Abstract
The aim of this study was to investigate the intracellular responses associated with the acquisition and expression of cocaine-context associations. ERK (extracellular regulated kinase), CREB (cAMP responsive element binding protein), FosB and ΔFosB proteins were of particular interest due to their involvement in cocaine reward and in synaptic plasticity underlying learning and memory. We used the conditioned place preference (CPP) paradigm, which employs a Pavlovian conditioning procedure to establish an association between a drug-paired environment and the drug’s rewarding effects, to study the role of these signaling pathways in cocaine-context associations. N-methyl-D-aspartate receptor (NMDAR) antagonism prior to cocaine administration during conditioning blocked the acquisition of cocaine CPP and reduced Nucleus Accumbens (NAc) phosphorylated-ERK (pERK) and phosphorylated CREB (pCREB) levels following the CPP test (drug-free). We also show that cocaine-induced increases in Caudate Putamen (CPu) FosB and ΔFosB levels are decreased after MK-801 pre-treatment during conditioning. In addition, our results provide evidence for the involvement of striatal SIRT (Silent Information Regulator of Transcription) proteins in cocaine-CPP. These results will aid in the advancement of general knowledge about the molecular formation and retrieval of cocaine-associated memories that can be used in the future when designing treatments for cocaine addiction that target both prevention and relapse.
Keywords: Conditioned Place Preference, Cocaine, NMDARs, Nucleus Accumbens, Caudate Putamen
Introduction
Learned cocaine-environment associations play a major role in cocaine addiction and relapse. Cocaine increases synaptic concentrations of dopamine (DA) and glutamate, leading to the activation of molecular signaling cascades that cause functional changes in protein and gene expression and behavior [1] [2] [3]. The dorsal and ventral striatum, the caudate-putamen (CPu1) and nucleus accumbens (NAc) respectively, have been implicated in the regulation of habitual and reward-associated responses associated with addiction [4]. These brain regions undergo cocaine-induced neuroplastic changes in intracellular signaling similar to those underlying long-term memory processes [5] [6] [7]. For example, ERK, CREB and Fos proteins are signaling molecules that have been implicated in memory processes [5] [7] [8] [9] [10] [11] [12].
Activation of DA receptors (DARs) is required for the glutamatergic induction of LTP at synapses in the striatum [13]. The interaction between D1 DAR and the NR1 subunit of the NMDA receptor (NMDAR) forms a complex at striatal synapses and serves to maintain and strengthen synaptic activity in response to changes in synaptic DA concentrations [14]. Downstream, ERK, a signaling molecule of the MAPK signal transduction family, is phosphorylated after acute cocaine administration [8] [11] [12] [16]. The ERK pathway is an important regulator of phosphorylation of the transcription factor CREB and subsequent transcription [17] and much evidence supports a critical role of neuroadaptations produced by CREB and ERK signaling cascades in regulating synaptic plasticity through the alteration of gene activation [18] [19] [20]. Inhibition of ERK activation (pharmacologically or genetically) blocks cocaine CPP, indicating that the ERK pathway may be an essential requirement for the development of cocaine-associated memories [5] [11] [21] [22] [23] [24] .
Downstream of ERK and CREB signaling, SIRT1 and 2 (Silent Information Regulator of Transcription) are class III histone deacetylases (HDACs), recently implicated in the epigenetic changes underlying plasticity mechanisms including those associated with drug abuse [25]. Specifically, SIRTs are enzymes that play a role in the modification chromatin structure, which leads to long-term epigenetic changes in gene transcription and expression [26] [27]. Recent evidence suggests that an increase in SIRT1/2 subtypes enhances the rewarding effects of cocaine [25] and mediates drug-induced neuroplasticity. NAc SIRT 1 and 2 protein levels increase following cocaine administration [28] and Resveratrol (a SIRT1 and 2 agonist) increases the rewarding effects of cocaine [26]. Local inhibition of SIRT1 and SIRT2 in the NAc decreases cocaine reward exemplified by attenuated CPP [28]. Inhibition of SIRT1 has been found to decrease ERK phosphorylation and resveratrol increases ERK phosphorylation suggesting that SIRTs may play a role in regulating ERK activity [29]. SIRT activity may also regulate CREB phosphorylation and FosB overexpression after cocaine exposure [28] [30] [28] [31].
Given the extensive role of NMDARs in memory formation and cocaine CPP [32] [33] [34] [35] we aimed to investigate changes in NMDAR-dependent intracellular signaling cascades associated with cocaine-context associations. Specifically, we used the non-competitive NMDAR antagonist MK-801 to block the acquisition of a cocaine-environment association using a CPP model. We hypothesized that striatal ERK phosphorylation induced by cocaine is dependent on glutamate signaling through NMDARs and that MK-801 administration prior to cocaine administration would block CPP acquisition and subsequent ERK phosphorylation during the CPP expression test. We also expected to see similar NMDAR/ERK-dependent changes in pCREB, FosB, and SIRT1/2 protein levels.
