Abstract
Background
Exposure to aeroallergens induces eosinophilic airway inflammation in patients with asthma and allergic airway diseases. The circulating number of eosinophils in peripheral blood is relatively small, leading us to hypothesize that bone marrow needs to be engaged quickly to meet the demands of the tissues.
Methods
To investigate the communication between the lungs and bone marrow, we used acute allergen exposure and airway inflammation models in mice. Gene-deficient mice and cytokine reporter mice as well as in vitro cell culture models were used to dissect the mechanisms.
Results
Naïve BALB/c mice produced increased numbers of eosinophil precursors and mature eosinophils in the bone marrow when their airways were exposed to a common fungal allergen, Alternaria alternata. Expression of IL-5 and IL-33 increased rapidly in the lungs, but not in the bone marrow. Sera from allergen-exposed mice promoted eosinophilopoiesis in bone marrow cells from naïve mice, which was blocked by anti-IL-5 antibody. Mice deficient in the IL-33 receptor ST2 (i.e., Il1rl1−/− mice) were unable to increase their serum levels of IL-5 and allergen-induced eosinophilopoiesis in the bone marrow after allergen exposure. Finally, group 2 innate lymphoid cells (ILC2s) in the lungs showed robust expression of IL-5 after Alternaria exposure.
Conclusions
These finding suggests that lung IL-33, through innate activation of ILC2s and their production of IL-5, plays a key role in promoting acute reactive eosinophilopoiesis in the bone marrow when naïve animals are exposed to airborne allergens. Therefore, bone marrow eosinophilopoiesis may be affected by atmospheric environmental conditions.
Keywords: eosinophilopoiesis, eosinophils, IL-33, IL-5
Eosinophils and other immune cells are recruited to the lungs of patients with asthma and allergic airway diseases, where they produce inflammatory mediators and pathologic changes of the respiratory mucosa (1, 2). In patients with sudden-onset asthma, acute exacerbation of their clinical symptoms is associated with rapid infiltration of neutrophils to the lungs, followed by eosinophils at a later time point (3). Unlike neutrophils, which consist of greater than 50% of the peripheral blood leukocytes, the peripheral pool of eosinophils is relatively small (2), suggesting that the bone marrow production of eosinophils needs to be engaged effectively to provide sufficient numbers of eosinophils for tissues. Although chemokines and receptor molecules that are responsible for recruitment of mature eosinophils from peripheral blood into the lungs have been well characterized (4, 5), the mechanism by which the production of eosinophils is controlled in the bone marrow in asthma and allergic immune responses has yet to be elucidated. In the case of neutrophils, severe microbial infection in peripheral tissues affects the hematopoietic system and increases de novo production of neutrophils in the bone marrow, to meet the needs in the tissues (6). During acute gastrointestinal infection with Toxoplasma gondi, bone marrow NK cells respond to systemic IL-12 that is produced in the mucosal tissues and prime monocyte precursors for effective functions (7). It is unknown whether or not an eosinophil arm of such ‘emergency myelopoiesis’ in the bone marrow exists.
Lung epithelial cells, besides functioning as a physical barrier, play important roles in regulating immune responses by secreting damage-associated molecular patterns (DAMPs), cytokines, and chemokines (8, 9). One of the cytokines secreted by epithelial cells is interleukin (IL)-33. IL-33 affects type 2 immune responses in the lungs, by promoting production of IL-5 and IL-13 by innate and adaptive immune cells (10). IL-33 was originally characterized as a transcriptional repressor, but when secreted, it also signals through a receptor dimer of ST2 (i.e., a product of Il1rl1) and IL-1 receptor accessory protein (IL-1Rap) on the cell surface, to induce inflammatory responses in mice (11). Indeed, mice deficient in Il1rl1 show ablated IL-33 signaling in a number of cell types, including epithelial cells, high endothelial venules, innate lymphoid cells (ILCs), T cells, dendritic cells, and smooth muscle cells (10–13). One of the IL-33 targets is ILC2s, which quickly produce large quantities of IL-5 and IL-13 upon IL-33 simulation (14, 15). ILC2s are constitutively present in various mucosal organs, such as lungs and skin, as well as other organs, such as adipose tissues, suggesting their roles in innate immunity, regulation of adaptive immunity, and tissue homeostasis (16–20).
Exposure to Alternaria alternata is associated with acute exacerbation of asthma, sometimes fatal, in humans (21–23). In this study, to investigate eosinophilopoiesis during airborne allergen exposure, we used a mouse model of acute airway inflammation that was induced by exposure to this fungus. Naïve mice responded quickly to airway Alternaria alternata exposure, with an increase in bone marrow production of eosinophil precursors and mature eosinophils. This reactive eosinophilopoiesis was mediated by circulating IL-5 in the blood stream, which was derived from lung tissues. Furthermore, the source of IL-5 in the lungs was IL-33-responsive ILC2s, as demonstrated by analyzing gene knockout mice and cytokine reporter mice. Thus, early IL-33-dependent production of IL-5 in the lungs is likely a key innate mechanism for enhanced eosinophil production in the bone marrow when animals are exposed to potent airborne allergens.
Materials and methods
Mice
BALB/c, C57BL/6, Rag1−/− (B6.129S7-Rag1tm1Mom/J), and Il7r−/− (B6.129S7-Il7rtm1Imx/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice deficient in IL-33 receptor ST2 (Il1rl1−/−) on the BALB/c background were provided by Dr. Andrew N. McKenzie (Medical Research Council Laboratory of Molecular Biology, Cambridge, UK). IL-5venus reporter mice on the BALB/c background were provided by Dr. Kiyoshi Takatsu (University of Toyama, Toyama, Japan). Age-matched female mice (7- to 13-week-old) were used for all experiments. Animals were bred and maintained under pathogen-free conditions. All animal experiments were approved and were in accordance with regulatory guidelines and standards set by the Institutional Animal Care and Use Committee of Mayo Clinic Rochester.
Reagents
ELISA kits for mouse IL-3, IL-5, IL-33, and GM-CSF were purchased from R&D Systems (Minneapolis, MN, USA) and used according to the manufacturer’s instructions. Recombinant mouse IL-5 and IL-33, and FITC-conjugated anti-Gr-1 (Ly6G) Ab were purchased from eBioscience (San Diego, CA, USA). PE-anti-Siglec-F, APC-anti-CD44, PE-anti-CD25, anti-CD16/CD32 antibodies, and 7AAD were purchased from BD Biosciences (San Jose, CA, USA). V450-conjugated lineage cocktail consisting of antibodies to CD3, CD4, CD19, Terr-119, DX5, Gr-1, CD16/32, B220, CD8α, CD11b, CD11c, Ly-6C, and Ly-6G was purchased from BD Biosciences. Carrier-free recombinant mouse IL-5, low-endotoxin and azide-free (LEAF) purified anti-mouse/human IL-5 Ab (TRFK5), and LEAF-purified rat IgG1 isotype control were purchased from BioLegend (San Diego, CA, USA). For mRNA analysis, Trizol® (Sigma-Aldrich, St. Louis, MO, USA) and SYBR® Green Master Mix (Bio-Rad, Hercules, CA, USA) were used. An extract of Alternaria alternata (lot F19), containing 0.003 µg/mg endotoxin, was from Greer Laboratories (Lenoir, NC, USA). The BCA protein assay kit was purchased from Thermo Scientific (Waltham, MA, USA) and was used according to the manufacturer’s instructions.
