, Ensom MH. A critical review on the clinical pharmacokinetics, pharmacodynamics, and clinical trials of ceftaroline. Clin Pharmacokinet. 2015;54(9):915–931. doi: 10.1007/s40262-015-0281-3. [DOI] [PubMed] [Google Scholar]
- 33.Kemmerich B, Warns H, Lode H, Borner K, Koeppe P, Knothe H. Multiple-dose pharmacokinetics of ceftazidime and its influence on fecal flora. Antimicrob Agents Chemother. 1983;24(3):333–338. doi: 10.1128/AAC.24.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summary of product characteristics—MAXIPIMETM (cefepime hydrochloride, USP) for injection. May 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050679s031lbl.pdf. Accessed 31 Jul 2015.
- 35.Esmieu F, Guibert J, Rosenkilde HC, Ho I, Le Go A. Pharmacokinetics of cefotaxime in normal human volunteers. J Antimicrob Chemother. 1980;6(Suppl A):83–92. [DOI] [PubMed]
- 36.Harding SM, Ayrton J, Thornton JE, Munro AJ, Hogg MI. Pharmacokinetics of ceftazidime in normal subjects. J Antimicrob Chemother. 1981;8(Suppl B):261. [DOI] [PubMed]
- 37.Mouton JW, Horrevorts AM, Mulder PG, Prens EP, Michel MF. Pharmacokinetics of ceftazidime in serum and suction blister fluid during continuous and intermittent infusions in healthy volunteers. Antimicrob Agents Chemother. 1990;34(12):2307–2311. doi: 10.1128/AAC.34.12.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother. 2001;47(4):421–429. doi: 10.1093/jac/47.4.421. [DOI] [PubMed] [Google Scholar]
- 39.Patel IH, Kaplan SA. Pharmacokinetic profile of ceftriaxone in man. Am J Med. 1984;77(4C):17–25. [PubMed] [Google Scholar]
- 40.Summary of product characteristics—ceftriaxone 1 g powder for solution for injection. 11 Feb 2014. Available at: https://www.medicines.org.uk/emc/medicine/5469. Accessed 22 Oct 2015.
- 41.Rodvold KA, Nicolau DP, Lodise TP, Khashab M, Noel GJ, Kahn JB, et al. Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob Agents Chemother. 2009;53(8):3294–3301. doi: 10.1128/AAC.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52(1):24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodvold KA, George JM, Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet. 2011;50(10):637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 44.Boselli E, Breilh D, Rimmele T, Poupelin JC, Saux MC, Chassard D, et al. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 2004;30(5):989–991. doi: 10.1007/s00134-004-2171-2. [DOI] [PubMed] [Google Scholar]
- 45.Mazzei T, Novelli A, Esposito S, Periti P. New insight into the clinical pharmacokinetics of cefaclor: tissue penetration. J Chemother. 2000;12(1):53–62. doi: 10.1179/joc.2000.12.1.53. [DOI] [PubMed] [Google Scholar]
- 46.Boselli E, Breilh D, Duflo F, Saux MC, Debon R, Chassard D, et al. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit Care Med. 2003;31(8):2102–2106. doi: 10.1097/01.CCM.0000069734.38738.C8. [DOI] [PubMed] [Google Scholar]
- 47.Barbour A, Schmidt S, Sabarinath SN, Grant M, Seubert C, Skee D, et al. Soft-tissue penetration of ceftobiprole in healthy volunteers determined by in vivo microdialysis. Antimicrob Agents Chemother. 2009;53(7):2773–2776. doi: 10.1128/AAC.01409-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt-Hoffman A, Engelhardt M, Spickermann J, Jones M, Kaufhold A. Bone penetration of the new-generation cephalosporin ceftobiprole in patients following hip replacement surgery [abstract]. Presented at the 26th Annual European Congress of Clinical Microbiology and Infectious Diseases; 9–12 Apr 2016: Amsterdam.
- 49.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sorgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48(2):89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]
- 50.Yin LY, Calhoun JH, Thomas JK, Shapiro S, Schmitt-Hoffmann A. Efficacies of ceftobiprole medocaril and comparators in a rabbit model of osteomyelitis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(5):1618–1622. doi: 10.1128/AAC.00638-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roos B, Schmitt-Hoffmann A, Schleimer M, et al, editors. Safety and pharmacokinetics of BAL5788 in healthy subjects with normal or impaired renal function [abstract A-23]. 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 14–17 September 2003, Chicago.