2. Materials and Methods
2.1. Animals
Eight-week-old male Fischer rats (Charles River, Kingston, NY, USA) were individually housed in standard cages and maintained on a 12-hour light/dark cycle with free access to food and water. Behavioral testing took place during the light cycle. Rats were allowed to acclimate for 7 days before any experimental procedures began, and were handled once per day beginning 4 days prior to testing. Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, Bethesda, MD, USA) and approved by the Hunter College, CUNY, Institutional Animal Care and Use Committee.
2.2. Materials
2.2.1. Drugs and Antibodies
Cocaine hydrochloride and MK-801 were purchased from Sigma Chemical Co. (St. Louis, MO). Primary antibodies for pERK (9101), ERK (9102), SIRT1 (2493), SIRT2 (12672), FosB (5G4) and CREB (9197) were purchased from Cell Signaling Technologies (Beverly, MA). The primary antibody against pCREB (06–519) was purchased from Millipore (Billerica, MA, USA) and α-tubulin (sc-8035) was purchased from Santa Cruz Technologies (Santa Cruz, CA). Horseradish peroxidase-conjugated anti-rabbit (NA-934) and anti-mouse (NA-931) IgG were purchased from Amersham Phamacia (Piscataway, NJ).
2.2.2. CPP Apparatus
The place preference apparatus (purchased from Med Associates, Georgia, VT) previously described [7] [9] [36] consisted of a rectangular cage with three chambers: 2 square conditioning chambers (28cm in length) separated by a neutral rectangular chamber (12cm long and 4cm wide). The two conditioning chambers were differentiated by tactile and visual cues; in one, the floor was a stainless steel mesh and the walls were white, and in the other, the floor was made up of a grid of stainless steel rods and the walls were black. The middle chamber had grey walls and a smooth PVC floor. The chambers were separated by computer-automated guillotine doors, allowing free access among all three chambers during pre-test and preference testing. Locomotor responses were measured with a computerized photo-beam system and MED-PC software, which recorded time spent in each chamber, total locomotor behavior (sum of all horizontal counts), entrances into each chamber (multiple beams broken between two chambers) and exploratory behavior (a single broken beam between two chambers without entrance).
2.3. Procedures
2.3.1. CPP Procedure
After 4 days of handling, rats were placed into the neutral middle chamber of the CPP apparatus with the guillotine doors open and allowed to freely explore all three chambers for 15 minutes (pre-test). Rats were randomly assigned to one of three treatment groups as follows: saline/saline, saline/cocaine, or mk-801/cocaine treatment groups (n = 9–10 animals/group). Conditioning occurred over the next four days consisting of alternating drug/saline treatments on alternate days (2 cocaine/mk-801 treatments and 2 saline treatments on alternating days). On the first day of conditioning rats were pretreated with an i.p. injection of saline and received another saline injection 30 minutes later and were immediately confined to one of the conditioning chambers for 30 minutes. On the second day, rats were pretreated with i.p. injections of saline (0.9%) or MK-801 (.25 mg/kg) followed 30 minutes later by an i.p. injection of saline or cocaine (20 mg/kg) and immediately confined for 30 minutes to the chamber opposite from conditioning day one (counterbalanced so that half of the rats received cocaine in black and saline and white and vice versa). Control rats received both saline pretreatment and saline again 30 minutes later and were confined to alternating chambers on alternating days. CPP testing was conducted in a drug-free state the day after the last conditioning session and followed the same procedure as the preconditioning test.
2.3.2. Protein preparation
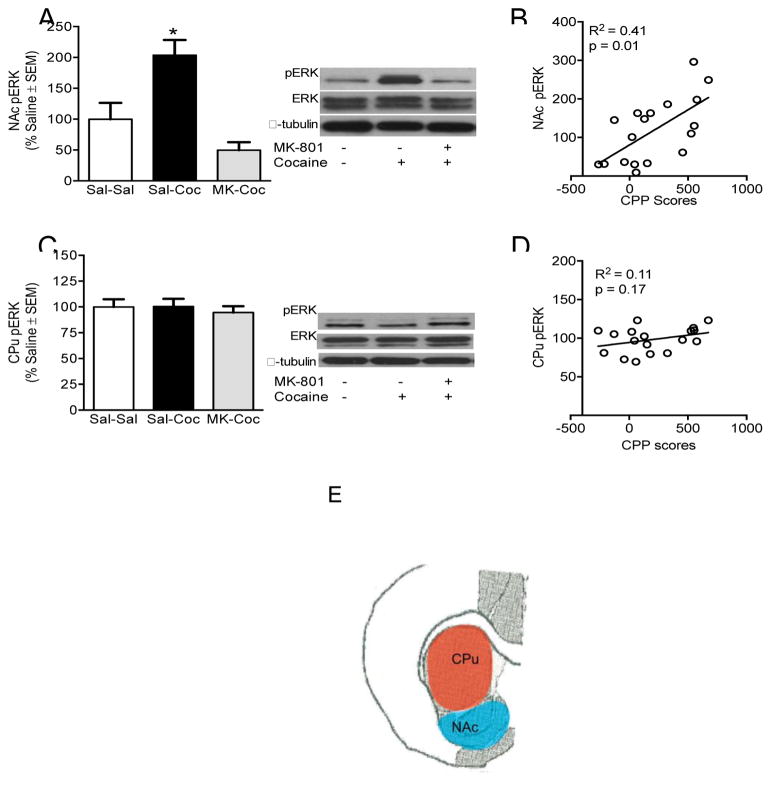
Immediately after the drug-free CPP test, rats were briefly exposed to CO2 (less than 30 seconds) and euthanized by rapid decapitation. Brains were removed and flash frozen in 2-methylbutane (−40 °C). Tissue punches of the NAc and CPu [Figure 3E, +2.0 to +1.8] were dissected out of each brain on a cold glass plate and homogenized with a Polytron handheld homogenizer (Kinematica, Luzern, Switzerland) in lysis buffer (50mM Tris-HCl, 150 mMNaCl, 2 mM EDTA, 10% Glycerol, 1% Triton X-100, 1% sodium deoxycholic acid) containing a phosphatase inhibitor cocktail. Homogenates were incubated for 30 minutes and then centrifuged for 15 minutes (13,000 rpm, 4°C). Supernatants were collected and stored at −80°C until used for western blot analysis.