Alternaria alternata-induced airway inflammation
To examine airway immune responses, Alternaria alternata extract [25 µg or 50 µg/dose in 50 µL endotoxin-free PBS] or PBS alone was administered i.n. once or every day for 6 days, to naïve mice anesthetized with isoflurane. Approximately 70% of the solution administered i.n. reached the lungs. For the kinetic study, Alternaria alternata extract or PBS alone was administered every 3 days (days 0, 3, and 6). In the blocking experiments, mice were injected intraperitoneally (i.p.) with 1 mg/kg anti-IL-5 (TRFK5) or isotype control antibody, 7 days and 1 day prior to the first i.n. administration of Alternaria alternata. In some experiments, mice were injected i.p. with 4 µg mouse IL-33. At the indicated time points, mice were euthanized by an overdose of pentobarbital. The trachea was cannulated, and the lungs were lavaged three times with Hank’s balanced salt solution (HBSS) (0.5, 0.25, and 0.25 ml). The cell number in the BAL fluid was counted with a hemocytometer, and differentials were determined in cytospin preparations stained with Wright–Giemsa stain. Using this protocol, ≥ 200 cells were analyzed using conventional morphological criteria. Femoral bones and peripheral blood were collected and processed for analyses of bone marrow cells and peripheral blood cells by FACS, as described below. Sera were collected and stored frozen for later analyses of cytokines. Lungs were also collected and homogenized in 1.0 ml of PBS for cytokine analyses. The homogenates were centrifuged at 10 000 × g at 4°C for 15 min, and the protein concentrations in the supernatant were quantified with the BCA protein assay kit. A portion of the lung was processed to obtain lung single-cell suspensions for analyses of cell surface molecules and cytokines by FACS, as described below.
Flow cytometry analyses
Bone marrow cells were collected from femoral and tibial bones by flushing once with RMPI 1640 media, from the ends of long bones after their removal. Bone marrow cells and peripheral blood cells were treated with ammonium chloride/ potassium (ACK) lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) to remove red blood cells. They were preincubated with an FcR blocker (anti-CD16.CD32 Ab) for 15 min at 4°C, followed by staining with FITC-anti-Gr-1(Ly6G) Ab and PE-anti-Siglec-F Ab.
To obtain single-cell suspensions of lung cells, lungs were minced and incubated in digestion medium with a cocktail of 25 µg/ml DNAse I and liberase (StemCell Technologies, Vancouver, Canada) for 60 min at 37°C. After a 60-min incubation, cells were gently dissociated using a MACS Dissociator™. Red blood cells were lysed with ACK lysing buffer, and lung cells were stained with a lineage cocktail, and anti-CD25 and anti-CD44 antibodies. Cells were fixed and analyzed immediately by flow cytometry by gating of live cell populations.
Bone marrow cell culture
Freshly isolated mouse bone marrow cells were treated with ACK lysis buffer and suspended in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum. Bone marrow cells were cultured at 4.0 × 106 cells/ml with 10 ng/ml IL-5 or sera (10%) from PBS- or Alternaria alternata-exposed mice, for 5 days at 37°C and 5% CO2. In some experiments, mouse sera were pretreated with anti-IL-5 Ab (TRFK5, 5 µg/ml) or control Ab for 20 min at room temperature. After culturing, bone marrow cells were stained with anti-Siglec-F Ab and anti-Gr-1 Ab and then analyzed by flow cytometry. In some experiments, the Siglec-F+Gr-1lo and Siglec-F+Gr-1+ populations were sorted, and cytospin preparations were stained with Wright–Giemsa stain for analyses of cell morphology.
Analyses of cytokine genes in lung and bone marrow
Expression of mRNA for IL-5 and IL-33 in lung and bone marrow single-cell suspensions was examined by quantitative real-time polymerase chain reaction (qRT-PCR). Briefly, total RNA was purified with TRIzol® (Life Technologies, Grand Island, NY, USA). The cDNAs were synthesized from 1 µg of purified RNA using an iScript cDNA synthesis kit (Bio-Rad). The reaction was incubated at 45°C for 60 min and was terminated by heating at 85°C for 5 min. The RT-PCRs contained 1 µL cDNA diluted 1 : 10 in nuclease-free water, 12.5 µL TaqMan™ Universal PCR Master Mix (Life Technologies), and 1.25 µL TaqMan® (Life Technologies), with the gene expression assay of the target genes involving IL-5, IL-33, and 18S (as a reference gene) (Life Technologies). The final reaction volume was 20.0 µL with sterile water. Amplification and detection of specific products were performed using the iQ™5 Multicolor Real-Time PCR Detection System (Bio-Rad) or the Applied Biosystems StepOne-Plus™ System. The protocol was as follows: denaturation by a hot start at 95°C for 10 min, followed by 40 cycles of a two-step program (denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 min). Cytokine mRNA expression was normalized to the expression of 18S mRNA.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) or mean ± range for the numbers of mice or experiments as indicated. The statistical significance of the differences between the various groups was assessed using a one-tailed Student’s t-test. P ≤ 0.05 was considered significant.
Results
Airborne allergen exposure induces IL-5-dependent eosinophilopoiesis in the bone marrow
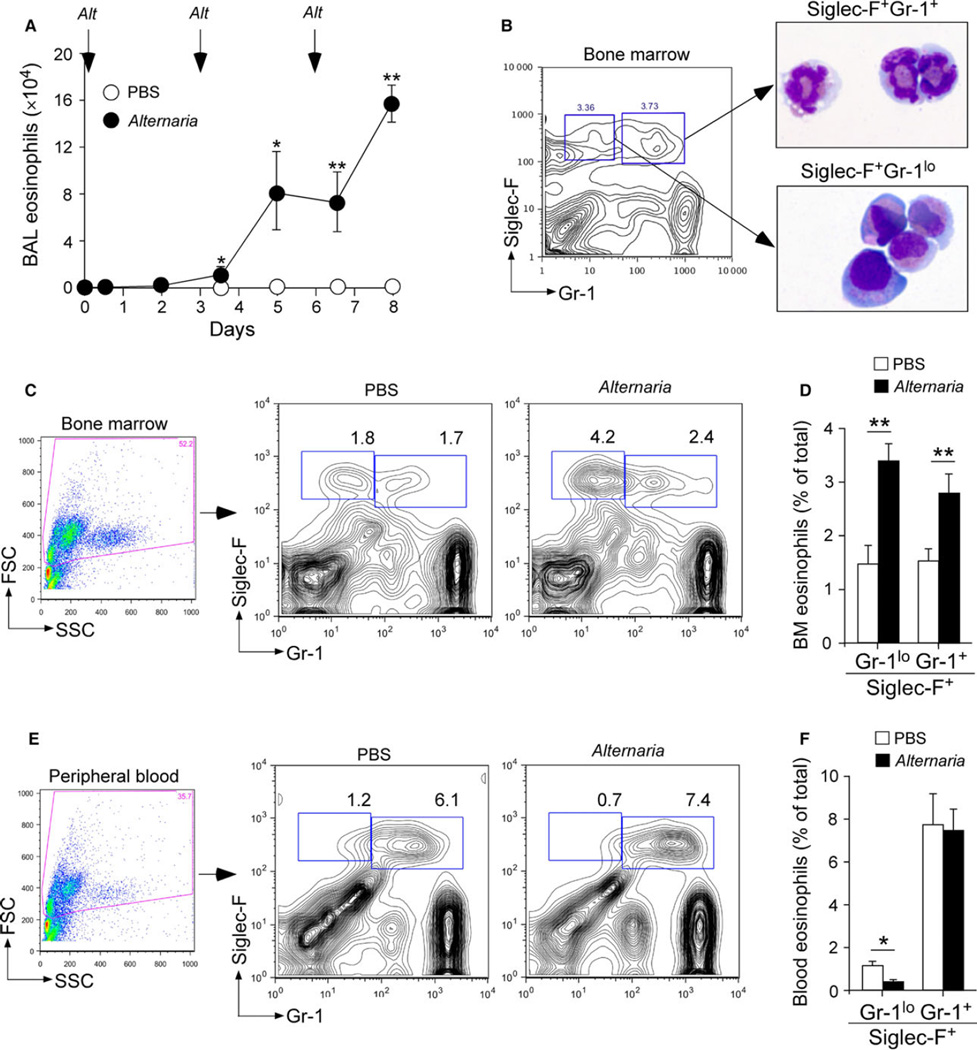
Increased bone marrow production of eosinophils was observed in mice that were sensitized and challenged with the model antigen, ovalbumin (OVA) (24, 25), and in patients with allergic asthma challenged with allergens (26). To establish a model to investigate the immunological mechanisms of bone marrow eosinophilopoiesis during acute allergic airway responses, we exposed naïve BALB/c mice to Alternaria alternata extract in vivo, every 3 days for 6 days. While airway eosinophilia was not detected during the first exposure, BAL eosinophil numbers clearly increased after the second exposure (Fig. 1A); eosinophil numbers continued to rise throughout the third exposure.
