Abstract
Objective
To assess the 1-year renal functional changes in patients undergoing partial nephrectomy with intra-operative renal biopsies.
Patients and Methods
A total of 40 patients with a single renal mass deemed fit for a partial nephrectomy were recruited prospectively between January 2009 and October 2010. We performed renal biopsies of normal renal parenchyma and collected serum markers before, during and after surgically induced renal clamp ischaemia during the partial nephrectomy. We then followed patients clinically with interval serum creatinine and physical examination.
Results
Peri-operative data from 40 patients showed a transient increase in creatinine levels which did not correlate with ischaemia time. Renal ultrastructural changes were generally mild and included mitochondrial swelling, which resolved at the post-perfusion biopsy. A total of 37 patients had 1-year follow-up data. Creatinine at 1 year increased by 0.121 mg/dL, which represents a 12.99% decrease in renal function from baseline (preoperative creatinine 0.823 mg/dL, estimated glomerular filtration rate = 93.9 mL/min/1.73 m2). The only factors predicting creatinine change on multivariate analysis were patient age, race and ischaemia type, with cold ischaemia being associated with higher creatinine level. Importantly, the duration of ischaemia did not show any significant correlation with renal function change, either as a continuous variable (P = 0.452) or as a categorical variable (P = 0.792).
Conclusions
Our data suggest that limited ischaemia is generally well tolerated in the setting of partial nephrectomy and does not directly correspond to long-term renal functional decline. For surgeons performing partial nephrectomy, the kidney can be safely clamped to ensure optimum oncological outcomes.
Keywords: ischaemia, nephrectomy, surgical clamp
Introduction
The direct response of human kidneys to clamp ischaemia has only recently been reported. The long-standing dogma of limiting warm ischaemia time to ≤30 min is based on animal studies and limited retrospective human studies. Our clinical practice has been driven by this dogma, culminating in the recent suggestion of completely eliminating renal ischaemia, even with the potential compromise of oncological outcomes because of the lack of a bloodless surgical field and increased peri-operative morbidity [1,2]. The widespread adoption of partial nephrectomy has been limited partly as a result of concerns about renal ischaemia and maintaining short ischaemia times; however, as diligent surgeons, we must fully understand the implications of ischaemia in renal surgery. We recently published the first and only prospective clinical study looking at the structural and functional response to controlled renal ischaemia in humans [3]. The data indicate significantly greater tolerance of human kidneys to clamp ischaemia and lesser structural changes compared with kidneys in animal models in the acute phase. In the present study, our primary aim was to evaluate prospectively and longitudinally renal functional outcomes at 1 year after surgery. We specifically examined the correlation between ischaemia duration and renal function at 1 year.
Patients and Methods
Study Design
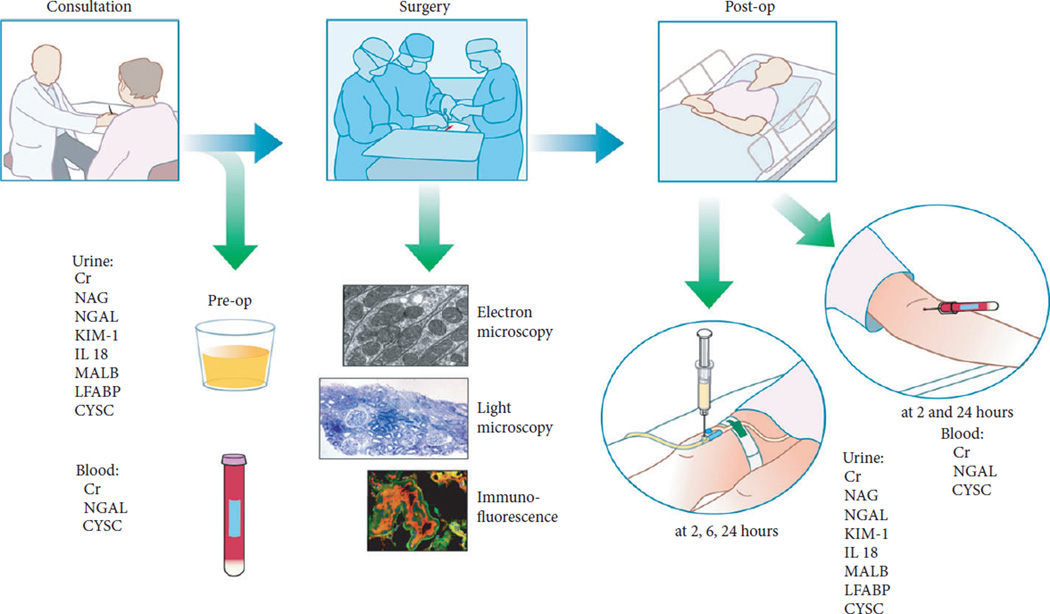
We recruited 40 patients into a prospective clinical trial from January 2009 to October 2010. Adult patients with two functioning kidneys and a single renal mass electing to undergo open partial nephrectomy were enrolled prospectively. Patients with small or minimally complex masses were referred for robot-assisted partial nephrectomy and were not included in the study. All surgeries were performed at University of Texas San Antonio by a single surgeon (D.J.P.). Demographic information was recorded. In the preoperative setting, urine and serum markers of renal function and renal injury were collected (Fig. 1).
