Abstract
Background
Because of the vulnerability and frailty of elderly adults, clinical drug development has traditionally been biased towards young and middle-aged adults. Recent efforts have begun to incorporate data from paediatric investigations. Nevertheless, the elderly often remain underrepresented in clinical trials, even though persons aged 65 years and older receive the majority of drug prescriptions. Consequently, a knowledge gap exists with regard to pharmacokinetic (PK) and pharmacodynamic (PD) responses in elderly subjects, leaving the safety and efficacy of medicines for this population unclear.
Objectives
The goal of this study was to extend a physiologically based pharmacokinetic (PBPK) model for adults to encompass the full course of healthy aging through to the age of 100 years, to support dose selection and improve pharmacotherapy for the elderly age group.
Methods
For parameterization of the PBPK model for healthy aging individuals, the literature was scanned for anthropometric and physiological data, which were consolidated and incorporated into the PBPK software PK-Sim®. Age-related changes that occur from 65 to 100 years of age were the main focus of this work. For a sound and continuous description of an aging human, data on anatomical and physiological changes ranging from early adulthood to old age were included. The capability of the PBPK approach to predict distribution and elimination of drugs was verified using the test compounds morphine and furosemide, administered intravenously. Both are cleared by a single elimination pathway. PK parameters for the two compounds in younger adults and elderly individuals were obtained from the literature. Matching virtual populations—with regard to age, sex, anthropometric measures and dosage—were generated. Profiles of plasma drug concentrations over time, volume of distribution at steady state (V ss) values and elimination half-life (t ½) values from the literature were compared with those predicted by PBPK simulations for both younger adults and the elderly.
Results
For most organs, the age-dependent information gathered in the extensive literature analysis was dense. In contrast, with respect to blood flow, the literature study produced only sparse data for several tissues, and in these cases, linear regression was required to capture the entire elderly age range. On the basis of age-informed physiology, the predicted PK profiles described age-associated trends well. The root mean squared prediction error for the prediction of plasma concentrations of furosemide and morphine in the elderly were improved by 32 and 49 %, respectively, by use of age-informed physiology. The majority of the individual V ss and t ½ values for the two model compounds, furosemide and morphine, were well predicted in the elderly population, except for long furosemide half-lifes.
Conclusion
The results of this study support the feasibility of using a knowledge-driven PBPK aging model that includes the elderly to predict PK alterations throughout the entire course of aging, and thus to optimize drug therapy in elderly individuals. These results indicate that pharmacotherapy and safety-related control of geriatric drug therapy regimens may be greatly facilitated by the information gained from PBPK predictions.
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-016-0422-3) contains supplementary material, which is available to authorized users.
Key Points
| The course of healthy aging can be accounted for in a physiologically based pharmacokinetic (PBPK) model. The age dependence of distribution and elimination processes is described by literature-informed re-parameterization of organ volumes and blood flow rates. |
| A knowledge-driven whole-body PBPK model is a valuable tool for assessment of drug exposure in the elderly, facilitating optimization of dosing regimens and clinical trials with regard to the safety and efficacy profile of a given compound. |
Introduction
In developed countries, the elderly population is growing rapidly. In the USA, the group of elderly above the age of 65 years is expected to double by 2040 [1]. Globally, the elderly population will exceed the number of children by that decade [2]. This is due not only to a decrease in birth rates but also to medical advances and global prosperity, which have resulted in an increase in life expectancy.
Elderly people receive the majority of drug prescriptions. In the UK, the elderly account for only one fifth of the population, yet they receive nearly 60 % of the prescribed drugs [3]. This population is more likely to experience adverse drug events, because of polymedication and differences in physiology between this age group and the younger adults who are typically recruited in clinical trials. Therefore, pharmacokinetic (PK) and pharmacodynamic (PD) in elderly patients are difficult to estimate [4–6]. Although this vulnerable patient group should receive more attention in clinical trials, this is difficult to realize, because of morbidity and frailty [7]. Alternative approaches to the conduct of clinical PK/PD studies in the elderly have to be considered to obtain the required information for proper dosing in the elderly. These approaches should incorporate understanding of the course of human aging and the ability to distinguish between age- and disease-related physiological alterations.
The power and applicability of physiologically based pharmacokinetic (PBPK) models have stimulated the demand for a PBPK model tailored to the elderly [8, 9]. Moreover, the value of successful PBPK modelling approaches in children [10, 11], pregnant women [12, 13] and diseased subjects [14, 15], in order to investigate and translate knowledge to special populations, has been recognized by regulatory authorities [16, 17]. This would improve pharmacotherapy and increase the safety of medications, thereby reducing costs resulting from adverse events caused by suboptimal medication. A number of PBPK models have already implemented parameters for aging, but all of these have been highly focused on special aspects such as enzyme activities [18] or volumetric changes in single organs and overall blood flow [19]. An overall understanding of the course of human aging and the ability to distinguish between age- and disease-related physiological alterations require a whole-body PBPK approach. Therefore, establishing realistic models will require incorporation of detailed descriptions of the age dependence of anatomical and (patho)physiological parameters. The aim of this study was to develop a whole-body PBPK approach to understand and correctly predict exposure to drugs in healthy, elderly Europeans between 65 and 100 years of age.
Methods
Collection of Data on Anthropometric, Anatomical and (Patho)physiological Changes with Age
The previously established database of Thompson et al. [20] provided a sound basis for establishing a PBPK model for the elderly age range but needed extension. Clear data on sex, race and disease diversification, as well as details regarding covariates, are pivotal for establishing predictive PBPK models. Body weight and height or body mass index (anthropometric measures), as well as anatomical and (patho)physiological parameters, were searched for in PubMed, using the filters ‘species-human’ and ‘ages-aged: 65+ years’. Additional terms such as ‘age’, ‘ag(e)ing’, ‘elderly’ and ‘old’ were added for refinement where needed. For parameters that yielded only sparse literature in this initial search, an additional screen was performed in Google Scholar and MedPilot. The inclusion criteria were (1) clear assignment of sex; (2) clear assignment of race; (3) a comprehensible statement of the method of analysis; and (4) ruling out of effects of medication or disease on subject physiology. Studies that included longitudinal surveillance were preferred. Since in vivo measurements of organ size are not directly comparable to measurements obtained from autopsy reports, the former were taken into consideration only for evaluation of muscle, fat and blood flow [21]. Furthermore, for each study cohort, the available details about body composition—for example, fat mass (FM), fat-free mass (FFM) and lean body mass (LBM), as well as total body water (TBW)—were extracted and used as covariates. A second literature screen was performed for the aging that occurs between 30 and 65 years of age (younger adults), in order to refine the existing physiological information and, thus, ensure that the data on aging were continuous for the purpose of modelling.
In total, 19 studies from the database of Thompson et al. [20] were used. Together with the second literature search, 97 additional studies were added to build the aging PBPK model.
Workflow for Elderly PBPK Model Development
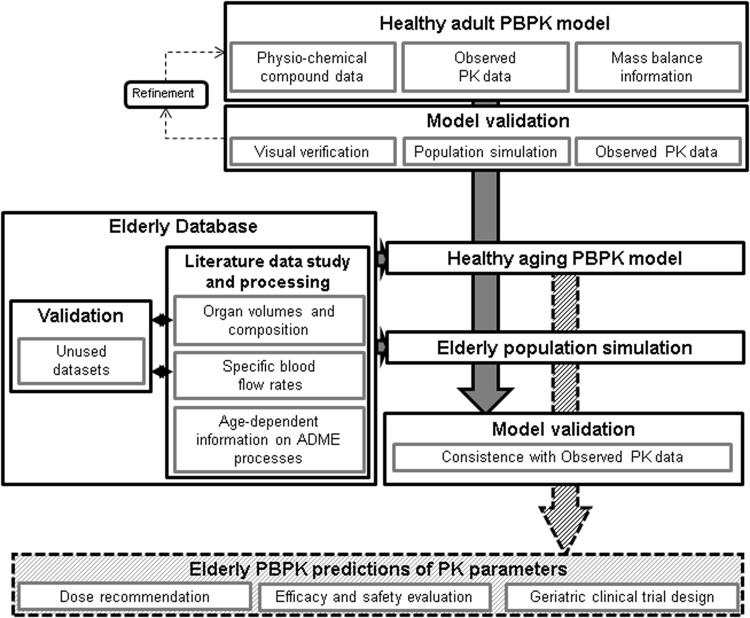
The workflow for this study is described in Fig. 1. Since biological and chronological aging differ, the data were analysed in 10-year age bins, as recommended by the World Health Organization (WHO) [22], and interpolated linearly. Thus, the data were pooled into seven age bins covering the age range of 30–100 years, using Matlab®, in order to derive the distinctive age-related body composition across the lifespan throughout the European data set. In cases where the pooling did not lead to an acceptable description of the organ aging process, because of insufficient data, additional data from North American and Australian subjects were included.
Fig. 1.
Proposed workflow for a knowledge-driven physiologically based pharmacokinetic (PBPK) aging approach. A solid line represents the work of the current study; a broken line depicts the potential usage of an aging PBPK model. ADME absorption, distribution, metabolism and excretion processes, PK pharmacokinetic
Body composition for the same age may also vary between different historical time periods because of variations in anthropometric measures or potential impacts of national nutrition [23]. In order to cope with these secular trends in study data published over the course of several decades, a reference anthropometric measure for the age range of the aging model that was considered was generated for each 10-year time period over the past 60 years. It was applied whenever study data were stated without the corresponding subjects’ anthropometric measures, to normalize organ weights for the scaling approach based on Willmann et al. [24].
The reported organ volumes originated mostly from autopsy studies and thus represented cellular mass. They were used mainly in determining respective organ volumes, specific vascular fractions, interstitial fractions and cytosolic proteins [10].
For each age bin, the processed organ masses were summed up and found to reach 91–95 % of body weight; the gap accounts for smaller organs not captured in this model—for example, the adrenal glands, thyroid, tongue and prostate. In order to reach the final body weights, muscle and FM were increased in proportion to this gap.
Age-related changes in blood flow distribution were accounted for as changes in cardiac output (CO). Blood flow rates and CO values were taken from the literature. The sum of all blood flows should be equal to CO. Thus, unknown age-dependent blood flows were scaled with regard to their relative CO contributions in adults, as postulated by the International Commission on Radiological Protection (ICRP) report [25] to match age-related changes in CO.
A virtual 30-year-old individual was created using the PBPK software tool PK-Sim® (version 5.5) [24]. On the basis of this individual, a virtual population (N = 5000) for each sex was generated by only predefining the desired age range from 30 to 100 years. This population was used for checks of physiological consistency. Body water distribution and single, simulated sizes of each organ by the newly age-informed virtual population were compared with unused data sets or nomograms.
