Abstract
Post-weaning social isolation (PSI) has been shown to increase aggressive behavior and alter medial prefrontal cortex (mPFC) function in social species such as rats. Here we developed a novel escapable social interaction test (ESIT) allowing for the quantification of escape and social behaviors in addition to mPFC activation in response to an aggressive or nonaggressive stimulus rat. Male rats were exposed to 3 weeks of PSI (ISO) or group (GRP) housing, and exposed to 3 trials, with either no trial, all trials, or the last trial only with a stimulus rat. Analysis of social behaviors indicated that ISO rats spent less time in the escape chamber and more time engaged in social interaction, aggressive grooming, and boxing than did GRP rats. Interestingly, during the third trial all rats engaged in more of the quantified social behaviors and spent less time escaping in response to aggressive but not nonaggressive stimulus rats. Rats exposed to nonaggressive stimulus rats on the third trial had greater c-fos and ARC immunoreactivity in the mPFC than those exposed to an aggressive stimulus rat. Conversely, a social encounter produced an increase in large PSD-95 punctae in the mPFC independently of trial number, but only in ISO rats exposed to an aggressive stimulus rat. The results presented here demonstrate that PSI increases interaction time and aggressive behaviors during escapable social interaction, and that the aggressiveness of the stimulus rat in a social encounter is an important component of behavioral and neural outcomes for both isolation and group-reared rats.
Keywords: medial prefrontal cortex, adolescence, social isolation, PSD-95, c-fos, Arc
1. Introduction
Social interaction during adolescence is critical for the development of competent and developmentally appropriate social behavior during adulthood. Social behavior is dependent upon a number of factors including the social history of the individual [1,2], the testing environment [3], and characteristics of social partners [4–6]. Post-weaning social isolation (PSI, also known as isolation rearing) in rats has been shown to produce altered social behavior that includes both increased social interaction as well as increased aggression [7,8]. This suggests that even though the motivation for social interaction is increased after PSI, the social interactions themselves may be unpleasant for individuals that have been deprived of normal social interaction during the adolescent period. Conversely, social conflict may be less aversive, or even reinforcing, for individuals that have been subjected to PSI and this social conflict may drive the PSI-induced increase in social interaction. One potential consequence of this is that isolates may be more likely to remain in abusive or maladaptive social situations.
PSI consists of depriving adolescent rats of social experience by housing them individually (ISO), as compared to housing in same-sex groups [GRP] for a period of 4 to 8 weeks after weaning. PSI of male rats produced increases in aggression [9], especially when testing occurred in an unfamiliar environment [3]. We have observed increases in social interaction after 4 weeks of PSI, and this increase was almost completely accounted for by increases in time spent engaging in aggressive grooming [7]. In spite of the finding that ISO rats spend more time in social interaction with a novel conspecific than GRP rats, we have not observed an increase in the reinforcing property of social interaction after PSI [10]. Conditioned place preference (CPP) studies in our laboratory have indicated that males, but not females, rapidly develop CPP to a context associated with a novel, same-sex, conspecific regardless of the experimental rat's rearing condition. Thus, even though isolation rearing dramatically increased the time spent interacting with a novel conspecific, it did not increase preference for a conspecific-associated context [10], consistent with the results of Douglas et al. [11].
The medial prefrontal cortex (mPFC) undergoes significant developmental fine-tuning during adolescence [12] and is crucial for the regulation of emotion [13] as well as for executive function [14]. In young men, executive function is negatively correlated with high frequency of physical aggression [15] thus changes in mPFC function produced by PSI may be directly related to the aggression observed in rats exposed to PSI. PSI leads to abnormalities in mPFC structure and function including alterations in dendritic spine morphology [16] as well as decreased expression of immediate early genes [7,17,18] and synaptic-associated proteins including PSD-95 [19]. PSD-95 expression was decreased in the mPFC after social isolation when assessed by Western blot [19]. However, reorganization of PSD-95 into large clusters (punctae) is associated with plasticity [20] independently of protein expression per se, and PSD-95 can be quantified by assessing the numbers of small, medium, and large punctae using immunohistochemistry [21].
Here we developed a novel procedure, the escapable social interaction test (ESIT), to quantify social preference and escape behavior in PSI-exposed rats in response to a novel stimulus rat. The ESIT provides the first method to assess social interaction in rats that allows for social preference to be examined with experimental rat-induced social interactions similar to the social approach task (SA) [22,23] while simultaneously monitoring social behaviors as can be done with standard social interaction tasks or CPP. The SA is limited in this regard as the stimulus animal is confined to a cage, and while CPP allows for behavioral monitoring during training, the later testing phase prohibits harvesting tissue within relevant time frames to examine immediate early gene expression in response to the social behavior, which occurs within a timeframe between 1 and 2 hours [24]. Additionally, the use of “escapable” social interaction may provide a direct measure of social interaction-seeking behavior reflective of motivational drive in the absence of a learned association with a specific environment as in CPP, which may be confounding in ISO rats as PSI induces learning deficits [25]. Furthermore, the ESIT allows for the manipulation of the behavioral phenotype of the stimulus rat. Here, we manipulated the aggressiveness of the stimulus rat by exposure to either group or PSI rearing. We hypothesized that because of their social incompetence and increased motivation for social interaction, PSI rats would be more likely to spend time interacting with an aggressive stimulus rat than GRP rats would. We assessed escape behavior as well as social and aggressive behavior after either 1 or 3 trials with either a nonaggressive or an aggressive stimulus rat. Finally, we assessed the immediate early genes c-Fos and Arc, as well as PSD-95 in the anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) subdivisions of the mPFC.
2. Materials and Methods
2.1. Animals
Male (n = 88) Sprague-Dawley rats (Harlan; Indianapolis, IN) were purchased at postnatal day (P) 28 and were housed in standard Plexiglas cages either individually or in groups of 4 with food and water freely available in a 12:12 light:dark cycle. Isolated rats were exposed to the sight, sound, and smell of other rats in the colony room but were deprived of physical contact. Rats were weighed weekly but were not otherwise handled. Experimentation took place after 3 weeks of either isolation (ISO) or group (GRP) rearing, between days P49 and P52, a period corresponding to late adolescence [26]). Rats were run in squads of 16 per day; all groups were run in a counterbalanced design. Experiments occurred at the same time each day between 10:00 a.m. and 1:00 p.m. All experiments were carried out in accordance with the NIH best practices for animal use and approved by the University of Colorado Denver Institutional Animal Care and Use Committee and by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
2.2. Apparatus
The apparatus was in a testing room separate from the animal colony, under dim lighting, and maintained at 23°C. It consisted of two rooms, the interaction room and the escape room. The interaction room was 30 cm × 30 cm square with 35 cm high silver Plexiglas walls, standard black bar type flooring, (5 mm diameter spaced 1.5 cm apart) with an 8 cm × 8 cm doorway in the lower rear corner leading into the escape room. The escape room was 12 cm × 30 cm × 35 cm high with a black Plexiglas floor and black walls. A tether was mounted via swivels to the top corner of the experimental room opposite to the escape door via an Irwin Quick grip 4” clamp (Irwin). Cable ties (Jansco Products) were placed directly posterior to the front limbs of the stimulus rat, but caudal to the top of the rib cage, at the narrowest point roughly above the shoulder blades. The tie was loose enough to allow for normal breathing and full range of motion as well as allowing normal movement of the stimulus rat throughout the experimental room of the apparatus, but not the escape room. The experimental rat had freedom to roam throughout both rooms. The tether prevented access to the escape room by the stimulus rat, and thus the experimental rat had the option to escape social interaction by entering the escape room.
2.3. Stimulus rats
Stimulus rats were either isolation or group housed for 4 to 5 weeks; stimulus rats that were slightly older and larger than the experimental rats were used to simulate bullying. Because not all isolation-reared stimulus rats appeared to be more aggressive than group-reared rats, the behavior of the stimulus rats during the social encounters was assessed for aggressive grooming (aggressive grooming by the stimulus rat of the experimental rat) by a blinded experimenter; stimulus rats were then designated as Aggressive or Nonaggressive using a median split of their aggressive grooming values. The median value was 1.25 sec per trial; the mean of the Nonaggressive stimulus rats was 0.12 (± 0.038 SEM) sec per trial (most showed no aggressive grooming) and the mean of the Aggressive stimulus rats was 11.53 (± 1.36 SEM) sec per trial. Thus while it is clear that many aspects of the social behavior of the stimulus rats exposed to PSI were abnormal, stimulus rats were specifically assigned to groups based on aggressive grooming behavior. Thus while it is clear that many aspects of the social behavior of the stimulus rats exposed to PSI were abnormal, stimulus rats were specifically assigned to groups based on aggressive grooming behavior. Aggressive grooming was first identified by Grant and Mackintosh [27], and is distinguished from normal social grooming by an increased use of the teeth, pulling of fur, and its vigorousness. Hurst et al. [28] has defined it as “vigorous grooming by the experimental rat of the novel conspecific when it is standing, crouching, supine, or trying to escape”. Although aggressive grooming does not typically include biting or lateral displays, it has been called an agonistic behavior [29]. Stimulus rats were subject to a maximum of 8 trials over two experimental cohorts totaling 10 days. Whenever possible exposure of the experimental rat to the same stimulus rat was avoided.
