Abstract
Introduction
Decreased sociability is a symptom of psychiatric conditions including autism-spectrum disorder and schizophrenia. Both of these conditions are associated with decreases in GABA function, particularly in the medial prefrontal cortex (PFC) and the basolateral amygdala (BLA); structures that are components of the social brain. Here, we determined if decreasing GABA transmission within either the PFC or the BLA decreases social behavior.
Methods
Rats were implanted with cannulae aimed at either the medial PFC or the BLA and then were tested on up to 4 behavioral tests following bilateral infusions of 0.5 μl bicuculline methiodide (BMI, a GABAA receptor antagonist) at doses of 0, 25, or 50 ng/μl. Rats were tested in the social interaction test, the social preference test, the sucrose preference test and for locomotor activity (BLA infusions only).
Results
Intra-BLA or PFC BMI infusions decreased the amount of time and the number of social interactions in the social interaction test. Further, in the social preference test, rats infused with 50 ng BMI no longer exhibited a preference to explore a social over a non-social stimulus. The change in sociability was not due to a change in reward processing or locomotor behavior.
Discussion
Decreasing GABA transmission in either the medial PFC or BLA decreased sociability. Thus, changes in GABA signaling observed in conditions such as autism or schizophrenia may mediate the social withdrawal characteristic of these conditions. Moreover, they suggest that social withdrawal may be treated by drugs that potentiate GABA transmission.
Keywords: sociability, prefrontal cortex, basolateral amygdala, GABAA receptor, schizophrenia, autism
1. Introduction
Sociability, or the tendency to seek social interactions, is disrupted in a number of psychiatric conditions including notable deficits in autism-spectrum disorder (ASD) [1,2,3,4] and schizophrenia [1,2,3,5,6,7,8]. Indeed, social dysfunction in psychiatric conditions is increasingly being recognized as a major obstacle for treatment of psychiatric conditions [5,9]. As such, understanding the neural systems mediating social behaviors is of the utmost importance.
A number of studies suggest that the corticolimbic circuitry, including the medial prefrontal cortex (PFC) and the basolateral amygdala (BLA), play a role in regulating social behaviors [2,10]. Indeed, optogenetic activation of medial PFC pyramidal neurons caused a reduction in social interactions in the social interaction test and reduced social preference in a 3-chamber social preference test in rodents, tests of affiliative social behaviors [11]. In contrast, lesions of the medial PFC lead to increases in social behavior in rats [12]. Similarly, activation of the BLA leads to a reduction in social behavior in the social interaction test [13], while suppressing glutamatergic transmission by blocking NMDA and/or AMPA receptors within the BLA leads to an increase in social interactions [14]. Finally, a recent study found that activation of the BLA projections to the PFC decreased social behaviors while concurrently increasing non-social behaviors related to exploration in a resident-juvenile intruder paradigm [15].
The inhibitory neurotransmitter GABA has been implicated as one of a number of neurotransmitter systems that regulate sociability. Indeed, widespread reductions in GABA transmission decrease sociability in the social preference test [16,17]. Furthermore, a selective reduction in NMDA receptor NR1 subunits on parvalbumin-containing GABA neurons (a manipulation that decreases activation of these neurons) also decreases social approach in a social preference test [18]. In contrast, potentiating GABA signaling using the benzodiazepine (BDZ) diazepam improves social approach in NMDA NR1 knockdown mice, mice that exhibit a deficit in social approach [19]. The proposed involvement of GABA in social behavior is confirmed by manipulations that decrease GABA transmission within specific brain areas. For example, reducing GABA signaling within either the striatum [20] or the hypothalamus [21] decreases sociability in the social preference test and social interaction test, respectively.
Notably, GABA dysfunction or alterations in excitatory/inhibitory balance have been implicated in a number of psychiatric conditions including schizophrenia [22] and ASD [23]. In schizophrenia, research investigating GABA dysfunction has mainly focused on changes in GABA function in cortical regions, but abnormalities have also been observed in limbic structures including the amygdala. Within the PFC, reductions in the GABA synthesizing enzyme GAD67— particularly in parvalbumin-containing GABA neurons [24,25]—as well as reductions in GAT1 [26], and changes in GABAA receptor subunit expression have all been observed and have been interpreted to suggest a reduction in GABA synthesis and release in this brain area in people with schizophrenia [27, 28; reviewed in 22]. Furthermore, polymorphisms in the GAD1 gene (which codes for GAD67) are associated with childhood-onset schizophrenia and with cortical gray matter loss [29]. In the amygdala, early studies suggest changes in GABA function in people with schizophrenia [30 and references therein] but there have not been follow-up analyses. That said, recent work suggests that first degree relatives of individuals with schizophrenia have greater BDZ-induced suppression of amygdala activity during an emotion identification task, a finding that implies that GABA is decreased in this brain area and thus there is a compensatory upregulation of GABA receptor number or function [31]. GABA dysfunction is likewise implicated in ASD [23]. Blatt and Fatemi [32] observed that GABAA α1 receptor subunits and GABAB1 receptors are significantly downregulated in frontal cortical areas. Similarly, reduced GABAA receptor binding has been observed within the anterior cingulate cortex [33] and reduced BDZ binding has also been observed in frontal cortical areas [34]. Moreover, reductions in the GABA synthesizing enzymes GAD67 and GAD65 were observed in cortical areas [35] and a preliminary report suggests that GABA concentrations are reduced in children with ASD [36]. Finally, the hypothesis that GABA dysfunction contributes to ASD symptomology comes from the observations of a high co-morbidity between epilepsy and ASD and the multitude of ASD mouse models that all exhibit changes in GABA signaling (reviewed in [23]).
The goal of the current experiment was to determine if decreasing GABA transmission specifically within the medial prefrontal cortex (PFC) or the basolateral amygdala (BLA) affects sociability in rats. GABA function was decreased in each structure by microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI), a manipulation that has been found to increase in neural activity [37,38]. Sociability was tested using both the social interaction test and the social preference test. Because positive social interactions are thought to be intrinsically rewarding [39], we also tested rats basic reward functioning using the sucrose preference test. Finally, because social interactions require intact locomotor activity, we also assessed locomotion in an open field following intra-BLA BMI administration. We have previously shown that intra-PFC BMI administration at doses used in the current experiment does not affect locomotor activity [37].