Predictive Performance
In order to assess the predictive ability of the knowledge-driven PBPK aging approach, two compounds with different major elimination pathways were selected: the high-extraction drug morphine, which is up to 90 % cleared by hepatic metabolism, and furosemide, which is cleared mainly by the kidney. Model-building parameters are listed in Table 1. The literature was screened for observed plasma concentration–time profiles and corresponding PK parameters in healthy European volunteers ranging from young adults to the extremely elderly. Group data were preferred, but whenever individual data with a description of the subject’s anthropometric measures were provided, corresponding virtual individuals were created. In cases of missing anthropometric information, age-respective mean values were used. Reported intrinsic clearance values were used for the young adult simulation. The predictability of the age-related impact on PK was then assessed in three steps. First, simulated plasma concentration–time profiles in the elderly were compared with observed profiles. In the second step, age-related changes in elimination were evaluated from shifts in elimination half-life (t ½) values. Finally, altered body composition and changes in the relationship between TBW and FM were assessed by calculation of the volume of distribution at steady state (V ss), with a non-compartmental analysis being performed. Two-sided 1.25-fold and 2-fold levels of prediction accuracy for the ratios of predicted to observed values of V ss, t ½ and plasma concentrations were applied for each compound.
Table 1.
Overview of input parameters for the physiologically based pharmacokinetic (PBPK) models of furosemide and morphine
| Parameter | Furosemide | Morphine |
|---|---|---|
| Physicochemistry | ||
| Molecular weight, g/mol | 330.74 | 285.30 |
| LogP | 1.75b | 0.89 [26] |
| pKa | 4.25 (acidic)b | 7.90 (basic)b |
| Fraction unbound | 0.03 [27] | 0.80 [28] |
| Distribution | ||
| Partition coefficient model | Willmann et al.c [29, 30] | Willmann et al.c [29, 30] |
| Cellular permeability model | Willmann et al.c [29, 30] | Willmann et al.c [29, 30] |
| Metabolism/elimination | ||
| Elimination pathways | ||
| GFR fractiona | 1 | 1 |
| Renal clearance, mL/min/kg | 1.61 [31] | |
| Hepatic clearance, mL/min/kg | 0.77 [31] | 19.10 [28] |
GFR glomerular filtration rate, LogP octanol–water partition coefficient, pKa acid dissociation constant (on a log scale)
aFraction of GFR used for passive renal elimination
bInformation obtained from Drugbank (http://www.drugbank.ca)
cWillmann et al. [29, 30] is defined as the PK-Sim® standard
Morphine
Morphine is a strong opioid analgesic and is indicated for cancer pain. Because of its basic alkaloid structure, morphine has low lipid solubility. It is metabolized by several pathways leading to dihydromorphinone and normorphine. Morphine binds to plasma proteins independently of its concentration and is distributed to albumin, globulin and glycoproteins. The drug has a high hepatic extraction ratio, which is not affected by the dose [32]. In the PBPK model, metabolism of morphine was implemented as an intrinsic hepatic elimination process and thus affected only by age-dependent changes in hepatic blood flow and tissue size.
Furosemide
The loop diuretic agent furosemide induces production of urine from water and sodium. It is moderately lipophilic and undergoes only minor metabolism in the liver, which is incorporated into the PBPK model as an non-specific intrinsic process. As furosemide is highly bound to albumin, renal elimination is driven mainly by tubular secretion, which delivers the compound to its site of action. Although clearance is transporter driven, the magnitude is accepted to be dose linear for the therapeutic range [33]. Thus, tubular secretion was implemented as an intrinsic process in addition to passive filtration. Changes in kidney tissue and the vasculature are known to alter renal elimination of furosemide and thus delivery to the target located at the luminal side of the sodium–potassium–chloride cotransporter in the loop of Henle [34].
Statistical Analysis
After the PBPK model was built using observed adult data, studies in the elderly were simulated by creating virtual individuals matching anthropometric measures of the clinical study populations or individuals. Virtual individuals were generated by two means: with use of either the physiology of a young adult as an uninformed simulation, or with use of physiological information for the elderly gathered within this study as an age-informed simulation. The precision of simulated PK parameters was evaluated with the root mean squared prediction error (RMSE) and the bias based on the mean prediction error (ME), using weighted residuals. In order to estimate the improvement with application of age-informed physiology for predictions in the elderly data set, a relative bias was calculated by subtraction of the informed from the uniformed predictions (ΔME). The same procedure was used to calculate the precision (ΔRMSE). The changes were also set in relation to the uninformed predictions and expressed as percentages.
Results
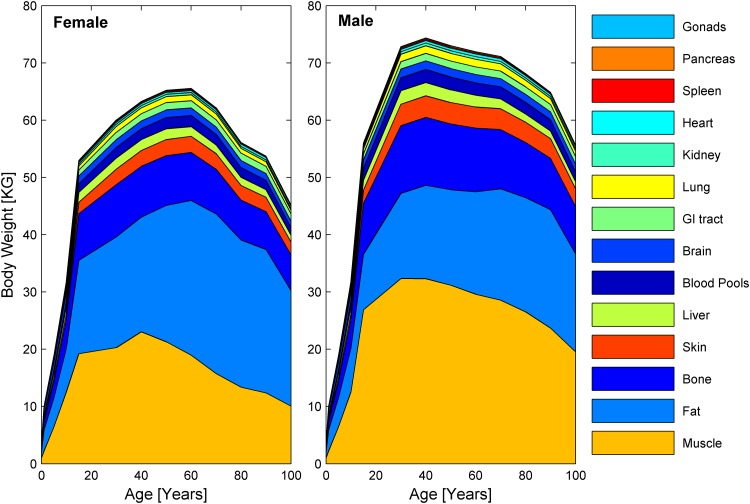
In the main part of this paper, the physiology dominating the PK in the model—including hepatic and renal changes, as well as the redistribution of fat and muscle mass—are reviewed. Also, cardiovascular and body water distribution are highlighted. A description of the physiology of less dominant organs within the whole-body aging PBPK approach is given in the Electronic Supplementary Material, including a summary of the literature used. The overall body composition over the course of aging, as implemented in the elderly PBPK approach, is listed in Table 2 and visualized in Fig. 2. The age-dependent volumetric development of all organs is described in detail in the Electronic Supplementary Material.
Table 2.
Anthropometric measures, organ weights and tissue weights, including blood content, as used in the elderly physiologically based pharmacokinetic (PBPK) models
| Parameter | Sex | Age, years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 30c | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||
| Body weight, kg | Female | 60.0 | 63.3 | 65.2 | 65.5 | 62.2 | 56.1 | 53.7 | 45.2 |
| Male | 73.0 | 74.4 | 73.0 | 72.0 | 71.1 | 68.1 | 64.9 | 55.6 | |
| Height, cm | Female | 163.0 | 160.9 | 160.0 | 158.3 | 155.4 | 151.7 | 150.0 | 147.9 |
| Male | 176.0 | 175.1 | 173.9 | 171.2 | 167.4 | 165.5 | 163.8 | 157.6 | |
| Organ masses, ga | |||||||||
| Blood poolsb | Female | 1899.4 | 1921.1 | 1961.2 | 1946.9 | 1847.7 | 1692.6 | 1634.5 | 1357.0 |
| Male | 2264.6 | 2269.5 | 2234.9 | 2180.2 | 2111.3 | 2042.5 | 1976.5 | 1739.5 | |
| Bone | Female | 9121.5 | 8886.3 | 8676.1 | 8401.8 | 7811.6 | 6981.4 | 6619.0 | 6263.5 |
| Male | 11,817.8 | 11,848.0 | 11,481.6 | 11,091.6 | 10,355.0 | 9637.1 | 8969.0 | 8300.9 | |
| Brain | Female | 1357.0 | 1352.3 | 1347.6 | 1315.1 | 1287.3 | 1241.4 | 1169.4 | 1097.4 |
| Male | 1508.8 | 1506.9 | 1502.9 | 1463.3 | 1434.3 | 1400.1 | 1372.1 | 1344.2 | |
| Fat | Female | 19,348.0 | 20,002.5 | 23,815.4 | 26,993.0 | 27,884.7 | 25,694.5 | 25,020.6 | 20,171.4 |
| Male | 14,868.0 | 16,309.7 | 16,676.5 | 17,920.7 | 19,447.4 | 19,947.7 | 20,691.0 | 17,082.3 | |
| Gonads | Female | 13.1 | 13.0 | 6.6 | 5.3 | 5.2 | 5.1 | 5.1 | 4.9 |
| Male | 40.3 | 40.8 | 35.0 | 33.5 | 31.9 | 30.6 | 30.3 | 29.5 | |
| Heart | Female | 328.4 | 340.0 | 355.6 | 377.1 | 399.1 | 413.5 | 431.2 | 412.9 |
| Male | 417.2 | 434.3 | 439.1 | 454.6 | 464.8 | 441.4 | 437.9 | 425.1 | |
| Kidney | Female | 403.4 | 401.7 | 400.5 | 383.4 | 364.2 | 325.2 | 308.8 | 295.8 |
| Male | 437.7 | 475.9 | 468.1 | 455.6 | 441.7 | 395.7 | 366.7 | 355.9 | |
| Liver | Female | 1905.5 | 1881.0 | 1867.7 | 1679.8 | 1504.9 | 1423.4 | 1329.5 | 1189.4 |
| Male | 2357.8 | 2324.5 | 2206.0 | 2041.3 | 1714.6 | 1417.3 | 1324.0 | 1202.0 | |
| Lung | Female | 1009.5 | 1021.4 | 1038.8 | 1023.8 | 1005.4 | 849.1 | 804.5 | 637.3 |
| Male | 1294.3 | 1334.7 | 1337.4 | 1349.0 | 1271.8 | 1138.0 | 1007.4 | 978.0 | |
| Muscle | Female | 20,276.2 | 23,058.3 | 21,300.1 | 18,971.2 | 15,746.4 | 13,367.8 | 12,394.3 | 10,060.6 |
| Male | 32,338.6 | 32,318.2 | 31,180.6 | 29,600.0 | 28,562.1 | 26,524.8 | 23,657.0 | 19,534.0 | |
| Pancreas | Female | 169.5 | 170.3 | 163.8 | 158.7 | 148.5 | 131.9 | 128.0 | 122.6 |
| Male | 190.3 | 190.8 | 183.6 | 178.2 | 165.4 | 149.9 | 143.7 | 139.5 | |
| Skin | Female | 2723.5 | 2773.2 | 2836.2 | 2829.5 | 2725.8 | 2559.1 | 2493.8 | 2223.5 |
| Male | 3760.9 | 3790.2 | 3745.1 | 3692.0 | 3635.2 | 3537.0 | 3439.7 | 3122.6 | |
| Spleen | Female | 219.2 | 197.7 | 190.7 | 182.4 | 164.5 | 149.7 | 99.4 | 78.7 |
| Male | 243.4 | 221.9 | 208.2 | 197.0 | 186.6 | 160.4 | 133.4 | 108.4 | |
aOrgan masses for gastrointestinal organs were kept constant for females (1274.5 g) and males (1304.8 g) over the investigated age range
bBlood pools represent arterial and venous blood, as well as blood in the portal vein
cPhysiological and anatomical information on the 30-year-old individuals is derived from the International Commission on Radiological Protection reference man [25]
Fig. 2.