2.4. Escapable social interaction test (ESIT)
On P49 experimental rats were handled for 2 min each. Experimental rats that were group housed were additionally briefly acclimated to a transfer cage after handling. The following day experimental rats were acclimated to the experimental apparatus for ten minutes. Stimulus rats were acclimated to the apparatus and tether for ten minutes on the two consecutive days prior to experimentation.
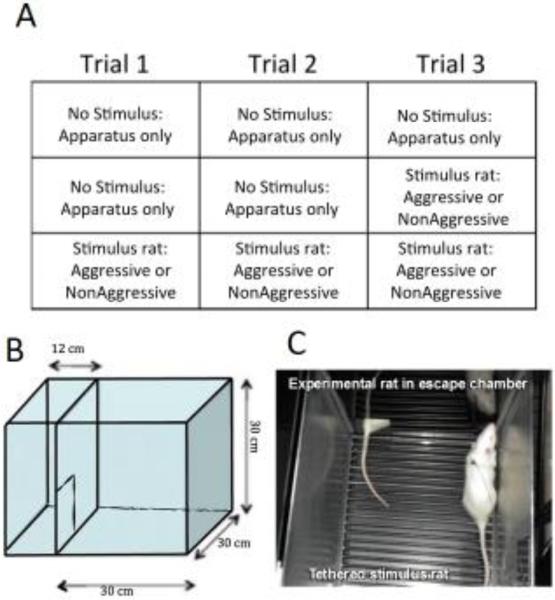
The ESIT began on P51 for the experimental rats. Testing sessions lasted 10 min, and there was one trial per day conducted 24 hours apart. All experimental rats were subject to 3 trials. All rats were exposed to the apparatus for each trial, with a tethered stimulus rat (either Aggressive or Nonaggressive) for either none of the trials (no-social control), the last trial only, or for all 3 trials. Rats were returned to their home cages immediately after each trial and were sacrificed 90–100 min after the final trial. This time point was chosen as an optimal time point to measure the protein products of activity-dependent genes based on the time of peak protein expression of the prototypical immediate-early gene, c-Fos [24]. The apparatus was washed with 70% alcohol between rats. The experimental design and apparatus are illustrated in Figure 1.
Figure 1.
The escapable social interaction test experimental design and apparatus. All rats were exposed to the apparatus for 3 trials, with either none of the trials, the last trial, or all 3 trials with a tethered stimulus rat that was either aggressive or non-aggressive (A). The apparatus consisted of 2 chambers, a larger social interaction chamber and a smaller escape chamber, separated by a door (B). In social trials the social interaction chamber contained a tethered stimulus rat that could not enter the escape chamber; the experimental rats had free access to the entire chamber (C).
Time spent in the escape chamber and number of entries into the escape chamber were recorded with AnyMaze software. Trials were video recorded and social behaviors were scored by experimenters blinded to the treatment conditions. The following behaviors were assessed: action: Overall time the experimental rat spends actively interacting (e.g. sniffing, following, grooming) with the novel conspecific [30]. Aggressive Grooming: Vigorous grooming by the experimental rat of the novel conspecific when it is standing, crouching, supine, or trying to escape [28]. Boxing: the experimental rat assumes an upright stereotyped boxing posture with orientation toward the introduced animal [31]. Pinning: Standing over/holding down the novel conspecific while it is in a supine posture [28].
2.5. Tissue harvest
Rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with cold 0.9% saline followed by 4% paraformaldehyde in 0.01M PBS. Brains were postfixed for 4 hours in the same paraformaldehyde solution, cryoprotected in 30% sucrose for three days, then rapidly frozen in −30°C isopentane just prior to cryosectioning. Sections were taken (40 μm) through the mPFC using the atlas of Paxinos and Watson [32]. Sections were stored at 4°C in cryoprotectant until immunohistochemistry was performed.
2.6. Immunohistochemistry
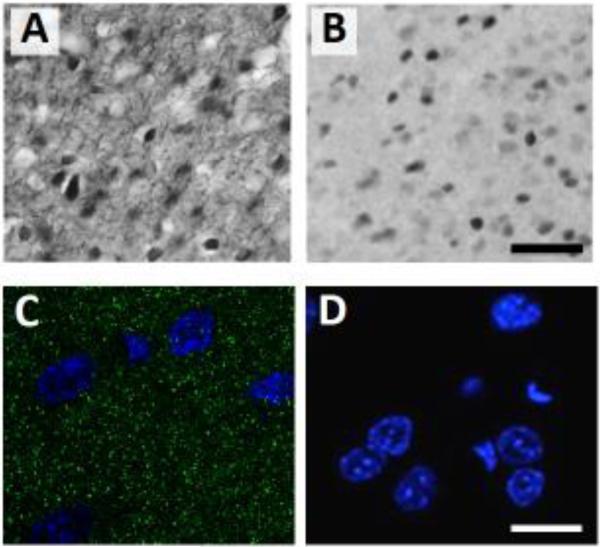
Immunohistochemistry was performed for c-Fos and Arc as previously reported [7]. Free-floating sections were first washed 3 times in 0.01 M PBS and between each subsequent step except as noted. Sections were incubated for in 0.3% hydrogen peroxide, followed by 5% normal goat serum (NGS) and 0.25% Triton X in PBS. Sections were incubated overnight at RT in either rabbit anti-Fos (1:10,000, Santa Cruz Biotechnology) or in rabbit anti-Arc, (1:3000, Synaptic Systems), in PBS with 5% NGS and 0.25% Triton X. Sections were then incubated in biotinylated goat anti-rabbit secondary antibody (1:200, Jackson Labs) for 2 h, followed by incubation in avidin biotin complex (ABC kit, Vector Laboratories) for 2 h. Sections were washed 3 times in 0.1 M PB, then immunoreactivity was visualized with 3,3’-diaminobenzidine (DAB substrate kit, Vector Laboratories) and nickel ammonium sulphate as chromogens. Sections were mounted on slides using a 0.15% gelatin solution, dehydrated using a series of ethanol solutions, defatted using Histoclear (Sigma-Aldrich), and coverslipped with Permount (Sigma-Aldrich). Representative photomicrographs are shown in Figure 2.
Figure 2.
Representative photomicrographs of immunohistochemistry in the PL showing light microscope images of Arc (A) and c-Fos (B), and a confocal image of PSD-95 (C, green punctae are PSD-95, blue is DAPI). A no-primary control assay was performed with goat anti-mouse Alexa-Flour 488 secondary antibody and DAPI (D). The scale bars are 50 μm (A) and 10 μm (B).
Fluorescent immunohistochemistry for PSD-95 was performed as follows. Sections (6-8 per rat) were washed in 0.01 M PBS 3 times for 10 minutes. Samples were incubated in 5% NGS solution with 0.25% Triton X in PBS for 1 hour at room temperature then incubated for 48 hours at 4°C in mouse anti-PSD-95 mAb antibody (1:1000, Calbiochem) in 5% blocking solution. Sections were then washed in 0.01 M PBS 3 times for 10 minutes. The remaining steps were performed in low light to help prevent bleaching of the fluorescent antibodies. Sections were incubated for 2 hours in goat anti-mouse Alexa-Flour 488 secondary antibody (1:200, Molecular Probes) in 5% blocking solution. Samples were washed in 0.01 M PBS 3 times for 10 minutes then mounted on Superfrost slides and coverslipped with Vectashield hard-set mounting medium with DAPI (Vector Labs). Representative photomicrographs are shown in Figure 2.
