Abstract
Opioids are effective at inhibiting responses to noxious stimuli in rodents, but have limited efficacy and many side effects in chronic pain patients. One reason for this disconnect is that nociception is typically assessed using withdrawal from noxious stimuli in animals, whereas chronic pain patients suffer from abnormal pain that disrupts normal activity. We hypothesized that assessment of home cage wheel running in rats would provide a much more clinically relevant method to assess opioid efficacy to restore normal behavior. Intraplantar injection of Complete Freund’s Adjuvant (CFA) into the right hindpaw depressed wheel running and caused mechanical allodynia measured with the von Frey test in both male and female rats. Administration of an ED50 dose of morphine (3.2 mg/kg) reversed mechanical allodynia, but did not reverse CFA-induced depression of wheel running. In contrast, administration of a low dose of morphine (1.0 mg/kg) restored running for one hour in both sexes, but had no effect on mechanical allodynia. Administration of the atypical opioid buprenorphine had no effect on inflammation-induced depression of wheel running in male or female rats, but attenuated mechanical allodynia in male rats. Administration of buprenorphine and higher doses of morphine depressed wheel running in non-inflamed rats, suggesting that the side effects of opioids interfere with restoration of function. These data indicate that restoration of pain-depressed function requires antinociception in the absence of disruptive side effects. The disruptive side effects of opioids are consistent with the major limitation of opioid use in human pain patients.
Keywords: pain-depressed behavior, morphine, buprenorphine, sex differences, antinociception
Introduction
Opioids such as morphine are the most effective treatment for most types of pain [26], but opioid efficacy is limited by unpleasant (e.g., nausea, constipation) and dangerous (e.g., sedation, addiction) side effects [32]. Opioids are very effective at inhibiting responses to noxious stimuli in laboratory animals, but their antinociceptive effects are rarely weighed against their disruptive side effects. The main problem with most analgesic drug development research in animals is that nociception is assessed using withdrawal from a noxious stimulus [20]. This approach fails to address the functional consequences of pain such as disruption of normal activity - the primary problem for chronic pain patients [9,27]. As such, the goal of pain treatments should be to promote and restore normal function, not to completely inhibit nociception as in most animal studies [13,16]. Restoration of normal activity requires treatments that attenuate abnormal pain without producing disruptive side effects. Despite their analgesic efficacy, the side effects produced by opioids are a major limitation.
A number of tests of pain-depressed behaviors in rodents have been developed to more closely mimic the functional consequences of pain in humans. Pain-depressed behaviors are defined as behaviors that decrease in rate, frequency, or intensity in response to a noxious stimulus or pain state [23]. Previous animal studies have examined pain-induced depression of feeding [17,30], intracranial self-stimulation [23], attention [12], nesting [24], and wheel running [6]. We have recently shown that home cage wheel running is an objective and sensitive method to assess the functional consequences of inflammatory pain in male and female rats [16]. Continuous assessment of home cage wheel running allows for the duration and magnitude of pain to be assessed in laboratory rats [16] and mimics the reduction in activity in chronic pain patients [5,31]. Thus, home cage wheel running may be an especially effective method to determine whether treatments can restore normal activity by inhibiting pain in the absence of disruptive side effects.
Opioid efficacy has been shown to vary between tests of pain-evoked and pain-depressed behavior [1,2]. Morphine and buprenorphine are two widely used opioids for treating chronic pain [26]. The primary goal of the present study was to test the hypothesis that morphine and buprenorphine will be more effective at inhibiting pain-evoked behavior (von Frey test of mechanical allodynia) than restoring pain-depressed wheel running. Given that opioids produce greater antinociception in male compared to female rats [19], we also hypothesized that morphine and buprenorphine will be more effective at restoring wheel running in male compared to female rats.
Materials and Methods
Subjects
Data were collected from 112 adult male (mean weight: 295 g) and 117 female (mean weight: 214 g) Sprague-Dawley rats bred at Washington State University Vancouver (Vancouver, WA, USA). All rats were 50–90 days old at the start of the study and randomly assigned to treatment groups. Prior to experimentation, rats were housed in pairs in a 22–24 °C colony room on a 12/12-hour light/dark cycle (lights off at 1800 h). Each rat was moved to an extra tall cage (36 × 24 × 40 cm) with a running wheel. Six to twelve rats were tested at a time in a large sound-attenuating booth (2.1 × 2.2 m; Industrial Acoustics Company, Inc., Bronx, NY, USA). Food and water were available ad libitum except for one hour each day when the rat was removed from the cage to assess mechanical allodynia, inject drugs, or induce inflammation. All procedures were approved by the Washington State University Animal Care and Use Committee and conducted in accordance with the International Association for the Study of Pain’s Policies on the Use of Animals in Research.