Figure 3.
Phosphorylated ERK 1/2 (44/42 kDa) in the NAc and CPu. (A) NAc pERK protein levels are reduced after pre-treatment with MK-801 and (B) positively correlated to CPP scores. (C & D) CPu pERK protein levels and correlation to CPP scores. Phosphorylated protein levels are expressed as a ratio to their respective total protein levels and expressed as percentage of saline controls (n = 6 per group). *Significant difference at p <0 .05. (E) Illustration indicating location of tissue from which brain punches used for protein analysis were taken.
2.3.3 Protein measurement and Western Blot analysis
The total protein content of each sample was determined with a Bradford kit from Bio-Rad laboratories (Hercules, CA). Protein extracts were boiled for 5 minutes in Lammeli buffer with 1% Beta-mercapthoethanol, followed by electrophoresis onto 10% Tris-HCl SDS-PAGE gels and then transfered onto PVDF membranes. Membranes were then blocked at room temperature with 5% nonfat dry milk in Tris-buffered saline with Tween-20 (TBST; pH = 7.4) for 1 hour. Membranes were then washed 3 times with TBST and incubated overnight at 4°C with the primary antibody for p-ERK, FosB, SIRT1, SIRT2, or p-CREB, (1:3000). Membranes were then washed with TBST 3 more times and incubated for 1 hour at room temperature with the appropriate secondary antibody (1:1000). After 3 more washes with TBST, a chemiluminescence kit (ECL; Amersham Pharmacia, Piscataway, NJ) was used and the membranes were exposed to x-ray film to detect antibody binding. All membranes were re-probed with the antibody for α-tubulin, which was used as a loading control. Phosphorylated proteins were also re-probed for their respective total protein in order to normalize the protein levels. Films were scanned and analyzed with ImageJ (NIH).
2.3.4. Data analysis
CPP scores were calculated by subtracting the time spent in the saline paired chamber from the time spent in the cocaine paired chamber during the drug-free preference test (CPP test). CPP scores and total locomotor responses during the CPP test were analyzed with one-way ANOVAs. Paired samples t-tests were used to test for differences in time spent, explorations, and entrances into the cocaine and saline-paired chambers during the CPP test. Locomotor behavior during conditioning sessions was analyzed with a mixed two way ANOVA (conditioning day x treatment). Western blot data were converted to a ratio of specific protein levels to total protein levels or α-tubulin using arbitrary densitometric units, expressed as a percentage of saline controls, and analyzed using one-way ANOVAs. Statistical significance was determined at p < 0.05. LSD post hoc analysis was used following all significant one-way ANOVAs.
3. Results
3.1 CPP and locomotor behavior during conditioning and drug-free CPP test
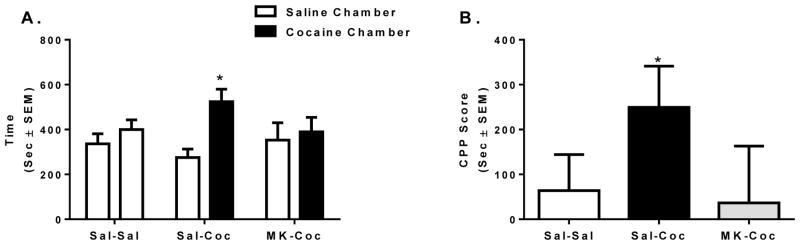
No differences were seen in time spent in either chamber or total locomotor activity during the preconditioning test (not shown). Cocaine-treated rats spent significantly more time in the cocaine paired chamber than the saline paired chamber during CPP testing [t(8) = 3.52, p < 0.01; Figure 1A]. No difference was observed in time spent in either chamber in saline controls or rats pre-treated with MK-801 [t(9) = 0.55, p = 0.96 and t(8) = 0.16, p = 0.88, respectively; Figure 1A]. Likewise, cocaine-treated rats had significantly higher CPP scores than MK-801 pretreated or saline treated rats [F(2,27) = 3.59, p < 0.05; Figure 1B).