2.7. Cell/puncta counts
2.7.1
Microscopy for Arc and c-Fos was performed using an Olympus BX51 microscope and VisioPharm software (VisioPharm, Hørsholm, Denmark). Immunolabeled cells were counted by centering a counting frame within each subregion at 10x then shifting to a 40x objective; cell counts were performed within the 22,406 μm2 counting frame in real-time throughout the z plane as previously reported [33]. Cell counts were performed in the anterior cingulate (AC), prelimbic (PL), and infralimbic (IL) subregions of the mPFC. For each rat, 6-8 individual hemisphere (both right and left) measurements were assessed.
2.7.2
Microscopy for PSD-95 was performed using a Zeiss Observer inverted confocal microscope with ZEN 2011 Laser Scanning Microscope software and a Plan-Apochromat 100x/1.40 Oil DIC M27 objective set at an aperture of 0.55. For each rat, four brain hemispheres were selected for imaging, and three images were collected per subregion. Frame positioning within each subregion was accomplished through the use of the EC Plan-Neofluar10x/0.30 M27 objective. An image was acquired within the center of each subregion, then the frame was shifted 70μm in the horizontal direction both laterally and medially to capture a total of 3 images/hemisphere/subregion; puncta counts were averaged across region and hemisphere. Quantification of PSD-95 punctae was performed from jpg image files using ImageJ 1.47v software (http://imagej.nih.gov/ij/). A plug-in was designed to perform both a broad pixel count that determined the numbers of PSD-95 punctae between 5.0-100.0 pixels and a size analysis of the punctae. Punctae were designated as small (less than or equal to 10 pixels), medium (between 11 and 25 pixels) or large (greater than 25 pixels). There were no significant differences between medium and large punctae in any group so they were pooled and titled “large”.
2.8. Statistics
Although all groups were run together in a counterbalanced fashion, behavioral data from the different groups were analyzed separately due to the different number of trials with a stimulus rat (the variable of interest). Escape data from No-Social controls were analyzed using 2 by 3 mixed ANOVA with Housing (Group or ISO) as the between groups variable and Trials as the within subjects variable. Escape and behavioral data from rats that were exposed to 1 trial of ESIT were analyzed using 2 by 2 factorial ANOVA with Housing and Stimulus type (Aggressive or NonAggressive) as variables; only data from the trial with the stimulus rat were analyzed. Escape and behavioral data from rats that were exposed to 3 trials of ESIT were analyzed using mixed ANOVA with Housing and Stimulus type as between groups variables and Trials as a within subjects variable. Immunohistochemistry data were analyzed separately for No-Social controls using one-way ANOVA with Housing (GRP or ISO) as the between groups variable. Immunohistochemistry data from rats exposed to 1 or 3 trials of ESIT were normalized as percent of their respectively housed No-Social controls (GRP or ISO) to reduce variability and were analyzed using 3-way factorial ANOVA with Housing, Stimulus type, and number of trials as variables. When significant interactions were obtained, Tukey's HSD post-hoc tests were performed to determine differences between groups. Alpha was set at .05. Statistical analyses were performed using Statview (SAS Institute) or SPSS (IBM SPSS Version 22, Chicago, IL).
3. Results
3.1. Escape behavior
We assessed Time spent in the escape chamber, Time per entry into the escape chamber, and Number of entries into the escape chamber separately for rats that were exposed to a stimulus rat for 0 (No Social controls), 1, or 3 trials.
3.1.1. No-Social controls
We first assessed time spent in the escape chamber, number of entries, and time per entry in rats that were exposed to the apparatus with no stimulus rat (No Social controls). This was done to determine if any differences between experimental groups were present in baseline preference for the two chambers of our apparatus. Although there was a trend for a main effect of Housing on time per entry (F [1, 28] = 4.29, p = 0.06; ISO rats spent somewhat less time per entry than GRP rats), we found no significant differences between Housing groups in either time spent in in the escape chamber, or in number of entries. Additionally, there were also no main effects or interactions for Trials. Together, these results demonstrate that GRP and ISO rats showed similar “escape behavior” in the absence of a social cue, and indicate that the experimental design is non-biased in regards to baseline preference. Escape behavior data for no-social controls, collapsed across trials, are shown in Table 1.
Table 1.
Escape behavior during exposure to 3 trials of the apparatus (no-social controls). Values are means ± SEMs. There were no significant differences between groups, although there was a trend for an effect of Housing on Time per escape (p = .06). There were no significant differences between trials, thus data are collapsed across trials.
| Group | Isolate | |
|---|---|---|
| 218.11 | ||
| Time spent in escape chamber (sec) | 250.65 (9.66) | |
| (14.54) | ||
| Time per escape (sec) | 20.24 (1.24) | 16.09 (1.12) |
| Entries into escape chamber | 12.88 (0.51) | 14.54 (0.89) |
3.1.2. One trial of escapable social interaction
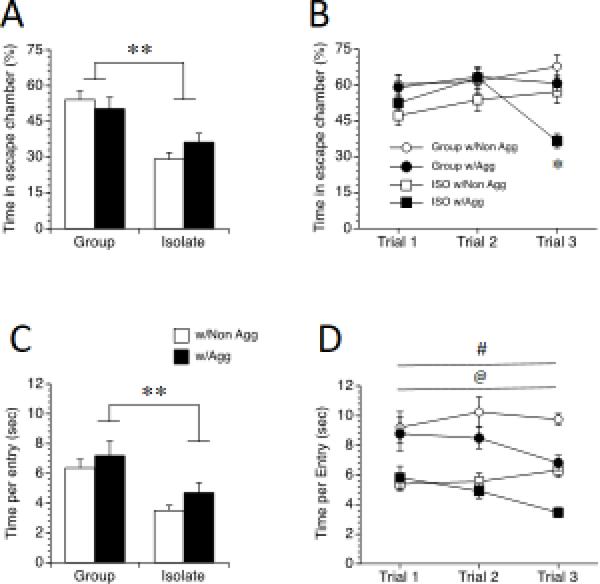
In the second condition, rats were exposed to the apparatus for two trials with no social cue, followed by one trial with an Aggressive or Non-aggressive stimulus rat (One-trial groups). Not surprisingly, ISO rats spent less time escaping the social situation than did their GRP counterparts, as revealed by a significant main effect of Housing, F(1, 32) = 44.51, p < 0.001 (Figure 3A); this was independent of the stimulus rat condition (no main effect of Stimulus). This difference was due to differences in total time per entry, as revealed by a significant main effect of housing, F(1, 32) = 14.94, p < 0.001 (Figure 3C), and not the number of entries to the escape chamber (no main effects or interactions for number of entries; data not shown). Thus, while the number of escapes did not differ between ISO and GRP rats in the one-trial condition, the time spent in the escape chamber (time between interactions) was greatly decreased for ISO rats.
Figure 3.
Escape behavior of rats in the escapable social interaction test. Rats were exposed to post-weaning group housing (GRP) or social isolation (ISO), then either 1 trial (left) or 3 trials (right) of escapable social interaction with a non-aggressive or an aggressive stimulus rat. Values are means ± SEMs. ** p < 0.01, significant difference between Group and Isolate rats. # p < 0.05, significant difference between Group and Isolate rats. @ p < 0.05, significant difference between rats with Aggressive and Non-Aggressive stimulus rats. * p < 0.05, Isolates with an Aggressive stimulus rat spent less time escaping than all other groups during Trial 3.
3.1.3. Three trials of escapable social interaction
In the third condition, rats were exposed to a social cue on all three trials (either Aggressive or Non-aggressive for all trials). Similar to the results from the one trial condition, overall, ISO rats spent less time in the escape chamber, as revealed by a significant main effect of Housing, F (1, 64) = 13.55, p < 0.01 (Figure 3B). All rats also spent less time in the escape chamber when the stimulus rat was Aggressive across trials, as revealed by a significant main effect of Stimulus, F (1, 64) = 5.91, p < 0.05. However, ISO rats with Aggressive stimulus rats spent less time escaping than all other groups in trial 3, as revealed by a significant Housing by Stimulus by Trial interaction, F (2, 64) = 5.18, p < 0.05 post-hoc test p < 0.05, suggesting a greater adaptation to the Aggressive stimulus condition across trials.