- 52.Backstrom T, Panagiotidis G, Beck O, Asker-Hagelberg C, Rashid MU, Weintraub A, et al. Effect of ceftobiprole on the normal human intestinal microflora. Int J Antimicrob Agents. 2010;36(6):537–541. doi: 10.1016/j.ijantimicag.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Nerandzic MM, Donskey CJ. Effect of ceftobiprole treatment on growth of and toxin production by Clostridium difficile in cecal contents of mice. Antimicrob Agents Chemother. 2011;55(5):2174–2177. doi: 10.1128/AAC.01612-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10 (quiz 1–2). [DOI] [PubMed]
- 55.Mouton JW. Breakpoints: current practice and future perspectives. Int J Antimicrob Agents. 2002;19(4):323–331. doi: 10.1016/S0924-8579(02)00028-6. [DOI] [PubMed] [Google Scholar]
- 56.Mouton JW, Punt N. Use of the t > MIC to choose between different dosing regimens of beta-lactam antibiotics. J Antimicrob Chemother. 2001;47(4):500–501. doi: 10.1093/jac/47.4.500. [DOI] [PubMed] [Google Scholar]
- 57.Craig WA, Andes DR. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob Agents Chemother. 2008;52(10):3492–3496. doi: 10.1128/AAC.01273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.European Committee on Antimicrobial Susceptibility Testing. Ceftobiprole: rationale for the clinical breakpoints, version 1.0, 2016. Available at: http://www.eucast.org. Accessed 28 Apr 2016.
- 59.Lodise TP, Jr, Pypstra R, Kahn JB, Murthy BP, Kimko HC, Bush K, et al. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob Agents Chemother. 2007;51(7):2378–2387. doi: 10.1128/AAC.01181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dudley MN, Ambrose PG. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr Opin Microbiol. 2000;3(5):515–521. doi: 10.1016/S1369-5274(00)00132-6. [DOI] [PubMed] [Google Scholar]
- 61.Drusano GL, Preston SL, Hardalo C, Hare R, Banfield C, Andes D, et al. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob Agents Chemother. 2001;45(1):13–22. doi: 10.1128/AAC.45.1.13-22.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller AE, Schmitt-Hoffmann AH, Punt N, Mouton JW. Monte Carlo simulations based on phase 1 studies predict target attainment of ceftobiprole in nosocomial pneumonia patients: a validation study. Antimicrob Agents Chemother. 2013;57(5):2047–2053. doi: 10.1128/AAC.02292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salem AH, Zhanel GG, Ibrahim SA, Noreddin AM. Monte Carlo simulation analysis of ceftobiprole, dalbavancin, daptomycin, tigecycline, linezolid and vancomycin pharmacodynamics against intensive care unit-isolated methicillin-resistant Staphylococcus aureus. Clin Exp Pharmacol Physiol. 2014;41(6):437–443. doi: 10.1111/1440-1681.12195. [DOI] [PubMed] [Google Scholar]
- 64.Muller AE, Punt N, Mouton JW. Exposure to ceftobiprole is associated with microbiological eradication and clinical cure in patients with nosocomial pneumonia. Antimicrob Agents Chemother. 2014;58(5):2512–2519. doi: 10.1128/AAC.02611-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Medicines and Healthcare Products Regulatory Agency. Public assessment report Zevtera 500 mg powder for concentrate for solution for infusion (UK/H/5304/001/DC). Available at: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con369256.pdf. Accessed 30 Jul 2015.
- 66.Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990;48(3):268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- 67.Summary of product characteristics—ceftazidime 1 g powder for solution for injection. 21 May 2015. Available at: http://www.medicines.org.uk/emc/medicine/21129. Accessed 31 Jul 2015.
- 68.Pea F. Plasma pharmacokinetics of antimicrobial agents in critically ill patients. Curr Clin Pharmacol. 2013;8(1):5–12. [PubMed] [Google Scholar]
- 69.Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Goncalves-Pereira J, Povoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of beta-lactams. Crit Care. 2011;15(5):R206. doi: 10.1186/cc10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Torres A, Sanchez-Garcia M, Demeyer I, Saulay M, Schmitt-Hoffmann A-H, Engelhardt M, et al. (eds). Pharmacokinetics, safety and tolerability of high-dose ceftobiprole medocaril administered as prolonged infusion in intensive-care-unit (ICU) patients [abstract O199]. 25th European Congress of Clinical Microbiology and Infectious Diseases; 25–28 Apr 2015: Copenhagen.
- 72.Blumer JL, Schmitt-Hoffman A, Engelhardt M, Spickermann J, Jones M, Kaufhold A. Pharmacokinetics of ceftobiprole in paediatric patients [abstract]. Presented at the 26th Annual European Congress of Clinical Microbiology and Infectious Diseases; 9–12 Apr 2016: Amsterdam.
- 73.Schmitt-Hoffman A, Engelhardt M, Spickermann J, Jones M, Kaufhold A. Pharmacokinetics and pharmacodynamics of ceftobiprole in adults who are severely obese [abstract]. Presented at the 26th Annual European Congress of Clinical Microbiology and Infectious Diseases; 9–12 Apr 2016: Amsterdam.
- 74.Summary of product characteristics—cefotaxime 2 g powder for solution for injection or infusion. 21 May 2015. Available at: https://www.medicines.org.uk/emc/medicine/12154. Accessed 30 Oct 2015.
- 75.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0, 2016. Available at: http://www.eucast.org. Accessed 16 Mar 2016.