Distributions of mean organ weights in females (left) and males (right), from newborns to individuals up to 100 years of age. GI gastrointestinal
Physiological Changes in the Elderly
Anthropometric Measures
Anthropometric measures were stated in almost every physiological study. In addition, comprehensive European anthropometric studies [35–46] were used, and coverage was dense for the age range of 30–100 years.
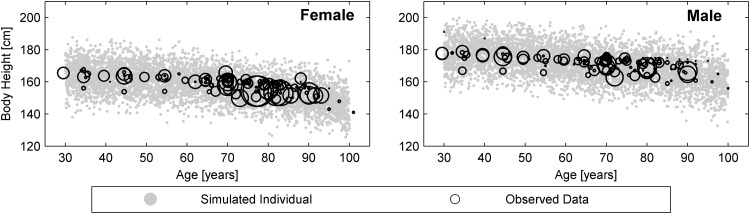
In general, men tended to be taller and heavier than women at any age stage. Both sexes showed an increase in body mass up to the age of 55 years, and then a progressive decrease. This decline started as a 1.5 % decrease per decade and rose to nearly 10 % per decade between 90 and 100 years of age. Body height started to decrease in early adulthood, after the age of 30 years, as visualized in Fig. 3. As with body weight, the decrease started with a low rate of 1 % per decade and was comparable for men and women. In women, this rate accelerated after menopause, culminating in a 4.2 % decrease in body height per decade in female nonagenarians. In males, height loss during the tenth decade averaged only about 3.5 %.
Fig. 3.
Age-dependent changes in body height: comparison between simulations (grey dots) [N = 5000] and observations (reference mean data; black circles) in females and males. The reference data were all gathered from anthropometric studies or studies where they appeared as covariates. The sizes of the black circles indicate the relative numbers of subjects
Muscle Mass
Loss of muscle mass is assessed as part of the screening test for sarcopenia, using the gold standard methods computer tomography (CT) and magnetic resonance imaging (MRI) [47], but also using dual-energy X-ray absorptiometry (DXA) [48–52] or bioimpedance analysis (BIA) [53–58], according to the European Working Group on Sarcopenia [59]. Janssen et al. [60] introduced an equation enabling calculation of skeletal muscle mass from BIA observations. As decreases in muscle mass vary between different parts of the body, whole-body analysis was preferred for the data pooling. The degressive decline in the maximum skeletal muscle mass from 37 % of body weight in adults to 22 % in the centenarian female opposed the progressive reduction from 44 % of body weight in adults to 34 % in extremely old males.
Fat Mass
Like muscle mass, FM can generally be assessed using CT [61], MRI, BIA [43, 53, 62–66] or DXA [41, 67–79]. Estimations based on skinfold thickness and whole-body count for total potassium content were not included, because of the varying methods of calculation [80].
During aging, FM in females increased to an absolute maximum at the age of 70 years, whereas in males, this peaked by the age of 65 years. Because of the diminished body weight and the severe loss of LBM from 70 to 100 years of life, the relative FM continued to increase, to as much as 45.8 % in female and 31.5 % in male centenarians. Here, the increases were constant in males, with 0.978 kg FM/decade. Females, however, experienced an accelerated gain of 3.50 kg FM/decade in the first two decades after menopause, and then a reduction to the same rate as in males of the same age group.
Kidney Weight
Four studies were included for model building [81–84]. These revealed that maximum kidney weight was achieved in the fourth decade and remained constant for almost 20 years before continuously declining. This decrease was associated with loss of renal tissue and a reduction in the number of nephrons, particularly in the renal cortex [85]. This resulted in a reduction in tubular and glomerular cells, and thus affected the glomerular filtration rate (GFR) [86, 87].
Liver Weight
The number of hepatocytes was reduced in the elderly, whereas the single-cell volume initially increased but subsequently decreased [88]. Thus, the number of hepatic lobules was constant over the course of aging, but the size of the liver declined, while collagen accumulation induced a widening of the perisinusoidal space [89]. In females, liver mass decreased almost linearly at the rate of 8.3 % per decade after the age of 40 years, whereas in males, it proceeded in two steps: an initial decline of 6.1 % per decade to the age of 60 years, followed by a more rapid loss of 11.4 % per decade [82–84, 90, 91]. This tendency was determined from autopsy analysis but was also detected in imaging studies [92–94].
Cardiac Output Distribution
Williams [95] conducted a comprehensive literature analysis on changes over the course of aging in CO and the cardiac index, a parameter that relates CO to body surface area (BSA). His study revealed that an initial decrease in cardiac index after the age of 20 years developed into a marked reduction after the sixth decade of life. Given that Williams summarized predominantly North American reports and a large Japanese trial, European studies were screened to control for potential racial variations. The studies taken into account for evaluating both changes in CO [96–99] over the course of aging and the underlying distributions are listed in Supplementary Table 1.
Females showed an almost linear decrease in cardiac index of 3.22 % per decade after the age of 30 years. The slowing of the cardiac blood circulation was more precipitous in males, where it started at 1.46 % per decade during early adulthood, followed by a stronger decrease of 8.30 % per decade in the elderly age range. These results were confirmed by studies on stroke volume [100–102], assuming that the heart rate remained constant over the lifespan. However, because of contradictory reports on changes in heart rate over the course of aging (some reports supported a decrease in heart rate, due to the dropout of pacemaker cells [103, 104] whereas others indicated that the heart rate increased in female subjects [105]), reports on isolated stroke volumes were not taken into account.
Kidney Blood Flow
In the kidney, the age-dependent decrease in the absolute perfusion rate was more severe than the weight loss. In comparison with the perfusion rate, the overall reduction in CO proceeded at a slower rate. Correlation of kidney perfusion and filtration performance was shown in a larger population ranging from 20 to nearly 90 years of age [106]. Para-aminohippuric acid (PAH) is the marker substance of choice for the assessment of renal blood flow; in the past, it was diodrast [107–109].
Hepatic and Splanchnic Blood Flow
In healthy adults, the pre-portal organs, including the stomach, intestines, pancreas and spleen, provided about 75 % of the portal blood flow [10]. This relationship seemed to be maintained over the course of aging, although the total flow rate decreased [110]. Additional shunting was negligible when the blood flow rate was reduced and portal pressure remained constant. The hepatic blood flow itself was measured using the dye dilution method, where a subject was catheterized in the portal and hepatic veins [93]. In order to eliminate potential anastomosis, additional studies based on Doppler measurements were integrated into this analysis.
Although no absolute information was obtainable for splanchnic blood flow, relative alterations could be analysed by laser Doppler flowmetry. Analysis of the blood flow in the superior mesenteric artery was the most common assessment of alterations in splanchnic perfusion [111–113]. Although the superior mesenteric artery is one of three vessels that contribute to the splanchnic blood supply, it is the major sustenance vessel of the small intestine, the duodenum and the colon. Alterations in perfusion of the stomach, pancreas and spleen could also be captured by this method. The ratio of splanchnic blood flow to CO gradually decreased from 22.5 % in adults to 17.5 % during the tenth decade of life [114–117].
In summary, information on blood flow alterations implemented in the elderly PBPK approach is listed in Table 3. The general lack of perfusion data impeded computation of robust variability for each blood flow rate, and so a 5 % coefficient of variation was assumed as accepted for other approaches [19].
Table 3.
Cardiac index and organ blood flow rates used in the elderly physiologically based pharmacokinetic (PBPK) models
| Parameter | Sex | Age, years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 30b | 40 | 50 | 60 | 70 | 80 | 90 | 100 | ||
| Cardiac index, L/m2 | Female | 3.34 | 3.33 | 3.08 | 2.89 | 2.75 | 2.68 | 2.52 | 2.41 |
| Male | 3.23 | 3.22 | 3.09 | 2.86 | 2.58 | 2.36 | 2.18 | 2.04 | |
| Organ blood flow, mL/min | |||||||||
| Adipose | Female | 501.1 | 528.2 | 578.0 | 655.3 | 684.7 | 630.2 | 607.0 | 491.5 |
| Male | 324.7 | 352.1 | 357.3 | 381.4 | 408.0 | 419.8 | 429.2 | 357.3 | |
| Cerebral | Female | 707.8 | 686.9 | 666.1 | 632.1 | 601.2 | 562.8 | 514.2 | 467.6 |
| Male | 779.7 | 747.6 | 727.9 | 692.9 | 650.6 | 623.8 | 594.4 | 536.1 | |
| Gonads | Female | 1.2 | 1.2 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.4 |
| Male | 3.2 | 3.3 | 2.8 | 2.7 | 2.6 | 2.5 | 2.4 | 2.4 | |
| Myocardial | Female | 295.0 | 311.2 | 313.2 | 363.1 | 376.3 | 445.8 | 464.8 | 445.2 |
| Male | 260.1 | 271.1 | 278.0 | 299.1 | 308.7 | 334.7 | 332.1 | 322.3 | |
| Renal | Female | 1121.0 | 1061.8 | 943.8 | 825.8 | 707.8 | 589.8 | 471.8 | 353.8 |
| Male | 1325.0 | 1339.3 | 1116.3 | 913.3 | 730.3 | 567.3 | 424.3 | 301.3 | |
| Splanchnica | Female | 1239.0 | 1254.3 | 1187.2 | 1015.3 | 862.6 | 771.5 | 679.1 | 570.5 |
| Male | 1235.0 | 1241.9 | 1237.7 | 1056.7 | 813.1 | 610.6 | 512.9 | 413.5 | |
| Hepatic | Female | 383.4 | 388.1 | 367.3 | 314.2 | 266.9 | 238.7 | 210.1 | 176.5 |
| Male | 423.0 | 425.4 | 423.9 | 361.9 | 278.5 | 209.1 | 175.7 | 141.6 | |
| Muscle | Female | 665.1 | 774.8 | 636.6 | 568.4 | 479.8 | 405.6 | 369.0 | 298.8 |
| Male | 1105.7 | 1086.8 | 1036.8 | 975.0 | 922.3 | 861.7 | 751.9 | 625.9 | |
| Skeleton | Female | 294.9 | 287.3 | 280.5 | 271.6 | 252.6 | 225.7 | 214.0 | 202.5 |
| Male | 324.9 | 325.8 | 315.7 | 305.0 | 284.7 | 265.0 | 246.6 | 228.2 | |
| Skin | Female | 295.7 | 301.1 | 307.9 | 307.2 | 295.9 | 277.8 | 270.7 | 241.4 |
| Male | 325.1 | 327.7 | 323.8 | 319.2 | 314.3 | 305.8 | 297.4 | 270.0 | |
aThe splanchnic blood flow rate combines gastrointestinal, pancreatic and splenic blood flow, and describes portal blood flow
bCardiac output distribution information on 30-year-old individuals is derived from the International Commission on Radiological Protection reference man [25]
Total Body Water Distribution
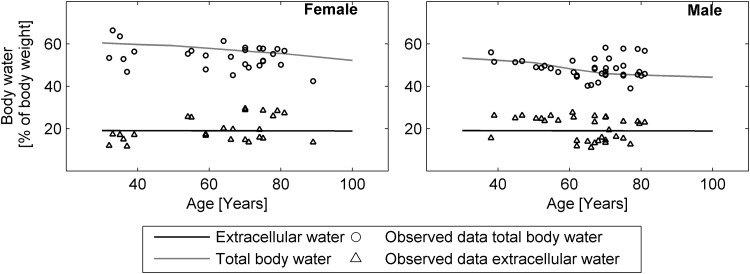
TBW remained stable throughout most of adulthood but decreased starting in the sixth decade of life. Race and sex have been associated with differences observed over the complete lifespan [118]. Normalization of TBW to body weight revealed that the ratio declined gradually during the aging process. This reduction was mainly due to loss of intracellular water; the ratio of extracellular water to body weight was maintained during aging. These changes are visualized in Fig. 4, together with observed data in healthy Caucasians [43, 64, 119–122].