Similarly to what was noted in the one-trial condition, the differences in escape behavior between GRP and ISO rats in the three trial condition were due in part to differences in time per entry, with ISO rats spending less time per entry in the escape chamber than their GRP counterparts, as revealed by a significant main effect of Housing, F (1, 64) = 60.32, p < 0.001 (Figure 3D). Similarly, all rats spent less time per entry in the escape chamber when the stimulus rat was Aggressive, as revealed by a significant main effect of Stimulus, F (1, 64) = 8.53, p < 0.01. However, although ISO rats spent less total time escaping Aggressive stimulus rats in the third trial when compared to all other groups, all rats spent less time per entry in the escape chamber during trial 3 when the stimulus rat was Aggressive, as revealed by a Trials by Stimulus interaction, F (2, 64) = 5.15, p < 0.01, post-hoc test p < 0.05.
3.2. Social behavior
In all trials with a stimulus rat, we assessed total social interaction, aggressive grooming, boxing, and pinning. Time (sec) was used as the dependent measure for social interaction and aggressive grooming as they are typically ongoing behaviors, and frequency was used as the dependent measure for boxing and pinning as they are discrete behaviors that are brief in duration.
3.2.1. One trial of escapable social interaction
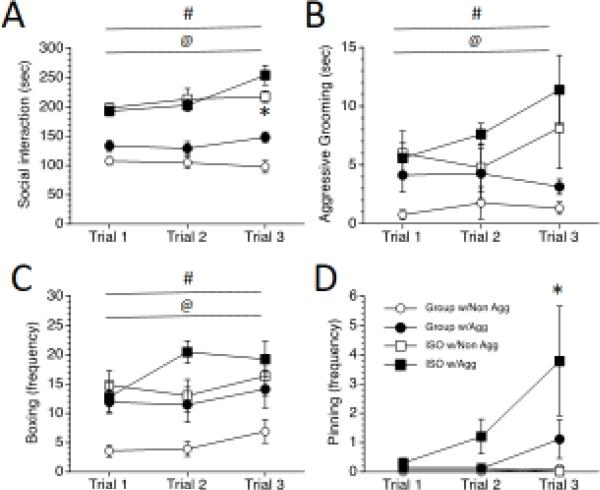
Overall, in the one-trial condition, housing had a profound effect on the assessed social behaviors. ISO rats engaged in more total social interaction, as revealed by a significant main effect of Housing, F (1, 32) = 79.33, p < 0.001 (Figure 4A), more aggressive grooming, as revealed by a significant main effect of Housing, F (1, 32) = 41.79, p < 0.001 (Figure 4B), and more boxing than GRP rats, as revealed by a significant main effect of Housing, F (1, 32) = 21.68, p < 0.001 (Figure 4C). However, while pinning did not differ between ISO and GRP rats (no main effect of housing), there was a tendency for all rats to pin Aggressive stimulus rats more, as revealed by a trend for a main effect of Stimulus, F (1, 32) = 2.25, p = 0.09 (Figure 4D).
Figure 4.
Social behavior during 1 trial in the escapable social interaction test. Rats were exposed to post-weaning group housing (GRP) or social isolation (ISO), then 1 trial of escapable social interaction with a non-aggressive or an aggressive stimulus rat. ** p < 0.01, significant difference between GRP and ISO rats. Values are means ± SEMs.
3.2.2. Three trials of escapable social interaction
For the three-trials condition, both Housing and Stimulus type affected social behaviors, and in some cases these behaviors changed across the 3 trials. As in the one-trial condition, ISO rats spent more time interacting with a stimulus rat across trials, as revealed by a significant main effect of Housing, F (1, 64) = 124.54, p < 0.0001 (Figure 5A). All rats also spent more time interacting with an Aggressive stimulus rat across trials, as revealed by a significant main effect of Stimulus, F (1, 64) = 7.43, p = 0.01. Moreover, the propensity to engage more with an Aggressive stimulus rat was further perpetuated in the third trial, as revealed by a significant Trials by Stimulus interaction, F (2, 64) = 5.41, p < 0.05, post-hoc test p < 0.05. However, despite the overall increase in social interaction seen in the third trial for all rats, ISO rats still spent more time interacting with a stimulus rat during the third trial than GRP rats, as revealed by a significant Trials by Housing interaction, F (2, 64) = 7.82, p < 0.05, post-hoc test p < 0.05.
Figure 5.
Social behavior during 3 trials in the escapable social interaction test. Rats were exposed to post-weaning group housing (GRP) or social isolation (ISO), then 3 trials of escapable social interaction with an aggressive or a non-aggressive stimulus rat. # p < 0.05, significant difference between GRP and ISO rats. @ p < 0.05, significant difference between rats with Aggressive and Non-Aggressive stimulus rats. * p < 0.05, Significant differences between rats with Aggressive and Non-Aggressive stimulus rats during Trial 3.
In accordance with what we have noted previously [7,34], in addition to an increase in total interaction, ISO rats also spent more time engaged in aggressive grooming behavior than did GRP rats, as revealed by a significant main effect of Housing, F (1, 64) = 16.23, p < 0.001 (Figure 5B). ISO rats also engaged more than GRP rats in the other aggressive behaviors, as revealed by significant main effects of Housing on boxing F (1, 64) = 15.47, p < 0.001 (Figure 5C), and pinning F (1, 64) = 3.88, p < 0.05 (Figure 5D). Thus, the increase in total interaction noted in ISO rats also included an increase in aggressive behaviors.
Interestingly, when paired with an Aggressive stimulus rat, both GRP and ISO experimental rats had tendencies to engage more in these aggressive behaviors, and some aggressive behaviors increased over trials. When paired with an Aggressive stimulus rat, there was a strong trend to engage in more aggressive grooming, as revealed by a marginal main effect of Stimulus, F (1, 64) = 4.11, p = 0.05, there were more bouts of boxing as revealed by a significant main effect of Stimulus, F (1, 64) = 7.63, p < 0.01, and there was more pinning, as revealed by a significant main effect of Stimulus, F (1, 64) = 7.33, p = 0.01. Additionally, there was a tendency for rats to engage in increased bouts of boxing over trials, as revealed by a marginal main effect of Trials on boxing, F (2, 64) = 3.08, p = 0.05, and pinning of Aggressive stimulus rats was greatest during the third trial, as revealed by a significant Trials by Stimulus interaction, F (2, 64) = 3.96, p < 0.05, post-hoc test p < 0.05. Thus, both the condition of the stimulus rat and the number of trials affected aggression by the experimental rat, with rats tending to engage in more aggressive behaviors when paired with an Aggressive stimulus rat, and with bouts of boxing and pinning increasing over trials.
3.3. Immediate early gene and PSD-95 expression in the mPFC
3.3.1. No-social controls
Separate ANOVAs were performed for Arc, c-Fos, and PSD-95 in the AC, PL, and IL of the mPFC of animals exposed to the apparatus for 3 trials. There were no effects of Housing on Arc, c-Fos, or PSD-95 expression in any of the subregions; data are shown in Table 2.
Table 2.
Expression of ARC, c-Fos, and PSD-95 (large punctae) in mPFC subregions of Group and Isolation reared rats exposed to 3 trials of the apparatus (no-social controls). Values are means ± SEMs of counts/mm2. There were no significant differences between groups.
| Protein | Anterior Cingulate | Prelimbic | Infralimbic | |||
|---|---|---|---|---|---|---|
| Group | Isolation | Group | Isolation | Group | Isolation | |
| ARC | 94.3 (20.3) | 100.7 (12.2) | 35.2 (5.3) | 27.9 (8.0) | 15.3 (7.7) | 15.1 (2.9) |
| c-Fos | 30.1 (6.2) | 38.9 (7.9) | 24.9 (7.3) | 36.8 (23.0) | 18.5 (12.2) | 13.7 (8.5) |
| PSD-95 | 367.8 (91.0) | 498.2 (122.7) | 148.11 (76.2) | 126.3 (92.9) | 131.8 (128.6) | 140.2 (111.5) |
3.3.2. Escapable social interaction
Values were expressed as a percentage of No-Social controls by the appropriate Housing condition (thus GRP rats were normalized by GRP controls and ISO rats were normalized by ISO controls). Two animals sustained tissue damage during processing and were not usable for immunohistochemistry.
