Fat embolism syndrome (FES), caused by the release of fat globules into the systemic circulation, is characterized by a constellation of cerebral, pulmonary, cutaneous, and hematological symptoms of variable severity. The presentation varies from asymptomatic or with mild respiratory distress to a catastrophic clinical scenario with a triad of symptoms that include dyspnea, petechial hemorrhages of the skin, and confusion that may lead to coma or death. The FES is usually prevalent in 1-5% of patients following trauma to the pelvis and long bones and rarely described in elective orthopedic knee procedures.1 A 71-year-old male patient with controlled diabetes mellitus and hypertension was admitted to King Abdulaziz University Hospital, Jeddah, Kingdom of Saudi Arabia for scheduled bilateral total knee replacement (TKR) in July 2014. He had an 8-year history of progressive bilateral knee pain and limitation of walking due to severe osteoarthritis that did not improve with repeated local injections and physical therapy. Before surgical intervention, his vital signs were: sinus rhythm pulse of 78/min, blood pressure of 130/80, respiratory rate of 22/min, a temperature of 37°C, and oxygen saturation on room air was 98%. His physical and neurological examination was within normal. A routine preoperative laboratory tests (complete blood count, renal, liver, and coagulation profiles) were within normal limits: there was mildly elevated random blood sugar and cholesterol levels. Electrocardiography (ECG) and preoperative chest radiograph were unremarkable. Surgery was performed under mild hypotensive epidural anesthesia and intravenous Propofol sedation (100 mcg/kg/min) in a supine position with the application of thigh tourniquet. Bilateral 1-stage TKR surgery was uneventful; there was no intraoperative incidence during the 2 and half-hour of surgery, particularly disturbance of blood pressure and oxygen saturation. He was transferred to recovery room alert and in a good condition and postoperative knee x-rays was satisfactory (Figure 1A). However, 10 minutes later, he complained of chest tightness and difficulty breathing and became restless and combative with a Glasgow Coma Score (GCS) of 8. His vital signs revealed sinus tachycardia (90/min) and tachypnea (28/min), a blood pressure of 108/70, and an oxygen saturation of 90% on facemask with100% oxygen of 5 L/min. General physical examination revealed cutaneous trunk petechial hemorrhages and bilateral basal lung crepitation. He was immediately transferred to the Intensive Care Unit (ICU) where he required endotracheal intubation and mechanical ventilation due to desaturation and deteriorated level of consciousness. A chest radiograph showed bilateral infiltrates (Figure 1B). A postoperative laboratory test was unremarkable apart from a drop in hemoglobin from 13.4 to 8.7 g/100 ml, despite an estimated blood loss was around 250 ml in addition to 120 cc bloody fluid was in the subcutaneous drains within 12 hours, for which he received 2 units of packed red blood cells. His platelets have dropped from 379 to 209 billion cells/L and blood gasses showed evidence of respiratory acidosis (pH=7.23, PCO2=48 mm Hg, PO2=61 mm Hg, HCO3=25, and O2 saturation of 76%). Immediate ECG revealed sinus tachycardia with no ischemic changes; cardiac enzymes were not elevated, and trans-esophageal echocardiogram revealed no evidence of cardiac ischemia, thrombus, or atrial septal defect. Carotid doppler study revealed no atherosclerotic stenosis, and lower extremities duplex study was negative for deep venous thrombosis. An emergency plain computed tomography (CT) scan of the brain revealed extensive bilateral cerebral and cerebellar periventricular low-attenuation foci. These findings raised the suspicion of acute embolic infarction. An urgent magnetic resonance imaging (MRI) scan was performed which was unremarkable on T1-weighted images, but on T2-Weighted images, there were multiple diffuse small hyperintense foci in both cerebral and cerebellar hemispheres with a symmetrical distribution. These showed restricted diffusion in diffusion-weighted (DWI) and apparent diffusion coefficient (ADC) images, in keeping with acute infarction (Figure 1C). On susceptibility weighted MRI sequences these areas of acute infarction showed susceptibility artifacts in keeping with petechial hemorrhagic foci. The distribution of these multiple small foci of hemorrhagic infarctions described as a ‘starfield pattern’ as it simulates the appearances of a cluster of stars in the night sky on MRI scan. In the patient’s clinical context, these imaging findings were for cerebral fat embolism. The patient was stable hemodynamically with GCS varied from 7 to 9 for the first 7 days then gradually improved. Electroencephalography revealed no epileptic discharges. Intravenous low molecular weight heparin was started at 72 hours after TKR. After 3-week of ICU admission, he gradually had improvement of the level of consciousness and weaned from mechanical ventilation. He remained in the hospital for 3 months with significant residual cognitive and motor impairment that required a special assistance for feeding and ambulation. A follow-up CT scan (Figure 1D) before discharged home, showed extensive small vessel ischemic changes in the central white matter in a watershed territory distribution.