2. Methods
2.1 Animals
Forty-five male Sprague-Dawley rats between 75–80 days of age at the time of surgery were used as experimental rats. An additional 14 male rats, between 50–60 days of age at the time of the social interaction test were used as stimulus rats. Rats were maintained on a 14-h/10-h light-dark cycle (lights on at 0700h) and were group housed until approximately post-natal day (PND) 55. Experimental rats were then housed in pairs or groups of up to 8 until the time of surgery and then were housed singly thereafter. Stimulus rats were housed in groups of 2–3 starting one week prior to the start of and throughout the experiment. To increase exposure of the stimulus rats to other rats, when possible, groups/pairs were changed every 2–3 days (stimulus rats were exposed to a maximum of 3 other stimulus rats during this time). Rats were allowed free access to food (Purina Rat Chow) and water when in their home cages, except when specified below. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [40] and Oberlin College policies.
2.2 Drugs and Administration
Bicuculline methiodide (BMI) was purchased from Sigma and dissolved in saline to doses of 25 ng/μl and 50 ng/μl. BMI (25 or 50 ng/ μl) or vehicle (saline) was infused into the medial prefrontal cortex (PFC) or basolateral amygdala (BLA) in a volume of 0.5 μl/hemisphere, at a rate of 0.25 μl/min and infusers were left in place for an additional 2 min. Following infusions, rats were returned to their home cage for a 5 min absorption period prior to testing. Rats were habituated to the infusion procedure using 0.5 μl/hemisphere saline infusions on the day preceding the first social interaction test (see below).
2.3 Surgery
One week prior to the start of the experimental protocol, experimental rats were anesthetized with 3% isoflurane (3% isoflurane with .75 l/min O2) and either implanted with a bilateral guide cannula (26-gauge; Plastics One, Roanoke, VA) aimed at the medial PFC near the border of infralimbic (IL) and prelimbic (PrL) cortices (relative to bregma: AP = + 2.8, ML = ± 0.75, DV = −3.0 mm from dura [41]) or two cannulae aimed at the BLA (relative to bregma: AP = −2.6, ML ± 5.2, DV = −5.0 mm from dura [Paxinos and Watson, 2009]). Nineteen rats were implanted with cannulae aimed at the PFC and 26 rats were implanted with cannulae aimed at the BLA. Ketprofen (5 mg/kg, SC, Sigma) was administered prior to surgery as an analgesic. Rats were allowed to recover for at least 7 days prior to the start of behavioral testing.
2.4 Behavioral Tests
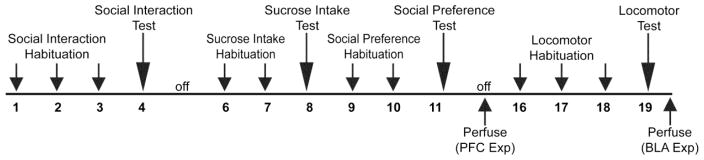
The experimental time line is depicted in Figure 1.
Figure 1.
Experimental timeline. Rats underwent surgery to implant cannulae into either the medial prefrontal cortex (PFC) or basolateral amygdala (BLA) and then were allowed approximately one week to recover. Following recovery rats underwent 3 (PFC group) or 4 (BLA group) behavioral tests over the course of ~3 weeks: the social interaction test, the sucrose preference test, the social preference test and finally a test of locomotor activity (BLA group only). Prior to each test, rats were infused with BMI (0 – 50 ng/μl/side). Following the completion of behavioral testing rats were perfused, their brains extracted and then their cannulae placements assessed.
2.4.1. Social Interaction Test
The social interaction test was based on the one described by Lapiz-Bluhm et al. [42] and occurred over 4 days in a 60 × 60 × 40 cm black Plexiglas open field; the floor of the arena was covered in pine bedding material.
On days 1–3, rats were handled and then placed individually (experimental rats) or in pairs (stimulus rats) in the center of the open field. After 5 min, rats were removed from the open field and the bedding mixed to redistribute odors that could affect social behavior. On day 4, the experimental rats were treated with the appropriate drug dose, returned to their home cage for a 5-min period absorption period and then placed in the open field with a “stranger” stimulus rat for 5 min. The social interaction was videotaped for off-line analysis of the number of experimental rat initiated social interactions (sniffing, climbing on/under, chasing, wrestling) and total time the experimental rat engaged in social behavior (based on procedures described in [42]). Aggressive or defensive behaviors were not observed during the social interaction test and thus were not scored.
2.4.2 Sucrose Intake Test
The sucrose preference test was conducted over 3 consecutive days and consisted of habituation days and a test day. On habituation day 1, rats were provided with 2 water bottles; one containing 1% sucrose and the other containing water. On habituation day 2 (24h after introduction), the left/right location of the sucrose and water bottles was reversed. On the test day (day 3), both water bottles were removed and the rats were water deprived for 4h. After 4h the 30-min test was administered. During the test the rats were provided with 2 water bottles: one contained 10% sucrose, the other contained water. At the end of the test, the volume of each liquid consumed was recorded and used to calculate the sucrose preference index [sucrose solution consumed (ml) − water consumed (ml)]/[sucrose solution consumed (ml) + water consumed (ml)].
2.4.3 Social Preference Test
Social preference was tested using a modified version of the 3-chamber social preference test described by Moy et al. [43] and was similar to the version used by Mielnik et al. [19]. Social preference testing took place in the same Plexiglas open field used in the social interaction test and contained two acrylic restrainers (Harvard Apparatus) located in opposite corners.
On days 1–2, experimental rats were individually habituated to the arena containing the restrainers for 5 min; during habituation the restrainers were empty. On day 3, the experimental rat was placed in the arena for a 5 min test. During the test, one restrainer contained the stimulus rat (i.e., the social stimulus) and the other contained the inanimate, plastic rat (i.e., the non-social stimulus). The location of the social and the non-social stimuli was counterbalanced across rats both within and between groups. Between tests the restrainers were cleaned with isopropyl alcohol and thoroughly rinsed with water and the social and non-social stimuli were rotated between different restraint tubes so as to minimize the impact of olfactory cues on the behavior of the experimental rat. The test session was videotaped and the time the experimental rat spent exploring the restrainer containing the social stimulus and the time spent exploring the restrainer containing the non-social stimulus across the 5 min test was measured. These data were used to calculate a social preference index: (*social stimulus (sec) − non-social stimulus (sec)]/ [social stimulus (sec) + non-social stimulus (sec)]*100).