Figure 1.
Alternaria alternata exposure accelerates eosinophilopoiesis in the bone marrow. (A) Naïve BALB/c mice were exposed intranasally (i.n.) to phosphate-buffered saline (PBS) (open circles) or 50 µg Alternaria alternata extract (Alt) (closed circles) on days 0, 3, and 6. After 12 h or 48 h of each exposure, numbers of eosinophils in bronchoalveolar lavage (BAL) fluids were determined by analyzing cytospin preparations. Data (mean ± SEM, n = 3) are representative of two experiments. *P < 0.05 and **P < 0.01, compared to baseline (i.e., day 0). (B) Gating strategy to identify bone marrow eosinophil populations by flow cytometry. Bone marrow cells from naïve mice were cultured with 10 ng/ml IL-5 for 5 days. Siglec-F+Gr-1+ and Siglec-F+Gr-1lo populations were sorted, and cytospin preparations were stained with Wright–Giemsa stain. (C–F) Naïve BALB.c mice were exposed i.n. to 25 µg Alternaria or PBS, once every day for 6 days. Six hours after the last exposure, bone marrow cells (C) and peripheral blood cells (E) were stained with anti-Siglec-F and anti-Gr-1 antibodies and analyzed as described in Panel B. A summary of results from bone marrow (D) and peripheral blood (F) is shown (mean ± SEM, n = 3 or 4). Data are representative of two independent experiments. *P < 0.05 and **P < 0.01, between the groups indicated by horizontal lines.
Next, we investigated whether airway eosinophilia is associated with increased production of eosinophils in the bone marrow. To optimize the procedure to analyze eosinophils in the bone marrow by flow cytometry, we cultured bone marrow cells from naïve mice with IL-5 for 5 days and analyzed them using a strategy modified from a previous study (27). Two populations of Siglec-F-positive cells, including Siglec-F+Gr-1+ cells and Siglec-F+Gr-1lo cells, were identified based on their expression of a granulocyte marker Gr-1 (Ly6G) (Fig. 1B). The Siglec-F+Gr-1+ cells displayed a characteristic circular and segmented nuclei and a cytoplasm rich with eosin-staining granules, consistent with fully differentiated mouse eosinophils. The Siglec-F+Gr-1lo cells displayed an oval- or kidney-shaped nuclei and perinuclear eosinophilic staining, consistent with the morphology of eosinophil precursors.
Using this gating strategy, we analyzed fresh bone marrow preparations from mice that had been exposed in vivo to phosphate-buffered saline (PBS) or Alternaria. The bone marrow of PBS-exposed mice showed two distinct populations of eosinophils, including Siglec-F+Gr-1+ and Siglec-F+Gr-1lo cells (Fig. 1C), suggesting the presence of both mature eosinophils and eosinophil precursors. When exposed to Alternaria, both Siglec-F+Gr-1lo cells and Siglec-F+Gr-1+ cells increased significantly (P < 0.01, Fig. 1D). In the peripheral blood, PBS-exposed mice showed mainly one population of eosinophils, which was Siglec-F+Gr-1+ mature cells, with a small contribution by Siglec-F+Gr-1lo immature cells (Fig. 1E). The numbers of Siglec-F+Gr-1+ cells did not change after Alternaria exposure, while Siglec-F+Gr-1lo cells decreased significantly (P < 0.05, Fig. 1F). Spleens were analyzed similarly for eosinophils after PBS or Alternaria exposure. However, eosinophils (a combination of Siglec-F+Gr-1lo cells and Siglec-F+Gr-1+ cells) consisted approximately 0.7% of total leukocytes, and no significant differences were observed between the two treatment groups (data not shown).
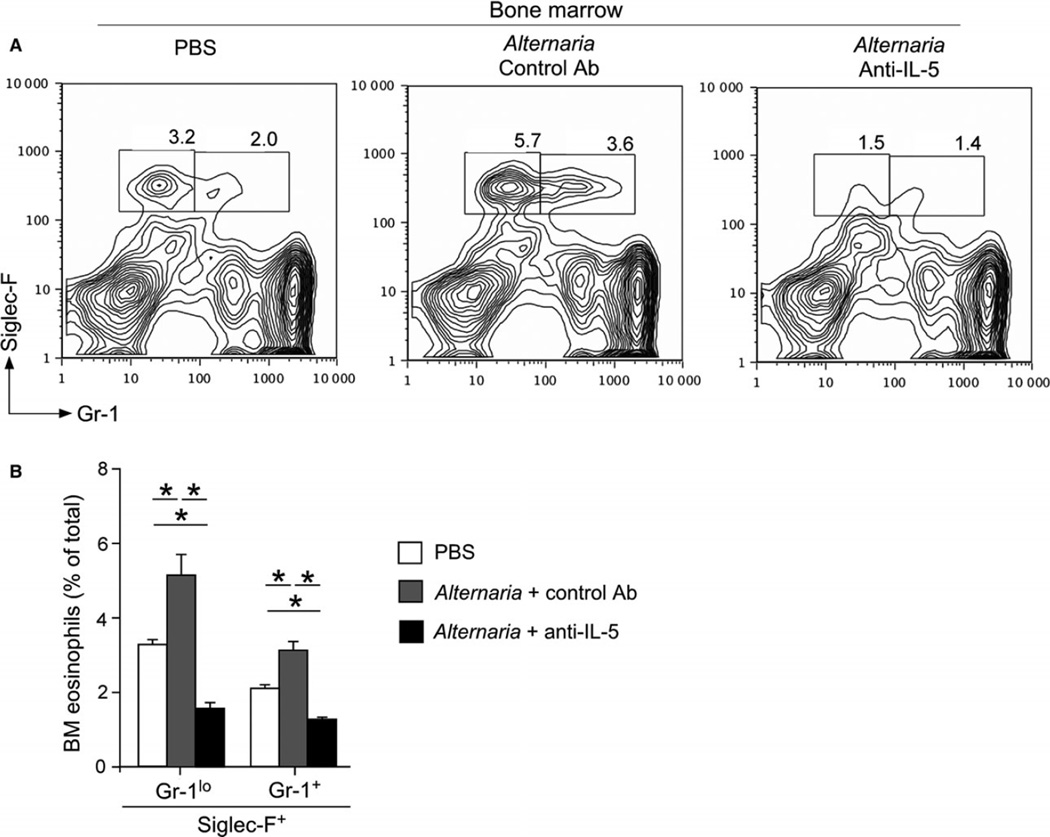
A Th2-type cytokine, IL-5, is involved in production, survival, and activation of eosinophils (28). Treatment with anti-IL-5 antibody decreases blood and tissue levels of eosinophils in mouse models of allergic airway inflammation and in patients with asthma and eosinophilia (29). Therefore, to examine the role of IL-5 in allergen-induced eosinophilopoiesis, mice were treated with anti-IL-5 antibody (Ab) (TRFK-5) prior to exposure to Alternaria. In the bone marrow of mice treated with control Ab, the numbers of both Siglec-F+Gr-1+ and Siglec-F+Gr-1lo populations significantly increased as compared to mice exposed to PBS (P < 0.05, Fig. 2A and B). The increase in both populations was abolished in mice treated with anti-IL-5 Ab. Indeed, the numbers of Siglec-F+Gr-1+ cells and Siglec-F+Gr-1lo cells in anti-IL-5-treated and Alternaria alternata-exposed mice were even lower than those exposed to PBS, suggesting that IL-5 was required for steady-state as well as reactive eosinophilopoiesis. Together, these findings suggest that airway exposure to fungus Alternaria affects a distant organ, namely the bone marrow, and promotes IL-5-dependent production of eosinophil precursors and mature eosinophils.