Fig. 4.
Distribution of body water in males (left) and females (right) over the course of aging. Extracellular body water (dark grey lines) and total body water (light grey lines) are shown as percentages of body weight. Values reported in the literature for extracellular water (triangles) and total body water (circles) are shown for comparison
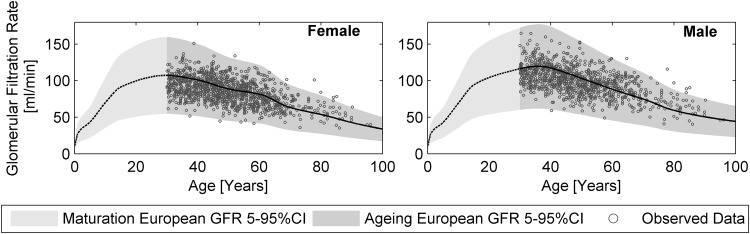
Glomerular Filtration Rate
In order to generate population samples large enough to investigate the GFR, most studies were carried out in prospective renal transplant donors, using exogenous markers [123–126]. An analysis of the gathered and BSA-adjusted data for 1213 females and 1081 males was performed. This resulted in a slight reduction starting at the age of 30 years and a more rapid reduction starting at the age of 40 years.
The GFR in PK-Sim® is linked to maturation of the kidney volume and is described by a Hill function [127]. To describe the effect of aging on the GFR, a new, reverse sigmoid hyperbolic maturation function was developed and optimized with the Matlab® function lsqnonlin, starting (for both sexes) at the age of 30 years.
| 1 |
Here, F PMA is the specific GFR derived from the sigmoid hyperbolic maturation function [127] after full maturation is achieved in the 40th week of gestation (26.6 mL/min/100 g kidney weight) [128]. In order to describe the effects of aging on kidney function, the maximal decreasing rate factor (V max) was 0.9 mL/min/100 g kidney weight, and the aging half-time (TA50) was 59 years for females and 54 years for males. The Hill coefficient was parameterized at 1.5. The resulting function over the aging period is visualized in Fig. 5. While specific GFR function was parameterized using the observed GFR values and assuming the mean kidney weight at the respective age, the variability was entirely dependent on the deviation in kidney weight.
Fig. 5.
Changes in kidney function in females (left) and males (right) over the course of aging. To describe the influence of aging on the glomerular filtration rate (GFR), a new reverse sigmoid hyperbolic maturation function was developed and applied to both sexes, starting at the age of 30 years. Changes in the GFR were investigated by analysis of exogenous markers in prospective renal transplant donors. A stepwise regression analysis of the gathered data, normalized to body surface area (BSA), was performed for 1213 females (upper panel) and 1081 males (lower panel). A solid black line represents the new GFR function in relation to kidney weight, and a dashed black line represents the maturation of GFR function according to Rhodin et al. [127]. The grey shaded areas represent the predicted 95 % percentile range based on kidney size variability. The black circles depict the observed GFR rates [123–126]
Predictability of PK Data in the Elderly
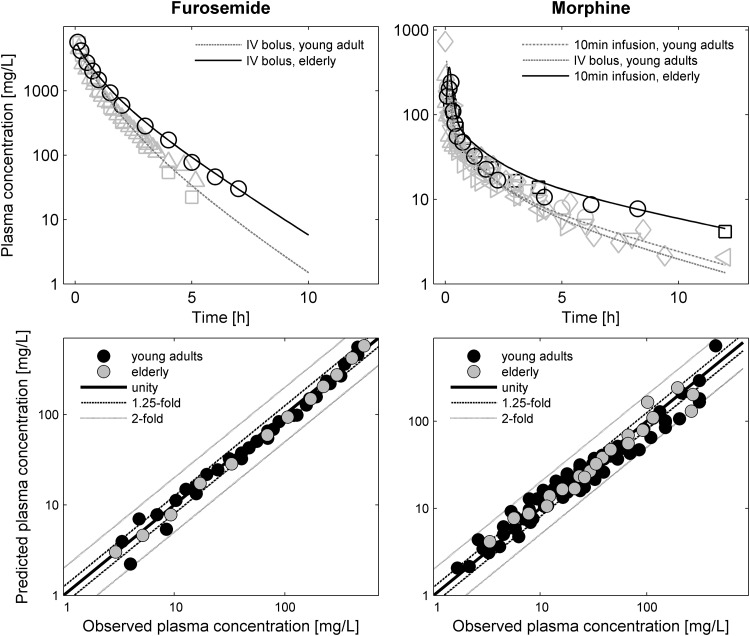
The literature screen for observed PK data led to inclusion of several studies of morphine [129–136] and furosemide [31, 137–140], as listed in Table 4. The simulated plasma concentration–time profiles of furosemide and morphine in healthy adults were consistent with the observed data, as visualized in Fig. 6. The scaling to older ages reported in the above-mentioned drug studies led to an accurate description of the estimated PK parameters, as shown in Figs. 7 and 8.
Table 4.
Summary of clinical studies after intravenous dosing of the test compounds used to compare the simulated data
| Dose | Age, years | N | Height, cm | Weight, kg | References |
|---|---|---|---|---|---|
| Morphine | |||||
| 0.125 mg/kg | 30.6 (24–40) | 11 | 62.6 (47–85) | [129] | |
| 10 mg | 27.4 (26–30) | 8 | 67.6 ± 4.5 | [130] | |
| 74.0 (68–90) | 9 | 66.4 ± 3.2 | |||
| 5 mg | 31 (26–40) | 6 | [131] | ||
| 5 mg | 30.2 (25–44) | 10 | 72 (63–83) | [132] | |
| 5 mg | 25.8 (20–40) | 6 | 71.4 (49.2–102.1) | [133] | |
| 0.05 mg/kg | 76.1 ± 4.5 | 16 | 167.4 ± 8.3 | 77.8 ± 16.9 | [134] |
| 10 mg | (22–29) | 12 | 76.4 ± 7.4 | [135] | |
| 10 mg | (20–39) | 14 | 74.9 ± 11.0 | [136] | |
| Furosemide | |||||
| 80 mg | 27.1 (20–35) | 10 | 71.4 ± 6.7 | [31] | |
| 80 mg | 27 ± 4.8 | 8 | 71 ± 6.7 | [137] | |
| 64 ± 4.0 | 8 | 76 ± 7.3 | |||
| 40 mg | 30 (20–45) | 7 | [138] | ||
| 20 mg | 76 | 1 | 70 | [139] | |
| 40 mg | 74 (64–84) | 20 | 73.1 ± 12.8 | [140] | |
Values are expressed with ranges (in parentheses) or ± standard deviations
Fig. 6.
Upper panel: predicted mean plasma concentration–time profiles of furosemide and morphine in younger adults (grey dashed line) versus the elderly (black line). The concentrations are normalized to a 40 mg dose for furosemide and to a 10 mg dose for morphine. Observed data are superimposed (black open symbols represent values for the elderly [130, 134, 137]; grey open symbols represent values for younger adults [129, 130, 133, 136–138, 141, 142]). Lower panel: goodness-of-fit plots for furosemide and morphine model predictions in the adult population (black dots) and the elderly population (grey dots). A solid line represents the line of identity; dashed lines represent the 1.25-fold error range and dotted lines represent the 2-fold error range
Fig. 7.
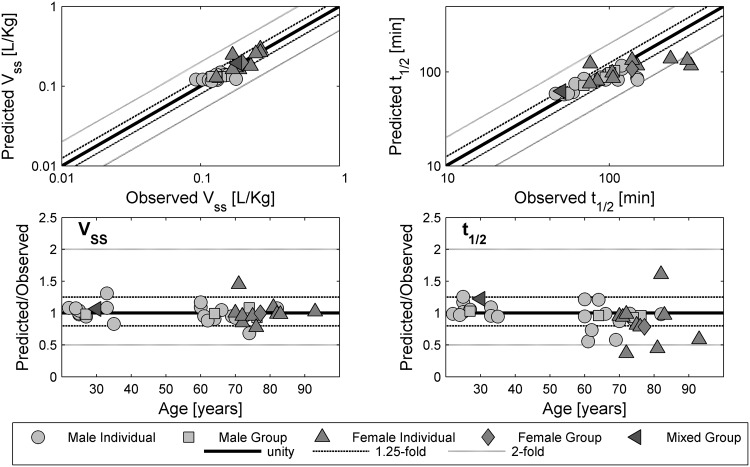
Observed versus predicted values for the volume of distribution at steady state (V ss) and elimination half-life (t ½), as well as the corresponding fold over-/underprediction, for the test compound furosemide. A solid line represents the line of unity; dashed black lines represent the 1.25-fold level and grey lines represent the 2-fold level of the predicted accuracy
Fig. 8.