Figure 1.
Effect of MK-801 pretreatment on cocaine-induced CPP acquisition. (A) Average time spent in the saline and cocaine paired chambers during the CPP test. (B) CPP scores: time spent in the cocaine paired chamber minus time spent in the saline paired chamber (in seconds ± SEM) (n = 9–10 animals per group). * Indicates statistically significant differences at p < 0.05.
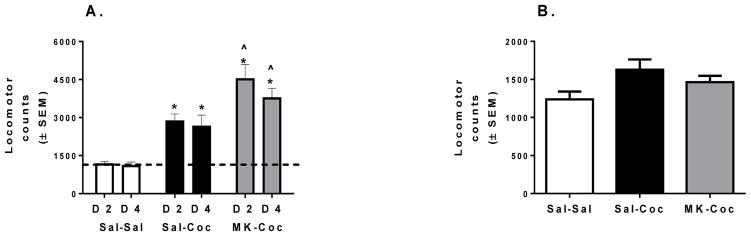
A significant interaction effect of treatment and conditioning day showed that although cocaine increased locomotor responses during conditioning regardless of pre-treatment compared to saline controls, MK-801 pretreatment significantly increased cocaine-induced locomotor responses [F(6,81) = 9.53, p < 0.01; Figure 2A]. Total locomotor behavior during the CPP test did not differ based on treatment [Figure 2B].
Figure 2.
Effect of cocaine and MK-801 pre-treatment on locomotor responses. (A) Total locomotor responses during cocaine (days 2 and 4) or saline conditioning days did not differ across all treatment groups. Dotted line represents average of locomotor activity during saline treatment days (days 1 and 3). (B) Total locomotor responses during the CPP test (drug-free). * Significant differences from saline controls at p < 0.05. ^Significant differences from cocaine only treated rats (Sal-Coc) at p < 0.05.
3.2. NAc and CPu pERK 1/2, protein levels after cocaine and MK801 pre-treatments
No changes were seen in total ERK or CREB protein levels in any brain area examined [not shown]. A one-way ANOVA revealed that cocaine-only treated rats (saline pretreatment) had significantly higher NAc pERK levels after CPP expression than saline controls and MK-801 pretreated rats [F(2,15) = 12.58, p < 0.01] . MK-801 pretreatment before cocaine injections during conditioning significantly reduced the cocaine-induced increase in NAc pERK levels after CPP expression [p < 0.05 for all comparisons; Figure 3A]. NAc pERK levels were significantly correlated to CPP scores, [r =0.64, p < 0.05; Figure 3B]. No changes were seen in CPu pERK levels (Figure 3C] and CPu pERK levels were not correlated to CPP scores (Figure 3D).
3.3. NAc and CPu pCREB protein levels after cocaine and MK801 pre-treatments
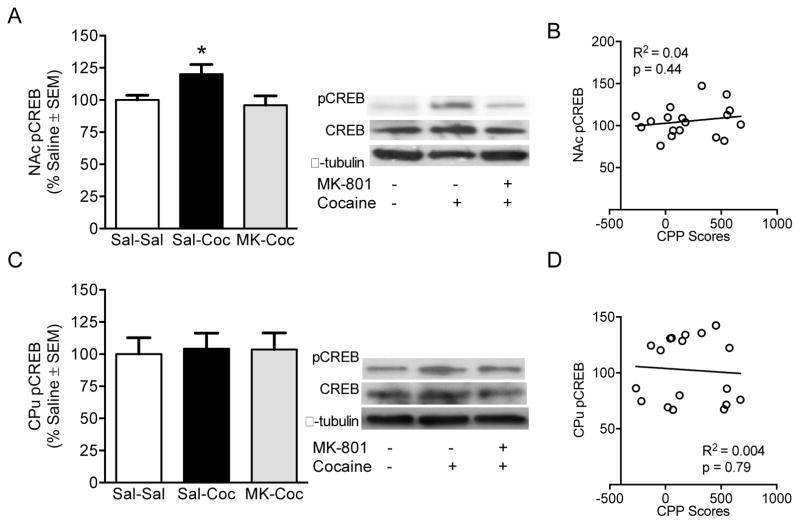
A one-way ANOVA revealed a significant effect of treatment on NAc pCREB levels [F(2,15) = 4.02, p < 0.05; Figure 4A]. Cocaine treated rats had significantly higher NAc pCREB levels than saline controls and MK-801 pretreated rats [p < 0.05 for all comparisons]. No changes were seen in CPu pCREB levels (Figure 4C]. Neither NAc nor CPu pCREB levels were correlated to CPP scores (Figure 4B & D respectively).
Figure 4.
Phosphorylated CREB in the NAc and CPu. (A) NAc pCREB protein levels are reduced after pre-treatment with MK-801, (B) but are not correlated to CPP scores. (C & D) CPu pCREB protein levels and correlation to CPP scores. Phosphorylated protein levels are expressed as a ratio to their respective total protein levels and expressed as percentage of saline controls (n = 6 per group). *Significant difference at p <0 .05.