Figure 2.
Anti-IL-5 Ab inhibits eosinophilopoiesis in response to Alternaria exposure. Naïve BALB/c mice were injected intraperitoneally (i.p.) with 100 µg anti-IL-5 antibody (Ab) or isotype-matched control Ab 7 days and 1 day prior to the first i.n. administration of Alternaria. Mice were then exposed i.n. to 25 µg Alternaria or PBS once every day for 6 days and analyzed as described in Fig. 1. (A) A representative FACS scattergram from each group is shown. (B) A summary of results is shown (mean ± SEM, n = 4). *P < 0.05, between the groups indicated by horizontal lines.
Lung-derived IL-5 mediates eosinophilopoiesis in the bone marrow
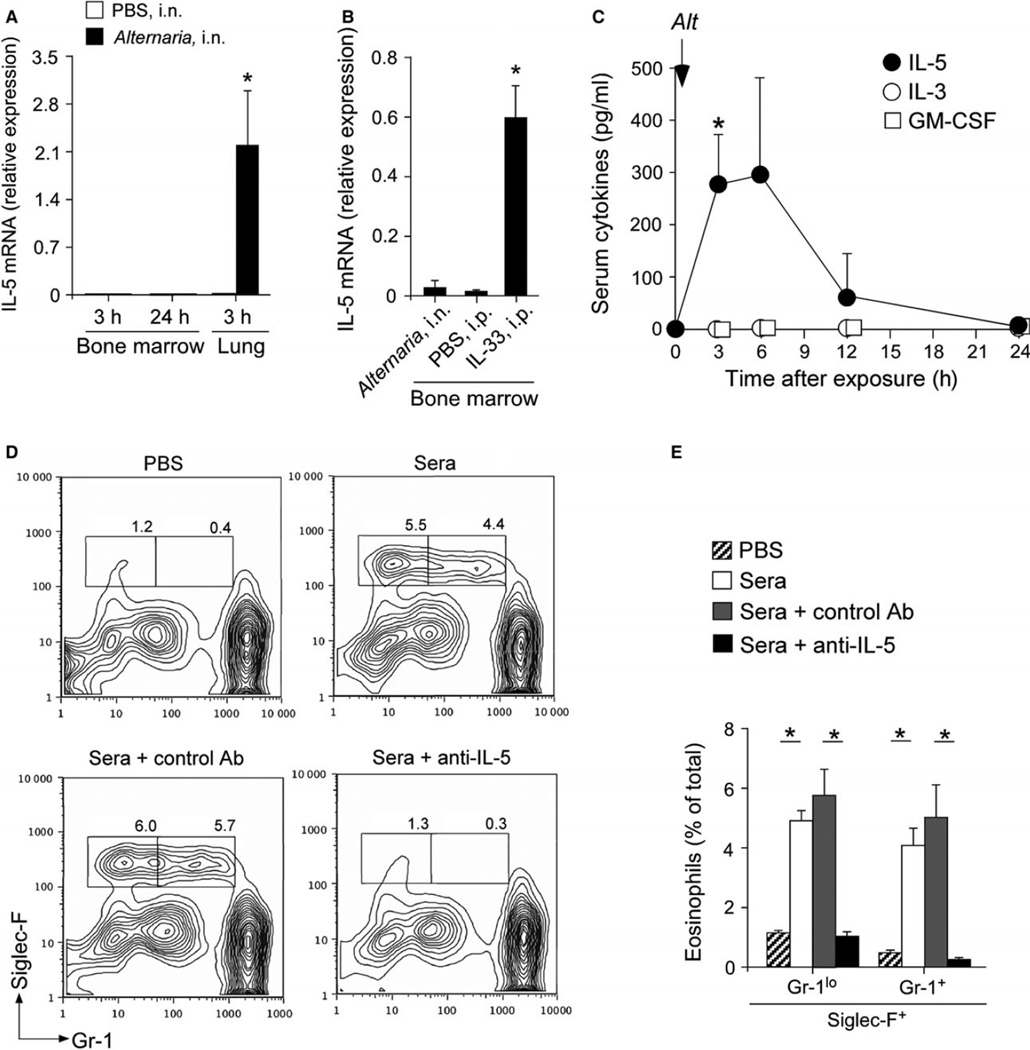
In individuals sensitized to airborne allergens, IL-5 is detectable in CD3+ T cells in the lungs as well as in bone marrow (30). Similarly, in mice sensitized and challenged with an allergen, IL-5 mRNA is detected in CD3+ T cells in the bone marrow (31). To determine the source of IL-5, we examined IL-5 mRNA expression in the lungs and bone marrow. In the bone marrow, no elevation of IL-5 mRNA was detectable 3 h or 24 h after a single exposure to Alternaria (Fig. 3A). In contrast, increased expression of IL-5 was observed in the lungs at 3 h. Recent findings suggest that epithelium-derived cytokines, such as IL-33, IL-25, and thymic stromal lymphopoietin (TSLP), play important roles in regulating production of Th2-type cytokines (8, 9, 32, 33). Indeed, bone marrow levels of IL-5 mRNA significantly increased in mice that were treated systemically with IL-33 by intraperitoneal injection (Fig. 3B), suggesting that the bone marrow is capable of expressing IL-5.
Figure 3.
IL-5 is expressed in the lungs, but not in the bone marrow. (A) Wild-type BALB/c mice were exposed once i.n. to either 50 µg Alternaria or PBS. The bone marrow and lungs were collected after 3 h or 24 h, and IL-5 mRNA expression was analyzed by qRT-PCR. Cytokine mRNA expression was normalized to the expression of 18S RNA. Data are shown as mean ± SEM (n = 3). Data are representative of two independent experiments. (B) Wild-type BALB/c mice were exposed i.n. to 50 µg Alternaria or injected i.p. with PBS or 4 µg IL-33. The bone marrow was collected after 6 h, and IL-5 mRNA expression was analyzed by qRT-PCR. Data are shown as mean ± SEM (n = 3). *P < 0.05, compared to mice given either PBS or Alternaria. Data are representative of two independent experiments. (C) Mice were exposed i.n. to 50 µg Alternaria, and sera were collected at the indicated times points. The levels of cytokines were determined by an ELISA. Data are shown as mean ± range (n = 2). *P < 0.05, compared to baseline (i.e., 0 h). (D and E) Mice were exposed i.n. to 50 µg Alternaria or PBS, and peripheral blood was collected at 6 h. Sera were untreated or preincubated with neutralizing anti-mouse IL-5 or control Ab and then added at 10% (v/v) to freshly isolated bone marrow cells from naïve mice. Cells were cultured for 5 days and analyzed as described in Figure 1. (D) A representative FACS scattergram from each group is shown. (E) A summary of results is shown (mean ± SEM, n = 4). *P < 0.05, between the groups indicated by horizontal lines.