Fig. 1.
Trial design.
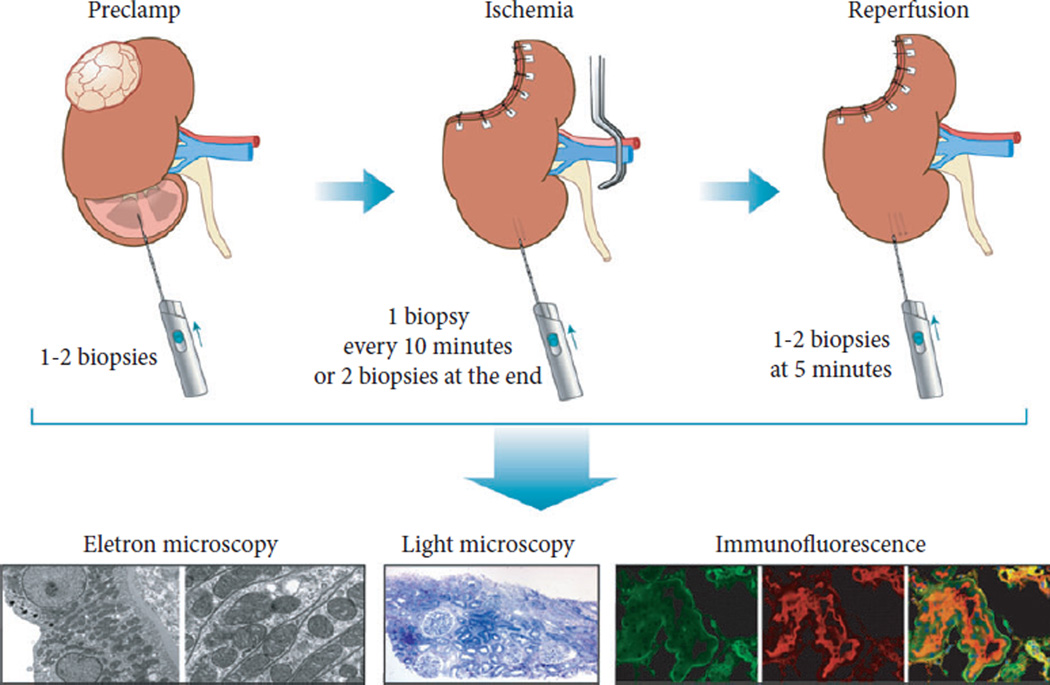
All patients underwent open partial nephrectomy. After exposure of the kidney and hilum, a clamp was placed across the renal artery, alone or the renal hilum en bloc. The decision regarding type and duration of hilar clamping was made by the surgeon. Initially, cold ischaemia was used for the most complex masses. After an interim analysis of the first 50% of patients, warm ischaemia was used for all patients. For patients undergoing cold ischaemia, the kidney was covered with ice slush for 10 min, increasing the overall ischaemia time. All patients underwent multiple intra-operative biopsies of the uninvolved parenchyma at predefined time points including: before hilar clamping (pre-clamp); every 10 min while the kidney was clamped; at the end of clamping (end-clamp); and 5 min after reperfusion (post-clamp; Fig. 2) Urine and serum markers were collected daily until discharge. Patients were seen at 1 month postoperatively and then every 3–6 months. Follow-up visits included serum creatinine measurements and physical examinations. Estimated GFR (eGFR) was calculated using the Modification of Diet in Renal Disease study equation and stages of chronic kidney disease (CKD) were defined according to guidelines reported by the National Kidney Foundation. Immunofluorescent, ultrastructural and histopathological tests were performed during the first phase of the study and are detailed in a previous manuscript [3].
Fig. 2.
Biopsy protocol.
Statistical Analyses
Creatinine and eGFR mean comparisons were analysed by repeated measures anova followed by prespecified pairwise comparisons with adjusted P value by Bonferroni’s method for multiple comparisons. The corresponding model included two factors: ischaemia duration as a fixed factor with two levels (≤30 min and >30 min) and the repeated factor time with three levels (preoperatively, and at 24 h and 1 year after surgery). We also performed univariate and multivariate regression analyses looking at selected variables that could cause renal functional decline at 1 year after surgery. These variables were ischaemia duration (as a continuous variable and as a categorical variable: ≤30 and >30 min), tumour size, age, comorbidities and histopathological data from the operation biopsies. Multivariate models were derived using a stepwise method with a 10% significance level to enter and to stay in the model. Tests were conducted at the 5% significance level. Statistical analyses were carried out in SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
From the initial 40 patients enrolled in the study, data on 37 patients were available for the 1-year follow-up. One patient died from an unrelated cardiac cause and two patients were lost to follow-up.
Demographic Data
Table 1 shows the demographic data for the entire cohort. The mean and median patient age were both 56 years. In all, 78% of the patients were of Hispanic origin, consistent with the local population, 67% of the patients were female and the mean tumour size was 4 cm. The median (range) ischaemia time was 32 (15–61) min. Two thirds of the patients underwent warm ischaemia and the remaining third had cold ischaemia.
Table 1.