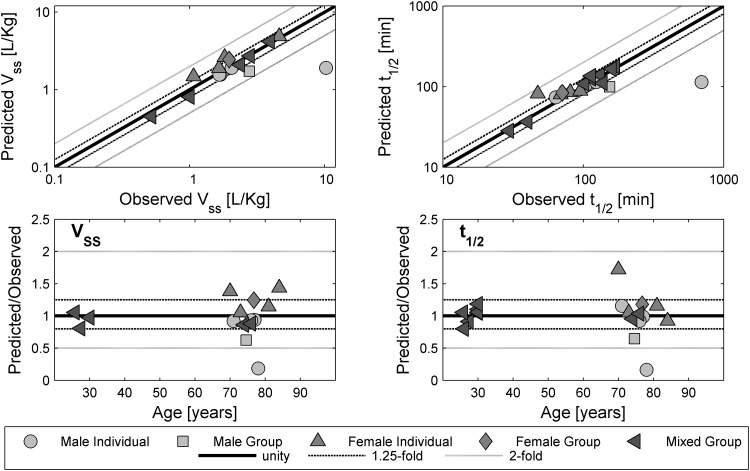
Observed versus predicted values for the volume of distribution at steady state (V ss) and elimination half-life (t ½), as well as the corresponding fold over-/underprediction, for the test compound morphine. A solid line represents the line of unity; dashed black lines represent the 1.25-fold level and grey lines represent the 2-fold level of the predicted accuracy
The predictive performance of the PBPK models of furosemide and morphine is summarized in Table 5. The precision of the predictions of plasma concentrations improved by 0.132 and 0.212 for furosemide and morphine, respectively, with use of age-informed physiology for the simulations of the elderly studies. This represented relative improvements in the predictions, by 32 % in the case of furosemide and by 49 % in the case of morphine. For both drugs, the plasma concentrations were underpredicted in the absence of consideration of age-informed physiology. The bias was considerably reduced when age-informed physiology was applied.
Table 5.
Predictive performance analysis of the furosemide and morphine physiologically based pharmacokinetic (PBPK) models with and without age-informed physiology
| Furosemide | Morphine | |||||
|---|---|---|---|---|---|---|
| Adult physiology | Age-informed physiology | Adult physiology | Age-informed physiology | |||
| Adult data | Elderly data | Elderly data | Adult data | Elderly data | Elderly data | |
| Bias | ||||||
| ME | 0.032 (−0.034 to 0.010) | −0.339 (−0.621 to −0.057) | 0.076 (−0.015 to 0.137) | 0.082 (0.001 to 0.163) | −0.093 (−0.231 to 0.045) | 0.050 (−0.072 to 0.172) |
| Precision | ||||||
| RMSE | 0.180 (0.141 to 0.212) | 0.408 (0.004 to 0.721) | 0.276 (0.185 to 0.343) | 0.286 (0.189 to 0.358) | 0.437 (0.001 to 0.656) | 0.224 (0.004 to 0.384) |
| ΔRMSE | 0.132 (0.005 to 0.695) | 0.212 (0.001 to 0.635) | ||||
| Relative ΔRMSE | 32 % | 49 % | ||||
Values are expressed in mg/L with 95 % confidence intervals (in parentheses)
ME mean prediction error, RMSE root mean squared prediction error, ΔRMSE difference in RMSE
The scaling to older ages reported in the above-mentioned drug studies led to an accurate description of the estimated PK parameters, as shown in Figs. 7 and 8. All but four of 36 predicted V ss values for furosemide were within 1.25-fold of the experimental values. The predicted t ½ of furosemide in individuals with a longer half-life was underpredicted. Here, eight of 36 values of t ½ were not within the 1.25-fold range. Reliable predictions were obtained for the V ss and t ½ of morphine. Only three individual values for V ss and two of eight individual values for t ½ were outside the 1.25-fold range without any age-related pattern.
Discussion
The PBPK aging approach described here summarizes anatomical and (patho)physiological changes from early adulthood to extreme old age. Data were extracted from the peer-reviewed literature for all relevant PBPK parameters and analysed in order to describe the systemic changes that occur with age.
Only a few studies have attempted geriatric PBPK modelling by including aging considerations in their databases. The recent approach by McNally et al. [19] included detailed considerations of age-related changes in brain weight and bone mass. Also, the implementation of muscle mass alterations according to Janssen et al. [47], as well as the analysis of changes in CO by Luisada et al. [143], have proven to be useful for PBPK modelling for the elderly. By informing bone and muscle mass, this approach considers changes in FM indirectly. However, alterations in CO distribution and changes in major organs (such as the liver and kidney) were not captured by data from the literature [19, 143].
The gathering of data from the literature for PBPK application is a serious challenge. Some organs, such as the brain, have been well described over the entire age range, because of advances in analytical methods and enhanced focus on research in these areas. This has enabled investigations into secular trends and tracking of longitudinal aging trends. For other organs, however, the available data are sparse or outdated. One of the main cited studies referring to changes in liver weight over the course of aging was performed by Boyd [144], dating from 1933; this study included severely diseased individuals. Generally, because of the higher survival rate of women, data yield for extreme old age was less representative for men. Another hurdle is the research on massive blood-containing organs such as the lung and the spleen. Here, an infection prior to death can cause great variability in the reported weight at autopsy. Furthermore, in contrast to gestation and maturation, which involve highly predictable biological changes over a defined time scale, classification of individuals beyond the age of 65 years has no physiological basis. This impedes generation of a clear reference interval for a certain chronological sequence and thus increases variability.
In contrast to studies on changes in organ volume during the aging process, studies on blood flow rates and CO distribution have been sparse or used questionable analytical methods. In these cases, our data collection included information from studies in North Americans and Australians, and linear regression was performed. Since no study covered the topic of gonadal perfusion, specific blood flow was kept constant and only volumetric change in these organs was taken into account. Moreover, the last data point for muscle blood flow was for a group of 73-year-old subjects, allowing only a linear regression from young adulthood up to 100 years of age. Similarly, no studies on body water distribution were available for females above the age of 80 years. Besides lack of data, the predicted variability in the GFR based on kidney size variability was too great throughout the course of aging, as can be seen in Fig. 5. Alternative approaches, such as correlation of GFR and renal plasma flow, have been proposed by others [106] because of the uncertain extent of glomerulosclerosis. The postulated blood flow rate variability would propagate to the GFR calculation to yield a narrower variability distribution in comparison with propagation of kidney size variability.
In the current study, parameters relevant to a PBPK approach were gathered by a comprehensive review of the literature and, for the first time, they were integrated into a knowledge-driven PBPK aging approach that captured whole-body physiology. This methodology enabled an age-dependent description of plasma concentration–time profiles for young adults and elderly (Fig. 6). Importantly, the clinically observed increasing plasma concentrations of both drugs in the elderly were reflected by the aging PBPK model. Along with this, as shown in Figs. 7 and 8, this approach allowed description of the shifts in V ss and t ½ for selected compounds. Unfortunately, only a few studies providing plasma concentration–time profiles were available; thus, an age-grouped analysis was not feasible. Especially for the age ranges of 40–60 years and 85–100 years, there were hardly any stratified PK data. When the underlying physiological changes were used, the predictions were reasonably precise over the entire age range. There was an underprediction of higher t ½ values for furosemide, which could have been due to temporary decreases in the kidneys’ hydrodynamics observed in several patients in the first 2–3 h after intravenous administration [34]. This dose–response relationship is considered to be dose dependent [145] and could be assessed with an additional PD model. Variability in morphine PK is associated with variation in protein concentrations. Perturbed levels of albumin and acidic glycoprotein may occur in patients suffering from acute or chronic pain, as a result of immobility. This, in turn, would strongly impact the extent of morphine binding to plasma proteins [28]. Because of the high extraction ratio, age-related changes in the vasculature and hepatic tissue are more descriptive of the fate of morphine [28, 146].
This paper supports specific considerations for the aged population in geriatric clinical trial designs, as this group receives the greatest number of drug prescriptions. The established PBPK aging approach represents a valuable tool for age-related PBPK modelling. With this approach, anatomical and (patho)physiological age dependence can be used to predict alterations in PK from early adulthood through extreme old age. This will improve prediction of effective starting drug dosages for healthy elderly patients, as depicted in Fig. 1 and emphasized by Jadhav et al. [147]. Further disease implementations are encouraged in order to distinguish between age- and/or disease-related physiological alterations in drug usage. In general, this knowledge-driven approach is expected to be effective for increasing the efficiency of clinical trial designs, as well as for optimizing drug therapy in the elderly population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 4 (TIFF 20682 kb)
Acknowledgments
This publication and the work involved were funded by Bayer Technology Services GmbH.
The authors would like to thank André Dallmann (University of Muenster, Muenster, Germany) for valuable discussions.
Compliance with Ethical Standards
Conflict of interest
Jan-Frederik Schlender is a PhD student at the University of Bonn and is employed on a grant from Bayer Technology Services GmbH. Michaela Meyer, Kirstin Thelen, Markus Krauss, Thomas Eissing and Stefan Willmann were employed by Bayer Technology Services GmbH during preparation of this manuscript and are potential stock holders of Bayer AG, the holding owning Bayer Technology Services GmbH. Ulrich Jaehde received a research a Grant from Bayer Technology Services between 2013 and 2015.
References
- 1.Administration on Aging. A profile of older Americans: 2014. US Department of Health Human Services—Administration for Community Living; 2014.
- 2.United Nations Department of Economic and Social Affairs. Population ageing and development: ten years after Madrid. Population facts no. 2012/4. New York: United Nations Department of Economic and Social Affairs—Population Division; 2012.
- 3.Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606–609. doi: 10.1136/bmj.39503.424653.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cusack BJ. Pharmacokinetics in older persons. Am J Geriatr Pharmacother. 2004;2:274–302. doi: 10.1016/j.amjopharm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Hammerlein A, Derendorf H, Lowenthal DT. Pharmacokinetic and pharmacodynamic changes in the elderly. Clinical implications. Clin Pharmacokinet. 1998;35:49–64. doi: 10.2165/00003088-199835010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diener L, Hugonot-Diener L, Alvino S, Baeyens JP, Bone MP, Chirita D, et al. Guidance synthesis. Medical research for and with older people in Europe: proposed ethical guidance for good clinical practice: ethical considerations. J Nutr Health Aging. 2013;17:625–627. doi: 10.1007/s12603-013-0340-0. [DOI] [PubMed] [Google Scholar]
- 8.Alberighi ODC, Barrett J, Läer S, Meibohm B. Response to “physiologically based pharmacokinetic modeling at the extremes of age”. Clin Pharmacol Therap. 2012;93:149. [DOI] [PubMed]
- 9.Shepard T, Scott G, Cole S, Nordmark A, Bouzom F. Physiologically based models in regulatory submissions: output from the ABPI/MHRA forum on physiologically based modeling and simulation. CPT Pharmacomet Syst Pharmacol. 2015;4:221. doi: 10.1002/psp4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–1034. doi: 10.2165/00003088-200645100-00005. [DOI] [PubMed] [Google Scholar]
- 11.Jiang XL, Zhao P, Barrett JS, Lesko LJ, Schmidt S. Application of physiologically based pharmacokinetic modeling to predict acetaminophen metabolism and pharmacokinetics in children. CPT Pharmacomet Syst Pharmacol. 2013;2:e80. doi: 10.1038/psp.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51:365–396. doi: 10.2165/11597440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Xia B, Heimbach T, Gollen R, Nanavati C, He H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013;15:1012–1024. doi: 10.1208/s12248-013-9505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edginton AN, Willmann S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet. 2008;47:743–752. doi: 10.2165/00003088-200847110-00005. [DOI] [PubMed] [Google Scholar]
- 15.Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189–206. doi: 10.2165/11318160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Sinha V, Zhao P, Huang SM, Zineh I. Physiologically based pharmacokinetic modeling: from regulatory science to regulatory policy. Clin Pharmacol Ther. 2014;95:478–480. doi: 10.1038/clpt.2014.46. [DOI] [PubMed] [Google Scholar]
- 17.Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with impaired hepatic function. European Medicines Agency—Committee for Medicinal Products for Human Use. London; 2005.