Because IL-5 mRNA was not detected in the bone marrow after Alternaria exposure, we hypothesized that cytokine(s) in the systemic circulation that originated from the lungs is responsible for increased eosinophilopoiesis in the bone marrow. To test this possibility, naïve mice were exposed with a single intranasal (i.n.) administration of 50 µg Alternaria, and kinetic changes in the levels of eosinophilopoietic cytokines, including IL-5, IL-3, and GM-CSF, were quantitated in sera. Serum levels of IL-5 increased shortly after Alternaria exposure and peaked at 3 h or 6 h (Fig. 3C), then the levels returned to baseline by 24 h. Other eosinophilopoietic cytokines, including IL-3 and GM-CSF, were undetectable.
To verify and characterize the biological activities of cytokine(s) in sera, we cultured bone marrow cells from naïve mice with sera collected from the mice 6 h after exposure to either PBS or Alternaria. Bone marrow cells cultured with sera from PBS-treated mice showed minimal eosinophilopoiesis (Fig. 3D). In contrast, bone marrow cells cultured with sera from Alternaria-exposed animals showed distinct populations of Siglec-F+Gr-1+ and Siglec-F+Gr-1lo cells (Fig. 3D). An increase in these cells was abolished when the sera from Alternaria-exposed mice were pretreated with neutralizing anti-IL-5 Ab (TRFK5), but not with control Ab (Fig. 3D and E). Taken together, lung-derived IL-5 that reaches peripheral circulation likely mediates eosinophilopoiesis in the bone marrow when animals are exposed to airborne allergens.
IL-33 in the lungs is involved in increased serum levels of IL-5
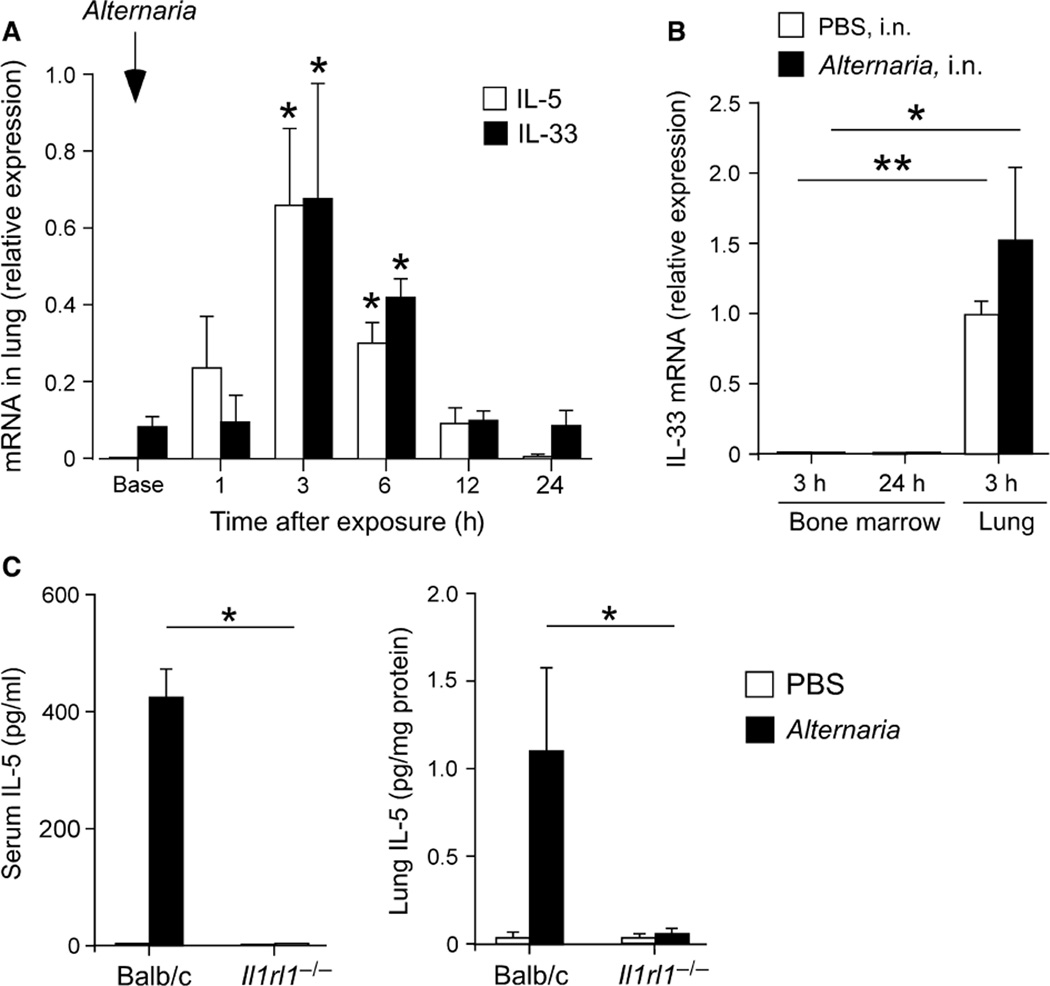
IL-33-producing cells, such as epithelial cells, and their targets, such as ILC2s, are strategically localized in mucosal tissues (16, 17, 34). To examine the relationship between IL-33 and IL-5, we examined kinetic changes in these cytokines in the lungs from naïve mice exposed to Alternaria. Expression of IL-5 mRNA was detectable within 1 h after allergen exposure and reached a peak at 3 h (Fig. 4A). IL-33 mRNA was clearly detectable before allergen exposure and further increased 3 h after Alternaria exposure. The kinetic changes in IL-5 and IL-33 mRNA were nearly identical. Similar to IL-5, no increase in IL-33 mRNA expression was observed in the bone marrow of Alternaria-exposed mice for up to 24 h (Fig. 4B).
Figure 4.
Increased serum IL-5 is dependent on the ST2/IL-33 pathway. (A) Naïve BALB/c mice were exposed once i.n. to 50 µg Alternaria, and the lungs were collected at the times indicated. Expression of mRNA for IL-5 and IL-33 was examined by qRT-PCR. Cytokine mRNA expression was normalized to the expression of 18S RNA. Data are shown as mean ± range (n = 2). *P < 0.05, compared to baseline (Base). (B) Naïve BALB/c mice were exposed once i.n. to 50 µg Alternaria, and the bone marrow and lungs were collected at 3 h or 24 h. IL-33 mRNA expression was determined by qRT-PCR. Data are shown as mean ± SEM (n = 3). Data are representative of two independent experiments. *P < 0.05 and **P < 0.01, between the groups indicated by horizontal lines. (C) Naïve wild-type BALB/c mice or Il1rl1−/− mice (BALB/c background) were exposed once i.n. to 50 µg Alternaria or PBS. IL-5 protein levels in sera and lung tissues were determined after 6 h. Data are shown as mean ± SEM (n = 4). *P < 0.05, between the groups indicated by horizontal lines.