The stimulus rats were habituated to confinement in the restrainers over 3 days; on each day they were confined to the tube for 5 min. The acrylic restrainer was suitable for use for rats weighing up to 500 g (Harvard Apparatus); stimulus rats typically weighed ~300 g.
2.4.4. Locomotor Activity
Because locomotor activity can affect the ability to interact socially and because some drug manipulations can affect locomotor activity, locomotor activity was assessed using 60 X 60 X 30 cm activity chambers (Med-Associates) over 4 days in rats infused in the BLA (previous research indicated that intra-PFC BMI infusions do not affect locomotor activity [37]). On days 1–3, rats were habituated to activity chamber for 30-min. On day 4, the rats were infused with vehicle or BMI (25 or 50 ng/μl), returned to their home cage for 5 min and then put in the activity chamber for 30 min. A computer program tracks the distance travelled (cm) by the rat during the sessions.
2.5 Histology
At the end of the experiment, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, IP) and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde. Following perfusions, brains were removed, post-fixed for 24 h and then cryoprotected in 30% sucrose prior to slicing on a microtome. Sections (40 μm) were mounted on slides, stained with cresyl violet and used to assess the location of cannulae.
2.6 Statistical Tests
A blind rater scored the videos of the social interaction and social preference tests. Data were analyzed with either one-way (condition) or two-way (condition X behavior/location/bottle/time) analyses of variance (ANOVAs); in all cases condition was a between-subjects factor while the other factor (behavior/location/bottle/time) was a within-subjects factor. Post-hoc analyses were conducted using an estimated marginal means procedure using a Bonferroni comparison. Preference Index data (social preference and sucrose preference tests) were also analyzed using a one-sample t-test to determine if the preference score was significantly greater than 0 (no preference).
3. Results
3.1 Histology
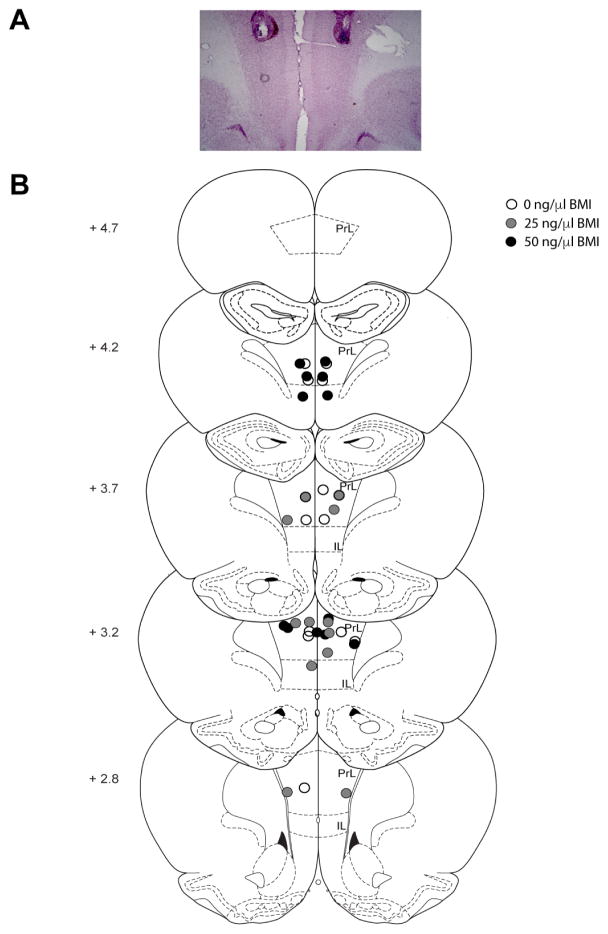
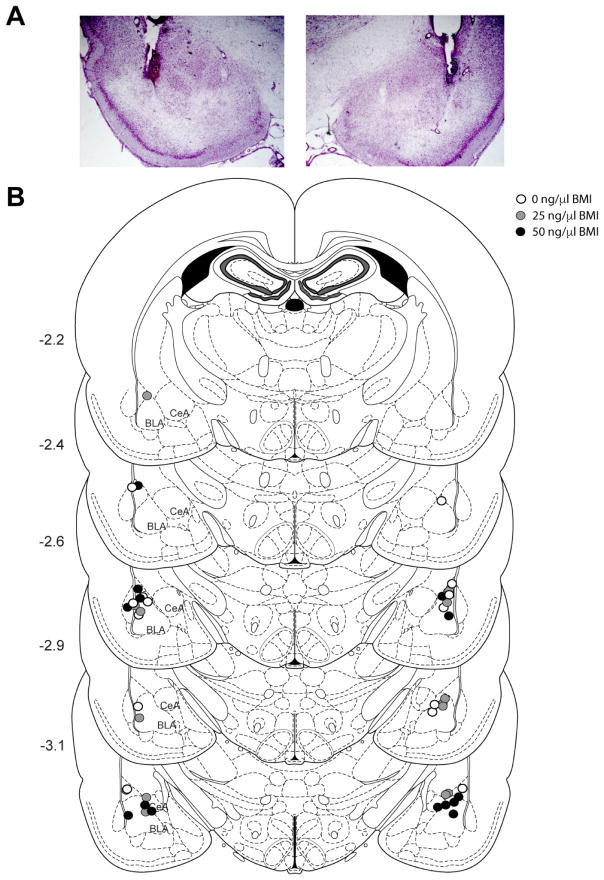
Cannulae placements are depicted in Figures 2 and 3. In the PFC experiment, one rat was excluded (n=1, 50 ng/μl) because its cannulae placements were located outside of the PrL or IL; this left a total of 6 rats per dose of BMI administered (Figure 2). In the BLA experiment, five rats were excluded (n=2, 0 ng/μl; n=3, 50 ng/μl) because cannulae placements were located outside of the BLA; this left a total of 6–7 rats per dose of BMI administered (Figure 3). An additional rat (BLA 0 ng/μl) was excluded from the analysis for the social interaction test due to a unilateral infusion; this rat was included in the analyses for the subsequent behavioral tests.
Figure 2.