Demographics and other patient characteristics.
| Total number of patients | 37 |
| Median (range) age, years | 56 (34–84) |
| Gender, n (%) | |
| Male | 12 (32.4) |
| Female | 25 (67.6) |
| Race/Ethnicity, n (%) | |
| White non-Hispanic | 8 (21.6) |
| Hispanic grouped | 29 (78.4) |
| Median (range) body mass index | 30.4 (20.1–43) |
| Charlson comorbidity score, n (%) | |
| 0 | 16 (43.2) |
| 1 | 10 (27.0) |
| 2 | 4 (10.8) |
| 3 | 6 (16.2) |
| 5 | 1 (2.7) |
| Median (range) ischaemia time, min | |
| Ischaemia (all) | 32 (15–61) |
| Warm ischaemia (n = 25) | 31 (15–53) |
| Cold ischaemia (n = 12) | 48 (30–61) |
| Median (range) estimated blood loss, mL | 200 (50–1000) |
| Median (range) total operative time, min | 155 (78–269) |
| Pathology lesion type, n (%) | |
| Clear-cell | 28 (75.7) |
| Papillary | 1 (2.7) |
| Oncocytoma | 2 (5.4) |
| Angiomyolipoma | 1 (2.7) |
| Benign simple cyst | 3 (8.1) |
| Benign parenchyma | 1 (2.7) |
| Sarcomatoid changes | 1 (2.7) |
| Median (range) tumour/lesion size, cm | 4 (2–7.2) |
| Margin status: negative, n (%) | 37 (100) |
Change in Renal Function
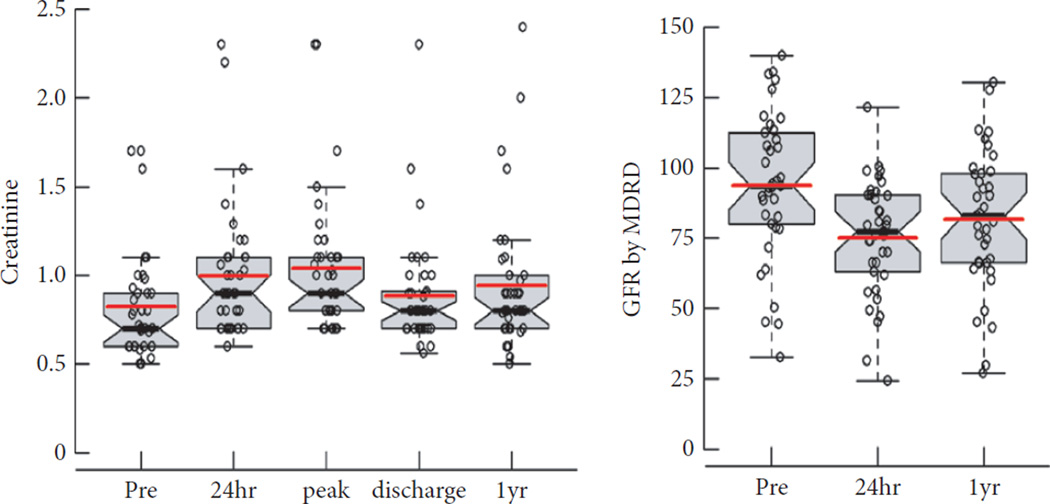
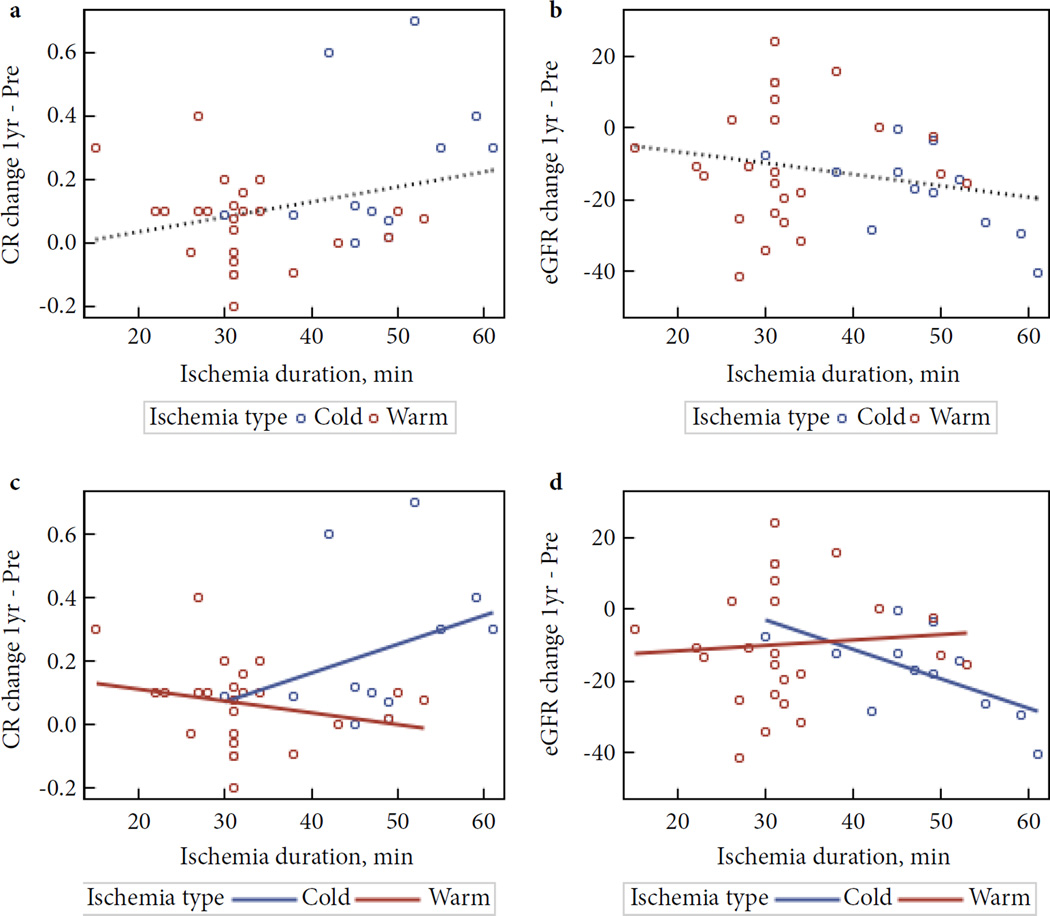
Table 2 and Fig. 3 summarize the behaviour of creatinine and eGFR during the entire course of the study. The largest change in creatinine level was in the peri-operative period (24 h after surgery): creatinine level increased by 21.1% in these 37 patients (26.3% in original 40 patients [3]). This change may not have been primarily attributable to decreased GFR because it was not accompanied by an increase in cystatin C and had largely recovered by the time of discharge [3]. The 1-year change in creatinine level for the entire group was 0.121 ng/mL (P = 0.003), corresponding to a decrease of 12.2 mL/min/1.73 m2 in eGFR. This is a 12.99% decline in renal function, which is above the 1% per year that can be attributed to aging alone, but a substantially smaller decline than would have been expected after partial nephrectomy. When patients were stratified by ischaemia type (cold or warm), most differences at 1 year were in those undergoing cold ischaemia (Fig. 4).
Table 2.
Comparison of creatinine and estimated GFR values, overall and stratified by ischaemia duration
| All patients (n = 37) |
Ischaemia duration ≤30 min (n = 9) |
Ischaemia duration > 30 min (n = 28) |
P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Mean | SD | SE | Mean | SD | SE | ||
| Preoperative creatinine | 0.823a,b | 0.301 | 0.049 | 0.889 | 0.344 | 0.115 | 0.802c,d | 0.289 | 0.055 | NS |