Figure 1.
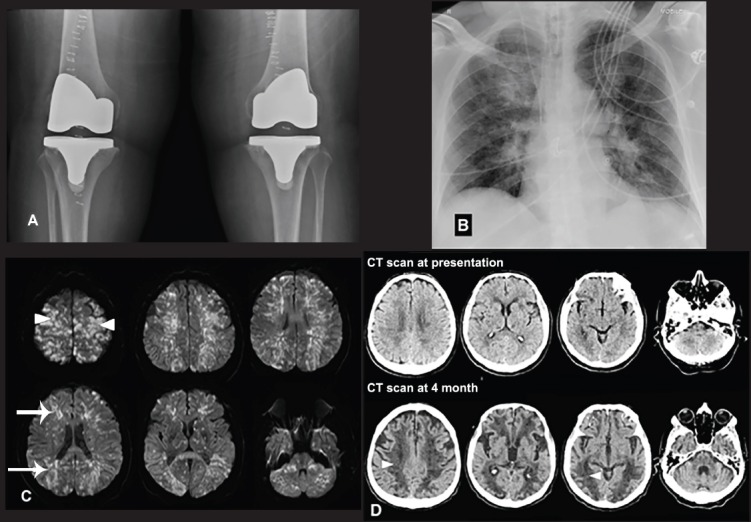
Radiological findings A) Postoperative knee radiograph is demonstrating metallic prosthesis in both knees, B) An immediate postoperative chest radiograph is showing acute interval development of bilateral infiltrated most pronounced in the respective peri-hilar regions and right upper lobe, C) Selected axial diffusion-weighted MRI images are demonstrating multiple small foci of diffusion restriction (confirmed by apparent diffusion coefficient maps) with a near symmetrical distribution predominantly regarding to the watershed territories (arrows). Some of the high bilateral parietal and frontal lesions were cortical based (arrow heads), D) Selected axial non-contrast enhanced CT scans of the brain at presentation and 4-month follow-up. The follow-up images (below) show extensive low attenuation areas in a watershed territory associated with accelerated central brain atrophy (arrow heads).
There is associated expedited central cerebral atrophy, which is pronounced compared to the initial CT scan at presentation. Fat embolism, a clinical manifestation caused by the release of fat globules into the systemic circulation, was first described in a rheumatoid patient one day after bilateral TKR using hinge prosthesis. They attributed the respiratory symptoms due to the poor respiratory condition from rheumatoid disease and the long-term use of steroid therapy. Subsequently, several cases of FES following bilateral TKR in 2 healthy patients.2 The incidence of FES in elective orthopedics joint operations is not well known. Recently, Lee et al2 retrospectively reviewed a series of 2345 patients underwent simultaneous bilateral TKA procedures and found that the overall incidence of FES with cerebral manifestations was 0.17%. The pathophysiology of fat embolism and its subsequent clinical manifestations is shrouded in controversy. However, 3 major theories have been described in the literature.2 First, the Mechanical theory which states that traumatic injury to long bones results in the release of microscopic fat droplets into circulation by the disruption of the fat cell in the traumatized bone or the surrounding adipose tissues. The fat droplets are thought to enter into circulation due to pre-existing pathological arterial-venous communication, like a patent foramen ovale. Alternatively, fat droplets entry into the torn veins in the vicinity of the trauma due to an inherent pressure differential that exists between the venous pressure and intramedullary pressure. The droplets in the order of 7-10-micron escape the pulmonary filter and reach the systemic circulation. Second, the Biochemical theory which postulated where there is hydrolyzation of the fat, over a few hours, in plasma to free fatty acids and other mediators are considered the most plausible, as shown in animal models. Agglutination of chylomicrons and low-density lipoproteins facilitated by C-reactive proteins was found and reviewed also to be responsible for FES in non-traumatic models as well. Last, the coagulation theory proposed that the release of tissue thromboplastin, following traumatic long bone injury, result in activation of the complement system and extrinsic coagulation pathways and factor VII activation that leads to intravascular coagulation and subsequently lead to an increase in pulmonary permeability of the fat globules.