Prefrontal cortex (PFC) cannulae placements. A) Photograph depicting cannula placements and typical amount of damage caused by the cannulae and obturator/infuser. B) Schematic drawing showing the location of injector tips within the medial PFC. Rats were excluded (not shown) if their tips were not within the boundary of either the prelimbic (PrL) or infralimbic (IL) cortex. Numbers on the left indicate location anterior from bregma. Adapted from Paxinos and Watson (2009).
Figure 3.
Basolateral amygdala (BLA) cannulae placements. A) Photograph depicting cannula placements and typical amount of damage caused by the cannulae and obturator/infuser. B) Schematic drawing showing the location of injector tips within the BLA. Rats were excluded (not shown) if their tips were not within or bordering the BLA. Numbers on the left indicate location posterior from bregma. Adapted from Paxinos and Watson (2009). BLA (basolateral amygdala); CeA (central amygdala).
3.2. Social Interaction Test
3.2.1 PFC
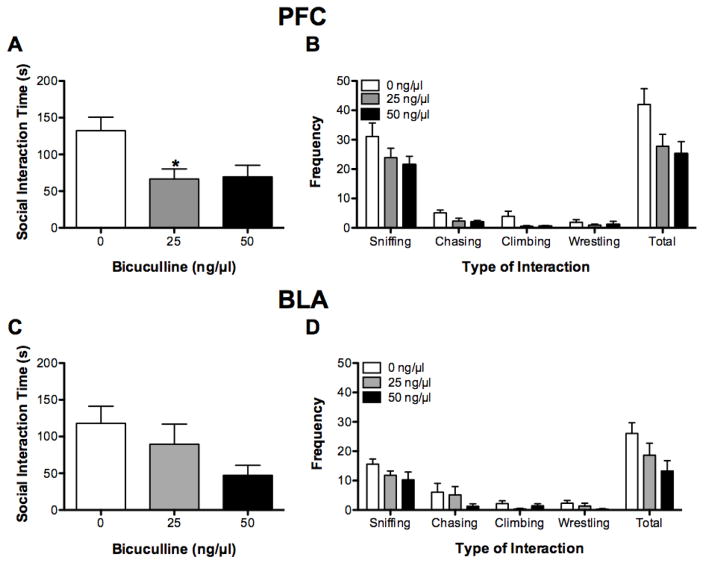
BMI administration affected the total time rats engaged in social interactions (F(2, 15) = 5.04, P < 0.05; see Figure 4A). Bonferroni comparisons showed that BMI (25 ng) significantly decreased the amount of time spent engaged in social interaction relative to vehicle (P = 0.04), and there was a trend for BMI (50 ng) to decrease social interaction time (P = 0.06).
Figure 4.
Social interaction test. Intra-PFC infusions of BMI decreased the total time engaged in social interaction (A) and the total number of social interactions (B). Intra-BLA infusions of BMI non-significantly decreased the time engaged in social interactions (C) and the number of social interactions (D). *P < 0.05, relative to 0 ng BMI.
Regardless of the dose of BMI administered, there were significant differences in the frequency that rats engaged in the different types of social behaviors measured (F(3, 45) = 120.05, P < 0.01; see Figure 4B); rats engaged in sniffing behavior more than any of the other behaviors measured (all P < 0.01). BMI administration significantly affected the total number of social interactions (F(2,15) = 3.92, P < 0.05) but did not affect the type of social behavior engaged in (F(6, 45) = 1.09, P > 0.05). Bonferroni comparisons showed that there was a trend for BMI (50 ng) to decrease the number of social interactions relative to vehicle (BMI 0 ng) (P = 0.06).
3.2.2 BLA
There was a trend for intra-BLA administration of BMI to affect the time engaged in social interactions (F(2, 18) = 2.83, P = 0.09; see Figure 4C). Subsequent Bonferroni comparisons showed that there was a trend for rats treated with BMI (50 ng) to spend less time engaged in social interactions than did rats treated with vehicle (BMI 0 ng) (P = 0.09).
Regardless of the dose of BMI administered, there were significant differences in the frequency that rats engaged in the different types of social behaviors measured (F(3, 51) = 33.03, P < 0.05; see Figure 4D); rats engaged in sniffing behavior more than any other type of behavior (all P < 0.01). There was a trend for dose of BMI administered to affect the number of social interactions (F(2,17) = 3.09, P = 0.07). Subsequent Bonferroni comparisons revealed that there was a trend for rats treated with the BMI (50 ng) to engage in fewer interactions than rats treated with vehicle (BMI 0 ng) (P = 0.07).
3.3 Social Preference Test
3.3.1. PFC
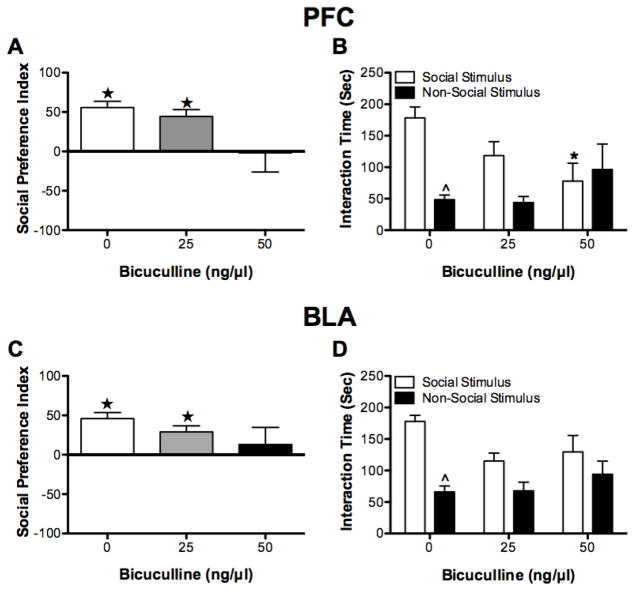
Intra-PFC BMI administration affected the size of the social preference index (F(2,15) = 3.80, P < 0.05; see Figure 5A). Subsequent Bonferroni comparisons showed that there was a trend for BMI (50 ng) to decrease the preference index (P = 0.06) relative to vehicle (BMI 0 ng). Furthermore, following BMI (0 ng and 25 ng) administration rats exhibited a preference for spending time with the social stimulus relative to the non-social stimulus (both t(5) > 5.12, P < 0.05). In contrast, following BMI (50 ng) administration rats did not exhibit a preference in where they spent their time (t(5) = −0.07, P > 0.05).
Figure 5.