| 24-h creatinine | 0.997a | 0.377 | 0.062 | 0.989 | 0.486 | 0.162 | 0.999c | 0.346 | 0.065 | NS |
| 1-year creatinine | 0.944b | 0.398 | 0.065 | 1.040 | 0.391 | 0.130 | 0.913d | 0.402 | 0.076 | NS |
| P | <0.001 | |||||||||
| Preoperative eGFR | 93.9a,b | 26.3 | 4.3 | 90.1c | 31.9 | 10.6 | 95.1d,e | 24.8 | 4.7 | NS |
| 1-year eGFR | 81.7b | 24.8 | 4.1 | 73.8c | 23.3 | 7.8 | 84.5e | 25.1 | 4.7 | NS |
eGFR, estimated GFR; NS, nonsignificant. Same lower case superscript letter indicates pairwise mean difference statistically significant at P < 0.05. Mean values are in bold.
Fig. 3.
Perioperative and long term creatinine change.
Fig. 4.
Relationship between creatinine/eGFR change at 1 year and ischemia duration overall (a & b) and by ischemia type (c & d). All 37 patients: No significant correlations. (a) Creatinine: intercept = −0.055 (P= 0.599), slope = 0.005 (P= 0.086), correlation r= 0.286 (P = 0.086), r2 = 0.082. (b) eGFR: intercept= −0.414 (P= 0.964), slope= −0.314 (P=0.179), r= −0.226 (P=0.179), r2=0.051. By ischemia type: (c) Creatinine: No significant correlations. Cold intercept = −0.204 (P= 0.604), slope = 0.009 (P= 0.272), r = 0.345 (P= 0.272), r2 = 0.119. Warm: intercept = 0.189 (P= 0.079), slope = −0.004 (P= 0.234), r= −0.247 (P= 0.234), r2 = 0.061. (d) eGFR: Significant correlation in COLD but not in WARM type. Cold: intercept = 22.20 (P= 0.201), slope = −0.833 (P= 0.032), r= −0.618 (P= 0.032), r2 = 0.382. Warm: intercept = −14.180 (P= 0.303), slope = 0.141 (P= 0.728), r= 0.073 (P= 0.728), r2 = 0.005.
Table 2 and Fig. 4 show the effect of ischaemia duration. Patients with ischaemia time of <30 min had a renal functional decline of 0.151 ng/mL (17%) at 1 year, while patients with an ischaemia time >30 min had a decline of 0.111 ng/mL (13.8%) in renal function. Figure 4a plots the change in 1-year creatinine level vs ischaemia time as a continuous variable. This graph shows no relationship between ischaemia time and the change in creatinine level (r = 0.123, P = 0.470). Figure 4c shows the same data, but stratified by type of ischaemia (cold vs warm). Again, the data show no significant relationship between ischaemia time and change in creatinine over 1 year, for either cold or warm ischaemia. Figures 4b and d show the eGFRs corresponding to these creatinine levels. Analysed in this fashion, there was a significant correlation between duration of cold ischaemia and decrease in eGFR (r = 0.618, P = 0.032), but this was still not the case for warm ischaemia (r = 0.073, P = 0.728).