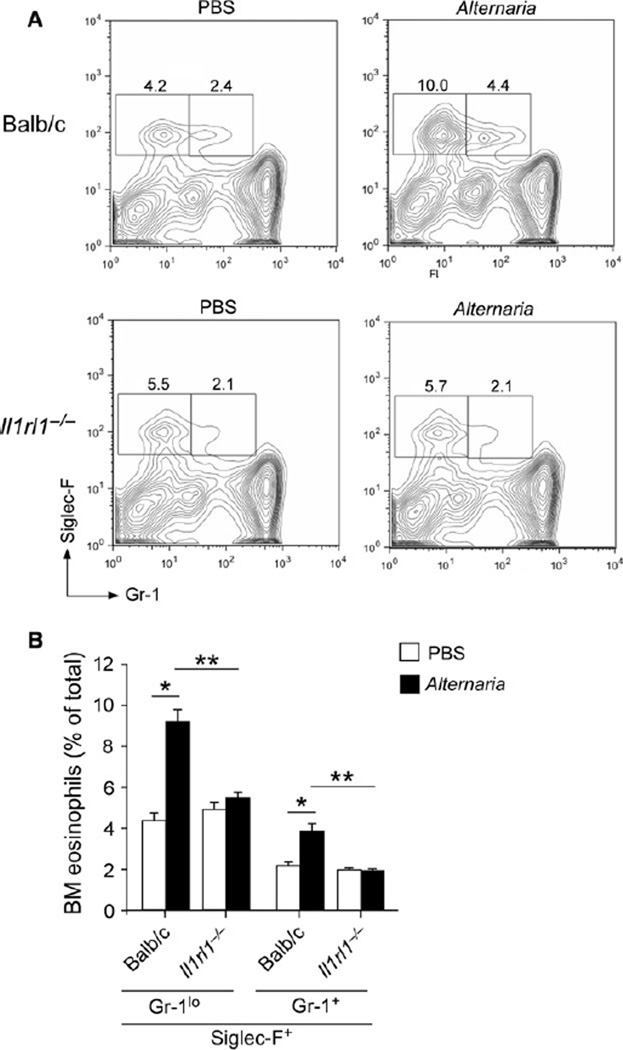
Next, we investigated the role of IL-33 in IL-5 expression and eosinophilopoiesis using mice (Il1rl1−/−) that were deficient in the IL-33 receptor, ST2. IL-5 protein was clearly detectable in sera and lung tissues of wild-type mice 6 h after Alternaria exposure, but was nearly undetectable in Il1rl1−/− mice (P < 0.05, Fig. 4C). In the bone marrow, production of both Siglec-F+Gr-1+ and Siglec-F+Gr-1lo eosinophil populations significantly decreased in Il1rl1−/− mice as compared to wild-type mice when they were exposed to Alternaria (P < 0.01, Fig. 5A and B). Interestingly, no apparent differences were observed in the Siglec-F+Gr-1+ and Siglec-F+Gr-1lo populations between Il1rl1−/− and wild-type mice when they were exposed to PBS, suggesting that IL-33 does not affect steady-state production of eosinophils.
Figure 5.
The ST2/IL-33 pathway is necessary for eosinophilopoiesis in response to Alternaria exposure. (A) Naïve wild-type BALB/c mice or Il1rl1−/− mice (BALB/c background) were exposed i.n. to 25 µg Alternaria or PBS every day for 6 days. Bone marrow was collected 6 h after the last exposure and analyzed by FACS. A representative FACS scattergram from each group is shown. (B) A summary of results is shown (mean ± SEM, n = 4). *P < 0.05 and **P < 0.01, between the groups indicated by horizontal lines.
Lung ILC2s contribute to IL-5 in sera
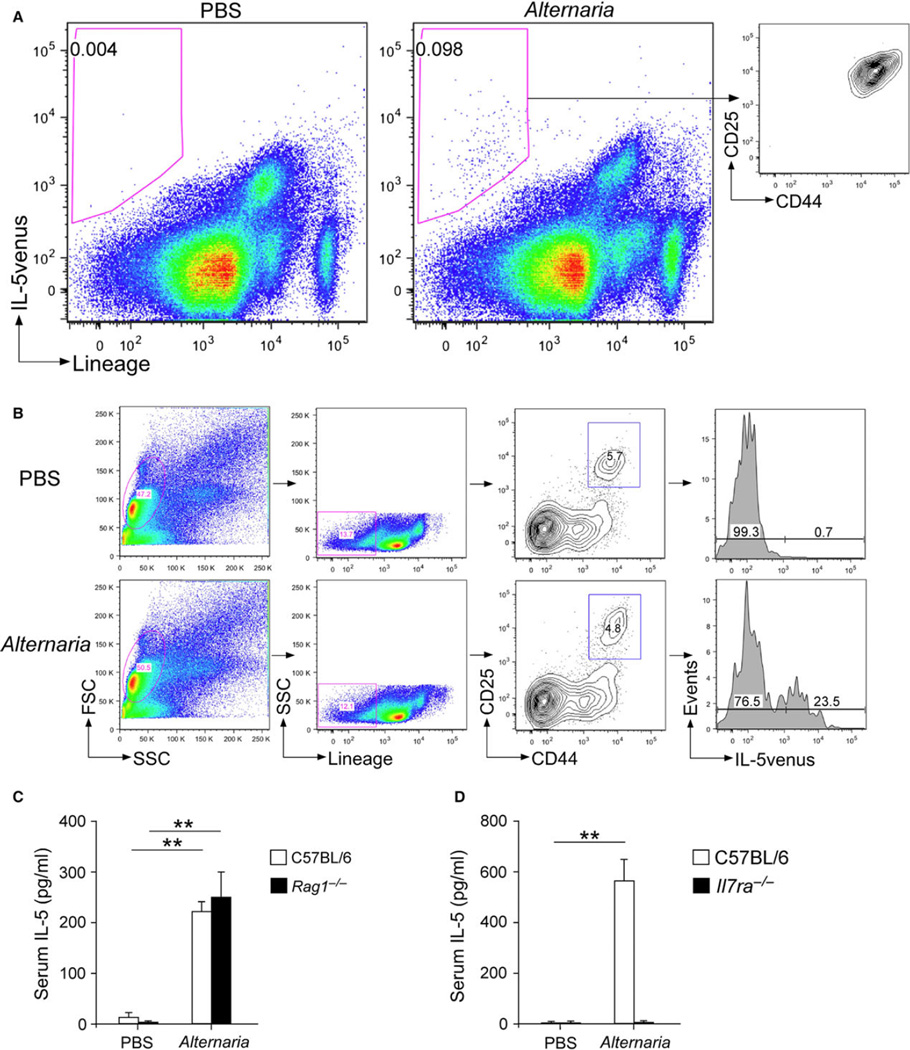
After identifying critical roles for the IL-33/ST2 pathway that induce bone marrow eosinophilopoiesis mediated by circulating IL-5, we determined the source of IL-5 in the lungs using cytokine reporter mice. We exposed IL-5venus reporter mice once to either PBS or Alternaria and harvested the lungs 24 h later. Side-by-side comparisons of mice exposed to PBS and those exposed to Alternaria showed that the IL-5venus signal was present mainly within the lineage-negative cell population(s) (Fig. 6A). Furthermore, IL-5venus-positive cells demonstrated a distinct CD25 and CD44 double-positive population (Fig. 6A, right panel), which is consistent with the phenotypes of lung ILC2s (15, 35). Conversely, analyses of IL-5venus expression by the gated ILC2 population (i.e., Lin-CD25+CD44hi) showed that approximately 23% of ILC2s expressed IL-5venus when mice were exposed to Alternaria (Fig. 6B); little or no IL-5venus was detectable in ILC2s from PBS-exposed mice. Finally, serum levels of IL-5 increased in both wild-type BALB/c mice and Rag1−/− mice that were exposed to Alternaria (Fig. 6C), suggesting the innate cell origin of serum IL-5. In contrast, this increase in IL-5 was abolished in Il7ra−/− mice (Fig. 6D) that were deficient in ILCs (15, 35). Taken together, these findings suggest that the interaction between IL-33 and ILC2s in the lung compartment plays a key role in mediating increased serum levels of IL-5 and subsequent eosinophilopoiesis in the bone marrow.