3.3.3. Arc
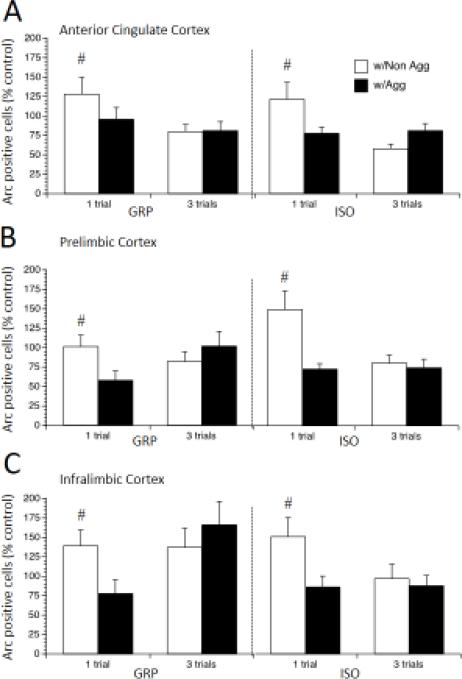
Arc is an immediate early gene often used as a measure of neuronal activation [35], and here we assessed neuronal activation in the AC, PL, and IL subdivisions of the mPFC of experimental rats exposed to the ESIT. Interestingly, the aggressiveness of the stimulus rat, but not the experimental rat's housing condition, impacted Arc immunoreactivity (there were no main effects or interactions for Housing). Arc positive cell numbers were greater overall in the AC in the one-trial condition, as revealed by significant main effect of Trials, F (1, 62) = 10.3, p < 0.01 (Figure 6A), and this was driven by an increase in rats exposed to a Nonaggressive stimulus rat, as revealed by a significant Trials by Stimulus interaction, F (1, 62) = 6.9, p < 0.01. There was also an increase in Arc positive cells in the PL of rats exposed to a Nonaggressive stimulus rat, as revealed by a significant main effect of Stimulus F (1, 62) = 7.1, p < 0.01; this was driven by the increase in the one-trial condition, as revealed by a significant Trials by Stimulus interaction, F (1, 62) = 11.25, p < 0.01, post-hoc test p < 0.05 (Figure 6B). Similarly, Arc positive cells were increased in the IL of rats in the one-trial condition, as revealed by a significant Trials by Stimulus interaction, F (1, 62) = 6.15, p < 0.05, post-hoc test p < 0.05 (Figure 6C). Therefore, all mPFC sub-regions showed an increase in Arc positive cells in response to a Non-aggressive stimulus rat in the one-trial condition, which was no longer noted when the experimental rat was exposed to three trials.
Figure 6.
Arc expression in subregions of the mPFC in rats exposed to post-weaning group housing (GRP) or social isolation (ISO) then 1 or 3 trials of escapable social interaction with an aggressive or a non-aggressive stimulus rat. Values are expressed as percentage of no-social controls (means ± SEMs). # p < 0.05, significant difference between rats with Aggressive and Non-Aggressive stimulus rats after 1 trial of escapable social interaction.
3.3.4. c-Fos
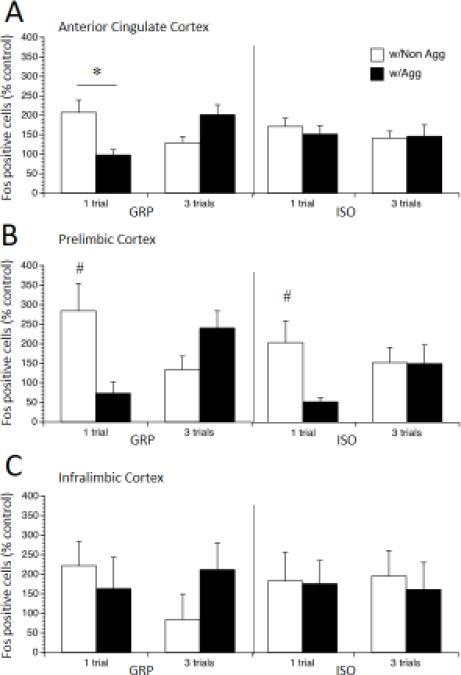
The immediate early gene c-Fos is also commonly used as a measure of neuronal activity [36], and we next sought to further corroborate our results with Arc by assessing c-Fos immuno-reactivity also in the AC, PL, and IL of the mPFC of rats exposed to the ESIT. In contrast to Arc, in which there were no effects of Housing on the increase in immuno-reactive cells in the AC in response to a single trial, the number of c-Fos positive cells in the AC was increased in GRP but not ISO rats exposed to 1 trial with a Nonaggressive stimulus rat as revealed by a significant Housing by Trials by Stimulus interaction, F (1, 62) = 5.27, p < 0.05, post-hoc test p < 0.05 (Figure 7A). In the PL, however, a similar effect was seen with c-Fos as was seen with Arc. Immuno-reactivity to c-Fos was increased in response to a Nonaggressive stimulus rat, as revealed by a significant main effect of Stimulus, F (1, 62) = 7.66, p < 0.01; this was also driven by a significant increase in the one-trial condition, as revealed by a significant Trials by Stimulus interaction, F (1, 62) = 19.06, p < 0.001, post-hoc test p < 0.05. However, in the PL, Housing had additional effects on c-Fos in the 3-trial condition not observed with Arc. Exposure to one trial with a Nonaggressive stimulus rat produced increased c-Fos expression in both GRP and ISO rats, while 3 trials with an Aggressive rat marginally increased c-Fos expression in GRP rats and had no effect on c-Fos expression in ISO rats, as revealed by a significant Housing by Trials by Stimulus interaction, F (1, 62) = 4.31, p < 0.05, post-hoc test p < 0.05 (Figure 7B). Immunoreactivity to c-Fos did not differ in the IL (no main effects or interactions, Figure 7C). Thus, while the trend toward increased immediate early gene expression in response to one-trial with a Non-aggressive stimulus was similar for c-Fos and Arc, our analysis of c-Fos revealed additional effects of Housing on neuronal activation in the AC, in which GRP but not ISO rats showed an increase in c-Fos in response to a Non-aggressive stimulus. Additionally, in the PL we noted additional effects of multiple trials, which were also housing dependent. However, in contrast to Arc expression, no differences in c-Fos expression reached significance in the IL.
Figure 7.
Fos expression in subregions of the mPFC in rats exposed to post-weaning group housing (GRP) or social isolation (ISO), then 1 or 3 trials of escapable social interaction with an aggressive or a non-aggressive stimulus rat. Values are expressed as percentage of no-social controls (means ± SEMs). # p < 0.05, significant difference between rats with Aggressive and Non-Aggressive stimulus rats after 1 trial of escapable social interaction. * p < 0.05, ISO rats with Aggressive stimulus rats significantly different than those with Non-aggressive stimulus rats after 3 trials of escapable social interaction.
PSD-95
While the immediate early genes Arc and c-Fos measure gross changes in neuronal activity and can thus be used to indicate overall network changes, here we sought to also evaluate synaptic changes indicative of learning and memory by examining the excitatory synapse scaffolding protein PSD-95. We examined the AC, PL and IL of the mPFC of the rats exposed to the ESIT. We found no changes in small PSD-95 punctae in any of the subregions (data not shown); in addition, we found no changes in large PSD-95 Punctae in the AC (Figure 8A). Interestingly, however, we found an increase in the number of large PSD-95 punctae in both the PL (Figure 8B) and IL (Figure 8C) of ISO but not GRP rats in response to interaction with an Aggressive stimulus rat, as revealed by significant Housing by Stimulus interactions in the PL, F (1, 62) = 7.99, p < 0.01 and IL, F (1, 62) = 6.62, p < 0.05, both post-hoc test p < 0.05. These results are in contrast to the expression of Arc and c-Fos, in which we found no group differences in the three-trial condition and therefore suggests an uncoupling of gross neuronal activity from the changes that occur at excitatory synapses.
Figure 8.
PSD-95 expression in subregions of the mPFC in rats exposed to post-weaning group housing (GRP) or social isolation (ISO), then 1 or 3 trials of escapable social interaction with an aggressive or a non-aggressive rat. Values are expressed as percentage of no-social controls (means ± SEMs). # p < 0.05, ISO rats with Aggressive stimulus rats significantly different than those with Non-aggressive stimulus rats.
4. Discussion
In the present study, a novel social interaction test, the ESIT, has been introduced. The ESIT is the first social test that allows for experimental rat-controlled social interaction, quantification of normal social behavior, and social motivation for a stimulus rat, while also providing a method for post-test detection of immediate early gene expression in response to the social interaction. A novel aspect of the ESIT is the ability to assess active avoidance of a social interaction, which may reflect social anxiety more accurately than the standard social interaction test. Rats that were exposed to either post-weaning social isolation or normal group housing were exposed to the ESIT apparatus for 3 trials; in either none of the trials, the last trial only, or all 3 trials the apparatus contained a stimulus rat that was either Aggressive or Non-aggressive. The results demonstrated that characteristics of both the experimental rat and the stimulus rat affected the motivation to escape a partner rat, and influenced the specific types of social behaviors that the rats engaged in. Moreover, mPFC function was sensitive to characteristics of the stimulus rat as well as the experimental rat.