Predictors of Long-Term Change in Creatinine Level
We performed univariate and multivariate analyses looking at potential factors influencing renal functional change. We looked specifically at ischaemia duration (as a continuous variable and as a categorical variable ≤30 vs >30 min), tumour size, patient age and comorbidities and histopathological data from the operation biopsies.
On univariate analysis (Table 3), the only factor that showed significant prediction of change in creatinine level was ischaemia type, with warm ischaemia showing less creatinine change than cold ischaemia. Interestingly, the duration of ischaemia showed no statistically significant relationship with long-term change in creatinine level. In addition, tumour size and patient factors, including hypertension and diabetes, also did not predict renal functional decline.
Table 3.
Univariate analysis of long-term change in creatinine (1 year minus preoperative value).
| Variable | Category | β estimate* (95% CI) | P | R2† |
|---|---|---|---|---|
| Ischaemia duration | 1-min increase | 0.005 (−0.001, 0.010) | 0.086 | 0.082 |
| Ischaemia duration | >30 vs ≤30 min (reference) | −0.040 (−0.184, 0.105) | 0.581 | 0.009 |
| Ischaemia type | Warm vs cold (reference) | −0.165 (−0.285, −0.044) | 0.009 | 0.181 |
| Creatinine 24-h after surgery minus preoperative | 1-unit increase | 0.210 (−0.063, 0.483) | 0.128 | 0.065 |
| End clamp thick section (n = 31) | 1-unit increase | 0.066 (−0.052, 0.185) | 0.264 | 0.043 |
| Tumour size (cm) | 1-cm increase | 0.004 (−0.048, 0.055) | 0.883 | 0.001 |
| Angiotensin-converting enzyme use | Yes vs no (reference) | 0.088 (−0.050, 0.225 | 0.203 | 0.046 |
| Body mass index | 1-unit increase | −0.002 (−0.011, 0.007) | 0.608 | 0.008 |
| Age (years) at surgery | 1-year increase | −0.003 (−0.009, 0.002) | 0.269 | 0.035 |
| Race/Ethnicity | White non-Hispanic vs Hispanic (reference) | 0.090 (−0.059, 0.238) | 0.229 | 0.041 |
| Gender | Male vs female (reference) | 0.084 (−0.047, 0.214) | 0.201 | 0.046 |
| Hypertension | Yes vs no (reference) | 0.086 (−0.037, 0.208) | 0.165 | 0.055 |
| Type 2 diabetes | Yes vs no (reference) | 0.067 (−0.064, 0.199) | 0.304 | 0.030 |
β estimate: estimated coefficient for the independent variable in predicting long-term change in creatinine.
Coefficient of determination: proportion of variation of the outcome explained by the simple regression model.
On multivariate analysis (Table 4), the ischaemia type was still a statistically significant predictor of renal function change. The only other factors which met statistical significance were patient factors including patient age and race. Most importantly, ischaemia duration did not correlate at all with change in renal function as a continuous variable (P = 0.452) or as a categorical variable (P = 0.792). Contributors to medical kidney disease, including diabetes, hypertension and obesity, did not predict long-term renal function change in our analysis. We did not find any significant correlation between patients who had a peri-operative decline in renal function (creatinine at 24 h minus preoperative creatine) and those with long-term decline in renal function (creatinine at 1 year minus preoperative creatinine; P > 0.30). This indicates that even patients with acute short-term decline in renal function can recover renal function in the long term.
Table 4.
Multivariate analysis of long-term change in creatinine (1 year minus pre-op).
| Variable | Category | Ischaemia duration as continuous variable |
Ischaemia duration <30 min vs >30 min |
||
|---|---|---|---|---|---|
| β estimate* (95% CI) | P | β estimate† (95% CI) | P | ||
| Intercept | 0.573 (0.029, 1.117) | 0.040 | 0.654 (0.158, 1.151) | 0.012 | |
| Ischaemia duration | 1-min increase | 0.003 (−0.004, 0.009) | 0.452 | – | |
| Ischaemia duration | >30 vs ≤30 min (reference) | – | 0.021 (−0.139, 0.181) | 0.792 | |
| Ischaemia type | Warm vs cold (reference) | −0.163 (−0.321, −0.005) | 0.044 | −0.195 (−0.325, −0.065) | 0.005 |
| Creatinine 24-h after surgery minus preoperative | 1-unit increase | 0.130 (−0.132, 0.393) | 0.316 | 0.126 (−0.146, 0.399) | 0.350 |
| Tumour size (cm) | 1-cm increase | 0.004 (−0.041, 0.048) | 0.869 | 0.008 (−0.035, 0.052) | 0.697 |
| Angiotensin-converting enzyme use | Yes vs no (reference) | 0.102 (−0.038, 0.242) | 0.148 | 0.091 (−0.060, 0.242) | 0.225 |
| Body mass index | 1-unit increase | −0.008 (−0.016, 0.000) | 0.061 | −0.008 (−0.016, 0.001) | 0.067 |
| Age (years) at surgery | 1-year increase | −0.006 (−0.011, −0.001) | 0.023 | −0.006 (−0.012, −0.001) | 0.031 |
| Race/Ethnicity | White non-Hispanic vs Hispanic | 0.167 (0.036, 0.299) | 0.015 | 0.172 (0.028, 0.315) | 0.021 |
| Gender | Male vs female | −0.005 (−0.126, 0.116) | 0.929 | 0.053 (−0.116, 0.126) | 0.929 |
| Hypertension | Yes vs no (reference) | 0.085 (−0.048, 0.218) | 0.200 | 0.086 (−0.049, 0.221) | 0.202 |
| Type 2 diabetes | Yes vs no (reference) | 0.029 (−0.102, 0.160) | 0.650 | 0.033 (−0.100, 0.167) | 0.614 |
| R2 | 0.554 | 0.545 | |||
β estimate: estimated variable coefficient in predicting long-term change in creatinine.