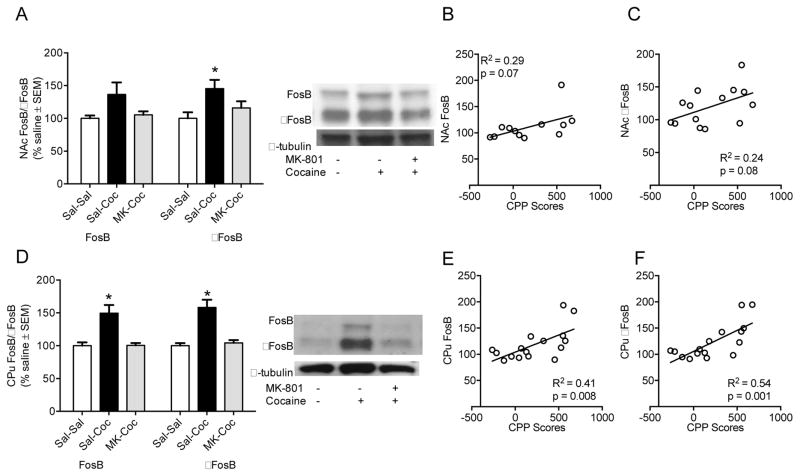
3.4. NAc and CPu FosB/ΔFosB protein levels after cocaine and MK801 pre-treatments
Although not statistically significant, we observed a trend towards an increase NAc FosB levels in cocaine-treated rats compared to saline control rats [FosB: F(2,13) = 3.40, p = 0.07; Figure 5A]. NAc FosB were significantly increased after cocaine treatment regardless of pre-treatment [F(2,15) = 16.40, p < 0.05; Figure 5A]. Although correlations between NAc FosB and FosB levels and CPP scores failed to reach significance, again, trends toward positive correlations were observed [r =0.53, p = 0.07; r =0.50, p = 0.08, Figure 5B and C]. In the CPu, increased FosB and FosB levels were observed and MK-801 pretreatment significantly reduced these increases [FosB: F(2,15) = 11.10, p < 0.01; FosB: F(2,15) = 15.11, p < 0.01; Figure 5D]. CPu FosB and FosB were significantly correlated to CPP scores, [r =0 .64, p < 0.05; r =0.73, p < 0.05, Figure 5E and F].
Figure 5.
(A) NAc FosB (left 3 bars, top band: 48 kDa) and ΔFosB (right 3 bars, bottom band: 38 kDa) protein levels and (B & C) correlation to CPP scores (FosB:B and ΔFosB:C). (D) CPu FosB (left 3 bars, top band: 48 kDa) and ΔFosB (right 3 bars, bottom band: 38 kDa) protein levels and (E & F) correlation to CPP scores (FosB:E and ΔFosB:F). Protein levels are expressed as a ratio to their respective α tubulin levels (55 kDa) (±SEM) (n = 6 animals per group). *Significant difference at p <0 .05.
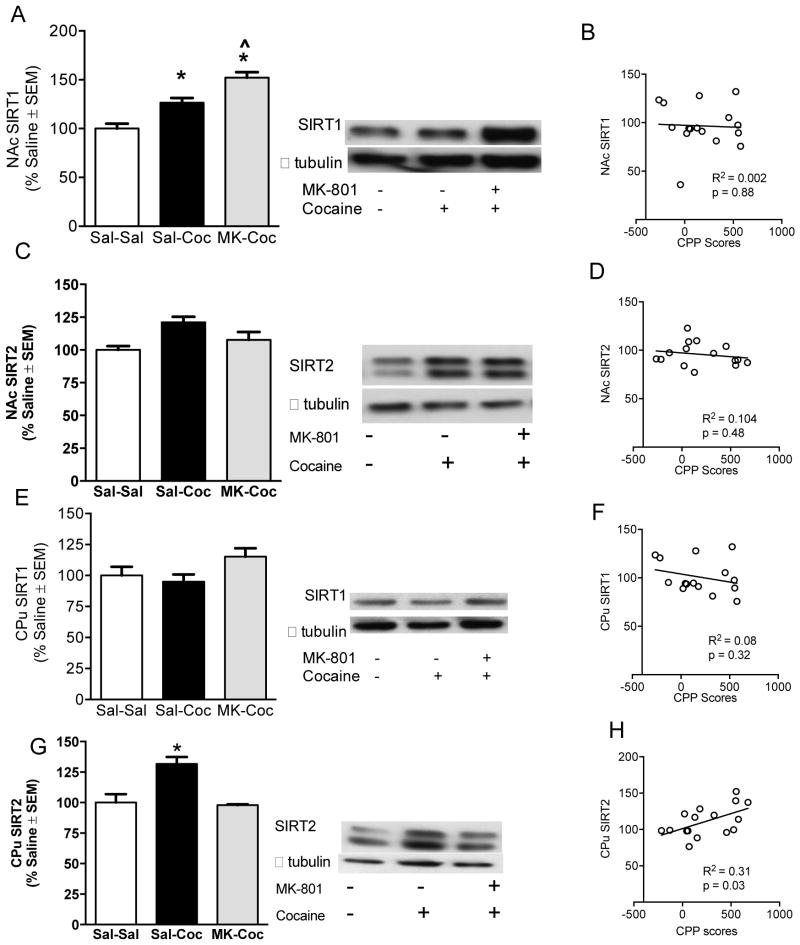
3.5. NAc and CPu SIRT1 and SIRT2 protein levels after cocaine and MK-801 pre-treatments
A significant one-way ANOVA revealed that NAc SIRT1 levels were higher in cocaine treated rats compared to saline controls [F(2,14) = 25.11, p < 0.05; Figure 6A]. Multiple comparisons also showed that MK-801 pre-treated rats had higher NAc SIRT1 levels than cocaine-only treat rats (p < 0.05 for all comparisons, Figure 6A). NAc SIRT2 levels remained unchanged based on treatment (Figure 6C). No changes were seen in CPu SIRT1 (Figure 6E). Cocaine-treated rats had increases in CPu SIRT2 levels, and the increase was reduced in rats pretreated with MK-801 [F(2,15) = 10.71, p < 0.01; Figure 6G). CPu SIRT2 protein levels were also significantly correlated to CPP scores [r =0.60, p < 0.05; Figure 6H].