Social preference test. Intra-PFC infusions of BMI tended to decrease the social preference index (A). Moreover, following BMI (50 ng) the social preference index was not significantly greater than zero meaning that rats did not exhibit a preference for the social stimulus versus the non-social stimulus. B) Rats infused with vehicle (0 ng) or BMI (25 ng) spent more time exploring the social than the non-social stimulus, this was not true of rats infused with BMI (50 ng). Intra-BLA infusions of BMI did not affect the size of the social preference index but the social preference index of rats infused with BMI (50 ng) was not significantly greater than zero (C). D) Rats receiving intra-BLA vehicle infusions spent significantly more time exploring the social than the non-social stimulus; the same was not true for rats infused with BMI (25 ng or 50 ng). *P < 0.05, relative to 0 ng BMI. ★P < 0.05, relative to a social preference index of 0. ^P < 0.05, relative social stimulus.
Dose of BMI administered significantly affected the time spent in the vicinity of the stimulus and non-stimulus rats during the social preference test (F(2, 15) = 3.68, P < 0.05, see Figure 5B). Bonferroni comparisons showed that rats treated with vehicle (BMI 0 ng) spent more time in the vicinity of the restrainer containing the social stimulus relative to the time spent near the non-social stimulus (P < 0.05); there was a trend for rats treated with 25 ng BMI to do the same (P = 0.07). There was no difference in the amount of time that rats treated with BMI (50 ng) spent in the vicinity of the social and non-social stimuli (P > 0.05). Moreover, rats treated with BMI (50 ng) spent less time in the vicinity of the social stimulus than did vehicle-treated rats (P < 0.05).
3.3.2. BLA
Intra-BLA BMI administration did not affect the size of social preference index (F(2, 18) = 1.36, P > 0.05, see Figure 5C). However, the social preference index was only greater than 0 following administration of 0 ng and 25 ng BMI (both t(6) > 3.71, P < 0.05), not following administration of 50 ng BMI (t(6) = 0.59, P > 0.05).
Rats spent more time near the stimulus than the non-stimulus rat (F(1,18) = 14.20, P < 0.05; see Figure 5D); furthermore, the dose of BMI administered affected where rats spent their time (F(1,18) = 4.69, P < 0.05). There was not, however, an interaction between the dose of BMI administered and the location where rats spent their time (F(2,18) = 1.91, P > 0.05). Bonferroni comparisons showed that following administration of vehicle (BMI 0 ng) rats spent more time in the vicinity of the social stimulus than they did in the presence of the non-social stimulus (P < 0.05); the same was not true following BMI (25 ng and 50 ng) administration (both P > 0.05).
3.4 Sucrose Intake Test
3.4.1. PFC
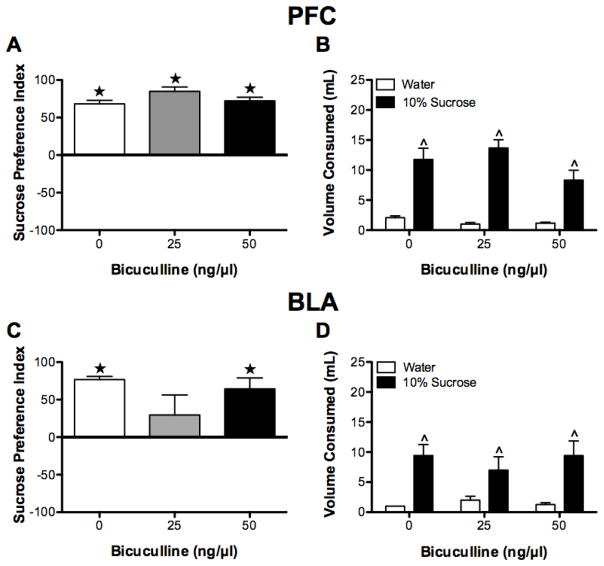
During the habituation phase, all rats displayed a preference index that was significantly greater than 0 (all t(5) > 3.0, P < 0.05), but there were no group differences in the size of the preference index (F(2,15) = 0.71, P > 0.05) (data not shown).
During the test phase, there was no significant group differences in size of the sucrose preference between groups, (F(2,15) = 2.81, P > 0.05). Furthermore, all groups exhibited a preference for the sucrose solution as indicated by the observation that all sucrose preference scores were significantly greater than 0 (no preference) (all t > 14.47, P < 0.05; see Figure 6A). The sucrose preference was confirmed by the observation that rats, regardless of condition, consumed more sucrose (10%) solution than water (F(1, 15) = 104.01, P < 0.01, see Figure 6B); an effect that was not influenced by dose of BMI infused (no interaction or effect of condition, both F < 2.78, P > 0.05).
Figure 6.
Sucrose intake test. With one exception (intra-BLA BMI 25 ng), all rats exhibited a preference for sucrose as indicated by a sucrose preference score that was significantly greater than 0 (A, C). Furthermore, regardless of condition, all rats consumed significantly more 10% sucrose than water (B, D). ★P < 0.05, relative to a sucrose preference index of 0. ^P < 0.05, relative to water.
3.4.2 BLA
During the habituation phase, all rats displayed a preference index that was significantly greater than 0 (all t(6) > 2.52, P < 0.05), but there were no group differences in the size of the preference index (F(2,15) = 0.75, P > 0.05) (data not shown).
During the test phase, there was no effect of BMI on the size of the preference index (F(2,15) = 1.92, P > 0.05; see Figure 6C), subsequent tests indicated that the sucrose preference index was significantly greater than 0 for the 0 ng and 50 ng doses of BMI (both t(6) > 4.43, P < 0.05), but not for the 25 ng dose (t(6) = 1.10, P > 0.05). Furthermore, all rats consumed more sucrose (10%) solution than water (F(1,18) = 26.68, P < 0.05; see Figure 6D); an effect that was not influenced by dose of BMI (no interaction or effect of condition, both F < 0.62, P > 0.05).