Figure 6.
ILC2s are a major source of serum IL-5 in mice exposed to Alternaria. Naïve IL-5venus cytokine receptor mice (BALB/c background) were exposed once i.n. to PBS or 50 µg Alternaria. (A) Lung single-cell suspensions were stained with a lineage cocktail Ab and analyzed for the IL-5venus signal by FACS. Right panel depicts CD25 and CD44 expression by the lineage-negative and IL-5venus-positive cell population in the Alternaria-exposed mice. (B) Lung single-cell suspensions were stained with a lineage cocktail Ab and Abs for CD25 and CD44, and expression of IL-5venus in the ILC2 population (Lin−CD25+CD44hi) was analyzed by FACS. Data are presented as representative of 2–4 mice in each group. (C and D) Wild-type C57BL/6 or Rag1−/− mice (C57BL/6 background) (C) or wild-type C57BL/6 mice or Il7ra−/− mice (C57BL/6 background) (D) were exposed i.n. to PBS or 50 µg Alternaria. Sera were collected 6 h later, and IL-5 levels were determined by ELISA. Data are shown as mean ± SEM (n = 4). **P < 0.01, between the groups indicated by horizontal lines.
Discussion
One of the major findings in this study is that there is innate immunological communication from the lungs to the bone marrow to promote eosinophilopoiesis, when naïve animals are acutely exposed to an airborne allergen. Exposure to fungal Alternaria alternata is closely associated with exacerbation of asthma in humans (23, 36). Using Alternaria as a model, we found that production of eosinophil precursors and mature eosinophils was increased in the bone marrow as a result of airway allergen exposure. Increased eosinophilopoiesis was apparent after 5 days of exposure to Alternaria and was dependent on IL-5 in the peripheral blood circulation (Figs 1 and 2). Indeed, increased expression of IL-5 mRNA was observed within 1 h of a single exposure to Alternaria (Fig. 4A), and IL-5 protein was detectable in sera within 3 h (Fig. 3C), suggesting the presence of a rapid sequence of events that link allergen exposure in the lungs to eosinophilopoiesis in the bone marrow. This observation is analogous to the concept of ‘emergency myelopoiesis’, where neutrophil production in the bone marrow is increased in response to infection with microbes, which is mediated by circulating growth factors, including G-CSF and GM-CSF (6). Similar concept was also reported recently for NK cells and monocyte precursors, which is induced by a gastrointestinal parasite and mediated by circulating IL-12 (7). Thus, the ‘emergency myelopoiesis’ concept may also apply to this arm of the eosinophilic granulocyte, although it is mediated by a different triggering agent and factor, namely allergen and IL-5, respectively.
Another major finding in this study is that the series of events leading to eosinophilopoiesis is initiated by IL-33, an epithelial danger signaling molecule. IL-33 mRNA was constitutively expressed in the lungs of naïve animals and was upregulated soon after Alternaria exposure (Fig. 4). Il1rl1−/− mice that are deficient in the IL-33 receptor ST2 had nearly undetectable levels of IL-5 in their blood and lungs shortly after exposure to Alternaria, while a large quantity of IL-5 was observed in wild-type mice. In addition, eosinophilopoiesis in response to Alternaria was nearly abolished in Il1rl1−/− mice, while steady-state eosinophilopoiesis was sustained in these animals (Fig. 5). This observation is in contrast to animals treated with anti-IL-5 Ab, in which the steady-state production of eosinophils as well as reactive eosinophilopoiesis was diminished (Fig. 2). Thus, lung IL-33 may play a pivotal role to control the magnitude of eosinophilopoiesis in the bone marrow, when animals are exposed to airborne allergens and perhaps other environmental agents, while it plays a minimal role in eosinophilopoiesis during resting conditions. IL-33 expression can be regulated by signaling from the protease-activated receptor 2 and by Notch (22, 37, 38). It has been shown that IL-33 is upregulated upon stimulation by TLR5 and IL-1α (39), both of which signal through NF-κB. IL-33 is upregulated in vitro when airway epithelial cells are stimulated with a combination of IL-33 and IL-13 (40). Further studies are therefore still necessary to elucidate the cellular and molecular mechanisms involved in increased production of IL-33 in the lungs when animals are exposed to airborne allergens.
In this study, increased expression of IL-5 and IL-33 mRNA after exposure to Alternaria was observed in the lungs (Figs 3 and 4). While the cells in the bone marrow have the ability to increase expression of IL-5 in response to systemic administration of IL-33, no evidence for IL-5 expression was detected up to 24 h after exposure to Alternaria alternata (Fig. 3). In addition, ILC2s in the lungs produced IL-5 soon after Alternaria exposure (Fig. 6). These findings suggest that IL-5 production is compartmentalized to the target organ of exposure during allergic responses. Previously, in mice sensitized systemically and challenged i.n. with the model antigen OVA, IL-5 mRNA was detected in CD3+ T cells in the bone marrow as well as in the lungs (31). Several reasons could explain the differences in these observations. For example, systemic sensitization with antigens (i.e., intraperitoneal sensitization) may alter normal immune responses, such as homing processes, and allow antigen-specific T cells to distribute to various secondary lymphoid organs in the body. In this study, we administered the antigens exclusively from the intranasal route to mimic human exposure to airborne allergens. It is also important to note that the length of exposure to Alternaria was relatively short to mimic acute exposure. While 8 days is sufficient to induce initial adaptive immunity responses (15), it may not be optimal to examine the full effect of recall immune responses. Thus, we cannot eliminate the possibility that cells in the bone marrow also have the capacity to produce IL-5 under certain conditions.
Nonetheless, our observations suggest that peripheral blood serves as a key element to carry IL-5 protein from the point of exposure (i.e., the lungs) to the bone marrow. Indeed, IL-5 activity in the sera of Alternaria-exposed mice was sufficient to induce production of both eosinophil precursors and mature eosinophils by bone marrow cells from naïve nonexposed animals (Fig. 3). These findings are consistent with observations in patients with asthma, who are treated systemically with neutralizing anti-IL-5 antibody, and demonstrate decreased numbers of eosinophil precursors in the bone marrow (41). We should also note that the concentrations of IL-5 in sera from mice that had been exposed to Alternaria alternata were considerably low (i.e., ~50 pg/ml when used in 10% serum) as compared to those used generally to grow eosinophils from the bone marrow in vitro (i.e., 10 ng/ml) (42). However, the sera were sufficient to drive eosinophilopoiesis by naïve bone marrow cells (Fig. 3), suggesting the presence of co-factors, such as other cytokines and soluble receptors, in the sera to facilitate eosinophilopoiesis, together with IL-5. Indeed, IL-5 has been shown recently to provide a system to create a cooperative network with IL-4 and chemokines that effectively promotes eosinophil precursor maturation (43). Therefore, further analyses of sera from Alternaria-exposed animals may provide novel information to identify these known and novel ‘co-factor(s)’.