Figure 6.
NAc SIRT1 (120kDa) (A) and SIRT2 (43kda) (C) protein levels and correlation to CPP scores (B & D). CPu SIRT1 (120kda) (E) and SIRT2 (43kda) (F) protein levels and correlation to CPP scores (G & H). Protein levels are expressed as a ratio to their respective α tubulin levels (55 kDa) (±SEM) (n = 4–5 animals per group). *Significant difference at p <0 .05. ^Significant differences from cocaine only treated rats (Sal-Coc) at p < 0.05.
4. Discussion
As predicted, MK-801 blocked both the acquisition of cocaine CPP [5], [32] [37] [34] and subsequent increases in NAc pERK, pCREB and FosB protein levels during the CPP expression test [5] [38] [39] . However, NAc pERK levels, but not pCREB levels, during the post-test were positively correlated with CPP scores. These data are consistent with previous studies showing that NAc ERK phosphorylation is associated with cocaine CPP [5] [7] [9] [11] [12] [40]. Here, we extend these results and show that during CPP expression, different patterns of intracellular signaling responses in the NAc and CPu emerge based on whether or not cocaine-CPP behavior has been acquired/expressed. After CPP expression, NAc pERK, pCREB, and ΔFosB in the CPu and NAc were consistently increased. Whereas, rats pretreated with MK-801 (0.25 mg/kg. i.p.), 30 minutes prior to cocaine administration during conditioning showed decreases in NAc pERK and pCREB and NAc and CPu FosB/ΔFosB levels compared to cocaine-only treated rats that expressed CPP behavior. This suggests that NAc ERK and CREB phosphorylation occur after exposure to a cocaine-associated context and ΔFosB accumulation in the NAc and CPu may be dependent on cocaine-induced NMDAR activation during cocaine exposure. However, CPu pERK and pCREB levels were unaffected by cocaine CPP expression or prior NMDAR antagonism. Whether or not the protein levels measured here are working as part of the same pathway is still unclear.
In agreement with previous research [25] [30], SIRT1 was elevated in the NAc after cocaine treatment, while SIRT2 was unchanged relative to saline. Interestingly, MK-801 pretreatment further increased SIRT1 above already elevated cocaine-induced levels. We also observed that during conditioning, MK-801 increased cocaine-induced locomotor responses compared to cocaine-only treated rats as previously reported [41], indicating a potential role for NMDAR-related alterations in NAc SIRT1 in cocaine-induced locomotor responses. However, this postulate requires further study. In contrast, in the CPu, SIRT1 levels did not differ among saline, cocaine, and MK801-pretreated cocaine animals, but SIRT2 showed an increase in cocaine-only animals that was blocked by MK-801-treatment. This dissociation suggests cocaine differentially influences SIRT1 and SIRT2 expression in the NAc and CPu. Cocaine-induced increases in NAc and CPu ΔFosB expression have been shown to undergo region-specific histone modifications at the FosB promoter, resulting in different patterns of gene expression after subsequent cocaine exposure [42]. Our results are in consistent with this and may suggest that NMDAR-mediated signaling may contribute to this difference. Inconsistent with our results, Ferguson et al. [25] also found an increase in NAc SIRT2. However, cocaine was administered for seven days compared to only two administrations in the current study. It is possible a longer-term (i.e. chronic) administration paradigm is necessary to induce SIRT2 in NAc, while SIRT1 is induced by comparatively less exposure to the drug. A limitation of these studies is that passive cocaine administration does not model the active, voluntary administration of cocaine in abusers. Still, taken together with previous research showing an increase in the rewarding effects of cocaine following pharmacological SIRT activation [25] our results provide a compelling case for the involvement in SIRT proteins in the behavioral effects of cocaine administration and particularly cocaine-associated memory.