3.5 Locomotor Activity
3.5.1 BLA
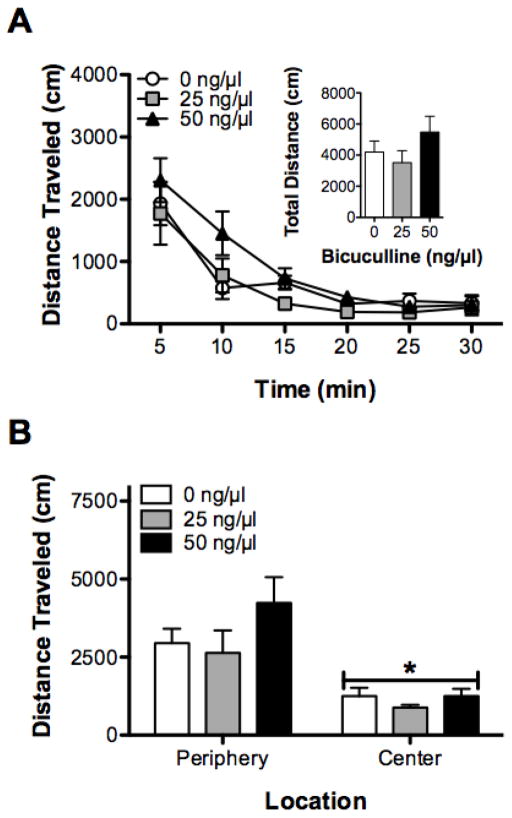
Locomotor activity decreased across the three habituation sessions (F(2, 36) = 33.24, P < 0.05), with activity being greater on habituation session 1 than habituation sessions 2 and 3. That said, there were no differences in locomotor activity between the different drug groups over the 3 days of habituation (both F < 1.54, P > 0.05, data not shown).
Locomotor activity decreased across the 30-min test session (F(5, 90) = 41.40, P < 0.05); this effect was not influenced by BMI administration (both F < 1.15, both P > 0.05; Figure 7A).
Figure 7.
Effects of Intra-BLA infusions of BMI on locomotor activity. A) Intra-BLA infusions of BMI did not affect locomotor activity across time (inset shows total activity) (A), or central zone activity (B). *P < 0.05, relative to periphery.
When total activity during the test was analyzed taking into account whether rats spent more time in center or the periphery of the locomotor activity chamber, there was significant effect of location (F(1,18) = 41.40, P < 0.05, Figure 7B), with rats spending more time in the periphery than in the center of the chamber (P < 0.01). There was not, however, any effect of BMI dose administered or an interaction (both F < 1.59, P > 0.05).
4. Discussion
In the current experiment, GABAA receptor blockade within the PFC caused a significant reduction in social behavior as measured by the social interaction and the social preference tests. Within the BLA, GABAA receptor blockade also reduced sociability in both behavioral tests, but the magnitude of the reduction in social behaviors was not, in all cases, statistically significant. In the social interaction test, BMI infusions reduced both the time the experimental rats engaged in social interactions with an unfamiliar rat and the total number of interactions with that rat. Notably, the decrease in social interactions cannot be accounted for by a reduction in any one specific behavior measured (sniffing, chasing, grooming, wrestling; aggressive and defensive encounters were not observed), rather it was due to a general decrease in affiliative social behaviors. It should be noted that in the current experimental context, only social interactions initiated by the experimental rat were assessed. That said, because of the reciprocal nature of the social interaction test, it is possible that the behavior of the stimulus rat was affected by the behavior of the experimental rat (and vice-versa); this could have impacted the total amount of time that the experimental rat engaged in social behaviors [44]. In the social preference test, BMI infusions caused a reduction in both the social preference index (PFC only) and the amount of time rats spent exploring the social versus the non-social stimulus. Notably, the social preference test measures social approach by the experimental rat and is less likely to be influenced by the behavior of the stimulus rat because there is no direct contact between the two rats [44]. Thus, the results of the two behavioral tests are consistent and suggest that decreasing GABA transmission within either the PFC or the BLA leads to a reduction in sociability.
The degree to which rats engage in social behavior is governed by both social and non-social factors. Thus, other than a change in sociability per se, there are a number of alternative explanations for why sociability scores may have decreased following BMI administration into the PFC or BLA. First, a reduction in social interactions could reflect an increase in general anxiety [45]. In the current experiment, care was taken to test animals under low anxiety conditions that promote the assessment of social behaviors [46]; rats were extensively handled and habituated to the arena prior to testing and both habituation and test sessions took place under red light conditions. This resulted in fairly high levels of social interaction for control animals (~40% time engaged in experimenter rat initiated social interactions). That said, it is possible that BMI infusions decreased social interaction due to an increase in general anxiety. Contrary to this possibility, intra-BLA BMI infusions did not increase anxiety as measured by locomotor activity in the current experiment. Nevertheless, intra-BLA infusions of BMI, at a dose comparable to the ones used here, have previously been found to increase anxiety as measured in a conflict test [13]. Moreover, intra-PFC infusions of BMI have been found to increase anxiety as measured in the elevated plus maze; however this effect was observed following administration of BMI doses that were ten times those used in the current experiment [47]. Thus, although data from the current experiment suggests that a general increase in anxiety did not contribute to the change in social behavior, this possibility cannot currently be ruled out. Second, positive social interactions are inherently rewarding [39], and thus it is possible that a change in basic reward processing could underlie the reduction in sociability. In the current experiment, rats that received either BLA or PFC infusions of BMI exhibited, in general, normal sucrose preference, a basic measure of reward processing [48]. The one exception to this were rats that received intra-BLA infusions of BMI (25 ng); in this group, the reduction in sucrose preference was primarily driven by two rats that also exhibited smaller preference index scores during the habituation phase (i.e., < 20). Alternatively, it is possible that a change in motivation, rather than a change in reward processing per se, could mediate the reduction in sociability. Previous research has found that intra-PFC infusions of BMI either enhanced motivation for brain stimulation reward [37] or did not alter motivation for food reward when rats were responding on a progressive ratio schedule [49]; thus it is unlikely following intra-PFC BMI administration rats were less motivated to interact with the stimulus rats. To our knowledge, it is not known how intra-BLA BMI infusions affect motivation to respond for reward; future research is needed to determine whether this manipulation caused a change in motivation that resulted in the reduction in social behavior. Finally, it is possible that impaired locomotor responses could affect measures of sociability. In the current experiment, we did not find evidence of altered locomotor activity following intra-BLA BMI infusions. Likewise, in previous research, intra-PFC infusions of BMI at the doses used in the current experiment did not affect locomotor activity [37,50]. In sum, although a number of non-social factors could affect social behavior, it appears that several of these factors are unlikely to account for the change in social behavior observed in the current experiment. Rather, the observed changes in social behavior likely reflect changes to a number of social factors that can influence sociability.