- 18.Polasek TM, Patel F, Jensen BP, Sorich MJ, Wiese MD, Doogue MP. Predicted metabolic drug clearance with increasing adult age. Br J Clin Pharmacol. 2013;75:1019–1028. doi: 10.1111/j.1365-2125.2012.04446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNally K, Cotton R, Hogg A, Loizou G. PopGen: a virtual human population generator. Toxicology. 2014;315:70–85. doi: 10.1016/j.tox.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Thompson CM, Johns DO, Sonawane B, Barton HA, Hattis D, Tardif R, et al. Database for physiologically based pharmacokinetic (PBPK) modeling: physiological data for healthy and health-impaired elderly. J Toxicol Environ Health B Crit Rev. 2009;12:1–24. doi: 10.1080/10937400802545060. [DOI] [PubMed] [Google Scholar]
- 21.Saisho Y, Butler AE, Meier JJ, Monchamp T, Allen-Auerbach M, Rizza RA, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933–942. doi: 10.1002/ca.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization expert committee. Am J Clin Nutr. 1996;64:650–658. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura H. Development of the Japanese reference man model for age-specific phantoms. Radiat Prot Dosim. 2012;149:28–34. doi: 10.1093/rpd/ncr362. [DOI] [PubMed] [Google Scholar]
- 24.Willmann S, Hohn K, Edginton A, Sevestre M, Solodenko J, Weiss W, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34:401–431. doi: 10.1007/s10928-007-9053-5. [DOI] [PubMed] [Google Scholar]
- 25.Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values: ICRP publication 89. Ann ICRP. 2002;32:1–277. [PubMed] [Google Scholar]
- 26.Avdeef A, Barrett DA, Shaw PN, Knaggs RD, Davis SS. Octanol-, chloroform-, and propylene glycol dipelargonat-water partitioning of morphine-6-glucuronide and other related opiates. J Med Chem. 1996;39:4377–4381. doi: 10.1021/jm960073m. [DOI] [PubMed] [Google Scholar]
- 27.Cutler RE, Blair AD. Clinical pharmacokinetics of frusemide. Clin Pharmacokinet. 1979;4:279–296. doi: 10.2165/00003088-197904040-00002. [DOI] [PubMed] [Google Scholar]
- 28.Glare PA, Walsh TD. Clinical pharmacokinetics of morphine. Ther Drug Monit. 1991;13:1–23. doi: 10.1097/00007691-199101000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Willmann S, Lippert J, Sevestre M, et al. PK-Sim: a physiologically based pharmacokinetic ‘whole-body’ model. Biosilico. 2003;1:121–124. [Google Scholar]
- 30.Willmann S, Lippert J, Schmitt W. From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin Drug Metab Toxicol. 2005;1:159–168. doi: 10.1517/17425255.1.1.159. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen F, Christensen CK, Jacobsen FK, Jansen J, Mogensen CE, Pedersen OL. The individual variation in pharmacokinetics and pharmacodynamics of furosemide in young normal male subjects. Eur J Clin Invest. 1982;12:247–255. doi: 10.1111/j.1365-2362.1982.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 32.Tiseo PJ, Thaler HT, Lapin J, Inturrisi CE, Portenoy RK, Foley KM. Morphine-6-glucuronide concentrations and opioid-related side effects: a survey in cancer patients. Pain. 1995;61:47–54. doi: 10.1016/0304-3959(94)00148-8. [DOI] [PubMed] [Google Scholar]
- 33.Waller ES, Massarella JW, Tomkiw MS, Smith RV, Doluisio JT. Pharmacokinetics of furosemide after three different single oral doses. Biopharm Drug Dispos. 1985;6:109–117. doi: 10.1002/bdd.2510060202. [DOI] [PubMed] [Google Scholar]
- 34.Ponto LL, Schoenwald RD. Furosemide (frusemide): a pharmacokinetic/pharmacodynamic review (part I) Clin Pharmacokinet. 1990;18:381–408. doi: 10.2165/00003088-199018050-00004. [DOI] [PubMed] [Google Scholar]
- 35.Perissinotto E, Pisent C, Sergi G, Grigoletto F. Anthropometric measurements in the elderly: age and gender differences. Br J Nutr. 2002;87:177–186. doi: 10.1079/bjn2001487. [DOI] [PubMed] [Google Scholar]
- 36.Wahren J. Average body weight and correlation weight in relation to age and sex [in German] Z Morphol Anthropol. 1981;72:65–76. [PubMed] [Google Scholar]
- 37.Rea IM, Gillen S, Clarke E. Anthropometric measurements from a cross-sectional survey of community dwelling subjects aged over 90 years of age. Eur J Clin Nutr. 1997;51:102–106. doi: 10.1038/sj.ejcn.1600370. [DOI] [PubMed] [Google Scholar]
- 38.Ravaglia G, Morini P, Forti P, Maioli F, Boschi F, Bernardi M, et al. Anthropometric characteristics of healthy Italian nonagenarians and centenarians. Br J Nutr. 1997;77:9–17. doi: 10.1017/s0007114500002841. [DOI] [PubMed] [Google Scholar]
- 39.Eiben G, Dey DK, Rothenberg E, Steen B, Bjorkelund C, Bengtsson C, et al. Obesity in 70-year-old Swedes: secular changes over 30 years. Int J Obes (Lond). 2005;29:810–817. doi: 10.1038/sj.ijo.0802940. [DOI] [PubMed] [Google Scholar]
- 40.Bartali B, Benvenuti E, Corsi AM, Bandinelli S, Russo CR, Di Iorio A, et al. Changes in anthropometric measures in men and women across the life-span: findings from the InCHIANTI study. Soz Praventivmed. 2002;47:336–348. doi: 10.1007/pl00012644. [DOI] [PubMed] [Google Scholar]
- 41.Gillette-Guyonnet S, Nourhashemi F, Andrieu S, Cantet C, Albarede JL, Vellas B, et al. Body composition in French women 75+ years of age: the EPIDOS study. Mech Ageing Dev. 2003;124:311–316. doi: 10.1016/s0047-6374(02)00198-7. [DOI] [PubMed] [Google Scholar]
- 42.Delarue J, Constans T, Malvy D, Pradignac A, Couet C, Lamisse F. Anthropometric values in an elderly French population. Br J Nutr. 1994;71:295–302. doi: 10.1079/bjn19940135. [DOI] [PubMed] [Google Scholar]
- 43.Dey DK, Bosaeus I, Lissner L, Steen B. Changes in body composition and its relation to muscle strength in 75-year-old men and women: a 5-year prospective follow-up study of the NORA cohort in Goteborg. Sweden. Nutrition. 2009;25:613–619. doi: 10.1016/j.nut.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 44.Health Survey for England—2012, trend tables: adult trend tables. England: Health and Social Care Information Centre (HSCIC); 2013.
- 45.de Groot LC, Sette S, Zajkas G, Carbajal A, Amorim JA. Nutritional status: anthropometry. Euronut SENECA Investigators. Eur J Clin Nutr. 1991;45(Suppl 3):31–42. [PubMed] [Google Scholar]
- 46.Health Survey for England—2012, trend tables: adult trend tables. England: Health and Social Care Information Centre; 2013.