Another limitation of our study is that we cannot rule out the potential that MK-801 induced state dependent learning that subsequently caused the lack of CPP expression [43]. It is possible that CPP might have been expressed had rats been primed with an MK-801 challenge prior to CPP testing. Due to the variability in behavioral responses to MK-801 and inconsistencies in previous research regarding MK-801 role in behavioral sensitization and state dependent learning [44] [45] [46], it is difficult to interpret the behavioral effects of MK-801 observed here. However MK-801 pre-treatment before a cocaine-primed CPP test has also been shown to block cocaine-CPP expression [47] indicating that cocaine-induced NMDAR activation is likely involved with assigning salience to contextual cues associated with cocaine-reward and subsequent expression of that association [48]. Interestingly Brown et al [47] also showed that MK-801 had no effect on lever pressing in a cocaine-self administration paradigm, further suggesting that MK-801 effects are specific to contextual-reward associations.
5. Conclusions
Our data provide further evidence for the role of ERK and CREB intracellular signaling pathways in mediating the neuronal plasticity involved with cocaine associated memories. Specifically, we show NMDAR dependent effects on NAc pERK and pCREB, and NAc and CPu ΔFosB after the expression of cocaine CPP behavior. Our results link NAc SIRT1 to cocaine exposure and provide evidence for an association between CPu SIRT2 levels and cocaine-CPP behaviors. However, our results are correlational and thus future studies are necessary to confirm whether these changes are necessary or sufficient for the formation of cocaine-associated memories.
Highlights.
Cocaine-CPP is blocked by NMDAR antagonism during conditioning
NMDAR antagonism reduces NAc pERK and pCREB and CPu ΔFosB and SIRT2 levels
NAc pERK levels during cocaine-CPP expression are positively correlated to CPP scores
Acknowledgments
This work was supported by NIDA DA12136 and MD007599 to S.J./V.Q.J. (Funding Sources had no involvement in the study design, collection, analysis and interpretation of data; in writing the report, and in the decision to submit the article for publication)
Footnotes
Author Contributions: SKN, VQJ, & SJ designed experiments. SKN performed experiments, statistical analysis, and wrote the manuscript. AK helped with behavioral testing, and western blot analysis. BB ran and analyzed western blots. All authors helped edit the manuscript and have approved the final article for submission.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anthony Klambatsen, Email: anthonyklambatsen@yahoo.com.
Bailey Balouch, Email: bbalouch@gmail.com.
Vanya Quinones-Jenab, Email: vaquinon@hunter.cuny.edu.
Shirzad Jenab, Email: sjenab@hunter.cuny.edu.
References
- 1.Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci Off J Soc Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritz M, Lamb R, Goldberg, Kuhar M. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 3.Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26. doi: 10.1038/sj/mp/4000964. [DOI] [PubMed] [Google Scholar]
- 4.Wickens JR, Budd CS, Hyland BI, Arbuthnott GW. Striatal Contributions to Reward and Decision Making: Making Sense of Regional Variations in a Reiterated Processing Matrix. Ann N Y Acad Sci. 2007;1104:192–212. doi: 10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]
- 5.Miller CA, Marshall JF. Molecular Substrates for Retrieval and Reconsolidation of Cocaine-Associated Contextual Memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci. 2014;35:374–383. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nygard SK, Klambatsen A, Balouch B, Quinones-Jenab V, Jenab S. Region and context-specific intracellular responses associated with cocaine-induced conditioned place preference expression. Neuroscience. 2015;287:1–8. doi: 10.1016/j.neuroscience.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenab S, Festa ED, Nazarian A, Wu HBK, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Nygard SK, Klambatsen A, Hazim R, Eltareb MH, Blank JC, Chang AJ, Quinones-Jenab V, Jenab S. Sexually dimorphic intracellular responses after cocaine-induced conditioned place preference expression. Brain Res. 2013;1520:121–133. doi: 10.1016/j.brainres.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of dopamine and NMDA receptors on cocaine-induced Fos expression in the striatum of Fischer rats. Brain Res. 2008;1243:1–9. doi: 10.1016/j.brainres.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci Off J Soc Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 13.Pawlak V, Kerr JND. Dopamine Receptor Activation Is Required for Corticostriatal Spike-Timing-Dependent Plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of Dopamine D1 Receptor Trafficking and Desensitization by Oligomerization with Glutamate N-Methyl-D-aspartate Receptors. J Biol Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- 15.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L. Cocaine-Induced Intracellular Signaling and Gene Expression Are Oppositely Regulated by the Dopamine D1 and D3 Receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 18.Berke JD, Hyman SE. Addiction, Dopamine, and the Molecular Mechanisms of Memory. Neuron. 2000;25:515–532. doi: 10.1016/S0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 19.Hyman SE. Addiction: A Disease of Learning and Memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 22.