Social motivation (motivation or drive to engage in social behavior), social anxiety (fear or avoidance of social interactions) and social cognition (ability to interpret both verbal and non-verbal social cues of other animals) [2] are essential social factors that govern sociability. Unfortunately, although the social interaction test and social preference test are both widely used behavioral tests of sociability, they do not allow researchers to clearly delineate between these social factors when determining why social behavior changed. Thus, future research will be needed to identify which of these social factors contributes to the reduction in social behavior observed following disruption of GABA transmission within either the PFC or BLA.
Our results support the notion that both the medial PFC and BLA are key components of the neural networks that comprise the social brain [2,10]. BMI is known to increase PFC neuron activity [37,38]. As such, our results are consistent with those of Yizhar et al. [11], who found that optogenetic activation of PFC pyramidal neurons lead to a reduction of social interactions and social preference. Moreover, lesions of the medial PFC activity have been shown to increase social behaviors in the social interaction test [12]. These data suggest that activation of the PFC decreases social behavior, while decreasing PFC activity increases social behavior. Similarly, activation of the BLA has previously been found to decrease sociability in the social interaction test [13]. Our results replicate and extend this finding, showing that increasing BLA activity also decreases social preference.
The PFC and BLA have dense reciprocal interactions [51,52,53,54,55,56] and thus it is possible that the effects of intra-PFC and intra-BLA BMI infusions observed in the current experiment are not independent. Rather, the effect of PFC activation on social behavior could be a result from PFC-mediated activation of the BLA or vice-versa. Indeed, the effects of both manipulations were remarkably similar across behavioral tests. It is noteworthy that Felix-Ortiz et al. [15] recently found that increasing activity of only the BLA neurons that project to the medial PFC was sufficient to cause decreased social interactions using a resident-juvenile intruder paradigm. It remains to be seen whether a similar manipulation would also decrease social behavior in the social interaction and social preference tests and whether activating only the PFC neurons that project to the BLA (i.e., the reciprocal connection) would also be sufficient to cause a change in social behavior.
Disruptions in social behavior are a key feature of a number of psychiatric conditions including ASD and schizophrenia (e.g., [1]). Notably, GABAergic dysfunction has been postulated to underlie the symptoms of both of these conditions (e.g., [22,23]). The current experiment suggests that GABAergic dysfunction specifically within either the medial PFC or the BLA could feasibly lead to observed changes in social behavior in these conditions. As such, it is possible that enhancing GABA tone could be a viable therapeutic strategy [23]. Indeed, BDZ have prosocial effects in the social interaction test under a variety of conditions [45,57] including when used in an animal model of schizophrenia [19]. However, classic BDZ are used somewhat reluctantly to treat conditions such as ASD and schizophrenia because of their sedating and hypnotic side effects. New research however, employing subunit selective or atypical BDZ, shows promise for alternative treatment strategies. Indeed, Han et al. [58] showed that enhancing GABA tone with low doses of clonazepam (a BDZ positive allosteric modulator (PAM)) or clobazam (an atypical BDZ PAM) enhanced social behavior in the BTBR mouse model of ASD, but not in wild-type mice. Further, L838,417, a BDZ PAM at α2/3-subunit containing GABAA receptors, improved sociability in BTBR mice, but not in WT mice [58]. In contrast, zolipdem, a BDZ PAM selective for a1-containing GABAA receptors, decreased social interactions, possibly due to its sedative effects [58]. Additionally, Richetto et al. [59] showed that SH-053-2′F-S-CH3, a BDZ PAM with partial selectivity at α2, α3, and α5 subunits of the GABAA receptor, improved social preference deficits in an immune-response mediated neurodevelopmental model of schizophrenia. Not only do these data suggest that subunit selective BDZ PAMs may be effective therapeutic strategies, but they also suggest that only a subset of GABAA receptors are involved in the regulation of social behaviors.
5. Conclusions
In summary, the data from the current experiment suggest that decreasing GABA transmission at GABAA receptors in either the medial PFC or the BLA is sufficient to lead to social withdrawal or avoidance as measured in the social interaction test and the social preference test. Moreover, the change in sociability is unlikely to result from changes in locomotor behavior, general anxiety or reward processing. Considering that changes in GABA signaling are observed in a number of psychiatric conditions that exhibit changes in social behavior, for example ASD and schizophrenia, these data suggest that strategies that potentiate GABA signaling could be explored as novel treatments for such disorders.
Highlights.
Decreasing GABA function within the PFC decreased social behavior
Decreasing GABA function within the BLA decreased social behavior
These manipulations did not change measures of anxiety, reward or locomotion
Acknowledgments
The authors would like to thank James Medina, Gabriel Hitchcock, Ted Hunter and Tania Mukherjee for their help with scoring the videotapes.