Coefficient of determination: proportion of variation of the outcome explained by the multivariate regression model. Statistically significant P values are in bold.
Evaluation of the pathological features from the renal biopsies showed that the end clamp thick-section score did not correlate with long-term creatinine value (P = 0.169) or change in creatinine (P = 0.264; Fig. 5); therefore, although these structural changes predicted decreased renal function in the peri-operative period, they did not predict change in the long term. Further study is warranted to fully elucidate the functional and structural renal response to clamp ischaemia.
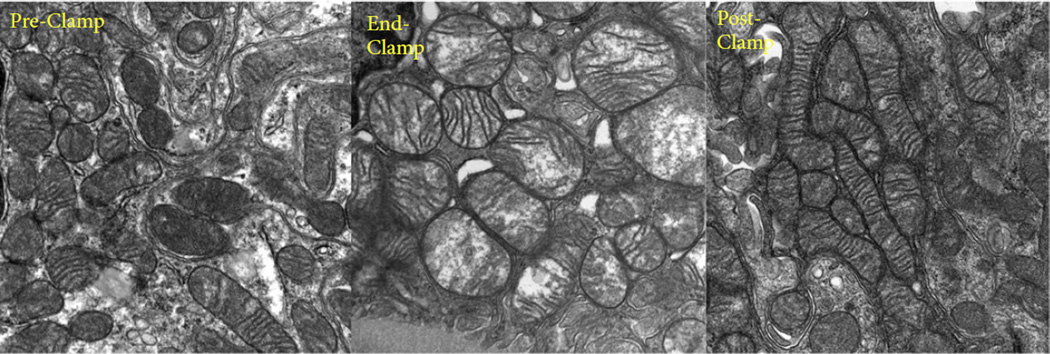
Fig. 5.
Examples of ultrastructural changes. Prominent mitochondrial swelling is seen at the end-clamp biopsy (middle), which largely resolves by the post-clamp biopsy (right).
Progression of Chronic Kidney Disease Stage
A total of 10 patients had an increased CKD severity classification at 1 year after surgery (Table 5): two patients changed from CKD 2 to CKD 3b and eight patients changed from CKD 1 to CKD 2. Most patients did not change classification. Of the 33 patients who had baseline eGFR ≥60 mL/min/1.73 m2, only two (5.4%) dropped below 60 mL/min/1.73 m2 (CKD stage 3). One of these patients had no identifiable aetiology. The other had an ischaemia time of 59 min and significant cardiovascular comorbidities, which may have played a role.
Table 5.
Chronic kidney disease classification of patients preoperatively and 1 year postoperatively.
| Preoperative GFR by MDRD | GFR by MDRD at 1 year after surgery |
|||
|---|---|---|---|---|
| CKD 3 <60 mL/min/1.73 m2 n(%) |
CKD 2 60–89 mL/min/1.73 m2 n(%) |
CKD 1 ≥90 mL/min/1.73 m2 n(%) |
Total n(%) |
|
| CKD 3 | 3 (8.1) | 1 (2.7) | – | 4 (10.8) |
| <60 mL/min/1.73 m2 | ||||
| CKD 2 | 2 (5.4) | 8 (21.6) | 1 (2.7) | 11 (29.7) |
| 60–89 mL/min/1.73 m2 | ||||
| CKD 1 | – | 8 (21.6) | 14 (37.8) | 22 (59.5) |
| ≥90 mL/min/1.73 m2 | ||||
| Total | 5 (13.5) | 17 (46.0) | 15 (40.5) | 37 (100.0) |
CKD, chronic kidney disease; MDRD, Modification of Diet in Renal Disease. Patients with increased CKD severity classification at 1 year after surgery are shown in bold.