4.1. Escape behavior
We assessed the amount of time that experimental rats spent in the escape chamber, the number of entries into the escape chamber, and the amount of time spent in the escape chamber per entry. Consistent with our prediction, ISO rats spent less time escaping from a social interaction than GRP rats during either 1 or 3 trials. A previous report demonstrated a decrease in social avoidance in ISO rats, but in that study both the experimental rat and the stimulus rat had free access to both sides of the two-chamber apparatus [37]. The present results are unique in that the ESIT paradigm is similar to those used in active avoidance; in line with this, male rats exposed to PSI were impaired in learning an active-avoidance task [38]. Interestingly, though we predicted that ISO rats would spend less time escaping an aggressive stimulus rat than GRP rats, we were surprised to observe that during the third trial, ISO rats escaped less from an aggressive stimulus rat than a nonaggressive one. ISO rats spent less time per entry than GRP rats, which may reflect an increase in locomotor behavior in ISO rats. Alternatively, this may represent hyper-reactivity to either the social stimulus or to the novel environment [39]. Evidence for hyper-reactivity to the novel environment is partially substantiated by a trend for a decrease in the amount of time per escape by ISO rats in the absence of a stimulus rat. However, the differences between GRP and ISO rats were profound when there was a stimulus rat present, and this held true for either 1 or 3 trials. This hyper-reactivity may be greater when the stimulus is more arousing. Time per entry decreased over the 3 trials when the stimulus rat was aggressive, regardless of the housing condition, suggesting that hyper-reactivity may occur in all rats as a result of the behavior of the stimulus rat, and that this may escalate over multiple trials.
4.2. Social behavior
Both housing condition and the aggressiveness of the stimulus rat increased social and aggressive behaviors, in particular during three trials of escapable social interaction. ISO rats engaged in more social interaction, aggressive grooming, and boxing, consistent with our earlier reports. We [7,10] and others [3,8,40] have previously observed PSI-induced increases in aggression, which may result from increased autonomic activation [8]. Interestingly, all rats engaged in more social interaction, aggressive grooming, and boxing when they were paired with aggressive stimulus rats, in parallel with decreases in escape behavior. We observed little pinning and less aggressive grooming than we have previously observed after PSI [7,10]. This may be due to the escapability of the social interaction, though there are a number of alternative explanations. The tethering of the stimulus rats may have made it more difficult for them to assume a supine position for pinning, and the tethers covered much of the nape area which is often the target in aggressive grooming. With respect to both aggressive grooming and pinning, the stimulus rats were slightly older and larger than the experimental rats and this may have discouraged these behaviors by the smaller experimental rats.
It is important to note that it can be difficult to distinguish between play and aggressive behavior at this age in the rat. Pinning and boxing may be considered as play fighting, rather than aggression. However, many of the behaviors that are manifested as play during adolescence can be interpreted as aggressive during adulthood, as “rough and tumble” social play serves as practice for behaviors that are aggressive in adults [41]. Playful attacks typically decline throughout puberty [42,43], suggesting that ISO rats may remain in an immature stage of social development. Play-like social interactions that vary too far from the expected behaviors are no longer considered playful [44] and may instead be interpreted as aggressive, thus provoking aggression from the experimental rats. Regardless of whether the interactions are agonistic or simply age-inappropriate, rats engaging in high levels of these behaviors may model social ineptness in adolescence. Recent clinical studies have confirmed that adolescents with poor social skills are much more likely to become victimized by their peers; victimization can include both social exclusion and overt bullying [45]. Olweus [46] has described the victims of bullying as socially isolated and anxious, but importantly noted that some victims become aggressive and provoke their victimizers. Thus a spiraling pattern of aggression and social exclusion can emerge. In support of this, in the current study aggressive acts appeared to be provoked by aggression by the stimulus rat, even when experimental rats were able to avoid the aggressor by escaping into a separate room. Escalation of the aggressive encounters was observed during exposure to multiple trials with an aggressive stimulus rat. In both GRP and ISO rats that had experienced 3 trials with an aggressive conspecific, aggressive grooming, boxing, and pinning increased in subsequent trials in comparison to the first trial (and to the 1-trial groups), in parallel with a decrease in escape. Consistent with this, when rats experienced only one trial with a conspecific, the aggressiveness of the stimulus rat did not impact the social or escape behavior of the experimental rat. Interestingly, aggressive interactions can be rewarding in a rodent model [47], and this may increase over trials as aggression escalates . Thus the level of aggression, and not just the amount of social interaction of the stimulus animal is an important factor that can modify social behaviors [4].
4.3. Immediate early gene and PSD-95 expression in subregions of the mPFC
Arc and Fos expression patterns within the AC, PL, and IL were similar, though not identical, and were dependent on both the experimental and the stimulus rat. In contrast to our previous report , we did not observe an attenuation of social interaction-induced Arc and Fos expression in the mPFC of ISO rats. It is possible that the escapability of the social interaction impacted the normal induction of Arc and Fos. Alternatively, multiple trials of habituation to the testing environment prior to the social interaction may have normalized the expression of immediate early gene protein products after PSI in the present study. In both GRP and ISO rats, a single trial with a nonaggressive stimulus rat produced elevated Arc levels in all subregions relative to 1 trial with an aggressive stimulus rat.
Increased Fos expression was most pronounced in the PL; after a single trial with a nonaggressive rat Fos was increased in the PL in both GRP and ISO rats. Although there was an increase in Fos in the PL of GRP rats exposed to three trials with an aggressive stimulus rat, this did not reach significance. In the AC a similar pattern was observed in GRP rats; a significant increase after 1 trial with a nonaggressive stimulus rat and a trend for an increase after 3 trials with an aggressive stimulus rat. Expression of Fos, as well as Arc, was greater after 1 trial than 3 trials, suggesting habituation of these responses; c-fos mRNA expression in the mPFC reflects habituation by decreasing over time in response to repeated stress [48]. Similarly, single-cell recording of neurons in the mPFC demonstrated that social approach activated a subset of these neurons during the first, but not the second session of social experience [49].
The mPFC may be important for regulating social interaction by facilitating interanimal coordination [50]; moreover, complex defensive behaviors may require an intact mPFC [51]. Impaired PFC function has been implicated in social dysfunction, including human violence and aggression. A study of blood oxygenation level dependent (BOLD) activation of the PFC of healthy adolescents and those with disruptive behavior disorders demonstrated that the modulation of PFC activation during retaliatory behavior observed in healthy adolescents was attenuated in adolescents with disruptive behavior disorders [52]. Greater activation of the dorsolateral PFC (the human analog to the rodent mPFC) was associated with less aggressive responding, suggesting that the PFC plays an important role in regulating social aggression [53]. A direct role for the mPFC in regulating aggressive behavior is provided by the finding that optogenetic stimulation of the mPFC inhibited escalated aggression in mice [54]; conversely, escalated aggression was increased by optogenetic silencing of the mPFC [54]. The mPFC is involved in the establishment of social dominance hierarchies [55], and dominant mice expressed greater amounts of c-Fos in the PL subregion of the mPFC [56]. This suggests that in the present study, the increased Fos in the PL after a single trial with a nonaggressive rat reflects an elevation in social rank when interacting with a nonaggressive, compared to an aggressive, stimulus rat.
A marked dissociation was observed between the expression of large PSD-95 punctae and the immediate early gene protein products Arc and Fos, effectively uncoupling changes in neuronal activity with lasting structural changes at dendritic spines. Although no effects of a social experience were observed on PSD-95 in the AC, increases in large PSD-95 punctae were observed in the PL and IL of ISO rats after 3 trials with an aggressive stimulus rat. Our results contrast with those of Hermes et al. [19], who reported decreased PSD-95 protein in the mPFC of isolates. However, there were several differences between the current experimental procedures and those of Hermes et al. [19]. First, the period of isolation was much longer in the Hermes et. al. study, from P19 to P73, compared to P28 to P52 in the current study, and longer lasting isolation has been accompanied by learning deficits [25] and amotivation for social interaction [57]. Second, the study of Hermes et al. used female rats, which could indicate sex differences in the effects of isolation rearing on mPFC PSD-95. In support of this we have previously published differences in social reward between male and female rats [10]. Third, in the Hermes et al., [19] study total protein expression was assessed using Western blot. PSD-95 clustering at the post-synaptic density is regulated in response to neuronal activity changes [58], and it is possible that the expression levels assessed by western blot may not accurately reflect the changes in PSD-95 occurring at individual spines that can be observed using IHC.