The triggering etiology of FES in TKR surgery was initially proposed to be due to cementing techniques and increased systemic monomer.3 This was demonstrated in animal models after simulated bilateral cemented arthroplasty. On the contrary, the development of FES was described after placement of the intramedullary femoral alignment guide during TKA, which released the bone marrow fat globules into the circulation before cement application, and advised canal marrow aspiration before alignment guide pin placement. The modification of surgical techniques to cementless TKR, and staging (unilateral versus bilateral) TKR procedures or the use of computer-assisted navigation has not been associated with a lower incidence of FES.3 The time onset of the clinical development of FES following knee arthroplasty was variable from an intraoperative cardiorespiratory collapse, before or after deflating the tourniquet, to the development of respiratory, cardiovascular and cerebral dysfunction minutes to hours in the postoperative period.2 The diagnosis of fat embolism is usually a clinical challenge and requires a vigilant high index of suspicion. The clinical symptoms and signs of FES are variable ranging from an asymptomatic patient, or the presence mild chest tightness and transient alteration of mental states, to coma and death. The basic triad of FES includes respiratory distress, neurological abnormality, and petechial rash, other associated features include thrombocytopenia, fever, and unexplained anemia. The diagnostic criteria by Gurd and Wilson since 1974 are the currently used.2 The pivotal role of imaging is 2 fold: first, it helps in early confirming the diagnosis and, second, in prognostication by describing the extent of ischemic damage and its reversal. Chest radiography is a simple and significant initial investigation in patients with pulmonary symptoms possibly due to FES: it reveals an increase in bilateral pulmonary markings suggestive of interstitial pulmonary edema, which is a consistent finding. A computed tomography (CT) scan would be useful to exclude intracranial hemorrhage. An MRI of the brain most accurately depicts the cerebral change in FES. Diffusion-weighted and susceptibility weighted imaging sequences are the mainstay of imaging sequences, with DWI picking up acute infarcts as early as 30 min after an onset of ischemia is documented.4 Multiple tiny non-confluent T2-weighted MRI demonstrates hyperintense lesions with similar diffusion restriction in the cerebral deep white matters, typically in the region of the centrum semiovale and corona radiata. These lesions are micro-infarction due to microscopic fat embolisms. This shows a characteristic ‘starfield’ pattern, an association has been seen between the degree of the neurological defect and the size and distribution of the lesions. In our patient, the characteristic appearance of T2 and DWI were seen. These MRI findings along with the clinical scenario were associated with high accuracy in making the diagnosis of cerebral FES. The treatment of FES is nonspecific largely supportive in nature with adequate oxygenation and mechanical ventilation, early resuscitation and minimizing the stress response and hypovolemia. The use of prophylactic steroid therapy has been advocated, but with limited results and no universal agreement regarding its role.5 Fortunately, the severe neurological symptoms of cerebral FES frequently resolve in most patients, but fatality and poor cognitive outcome, as in our unfortunate patient, may occur.
In conclusion, the occurrence of FES in the setting of elective orthopedics surgery, like TKR, is not a rare complication. We aim to raise the awareness and prediction among orthopedic surgeons, neurologists, and intensive care physicians. Early diagnosis needs a high degree of clinical suspicion and early radiological investigations, such as MRI, for confirmation and prognostication.
References
- 1.Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty:a case series. J Arthroplasty. 2012;27:409–414. doi: 10.1016/j.arth.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Yeo SH, Chang HW, Sohn SI, Cho CH, Bae KC. Pulmonary and cerebral fat embolism syndrome after total knee replacement. J Clin Med Res. 2013;5:239–242. doi: 10.4021/jocmr1251w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connor MI, Brodersen MP, Feinglass NG, Leone BJ, Crook JE, Switzer BE. Fat emboli in total knee arthroplasty:a prospective randomized study of computer-assisted navigation vs standard surgical technique. J Arthroplasty. 2010;25:1034–1040. doi: 10.1016/j.arth.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Parizel PM, Demey HE, Veeckmans G, Verstreken F, Cras P, Jorens PG, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern) Stroke. 2001;32:2942–2944. [PubMed] [Google Scholar]
- 5.Sen RK, Tripathy SK, Krishnan V. Role of corticosteroid as a prophylactic measure in fat embolism syndrome:a literature review. Musculoskelet Surg. 2012;96:1–8. doi: 10.1007/s12306-011-0156-1. [DOI] [PubMed] [Google Scholar]