Discussion
It is well documented that CKD is an independent risk factor for death from any cause, major cardiovascular events and hospitalizations [4]. Huang et al. [5] showed a marked increase in the development of CKD in patients undergoing radical nephrectomy compared with partial nephrectomy for pT1 renal cortical tumours [5]. As we transition to partial nephrectomy, the role of renal ischaemia has taken centre stage. Until recently, it was generally accepted that renal ischaemia should be limited to 20–30 min of warm ischaemia [6,7], but the duration of ischaemia that results in a permanent loss of kidney function has never been defined. Present guidelines are based entirely on animal model data and retrospective clinical data, which are subject to bias and short-term follow-up. The pitfalls of extrapolating results from animal models directly are well known. Although one must be very cautious when applying animal model data to humans, the pattern of renal recovery in larger animals can hint at a timeline to recovery in humans. In dogs undergoing 90 min of renal ischaemia, dogs with solitary kidneys showed full recovery at 2 weeks and dogs with two kidneys regained 75% function at 3–4 weeks [8]. In both groups, loss of renal function was seen within the first week, but significant recovery and regeneration of tubular function was seen in all subjects. Porcine models have also shown full recovery of renal function within 2 weeks after renal clamping for 90 min in a solitary kidney [9,10]. These data compel us to look beyond the peri-operative period for renal recovery and to assess the structural response of the human kidney to ischaemia.
We recently published a prospective clinical study, which was the first of its kind, with blinded investigators evaluating histopathological data in patients undergoing partial nephrectomy and found that the kidney showed ischaemic changes at a cellular level that recovered after reperfusion. Our data confirmed the human kidney’s tolerance to clamp renal ischaemia. In the present study, we have correlated the clinical and histopathological changes with long-term clinical follow-up. Clinically, we did not see a significant correlation between renal functional outcomes at 1 year with ischaemia time. In 37 patients with long-term follow-up data, ischaemia duration did not correlate with long-term eGFR loss. We investigated the relationship of long-term creatinine change with ischaemia both as a continuous variable (P = 0.086) and as a dichotomous variable (≤30 min or >30 min; P = 0.581) and neither showed a significant relationship. In multivariate analysis, there is even less of a statistical relationship. These data challenge the present dogma that every minute counts during renal ischaemia for partial nephrectomy and call into question the role of a finite value of ischaemia time being used as a dichotomous marker. Our data are consistent with others that have looked at creatinine changes over time after partial nephrectomy. Lane et al. [11] looked at GFR and creatinine changes in >1000 patients undergoing partial nephrectomy. They found the worst loss in GFR was in the peri-operative period (26% decrease from preoperative values); however, with long-term follow-up, significant renal recovery was found (7% decrease from preoperative values). Furthermore, others have shown that warm ischaemia time was not linked to GFR changes [12,13]. The present study results suggest that limited renal ischaemia is well tolerated in the setting of partial nephrectomy in patients with two functioning renal units.
We also assessed the association of structural changes of the kidney to long-term renal decline. Specifically, we assessed whether thick-section analysis (the only morphological change predicting renal loss in the peri-operative period) was predicative of long-term renal functional decline. Statistically, there was no association. The relative preservation of long-term function suggests that morphological changes from ischaemia recover rapidly after reperfusion and have little long-term consequence.
In our dataset, tumour size did not correlate with change in creatinine level in univariate (P = 0.883) or multivariate (P = 0.869) modelling. This finding was to be expected as the functional tumour volume does not contribute to GFR, and excision of normal parenchyma surrounding the tumour was kept to a minimum. What may be more important is the volume of preserved parenchyma [12,13]. In a multi-institutional study, Lane et al. (comparison of cold and warm ischaemia during partial nephrectomy in 660 patients) [14], found that that ultimate renal function was determined by percentage of parenchyma spared, while ischaemia time did not have statistical significance.
The median tumour size in the present study was 4 cm, which is significantly larger than most contemporary partial nephrectomy studies. The larger size and the more complex tumour location was primarily responsible for all patients undergoing an open approach in this study because patients with smaller tumours in less challenging locations underwent a robot-assisted approach. Although we did not assign a nephrometry score while designing the study, every patient in the study was selected for inclusion, in part, based on the large tumour size and complexity. Cold vs warm ischaemia allocation was also not randomized. Initially our approach was to subject the patients with extremely complex tumours to cold ischaemia as a means of renal protection; however, after 50% of the patients had been enrolled, we conducted a preliminary review that highlighted the safety of the warm ischaemia approach, even at durations that were previously considered potentially deleterious to the clamped kidney. Because we were aware of the paradigm shift towards a minimally invasive (robot-assisted) approach, in which achieving reliable hypothermia is challenging, we obtained permission from the Data Safety Monitoring Committee to allow us to perform warm ischaemia for the remaining patients regardless of the tumour size and complexity.
Patients undergoing cold ischaemia had significantly longer ischaemia times (48 vs 31 min), which is consistent with other partial nephrectomy series [14,15]. In our dataset, cold ischaemia predicted worsened renal function at 1 year, but warm ischaemia time did not, probably because of tumour complexity.
In the present study, significantly more women were enrolled than men. This finding was unexpected given national data for small renal masses and may reflect local referral patterns, but the relatively small sample size precludes any major conclusions from this finding. In a recent multi-institutional review of partial nephrectomy, Eggener et al. [15] found that female gender predicted worse renal functional decline in comparison with male gender. Previous studies have reported conflicting results regarding the role of gender as a predictor of renal functional decline with time [16]. In the present study, no statistically significant difference was observed with regard to gender as a predictor of renal function at 1 year in either univariate or multivariate analyses (Tables 3 and 4).
Remarkably, patients with obesity, diabetes and hypertension did not have more renal functional loss than those without. Long-term follow-up may ultimately show that these factors play a role in renal function [17]. We continue to follow these patients longitudinally and will report on long-term outcomes in the future.