- 47.Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 48.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 49.Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci. 2002;57:M772–M777. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher D, Visser M, De Meersman RE, Sepúlveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 51.Rolland Y, Lauwers-Cances V, Cournot M, Nourhashemi F, Reynish W, Riviere D, et al. Sarcopenia, calf circumference, and physical function of elderly women: a cross-sectional study. J Am Geriatr Soc. 2003;51:1120–1124. doi: 10.1046/j.1532-5415.2003.51362.x. [DOI] [PubMed] [Google Scholar]
- 52.Gouveia E, Blimkie CJ, Maia JA, Lopes C, Gouveia BR, Freitas DL. Multivariate analysis of lifestyle, constitutive and body composition factors influencing bone health in community-dwelling older adults from Madeira, Portugal. Arch Gerontol Geriatr. 2014;59:83–90. doi: 10.1016/j.archger.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17:534–541. doi: 10.1016/s0899-9007(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 54.Dittmar M. Reliability and variability of bioimpedance measures in normal adults: effects of age, gender, and body mass. Am J Phys Anthropol. 2003;122:361–370. doi: 10.1002/ajpa.10301. [DOI] [PubMed] [Google Scholar]
- 55.Tichet J, Vol S, Goxe D, Salle A, Berrut G, Ritz P. Prevalence of sarcopenia in the French senior population. J Nutr Health Aging. 2008;12:202–206. doi: 10.1007/BF02982621. [DOI] [PubMed] [Google Scholar]
- 56.Zoico E, Di Francesco V, Guralnik JM, Mazzali G, Bortolani A, Guariento S, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes Relat Metab Disord. 2004;28:234–241. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 57.Legrand D, Adriaensen W, Vaes B, Mathei C, Wallemacq P, Degryse J. The relationship between grip strength and muscle mass (MM), inflammatory biomarkers and physical performance in community-dwelling very old persons. Arch Gerontol Geriatr. 2013;57:345–351. doi: 10.1016/j.archger.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Masanes F, Culla A, Navarro-Gonzalez M, Navarro-Lopez M, Sacanella E, Torres B, et al. Prevalence of sarcopenia in healthy community-dwelling elderly in an urban area of Barcelona (Spain) J Nutr Health Aging. 2012;16:184–187. doi: 10.1007/s12603-011-0108-3. [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 61.Seidell JC, Oosterlee A, Deurenberg P, Hautvast JG, Ruijs JH. Abdominal fat depots measured with computed tomography: effects of degree of obesity, sex, and age. Eur J Clin Nutr. 1988;42:805–815. [PubMed] [Google Scholar]
- 62.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes Relat Metab Disord. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 63.Yliharsila H, Kajantie E, Osmond C, Forsen T, Barker DJ, Eriksson JG. Body mass index during childhood and adult body composition in men and women aged 56–70 y. Am J Clin Nutr. 2008;87:1769–1775. doi: 10.1093/ajcn/87.6.1769. [DOI] [PubMed] [Google Scholar]
- 64.Vache C, Rousset P, Gachon P, Gachon AM, Morio B, Boulier A, et al. Bioelectrical impedance analysis measurements of total body water and extracellular water in healthy elderly subjects. Int J Obes Relat Metab Disord. 1998;22:537–543. doi: 10.1038/sj.ijo.0800622. [DOI] [PubMed] [Google Scholar]
- 65.Deurenberg P, van der Kooij K, Evers P, Hulshof T. Assessment of body composition by bioelectrical impedance in a population aged greater than 60 y. Am J Clin Nutr. 1990;51:3–6. doi: 10.1093/ajcn/51.1.3. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Cabello A, Pedrero-Chamizo R, Olivares PR, Luzardo L, Juez-Bengoechea A, Mata E, et al. Prevalence of overweight and obesity in non-institutionalized people aged 65 or over from Spain: the elderly EXERNET multi-centre study. Obes Rev. 2011;12:583–592. doi: 10.1111/j.1467-789X.2011.00878.x. [DOI] [PubMed] [Google Scholar]
- 67.Gillette-Guyonnet S, Nourhashemi F, Lauque S, Grandjean H, Vellas B. Body composition and osteoporosis in elderly women. Gerontology. 2000;46:189–193. doi: 10.1159/000022158. [DOI] [PubMed] [Google Scholar]
- 68.Dey DK, Bosaeus I, Lissner L, Steen B. Body composition estimated by bioelectrical impedance in the Swedish elderly: development of population-based prediction equation and reference values of fat-free mass and body fat for 70- and 75-y olds. Eur J Clin Nutr. 2003;57:909–916. doi: 10.1038/sj.ejcn.1601625. [DOI] [PubMed] [Google Scholar]
- 69.Santana H, Zoico E, Turcato E, Tosoni P, Bissoli L, Olivieri M, et al. Relation between body composition, fat distribution, and lung function in elderly men. Am J Clin Nutr. 2001;73:827–831. doi: 10.1093/ajcn/73.4.827. [DOI] [PubMed] [Google Scholar]
- 70.Tanko LB, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL. Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism. 2002;51:69–74. doi: 10.1053/meta.2002.28960. [DOI] [PubMed] [Google Scholar]
- 71.Bedogni G, Pietrobelli A, Heymsfield SB, Borghi A, Manzieri AM, Morini P, et al. Is body mass index a measure of adiposity in elderly women? Obes Res. 2001;9:17–20. doi: 10.1038/oby.2001.3. [DOI] [PubMed] [Google Scholar]
- 72.Movsesyan L, Tanko LB, Larsen PJ, Christiansen C, Svendsen OL. Variations in percentage of body fat within different BMI groups in young, middle-aged and old women. Clin Physiol Funct Imaging. 2003;23:130–133. doi: 10.1046/j.1475-097x.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 73.Sardinha LB, Teixeira PJ, Guedes DP, Going SB, Lohman TG. Subcutaneous central fat is associated with cardiovascular risk factors in men independently of total fatness and fitness. Metabolism. 2000;49:1379–1385. doi: 10.1053/meta.2000.17716. [DOI] [PubMed] [Google Scholar]
- 74.Maden-Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age-related atrophy in thigh muscles. J Musculoskelet Neuronal Interact. 2013;13:320–328. [PubMed] [Google Scholar]
- 75.Bazzocchi A, Diano D, Ponti F, Andreone A, Sassi C, Albisinni U, et al. Health and ageing: a cross-sectional study of body composition. Clin Nutr. 2013;32:569–578. doi: 10.1016/j.clnu.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Horber FF, Gruber B, Thomi F, Jensen EX, Jaeger P. Effect of sex and age on bone mass, body composition and fuel metabolism in humans. Nutrition. 1997;13:524–534. doi: 10.1016/s0899-9007(97)00031-2. [DOI] [PubMed] [Google Scholar]
- 77.Puggaard L, Larsen JB, Ebbesen E, Jeune B. Body composition in 85 year-old women: effects of increased physical activity. Aging (Milano). 1999;11:307–315. doi: 10.1007/BF03339805. [DOI] [PubMed] [Google Scholar]
- 78.Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Gasbarrini G. Measurement of body fat in healthy elderly men: a comparison of methods. J Gerontol A Biol Sci Med Sci. 1999;54:M70–M76. doi: 10.1093/gerona/54.2.m70. [DOI] [PubMed] [Google Scholar]
- 79.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 80.Wang ZM, Deurenberg P, Guo SS, Pietrobelli A, Wang J, Pierson RN, Jr, et al. Six-compartment body composition model: inter-method comparisons of total body fat measurement. Int J Obes Relat Metab Disord. 1998;22:329–337. doi: 10.1038/sj.ijo.0800590. [DOI] [PubMed] [Google Scholar]
- 81.Tauchi H, Tsuboi K, Okutomi J. Age changes in the human kidney of the different races. Gerontologia. 1971;17:87–97. doi: 10.1159/000211811. [DOI] [PubMed] [Google Scholar]
- 82.Meyer WW, Peter B, Solth K. The weight of organs in the older age groups (70–92 years) and their relation to age and body weight [in German] Virchows Arch Pathol Anat Physiol Klin Med. 1963;337:17–32. [PubMed] [Google Scholar]
- 83.Puggaard L, Bjornsbo KS, Kock K, Luders K, Thobo-Carlsen B, Lammert O. Age-related decrease in energy expenditure at rest parallels reductions in mass of internal organs. Am J Hum Biol. 2002;14:486–493. doi: 10.1002/ajhb.10066. [DOI] [PubMed] [Google Scholar]
- 84.Raab F. Post-mortal organweights of spleen, liver, kidney and lung in relation to age, body weight and body height [PhD thesis; in German]. Heidelberg: University of Heidelberg; 1984.
- 85.Griffiths GJ, Robinson KB, Cartwright GO, McLachlan MS. Loss of renal tissue in the elderly. Br J Radiol. 1976;49:111–117. doi: 10.1259/0007-1285-49-578-111. [DOI] [PubMed] [Google Scholar]
- 86.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 87.Goyal VK. Changes with age in the human kidney. Exp Gerontol. 1982;17:321–331. doi: 10.1016/0531-5565(82)90032-8. [DOI] [PubMed] [Google Scholar]
- 88.Schmucker DL. Age-related changes in liver structure and function: implications for disease? Exp Gerontol. 2005;40:650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Grasedyck K, Jahnke M, Friedrich O, Schulz D, Lindner J. Aging of liver: morphological and biochemical changes. Mech Ageing Dev. 1980;14:435–442. doi: 10.1016/0047-6374(80)90014-7. [DOI] [PubMed] [Google Scholar]
- 90.Chouker A, Martignoni A, Dugas M, Eisenmenger W, Schauer R, Kaufmann I, et al. Estimation of liver size for liver transplantation: the impact of age and gender. Liver Transpl. 2004;10:678–685. doi: 10.1002/lt.20113. [DOI] [PubMed] [Google Scholar]
- 91.Thompson EN, Williams R. Effect of age on liver function with particular reference to bromsulphalein excretion. Gut. 1965;6:266–269. doi: 10.1136/gut.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swift CG, Homeida M, Halliwell M, Roberts CJ. Antipyrine disposition and liver size in the elderly. Eur J Clin Pharmacol. 1978;14:149–152. doi: 10.1007/BF00607447. [DOI] [PubMed] [Google Scholar]
- 93.Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297–301. doi: 10.1002/hep.1840090222. [DOI] [PubMed] [Google Scholar]
- 94.Marchesini G, Bua V, Brunori A, Bianchi G, Pisi P, Fabbri A, et al. Galactose elimination capacity and liver volume in aging man. Hepatology. 1988;8:1079–1083. doi: 10.1002/hep.1840080516. [DOI] [PubMed] [Google Scholar]
- 95.Williams LR. Reference values for total blood volume and cardiac output in humans: Dosimetry Research Goup. Oak Ridge: Oak Ridge National Laboratory; 1994.