PSD-95 is an excitatory post-synaptic scaffolding protein that regulates synaptic strength through the anchoring of glutamate receptors in the post-synaptic density, and accordingly its expression, degradation, and localization is highly regulated in vitro by changes in neuronal activity as well as in vivo by experience [59,60]. As dendritic spine size is proportional to PSD area [61], it is likely that the increase in large PSD-95 punctae observed here represent an increase in dendritic spine size consistent with structural changes associated with learning [62]. Therefore the alterations in PSD-95 punctae in the ventral mPFC may reflect differences in social learning in ISO rats, consistent with the increased time spent in social interaction with aggressive rats by ISO rats.
Consistent with a role in the regulation of synaptic homeostasis, Arc may be important for synaptic stability [63], rather than increased synaptic strength, which may be more associated with PSD-95 expression [64]. It is worth noting that alterations in Arc and PSD-95 expression are not always congruent; Abad et al. [65] have observed change in Arc, but not PSD-95 expression after MDMA. Interestingly, Arc-activated neurons were recently shown by Gruene et al. [66] to have reduced spine density after fear conditioning, suggesting that Arc may weaken inactive synapses in activated neurons [66]. Increased Arc expression has been shown to increase AMPA receptor endocytosis and reduce AMPA receptor-mediated excitability [67]. Thus, different patterns of Arc and PSD-95 expression observed here may reflect weakening of inactive synapses with normal social interactions, and synaptic strengthening during maladaptive social learning by ISO rats. The mPFC is emerging as a key structure regulating both memory consolidation [68] and social behavior [69], and in accordance with these two functional roles, mPFC dysfunction is noted a number of disorders that include social impairments, including schizophrenia, bi-polar disorder, and autism spectrum disorder [70–72]. It is thus important to note that the increase in large PSD-95 punctae in the mPFC of ISO rats noted here was in response to maladaptive social learning; i.e. spending more time interacting with an aggressive stimulus rat.
5. Conclusions
The ESIT allows for experimental rat-induced social encounters while additionally allowing normal social behaviors to be monitored. Moreover, escape behavior can be used to assess preference for social interaction without confounds of learning deficits that may affect other preference tasks including CPP. Using the ESIT, we found that immediate early gene expression and changes in large PSD-95 punctae in the mPFC as well as social motivation and social behaviors depended not only on the rearing condition of the experimental rat, but also on the aggressiveness of the stimulus rat. Furthermore, the results presented here have implications for our understanding of how social isolation may increase the likelihood of remaining in an abusive social situation.
Highlights.
Male rats were exposed to post-weaning social isolation or control conditions.
A novel escapable social interaction test was used to assess social behavior or escape.
Stimulus rats were either aggressive or non-aggressive.
Characteristics of both the experimental and stimulus rats determined social and escape behavior.
Characteristics of both the experimental and stimulus rats determined expression of immediate early gene and PSD-95 expression in the medial prefrontal cortex.
Acknowledgements
This work was supported by NIH Grant R15MH102717. Dayton Goodell and Jessica Baynard were supported by NIH BP-ENDURE undergraduate training grant R25GM097633.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- 1.Fone KCF, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents—Relevance to developmental neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Haller J, Harold G, Sandi C, Neumann ID. Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J. Neuroendocrinol. 2014;26:724–738. doi: 10.1111/jne.12182. [DOI] [PubMed] [Google Scholar]
- 3.Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav. Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- 4.Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol. Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa H, Arakawa K, Deak T. Acute illness induces the release of aversive odor cues from adult, but not prepubertal, male rats and suppresses social investigation by conspecifics. Behav. Neurosci. 2009;123:964–978. doi: 10.1037/a0017114. doi:10.1037/a0017114. [DOI] [PubMed] [Google Scholar]
- 6.Smith MA. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology (Berl.) 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall VL, Fischer EK, Bland ST. Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol. Behav. 2012;107:440–450. doi: 10.1016/j.physbeh.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toth M, Mikics E, Tulogdi A, Aliczki M, Haller J. Post-weaning social isolation induces abnormal forms of aggression in conjunction with increased glucocorticoid and autonomic stress responses. Horm. Behav. 2011;60:28–36. doi: 10.1016/j.yhbeh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, He S. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1173–1177. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Grotewold SK, Wall VL, Goodell DJ, Hayter C, Bland ST. Effects of cocaine combined with a social cue on conditioned place preference and nucleus accumbens monoamines after isolation rearing in rats. Psychopharmacology (Berl.) 2014;231:3041–3053. doi: 10.1007/s00213-014-3470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. doi:10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 12.Spear LP, Adolescent Neurodevelopment J. Adolesc. Health. 2013;52:S7–S13. doi: 10.1016/j.jadohealth.2012.05.006. doi:10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poldrack RA, Wagner AD, Ochsner KN, Gross JJ. Cognitive Emotion Regulation Insights From Social Cognitive and Affective Neuroscience. Curr. Dir. Psychol. Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. doi:10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins TW, Weinberger D, Taylor JG, Morris RG. Dissociating Executive Functions of the Prefrontal Cortex [and Discussion] Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. doi:10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 15.Barker ED, Séguin JR, White HR, Bates ME, Lacourse E, Carbonneau R, Tremblay RE. Developmental trajectories of male physical violence and theft: relations to neurocognitive performance. Arch. Gen. Psychiatry. 2007;64:592–599. doi: 10.1001/archpsyc.64.5.592. doi:10.1001/archpsyc.64.5.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav. Brain Res. 2007;180:174–182. doi: 10.1016/j.bbr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Levine JB, Youngs RM, MacDonald ML, Chu M, Leeder AD, Berthiaume F, Konradi C. Isolation rearing and hyperlocomotion are associated with reduced immediate early gene expression levels in the medial prefrontal cortex. Neuroscience. 2007;145:42–55. doi: 10.1016/j.neuroscience.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 18.Day-Wilson KM, Jones DNC, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141:1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. doi:10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 19.Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol. Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao CY, Sondhi R, van de Nes PS, Sacktor TC. PKMζ is necessary and sufficient for synaptic clustering of PSD-95. Hippocampus. 2011;22:1501–1507. doi: 10.1002/hipo.20996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forlano PM, Woolley CS. Quantitative analysis of pre-and postsynaptic sex differences in the nucleus accumbens. J. Comp. Neurol. 2010;518:1330–1348. doi: 10.1002/cne.22279. doi:10.1002/cne.22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. doi:10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 23.Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, Young NB, Nadler JJ, Moy SS, Young LJ, Caldwell HK, Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. doi:10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Kovács KJ. Measurement of immediate-early gene activation- c-fos and beyond. J. Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. doi:10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh AL, Ballard TM, Steward LJ, Moran PM, Fone KCF. The atypical antipsychotic risperidone reverses the recognition memory deficits induced by post-weaning social isolation in rats. Psychopharmacology (Berl.) 2013;228:31–42. doi: 10.1007/s00213-013-3011-2. doi:10.1007/s00213-013-3011-2. [DOI] [PubMed] [Google Scholar]
- 26.Saalfield J, Spear L. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev. Cogn. Neurosci. 2015;16:174–182. doi: 10.1016/j.dcn.2015.01.004. doi:10.1016/j.dcn.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant EC, Mackintosh JH. A Comparison of the Social Postures of Some Common Laboratory Rodents. Behaviour. 1963;21:246–259. doi:10.1163/156853963X00185. [Google Scholar]
- 28.Hurst J, Barnard C, Tolladay U, Nevision C, West C. Housing and welfare in laboratory rats: effects of cage stocking density and behavioural predictors of welfare. Anim. Behav. 1999;58:563–586. doi: 10.1006/anbe.1999.1165. doi:10.1006/anbe.1999.1165. [DOI] [PubMed] [Google Scholar]