There are a number of cell types reported to be capable of producing IL-5, including CD4+ Th2-type T cells, mast cells, ILC2s, γδ+ T cells, and others. For example, during the Th2-type adaptive immune response, CD4+ T cells in the lungs and bone marrow secrete IL-5, providing survival and maturation stimuli for eosinophils (30, 31). Mast cells also produce IL-5 in an IgE-dependent manner (44). Therefore, we used IL-5 reporter mice and gene knockout mice to determine the source of IL-5 in our model. PBS-treated mice contained ILC2s, but they were nearly negative for the IL-5venus signal (Fig. 6). Once exposed to Alternaria, a proportion of ILC2s became positive for IL-5venus. Visual inspection of IL-5venus-positive cells in a fluorescence-activated cell sorting (FACS) scattergram showed that ILC2s are likely the only cell population producing IL-5 in this model. The results of gene-deficient animals, such as Rag1−/− and Il7ra−/− mice, are also consistent with the ILC2 origin of serum IL-5. In ‘emergency myelopoiesis’ as discussed above (6), tissue-resident macrophages and monocytes likely play a key role in providing G-CSF and GM-CSF, to initiate neutrophil production in the bone marrow when exposed to microbes. Similarly, we propose lung ILC2s acts as a sentinel, to recognize exposure to environmental factor(s) in order to initiate eosinophilopoiesis. Recent studies suggest that ILC2s control homeostasis of eosinophils in the gastrointestinal tract in response to circadian regulation and caloric intake (16). Our observations add to this knowledge, by suggesting that ILC2s may regulate eosinophil dynamics at both local and systemic levels, depending on the magnitudes of environmental cues.
We conclude that IL-33, through activation of ILC2s and subsequent production of circulating IL-5, mediates reactive eosinophilopoiesis when animal is exposed to airborne allergens. Notably, the expression of IL-5 and IL-33 in the lungs was similar after airway exposure to Alternaria (Fig. 4). Deficiency in the IL-33 receptor resulted in undetectable serum levels of IL-5 (Fig. 4). Furthermore, deficiency in IL-33 resulted in ablated reactive eosinophilopoiesis in the bone marrow, while steady-state eosinophilopoiesis was not compromised (Fig. 5). Thus, the strategies that intervene in the IL-33 pathway by neutralizing IL-33 protein, blocking its receptor, or inhibiting production and/or secretion of IL-33, may be as effective as anti-IL-5 antibody to normalize eosinophilopoiesis in patients with airway eosinophilia, such as those with asthma. Future studies will therefore be necessary to determine which one, either IL-5 or IL-33, is more effective and safe as a therapeutic target to treat patients.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI34486, HL117823), from the Mayo Graduate School, and the Mayo Foundation. We thank Dr. Andrew N. McKenzie and Dr. Kiyoshi Takatsu for providing the Il1rl1−/− mice and IL-5venus mice, respectively. We thank LuRaye S. Eischens for secretarial help and Gail M. Kephart for technical assistance.
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
Author contributions
E.LA. and H.K. designed the studies and experiments; E.LA., T.K., and K.I. performed the experiments; E.LA., K.R.B., C-C.C., and H.K. interpreted the data; and E.LA., T.K., K.I., K.R.B., C-C.C., and H.K. wrote and approved the manuscript.
References
- 1.Wenzel SE. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 3.Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148:713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–1172. doi: 10.1006/bbrc.1993.2599. [DOI] [PubMed] [Google Scholar]
- 5.Bochner BS, Schleimer RP. Mast cells, basophils, and eosinophils: distinct but overlapping pathways for recruitment. Immunol Rev. 2001;179:5–15. doi: 10.1034/j.1600-065x.2001.790101.x. [DOI] [PubMed] [Google Scholar]
- 6.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 7.Askenase MH, Han SJ, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, et al. Bone-Marrow-Resident NK cells prime monocytes for regulatory function during infection. Immunity. 2015;42:1130–1142. doi: 10.1016/j.immuni.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambrecht BN, Hammad H. The airway epitheliumin asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 9.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 12.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–1054. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, et al. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J Immunol. 2012;188:2622–2629. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage-CD25+ CD44 (hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503– 1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roediger B, Kyle R, Le Gros G, Weninger W. Dermal group 2 innate lymphoid cells in atopic dermatitis and allergy. Curr Opin Immunol. 2014;31:108–114. doi: 10.1016/j.coi.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MW, Odegaard JI, Mukundan L, Qiu L, Molofsky AB, Nussbaum JC, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snelgrove RJ, Gregory LG, Peirό T, Akthar S, Campbell GA, Walker SA, et al. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, et al. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am J Physiol Lung Cell Mol Physiol. 2011;300:L605–L614. doi: 10.1152/ajplung.00359.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 24.Inman MD, Ellis R, Wattie J, Denburg JA, O’Byrne PM. Allergen-induced increase in airway responsiveness, airway eosinophilia, and bone-marrow eosinophil progenitors in mice. Am J Respir Cell Mol Biol. 1999;21:473–479. doi: 10.1165/ajrcmb.21.4.3622. [DOI] [PubMed] [Google Scholar]
- 25.Gaspar Elsas MI, Joseph D, Elsas PX, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Respir Cell Mol Biol. 1997;17:404–413. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- 26.Wood LJ, Inman MD, Watson RM, Foley R, Denburg JA, O’Byrne PM. Changes in bone marrow inflammatory cell progenitors after inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med. 1998;157:99–105. doi: 10.1164/ajrccm.157.1.9704125. [DOI] [PubMed] [Google Scholar]
- 27.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81:343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol. 2008;20:288–294. doi: 10.1016/j.coi.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Busse WW, Ring J, Huss-Marp J, Kahn JE. A review of treatment with mepolizumab, an anti-IL-5 mAb in hypereosinophilic syndromes and asthma. J Allergy Clin Immunol. 2010;125:803–813. doi: 10.1016/j.jaci.2009.11.048. [DOI] [PubMed] [Google Scholar]
- 30.Wood LJ, Sehmi LJ, Dorman S, Hamid Q, Tulic MK, Watson RM, et al. Allergen-induced increases in bone marrow T lymphocytes and interleukin-5 expression in subjects with asthma. Am J Respir Crit Care Med. 2002;166:883–889. doi: 10.1164/rccm.2108015. [DOI] [PubMed] [Google Scholar]
- 31.Minshall EM, Schleimer R, Cameron L, Minnicozzi M, Egan RW, Gutierrez-Ramos JC, et al. Interleukin-5 expression in the bone marrow of sensitized Balb/c mice after allergen challenge. Am J Respir Crit Care Med. 1998;158:951–957. doi: 10.1164/ajrccm.158.3.9709114. [DOI] [PubMed] [Google Scholar]
- 32.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur J Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells–how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 36.Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J. 2006;27:615–626. doi: 10.1183/09031936.06.00074705. [DOI] [PubMed] [Google Scholar]
- 37.Matsuwaki Y, Wada K, Moriyama H, Kita H. Human eosinophil innate response to Alternaria fungus through protease-activated receptor-2. Int Arch Allergy Immunol. 2011;155(Suppl. 1):123–128. doi: 10.1159/000327498. [DOI] [PubMed] [Google Scholar]
- 38.Sundlisaeter E, Edelmann RJ, Hol J, Sponheim J, Küuchler AM, Weiss M, et al. The alarmin IL-33 is a notch target in quiescent endothelial cells. Am J Pathol. 2012;181:1099–1111. doi: 10.1016/j.ajpath.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christianson CA, Goplen NP, Zafar I, Irvin C, Goot JT, Jr, Rollins DR, et al. Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33. J Allergy Clin Immunol. 2015;136:59–68. doi: 10.1016/j.jaci.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menzies-Gow A, Flood-Page P, Sehmi R, Murman J, Hamid Q, Robinson DS, et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–719. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 42.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulkerson PC, Schollaert KL, Bouffi C, Rothenberg ME. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J Immunol. 2014;193:4043–4052. doi: 10.4049/jimmunol.1400732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorentz A, Schwengberg S, Mierke C, Manns MP, Bischoff SC. Human intestinal mast cells produce IL-5 in vitro upon IgE receptor cross-linking and in vivo in the course of intestinal inflammatory disease. Eur J Immunol. 1999;29:1496–1503. doi: 10.1002/(SICI)1521-4141(199905)29:05<1496::AID-IMMU1496>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]