- 96.Kuikka JT, Lansimies E. Effect of age on cardiac index, stroke index and left ventricular ejection fraction at rest and during exercise as studied by radiocardiography. Acta Physiol Scand. 1982;114:339–343. doi: 10.1111/j.1748-1716.1982.tb06993.x. [DOI] [PubMed] [Google Scholar]
- 97.Granath A, Strandell T. Relationships between cardiac output, stroke volume and intracardiac pressures at rest and during exercise in supine position and some anthropometric data in healthy old men. Acta Med Scand. 1964;176:447–466. doi: 10.1111/j.0954-6820.1964.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 98.Mezzani A, Grassi B, Giordano A, Corra U, Colombo S, Giannuzzi P. Age-related prolongation of phase I of VO2 on-kinetics in healthy humans. Am J Physiol Regul Integr Comp Physiol. 2010;299:R968–R976. doi: 10.1152/ajpregu.00739.2009. [DOI] [PubMed] [Google Scholar]
- 99.Podlesch I, Ulmer WT. On the dependence of heart minute volume, heart index, stroke volume, stroke volume index and oxygen consumption on age [in German] Arch Kreislaufforsch. 1965;48:232–248. [PubMed] [Google Scholar]
- 100.Germing A, Gotzmann M, Rausse R, Brodherr T, Holt S, Lindstaedt M, et al. Normal values for longitudinal function of the right ventricle in healthy women >70 years of age. Eur J Echocardiogr. 2010;11:725–728. doi: 10.1093/ejechocard/jeq053. [DOI] [PubMed] [Google Scholar]
- 101.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Population-based reference values for 3D echocardiographic LV volumes and ejection fraction. JACC Cardiovasc Imaging. 2012;5:1191–1197. doi: 10.1016/j.jcmg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 102.Cain PA, Ahl R, Hedstrom E, Ugander M, Allansdotter-Johnsson A, Friberg P, et al. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging. 2009;9:2. doi: 10.1186/1471-2342-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheitlin MD. Cardiovascular physiology—changes with aging. Am J Geriatr Cardiol. 2003;12:9–13. doi: 10.1111/j.1076-7460.2003.01751.x. [DOI] [PubMed] [Google Scholar]
- 104.Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. doi: 10.1016/s0735-1097(97)00554-8. [DOI] [PubMed] [Google Scholar]
- 105.Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 106.Hoang K, Tan JC, Derby G, Blouch KL, Masek M, Ma I, et al. Determinants of glomerular hypofiltration in aging humans. Kidney Int. 2003;64:1417–1424. doi: 10.1046/j.1523-1755.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 107.Bauer JH, Brooks CS, Burch RN. Renal function and hemodynamic studies in low- and normal-renin essential hypertension. Arch Intern Med. 1982;142:1317–1323. [PubMed] [Google Scholar]
- 108.Fuiano G, Sund S, Mazza G, Rosa M, Caglioti A, Gallo G, et al. Renal hemodynamic response to maximal vasodilating stimulus in healthy older subjects. Kidney Int. 2001;59:1052–1058. doi: 10.1046/j.1523-1755.2001.0590031052.x. [DOI] [PubMed] [Google Scholar]
- 109.Ghose K, Burch A. Measurement of renal functions by double isotope techniques in elderly patients during tenoxicam therapy. Arch Gerontol Geriatr. 1989;9:115–122. doi: 10.1016/0167-4943(89)90032-0. [DOI] [PubMed] [Google Scholar]
- 110.Zoli M, Magalotti D, Bianchi G, Gueli C, Orlandini C, Grimaldi M, et al. Total and functional hepatic blood flow decrease in parallel with ageing. Age Ageing. 1999;28:29–33. doi: 10.1093/ageing/28.1.29. [DOI] [PubMed] [Google Scholar]
- 111.Gentilcore D, Hausken T, Meyer JH, Chapman IM, Horowitz M, Jones KL. Effects of intraduodenal glucose, fat, and protein on blood pressure, heart rate, and splanchnic blood flow in healthy older subjects. Am J Clin Nutr. 2008;87:156–161. doi: 10.1093/ajcn/87.1.156. [DOI] [PubMed] [Google Scholar]
- 112.Gentilcore D, Nair NS, Vanis L, Rayner CK, Meyer JH, Hausken T, et al. Comparative effects of oral and intraduodenal glucose on blood pressure, heart rate, and splanchnic blood flow in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2009;297:R716–R722. doi: 10.1152/ajpregu.00215.2009. [DOI] [PubMed] [Google Scholar]
- 113.Gentilcore D, Vanis L, Wishart JM, Rayner CK, Horowitz M, Jones KL. The alpha (alpha)-glucosidase inhibitor, acarbose, attenuates the blood pressure and splanchnic blood flow responses to intraduodenal sucrose in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:917–924. doi: 10.1093/gerona/glr086. [DOI] [PubMed] [Google Scholar]
- 114.Dunbar SL, Kenney WL. Effects of hormone replacement therapy on hemodynamic responses of postmenopausal women to passive heating. J Appl Physiol. 2000;89:97–103. doi: 10.1152/jappl.2000.89.1.97. [DOI] [PubMed] [Google Scholar]
- 115.Ho CW, Beard JL, Farrell PA, Minson CT, Kenney WL. Age, fitness, and regional blood flow during exercise in the heat. J Appl Physiol. 1997;82:1126–1135. doi: 10.1152/jappl.1997.82.4.1126. [DOI] [PubMed] [Google Scholar]
- 116.Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- 117.Vanis L, Gentilcore D, Lange K, Gilja OH, Rigda RS, Trahair LG, et al. Effects of variations in intragastric volume on blood pressure and splanchnic blood flow during intraduodenal glucose infusion in healthy older subjects. Am J Physiol Regul Integr Comp Physiol. 2012;302:R391–R399. doi: 10.1152/ajpregu.00464.2011. [DOI] [PubMed] [Google Scholar]
- 118.Chumlea WC, Guo SS, Zeller CM, Reo NV, Baumgartner RN, Garry PJ, et al. Total body water reference values and prediction equations for adults. Kidney Int. 2001;59:2250–2258. doi: 10.1046/j.1523-1755.2001.00741.x. [DOI] [PubMed] [Google Scholar]
- 119.Bruce A, Andersson M, Arvidsson B, Isaksson B. Body composition: prediction of normal body potassium, body water and body fat in adults on the basis of body height, body weight and age. Scand J Clin Lab Invest. 1980;40:461–473. doi: 10.3109/00365518009101869. [DOI] [PubMed] [Google Scholar]
- 120.Lesser GT, Markofsky J. Body water compartments with human aging using fat-free mass as the reference standard. Am J Physiol. 1979;236:R215–R220. doi: 10.1152/ajpregu.1979.236.3.R215. [DOI] [PubMed] [Google Scholar]
- 121.Sergi G, Lupoli L, Volpato S, Bertani R, Coin A, Perissinotto E, et al. Body fluid distribution in elderly subjects with congestive heart failure. Ann Clin Lab Sci. 2004;34:416–422. [PubMed] [Google Scholar]
- 122.Steen B, Bruce A, Isaksson B, Lewin T, Svanborg A. Body composition in 70-year-old males and females in Gothenburg, Sweden: a population study. Acta Med Scand Suppl. 1977;611:87–112. doi: 10.1111/j.0954-6820.1977.tb18070.x. [DOI] [PubMed] [Google Scholar]
- 123.Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 124.Peters AM, Perry L, Hooker CA, Howard B, Neilly MD, Seshadri N, et al. Extracellular fluid volume and glomerular filtration rate in 1878 healthy potential renal transplant donors: effects of age, gender, obesity and scaling. Nephrol Dial Transplant. 2012;27:1429–1437. doi: 10.1093/ndt/gfr479. [DOI] [PubMed] [Google Scholar]
- 125.Barnfield M. BM. Reference data for Tc99m-DTPA measurements of the GFR derived from live kidney donors. Spring BNMS; 2010: Nuc Med Comm; 2010. p. 471.
- 126.Grewal GS, Blake GM. Reference data for 51Cr-EDTA measurements of the glomerular filtration rate derived from live kidney donors. Nucl Med Commun. 2005;26:61–65. doi: 10.1097/00006231-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 127.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 128.Claassen K, Thelen K, Coboeken K, Gaub T, Lippert J, Allegaert K, et al. Development of a physiologically-based pharmacokinetic model for preterm neonates: evaluation with in vivo data. Curr Pharm Des. 2015;21:5688–5698. doi: 10.2174/1381612821666150901110533. [DOI] [PubMed] [Google Scholar]
- 129.Aitkenhead AR, Vater M, Achola K, Cooper CM, Smith G. Pharmacokinetics of single-dose i.v. morphine in normal volunteers and patients with end-stage renal failure. Br J Anaesth. 1984;56:813–819. doi: 10.1093/bja/56.8.813. [DOI] [PubMed] [Google Scholar]
- 130.Baillie SP, Bateman DN, Coates PE, Woodhouse KW. Age and the pharmacokinetics of morphine. Age Ageing. 1989;18:258–262. doi: 10.1093/ageing/18.4.258. [DOI] [PubMed] [Google Scholar]
- 131.Hoskin PJ, Hanks GW, Aherne GW, Chapman D, Littleton P, Filshie J. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27:499–505. doi: 10.1111/j.1365-2125.1989.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Osborne R, Joel S, Trew D, Slevin M. Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin Pharmacol Ther. 1990;47:12–19. doi: 10.1038/clpt.1990.2. [DOI] [PubMed] [Google Scholar]
- 133.Stuart-Harris R, Joel SP, McDonald P, Currow D, Slevin ML. The pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous bolus injection and subcutaneous infusion of morphine. Br J Clin Pharmacol. 2000;49:207–214. doi: 10.1046/j.1365-2125.2000.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Villesen HH, Banning AM, Petersen RH, Weinelt S, Poulsen JB, Hansen SH, et al. Pharmacokinetics of morphine and oxycodone following intravenous administration in elderly patients. Ther Clin Risk Manag. 2007;3:961–967. [PMC free article] [PubMed] [Google Scholar]
- 135.Westerling D, Hoglund P, Lundin S, Svedman P. Transdermal administration of morphine to healthy subjects. Br J Clin Pharmacol. 1994;37:571–576. doi: 10.1111/j.1365-2125.1994.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Westerling D, Persson C, Hoglund P. Plasma concentrations of morphine, morphine-3-glucuronide, and morphine-6-glucuronide after intravenous and oral administration to healthy volunteers: relationship to nonanalgesic actions. Ther Drug Monit. 1995;17:287–301. doi: 10.1097/00007691-199506000-00013. [DOI] [PubMed] [Google Scholar]
- 137.Andreasen F, Hansen U, Husted SE, Jansen JA. The pharmacokinetics of frusemide are influenced by age. Br J Clin Pharmacol. 1983;16:391–397. doi: 10.1111/j.1365-2125.1983.tb02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Keller E, Hoppe-Seyler G, Mumm R, Schollmeyer P. Influence of hepatic cirrhosis and end-stage renal disease on pharmacokinetics and pharmacodynamics of furosemide. Eur J Clin Pharmacol. 1981;20:27–33. doi: 10.1007/BF00554663. [DOI] [PubMed] [Google Scholar]
- 139.Kerremans AL, Tan Y, van Baars H, van Ginneken CA, Gribnau FW. Furosemide kinetics and dynamics in aged patients. Clin Pharmacol Ther. 1983;34:181–189. doi: 10.1038/clpt.1983.150. [DOI] [PubMed] [Google Scholar]
- 140.Muhlberg W, Platt D, Neubig E. Pharmacokinetics and pharmacodynamics of furosemide in geriatric patients. Arch Gerontol Geriatr. 1986;5:249–263. doi: 10.1016/0167-4943(86)90026-9. [DOI] [PubMed] [Google Scholar]
- 141.Patwardhan RV, Johnson RF, Hoyumpa A, Jr, Sheehan JJ, Desmond PV, Wilkinson GR, et al. Normal metabolism of morphine in cirrhosis. Gastroenterology. 1981;81:1006–1011. [PubMed] [Google Scholar]
- 142.Skarke C, Schmidt H, Geisslinger G, Darimont J, Lotsch J. Pharmacokinetics of morphine are not altered in subjects with Gilbert’s syndrome. Br J Clin Pharmacol. 2003;56:228–231. doi: 10.1046/j.1365-2125.2003.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Luisada AA, Bhat PK, Knighten V. Changes of cardiac output caused by aging: an impedance cardiographic study. Angiology. 1980;31:75–81. doi: 10.1177/000331978003100201. [DOI] [PubMed] [Google Scholar]
- 144.Boyd E. Normal variability in weight of the adult human liver and spleen. Arch Pathol Lab Med. 1933;16:350–372. [Google Scholar]
- 145.Benet LZ. Pharmacokinetics/pharmacodynamics of furosemide in man: a review. J Pharmacokinet Biopharm. 1979;7:1–27. doi: 10.1007/BF01059438. [DOI] [PubMed] [Google Scholar]
- 146.Macintyre PE, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1996;64:357–364. doi: 10.1016/0304-3959(95)00128-X. [DOI] [PubMed] [Google Scholar]
- 147.Jadhav PR, Cook J, Sinha V, Zhao P, Rostami-Hodjegan A, Sahasrabudhe V, et al. A proposal for scientific framework enabling specific population drug dosing recommendations. J Clin Pharmacol. 2015;55:1073–1078. doi: 10.1002/jcph.579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 4 (TIFF 20682 kb)