- 29.Clipperton Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E. Agonistic behavior in males and females: effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 2010;35:1008–22. doi: 10.1016/j.psyneuen.2010.01.002. doi:10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.File SE, Seth P. A review of 25 years of the social interaction test. Eur. J. Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard RJ, Fukunaga K, Blanchard DC, Kelley MJ. Conspecific aggression in the laboratory rat. J. Comp. Physiol. Psychol. 1975;89:1204–1209. doi: 10.1037/h0077177. [DOI] [PubMed] [Google Scholar]
- 32.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition. Academic Press; 2006. [Google Scholar]
- 33.Ahern M, Goodell DJ, Adams J, Bland ST. Brain regional differences in social encounter-induced Fos expression in male and female rats after post-weaning social isolation. Brain Res. 2016;1630:120–133. doi: 10.1016/j.brainres.2015.11.006. doi:10.1016/j.brainres.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural Neural Projection Dynamics Underlying Social Behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinaud R. Experience-dependent immediate early gene expression in the adult central nervous system: evidence from enriched-environment studies. Int. J. Neurosci. 2004;114:321–333. doi: 10.1080/00207450490264142. doi:10.1080/00207450490264142. [DOI] [PubMed] [Google Scholar]
- 36.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 37.Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav. Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. doi:10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viveros MP, Hernandez R, Gallego A. Effects of social isolation and crowding upon active-avoidance performance in the rat. Anim. Learn. Behav. 18:90–96. n.d. doi:10.3758/BF03205243. [Google Scholar]
- 39.Gentsch C, Lichtsteiner M, Feer H. Behavioural comparisons between individually- and group-housed male rats: effects of novel environments and diurnal rhythm. Behav. Brain Res. 1982;6:93–100. doi: 10.1016/0166-4328(82)90084-5. [DOI] [PubMed] [Google Scholar]
- 40.Toth M, Halász J, Mikics E, Barsy B, Haller J. Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav. Neurosci. 2008;122:849–854. doi: 10.1037/0735-7044.122.4.849. [DOI] [PubMed] [Google Scholar]
- 41.Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall'Olio S, Fouts HN, Řeháková-Petrů M, Siviy SM, Pellis SM. Rough-and-tumble play as a window on animal communication. Biol. Rev. Camb. Philos. Soc. 2015 doi: 10.1111/brv.12172. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 42.Panksepp J. The ontogeny of play in rats. Dev. Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- 43.Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev. Psychobiol. 1990;23:215–31. doi: 10.1002/dev.420230303. doi:10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- 44.Pellis SM, Pellis VC. To whom the play signal is directed: a study of headshaking in black-handed spider monkeys (Ateles geoffroyi) J. Comp. Psychol. Wash. DC 1983. 2011;125:1–10. doi: 10.1037/a0020547. doi:10.1037/a0020547. [DOI] [PubMed] [Google Scholar]
- 45.Braddock HJBA, Twyman KA, Garrity MR, Wang T, Neary MK, Ezzelgot J. A Few Close Friends: The Pediatrician's Role in the Management of Social Skills Deficits in Adolescent Children. Clin. Pediatr. (Phila.) 2015;54:1192–11. doi: 10.1177/0009922815570619. [DOI] [PubMed] [Google Scholar]
- 46.Olweus D. Stability of aggressive reaction patterns in males: a review. Psychol. Bull. 1979;86:852–875. [PubMed] [Google Scholar]
- 47.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, Aleyasin H, Menard C, Zhang H, Hodes GE, Bregman D, Khibnik L, Tai J, Rebusi N, Krawitz B, Chaudhury D, Walsh JJ, Han M-H, Shapiro ML, Russo SJ. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. doi: 10.1038/nature18601. doi:10.1038/nature18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kearns RR, Spencer RL. An unexpected increase in restraint duration alters the expression of stress response habituation. Physiol. Behav. 2013;122:193–200. doi: 10.1016/j.physbeh.2013.03.029. doi:10.1016/j.physbeh.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee E, Rhim I, Lee JW, Ghim J-W, Lee S, Kim E, Jung MW. Enhanced Neuronal Activity in the Medial Prefrontal Cortex during Social Approach Behavior. J. Neurosci. 2016;36:6926–6936. doi: 10.1523/JNEUROSCI.0307-16.2016. doi:10.1523/JNEUROSCI.0307-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Himmler BT, Bell HC, Horwood L, Harker A, Kolb B, Pellis SM. The role of the medial prefrontal cortex in regulating interanimal coordination of movements. Behav. Neurosci. 2014;128:603–13. doi: 10.1037/bne0000010. doi:10.1037/bne0000010. [DOI] [PubMed] [Google Scholar]
- 51.Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The role of the medial prefrontal cortex in the play fighting of rats. Behav. Neurosci. 2009;123:1158–68. doi: 10.1037/a0017617. doi:10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- 52.White SF, VanTieghem M, Brislin SJ, Sypher I, Sinclair S, Pine DS, Hwang S, Blair RJR. Neural Correlates of the Propensity for Retaliatory Behavior in Youths With Disruptive Behavior Disorders. Am. J. Psychiatry. 2016;173:282–90. doi: 10.1176/appi.ajp.2015.15020250. doi:10.1176/appi.ajp.2015.15020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Achterberg M, van Duijvenvoorde ACK, Bakermans-Kranenburg MJ, Crone EA. Control your anger! The neural basis of aggression regulation in response to negative social feedback. Soc. Cogn. Affect. Neurosci. 2016 doi: 10.1093/scan/nsv154. doi:10.1093/scan/nsv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi A, Nagayasu K, Nishitani N, Kaneko S, Koide T. Control of intermale aggression by medial prefrontal cortex activation in the mouse. PloS One. 2014;9:e94657. doi: 10.1371/journal.pone.0094657. doi:10.1371/journal.pone.0094657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F, Kessels HW, Hu H. The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci. 2014;37:674–682. doi: 10.1016/j.tins.2014.07.005. doi:10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. doi:10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 57.Möller M, Du Preez JL, Viljoen FP, Berk M, Emsley R, Harvey BH. Social isolation rearing induces mitochondrial, immunological, neurochemical and behavioural deficits in rats, and is reversed by clozapine or N-acetyl cysteine. Brain. Behav. Immun. 2013;30:156–67. doi: 10.1016/j.bbi.2012.12.011. doi:10.1016/j.bbi.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 58.Vallejo D, Codocedo JF, Inestrosa NC. Posttranslational Modifications Regulate the Postsynaptic Localization of PSD-95. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-9745-1. doi:10.1007/s12035-016-9745-1. [DOI] [PubMed] [Google Scholar]
- 59.Okabe S, Kim HD, Miwa A, Kuriu T, Okado H. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat. Neurosci. 1999;2:804–811. doi: 10.1038/12175. doi:10.1038/12175. [DOI] [PubMed] [Google Scholar]
- 60.Gray NW, Weimer RM, Bureau I, Svoboda K. Rapid Redistribution of Synaptic PSD-95 in the Neocortex In Vivo. PLOS Biol. 2006;4:e370. doi: 10.1371/journal.pbio.0040370. doi:10.1371/journal.pbio.0040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. Off. J. Soc. Neurosci. 1989;9:2982–97. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bailey CH, Kandel ER, Harris KM. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb. Perspect. Biol. 2015;7:a021758. doi: 10.1101/cshperspect.a021758. doi:10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. doi:10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Béïque J-C, Lin D-T, Kang M-G, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc. Natl. Acad. Sci. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. doi:10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abad S, Camarasa J, Pubill D, Camins A, Escubedo E. Adaptive Plasticity in the Hippocampus of Young Mice Intermittently Exposed to MDMA Could Be the Origin of Memory Deficits. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9618-z. doi:10.1007/s12035-015-9618-z. [DOI] [PubMed] [Google Scholar]
- 66.Gruene T, Flick K, Rendall S, Cho JH, Gray J, Shansky R. Activity-dependent structural plasticity after aversive experiences in amygdala and auditory cortex pyramidal neurons. Neuroscience. 2016;328:157–164. doi: 10.1016/j.neuroscience.2016.04.045. doi:10.1016/j.neuroscience.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased Expression of the Immediate-Early Gene Arc/Arg3.1 Reduces AMPA Receptor-Mediated Synaptic Transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. doi:10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cohen MX. Hippocampal-prefrontal connectivity predicts midfrontal oscillations and long-term memory performance. Curr. Biol. CB. 2011;21:1900–1905. doi: 10.1016/j.cub.2011.09.036. doi:10.1016/j.cub.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 69.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. doi:10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res. Brain Res. Rev. 2000;31:138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 71.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. doi:10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Li M, Long C, Yang L. Hippocampal-prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. BioMed Res. Int. 2015;2015:810548. doi: 10.1155/2015/810548. doi:10.1155/2015/810548. [DOI] [PMC free article] [PubMed] [Google Scholar]