A 2015–16 Research Status award from Oberlin College and NIH grant R15MH098246 supported this work; both were awarded to TAP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- 2.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 5.Green MF. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J Clin Psychiatry. 2016;77(Suppl 2):8–11. doi: 10.4088/JCP.14074su1c.02. [DOI] [PubMed] [Google Scholar]
- 6.Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neurosci Biobehav Rev. 2013;37:2166–2180. doi: 10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Corcoran CM, Kimhy D, Parrilla-Escobar MA, Cressman VL, Stanford AD, Thompson J, David SB, Crumbley A, Schobel S, Moore H, Malaspina D. The relationship of social function to depressive and negative symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2011;41:251–261. doi: 10.1017/S0033291710000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewandowski KE, Whitton AE, Pizzagalli DA, Norris LA, Ongur D, Hall MH. Reward Learning, Neurocognition, Social Cognition, and Symptomatology in Psychosis. Front Psychiatry. 2016;7:100. doi: 10.3389/fpsyt.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Fossati P. Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol. 2012;22(Suppl 3):S487–S491. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–194. doi: 10.1016/s0006-8993(03)02299-6. [DOI] [PubMed] [Google Scholar]
- 13.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 14.Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 1997;764:262–264. doi: 10.1016/s0006-8993(97)00594-5. [DOI] [PubMed] [Google Scholar]
- 15.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandhu KV, Lang D, Müller B, Nullmeier S, Yanagawa Y, Schwegler H, Stork O. Glutamic acid decarboxylase 67 haplodeficiency impairs social behavior in mice. Genes Brain Behav. 2014;13:439–450. doi: 10.1111/gbb.12131. [DOI] [PubMed] [Google Scholar]
- 17.Hanks AN, Dlugolenski K, Hughes ZA, Seymour PA, Majchrzak MJ. Pharmacological disruption of mouse social approach behavior: relevance to negative symptoms of schizophrenia. Behav Brain Res. 2013;252:405–414. doi: 10.1016/j.bbr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S, Featherstone RE, Ortinski PI, Gandal MJ, Lin R, Liang Y, Gur RE, Carlson GC, Hahn CG, Siegel SJ. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39:1603–1613. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielnik CA, Horsfall W, Ramsey AJ. Diazepam improves aspects of social behaviour and neuron activation in NMDA receptor-deficient mice. Genes Brain Behav. 2014;13:592–602. doi: 10.1111/gbb.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang K, Hill K, Labak S, Blatt GJ, Soghomonian JJ. Loss of glutamic acid decarboxylase (Gad67) in Gpr88-expressing neurons induces learning and social behavior deficits in mice. Neuroscience. 2014;275:238–247. doi: 10.1016/j.neuroscience.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Shekhar A, Katner JS. Dorsomedial hypothalamic GABA regulates anxiety in the social interaction test. Pharmacol Biochem Behav. 1995;50:253–258. doi: 10.1016/0091-3057(94)00307-5. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cellot G, Cherubini E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr. 2014;2:70. doi: 10.3389/fped.2014.00070. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bitanihirwe BK, Woo TU. Transcriptional dysregulation of γ-aminobutyric acid transporter in parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. Psychiatry Res. 2014;220:1155–1159. doi: 10.1016/j.psychres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curley AA, Eggan SM, Lazarus MS, Huang J, Volk DW, Lewis DA. Role of glutamic acid decarboxylase 67 in regulating cortical parvalbumin and GABA membrane transporter 1 expression: Implications for schizophrenia. Neurobiol Dis. 2013;50:179–186. doi: 10.1016/j.nbd.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, Greenstein D, Lenane M, Gochman P, Baker N, Balkissoon R, Vakkalanka RK, Weinberger DR, Rapoport JL, Straub RE. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Mol Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- 30.Benes FM. Amygdalocortical circuitry in schizophrenia: from circuits to molecules. Neuropsychopharmacology. 2010;35:239–257. doi: 10.1038/npp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf DH, Satterthwaite TD, Loughead J, Pinkham A, Overton E, Elliott MA, Dent GW, Smith MA, Gur RC, Gur RE. Amygdala abnormalities in first-degree relatives of individuals with schizophrenia unmasked by benzodiazepine challenge. Psychopharmacology (Berl) 2011;218:503–512. doi: 10.1007/s00213-011-2348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec (Hoboken) 2011;294:1646–1652. doi: 10.1002/ar.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. Erratum in: Autism Res. 2009 Aug;2(4):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori T, Mori K, Fujii E, Toda Y, Miyazaki M, Harada M, Hashimoto T, Kagami S. Evaluation of the GABAergic nervous system in autistic brain: (123)I-iomazenil SPECT study. Brain Dev. 2012;34:648–654. doi: 10.1016/j.braindev.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 36.Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, Matsuda T. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 2011;41:447–454. doi: 10.1007/s10803-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 37.Paine TA, Slipp LE, Carlezon WA., Jr Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parthoens J, Servaes S, Verhaeghe J, Stroobants S, Staelens S. Prelimbic Cortical Injections of a GABA Agonist and Antagonist: In Vivo Quantification of the Effect in the Rat Brain Using [(18)F] FDG MicroPET. Mol Imaging Biol. 2015;17:856–864. doi: 10.1007/s11307-015-0859-z. [DOI] [PubMed] [Google Scholar]
- 39.Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011;1:444–458. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council of the National Academies. Guide for the Care and Use of Laboratory Animals. 8. Washington (DC): National Academy Press; 2011. [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Amsterdam, Netherlands: Academic Press; 2009. [Google Scholar]
- 42.Lapiz-Bluhm MD, Bondi CO, Doyen J, Rodriguez GA, Bédard-Arana T, Morilak DA. Behavioural assays to model cognitive and affective dimensions of depression and anxiety in rats. J Neuroendocrinol. 2008;20:1115–1137. doi: 10.1111/j.1365-2826.2008.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- 44.Wöhr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 45.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 46.Wilson CA, Koenig JI. Social interaction and social withdrawal in rodents as readouts for investigating the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:759–773. doi: 10.1016/j.euroneuro.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solati J, Hajikhani R, Golub Y. Activation of GABAA receptors in the medial prefrontal cortex produces an anxiolytic-like response. Acta Neuropsychiatr. 2013;25:221–226. doi: 10.1111/acn.12016. [DOI] [PubMed] [Google Scholar]
- 48.Barnes SA, Der-Avakian A, Markou A. Anhedonia, avolition, and anticipatory deficits: assessments in animals with relevance to the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:744–758. doi: 10.1016/j.euroneuro.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piantadosi PT, Khayambashi S, Schluter MG, Kutarna A, Floresco SB. Perturbations in reward-related decision-making induced by reduced prefrontal cortical GABA transmission: Relevance for psychiatric disorders. Neuropharmacology. 2016;101:279–290. doi: 10.1016/j.neuropharm.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 51.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 52.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 53.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 54.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 55.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 56.Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull. 1986;17:321–333. doi: 10.1016/0361-9230(86)90237-6. [DOI] [PubMed] [Google Scholar]
- 57.Nicolas LB, Prinssen EP. Social approach-avoidance behavior of a high-anxiety strain of rats: effects of benzodiazepine receptor ligands. Psychopharmacology (Berl) 2006;184:65–74. doi: 10.1007/s00213-005-0233-y. [DOI] [PubMed] [Google Scholar]
- 58.Han S, Tai C, Jones CJ, Scheuer T, Catterall WA. Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–1289. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richetto J, Labouesse MA, Poe MM, Cook JM, Grace AA, Riva MA, Meyer U. Behavioral effects of the benzodiazepine-positive allosteric modulator SH-053-2′F-S-CH3 in an immune-mediated neurodevelopmental disruption model. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu055. pii: pyu055. [DOI] [PMC free article] [PubMed] [Google Scholar]