It is critical to note that our patient population included patients with two functional kidneys undergoing unilateral partial nephrectomy with uncompromised baseline renal function. Our results should not be extrapolated to patients with a solitary kidney, pre-existing renal insufficiency or those with multiple renal masses. In patients with two functional kidneys, it is unknown how much compensation the contralateral kidney provided in the long-term. In addition, there are concerns that current markers of renal injury, including creatinine and GFR, may be masked by a normal contralateral kidney. In our peri-operative analysis, we were able to mitigate this limitation by including assessment of additional markers of functional renal injury including serum markers such as cystatin C that remain unchanged, as well as direct examination of the renal ultrastructure under ischaemic conditions. Furthermore, because the current basis for identifying patients with CKD is heavily reliant on creatinine and GFR, we believe these measures are the most clinically relevant. Unfortunately, we were not able to collect biopsies in the delayed postoperative or long-term setting, as they are invasive and represented undue risk. Special serum and urinary markers were also not repeated at follow-up visits, although this represents an arena for future investigation. Lastly, renal nephrometry scoring, as a measure of mass complexity, was not captured in our dataset and may have added more information related to our surgical approach and ultimate kidney function.
We do not suggest that renal ischaemia should not be taken seriously. We believe, however, that the current practice of using ischaemia duration threshold as a dichotomous marker and the commonly suggested ‘safe’ ischaemia values of 20 or 30 min and recently, zero ischaemia, are flawed. Most urologists are able to perform renal tumour excision and parenchymal reconstruction in a timely manner using renal hilar clamping.
In conclusion, limited ischaemia is well tolerated in the setting of partial nephrectomy and does not directly correspond to long-term renal functional decline. Short-term structural changes appear to resolve in the peri-operative period. The present study highlights the safety of clamp renal ischaemia within a reasonable time frame in patients with two functional kidneys. Further investigation is required to fully understand the complex pathways that contribute to long-term functional renal decline.
Abbreviations
- eGFR
estimated GFR
- CKD
chronic kidney disease
Footnotes
Conflict of Interest
None declared.
References
- 1.Gill IS, Patil MB, de Castro AA, et al. Zero ischemia anatomical partial nephrectomy: a novel approach. J Urol. 2012;187:807. doi: 10.1016/j.juro.2011.10.146. [DOI] [PubMed] [Google Scholar]
- 2.Ng CK, Gill IS, Patil MB, et al. Anatomic renal artery branch microdissection to facilitate zero-ischemia partial nephrectomy. Eur Urol. 2012;61:67. doi: 10.1016/j.eururo.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, McCulloch C, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:129. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RH, Frank I, Lohse CM, et al. The impact of ischemia time during open nephronsparing surgery on solitary kidneys: a multi-institutional study. J Urol. 2007;177:471. doi: 10.1016/j.juro.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Thompson RH, Lane BR, Lohse CM, et al. Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol. 2010;58:340. doi: 10.1016/j.eururo.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Osman Y, Hamed SM, Moustafa FE, et al. Is solitary kidney really more resistant to ischemia? an experimental canine study. J Urol. 2013;190:1110. doi: 10.1016/j.juro.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Laven BA, Orvieto MA, Chuang MS, et al. Renal tolerance to prolonged warm ischemia time in a laparoscopic versus open surgery porcine model. J Urol. 2004;172:2471. doi: 10.1097/01.ju.0000138158.16968.8d. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin DD, Maynes LJ, Berger KA, et al. Laparoscopic warm renal ischemia in the solitary porcine kidney model. Urology. 2004;64:592. doi: 10.1016/j.urology.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Lane BR, Babineau DC, Poggio ED, et al. Factors predicting renal functional outcome after partial nephrectomy. J Urol. 2008;180:2363. doi: 10.1016/j.juro.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Simmons MN, Hillyer SP, Lee BH, Fergany AF, Kaouk J, Campbell SC. Functional recovery after partial nephrectomy: effects of volume loss and ischemic injury. J Urol. 2012;187:1667. doi: 10.1016/j.juro.2011.12.068. [DOI] [PubMed] [Google Scholar]
- 13.Song C, Bang JK, Park HK, Ahn H. Factors influencing renal function reduction after partial nephrectomy. J Urol. 2009;181:48. doi: 10.1016/j.juro.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Lane BR, Russo P, Uzzo RG, et al. Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol. 2011;185:421. doi: 10.1016/j.juro.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 15.Eggener SE, Clark MA, Shikanov S, et al. Impact of warm versus cold ischemia on renal function following partial nephrectomy. World J Urol. 2015;33:351–357. doi: 10.1007/s00345-014-1315-4. [DOI] [PubMed] [Google Scholar]
- 16.Hoyosa T, Toshima R, Icida K, Tabe A, Sakai O. Changes in renal function with aging among Japanese. Intern Med. 1995;34:520–527. doi: 10.2169/internalmedicine.34.520. [DOI] [PubMed] [Google Scholar]
- 17.Lane BR, Campbell SC, Demirjian S, Fergany AF. Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol. 2013;189:1649. doi: 10.1016/j.juro.2012.11.121. [DOI] [PubMed] [Google Scholar]