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, Rossi-Arnaud C, Grant SGN, Chapman PF, Lipp HP, Sturani E, Klein R. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 23.Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pagès G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouysségur J, Brambilla R. Knockout of ERK1 MAP Kinase Enhances Synaptic Plasticity in the Striatum and Facilitates Striatal-Mediated Learning and Memory. Neuron. 2002;34:807–820. doi: 10.1016/S0896-6273(02)00716-X. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 Enhances Cocaine-Evoked Immediate Early Gene Expression and Behavioral Plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, Renthal W, Neve R, Liu X, Shao N, Sartorelli V, Shen L, Nestler EJ. Essential Role of SIRT1 Signaling in the Nucleus Accumbens in Cocaine and Morphine Action. J Neurosci. 2013;33:16088–16098. doi: 10.1523/JNEUROSCI.1284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engel GL, Marella S, Kaun KR, Wu J, Adhikari P, Kong EC, Wolf FW. Sir2/Sirt1 Links Acute Inebriation to Presynaptic Changes and the Development of Alcohol Tolerance, Preference, and Reward. J Neurosci. 2016;36:5241–5251. doi: 10.1523/JNEUROSCI.0499-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect Publ Natl Inst Drug Abuse Natl Inst Health. 2005;3:4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ, Vialou V, Chakravarty S, Kodadek TJ, Stack A, Kabbaj M, Nestler EJ. Genome-wide Analysis of Chromatin Regulation by Cocaine Reveals a Role for Sirtuins. Neuron. 2009;62:335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson D, Shao N, Heller E, Feng J, Neve R, Kim HD, Call T, Magazu S, Shen L, Nestler EJ. SIRT1-FOXO3a Regulate Cocaine Actions in the Nucleus Accumbens. J Neurosci. 2015;35:3100–3111. doi: 10.1523/JNEUROSCI.4012-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renthal W, Nestler EJ. Histone acetylation in drug addiction. Semin Cell Dev Biol. 2009;20:387–394. doi: 10.1016/j.semcdb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Park WK, Jang CG, Oh S. Inhibition by MK-801 of cocaine-induced sensitization, conditioned place preference, and dopamine-receptor supersensitivity in mice. Brain Res Bull. 1996;40:201–207. doi: 10.1016/0361-9230(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 34.Alaghband Y, Marshall JF. Common influences of non-competitive NMDA receptor antagonists on the consolidation and reconsolidation of cocaine-cue memory. Psychopharmacology (Berl) 2013;226:707–719. doi: 10.1007/s00213-012-2793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. NeuroReport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- 36.Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- 37.Alaghband Y, O’Dell SJ, Azarnia S, Khalaj AJ, Guzowski JF, Marshall JF. Retrieval-induced NMDA receptor-dependent Arc expression in two models of cocaine-cue memory. Neurobiol Learn Mem. 2014;116:79–89. doi: 10.1016/j.nlm.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Ge S, Li N, Chen L, Zhang S, Wang J, Wu H, Wang X, Wang X. NMDA and dopamine D1 receptors within NAc-shell regulate IEG proteins expression in reward circuit during cocaine memory reconsolidation. Neuroscience. 2016;315:45–69. doi: 10.1016/j.neuroscience.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 39.Torres G, Rivier C. Cocaine-induced expression of striatal c-fos in the rat is inhibited by NMDA receptor antagonists. Brain Res Bull. 1993;30:173–176. doi: 10.1016/0361-9230(93)90055-g. [DOI] [PubMed] [Google Scholar]
- 40.Xu Y, Lv XF, Cui CL, Ge FF, Li YJ, Zhang HL. Essential role of NR2B-containing NMDA receptor-ERK pathway in nucleus accumbens shell in morphine-associated contextual memory. Brain Res Bull. 2012;89:22–30. doi: 10.1016/j.brainresbull.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- 42.Damez-Werno D, LaPlant Q, Sun H, Scobie KN, Dietz DM, Walker IM, Koo JW, Vialou VF, Mouzon E, Russo SJ, Nestler EJ. Drug Experience Epigenetically Primes Fosb Gene Inducibility in Rat Nucleus Accumbens. J Neurosci. 2012;32:10267–10272. doi: 10.1523/JNEUROSCI.1290-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlezon WA, Mendrek A, Wise RA. MK-801 Disrupts the expression but not the development of bromocriptine sensitization: A state-dependency interpretation. Synapse. 1995;20:1–9. doi: 10.1002/syn.890200102. [DOI] [PubMed] [Google Scholar]
- 44.Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- 45.Schenk S, Valadez A, McNamara C, House DT, Higley D, Bankson MG, Gibbs S, Horger BA. Development and expression of sensitization to cocaine’s reinforcing properties: role of NMDA receptors. Psychopharmacology (Berl) 1993;111:332–338. doi: 10.1007/BF02244949. [DOI] [PubMed] [Google Scholar]
- 46.Sripada S, Gaytan O, Swann A, Dafny N. The role of MK-801 in sensitization to stimulants. Brain Res Rev. 2001;35:97–114. doi: 10.1016/S0165-0173(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 47.Brown TE, Lee BR, Sorg BA. The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem. 2008;15:857–865. doi: 10.1101/lm.1152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Wolf ME. Can the “state-dependency” hypothesis explain prevention of amphetamine sensitization in rats by NMDA receptor antagonists? Psychopharmacology (Berl) 1999;141:351–361. doi: 10.1007/s002130050844. [DOI] [PubMed] [Google Scholar]