Running wheel
A Kaytee Run-Around Giant Exercise Wheel (Kaytee Products, Inc., Chilton, WI) with a diameter of 27.9 cm was suspended from the top of the rat’s home cage. The floor of the cage was covered with cellulose bedding (BioFresh™, Ferndale, WA, USA). A 0.8 mm thick aluminum plate (5.08 cm × 3.81 cm; K&S Precision Metals, Chicago, IL, USA) was attached to one spoke of the running wheel to interrupt a photobeam projecting across the cage with each rotation. The beam was set 18 cm above the floor of the cage so that only the rotation of the wheel, not the normal activity of the rat, would interrupt the beam. The number of wheel revolutions were summed over 5 min bins for 23 hrs each day using Multi-Varimex software (Columbus Instruments, Columbus, OH, USA). Wheel running was assessed 23 hr/day beginning at 1700 h. The active dark phase of the light cycle began at 1800 h. A full description of the running wheel with video is available in a previous publication [16].
Opioid effects on allodynia and wheel running
Rats were allowed unrestricted access to the wheel for 23 hr/day for 8 days prior to induction of inflammation. Rats that ran less than 400 revolutions on the baseline day were not included in further testing [16]. Rats were removed from their home cage for approximately 50 min each day (1600 h to 1650 h) to assess mechanical allodynia using an electronic von Frey anesthesiometer (IITC Inc., ALMEMO® 2450, Woodland Hills, CA). The rat was placed in a Plexiglas chamber (22 cm × 22 cm × 12.8 cm) on an elevated mesh surface and allowed to habituate for approximately 15 min. Baseline von Frey measurements from the right hindpaw were obtained immediately before induction of hindpaw inflammation. The threshold at which a rat withdrew its hindpaw when the von Frey filament was applied to the plantar surface of the hindpaw was recorded in grams. The paw was tested 2 times with approximately one minute separating each trial. Nociceptive sensitivity was defined as the mean of 2 trials/hindpaw.
The number of wheel revolutions that occurred during the 23 hrs prior to induction of hindpaw inflammation was used as the baseline activity. At the end of the eighth day, the rat was removed from its home cage, briefly anesthetized with isoflurane, and injected with Complete Freund’s Adjuvant (CFA; 0.1 mL; Sigma-Aldrich, St. Louis, MO, USA) into the right hindpaw using a 30-gauge needle. Control animals were anesthetized and injected with saline (Hospira Inc, Lake Forest, IL, USA) into the right hindpaw. The rat was returned to its home cage at 1650 h and wheel running was measured for 23 hrs beginning at 1700 h.
Twenty-three hours later, the rat was removed from its home cage and mechanical allodynia was assessed using the von Frey test. Immediately after, the rat was injected with one of the third log doses of morphine (0.32, 1.0, or 3.2 mg/kg, s.c.; n = 6–14 per group), buprenorphine (0.032, 0.1, or 0.32 mg/kg, s.c.; n = 6–11 per group), or saline (1 mL/kg; n = 9–14 per group). Mechanical allodynia was assessed again 30 min after opioid administration. The rat was returned to its home cage at approximately 1650 h and wheel running was measured for 23 hrs beginning at 1700 h.
Data analysis
All data are expressed as mean ± SEM except where stated. Baseline activity was defined as the total number of wheel revolutions during the 23 h preceding injection of CFA. Given individual differences in wheel running, subsequent wheel running data are presented as a percent change from each rat’s baseline value. The percent change in wheel running following CFA or saline administration was analyzed using a 2-way repeated measures ANOVA (CFA/saline × day). Given that the peak time for morphine antinociception is 30 – 60 min in the rat, a one-way ANOVA was used to compare the effects of opioid dose on wheel running during the hour following administration. CFA and opioid administration did not alter withdrawal thresholds in the uninjected (left) paw so only data obtained from the injected (right) paw are presented. The mean thresholds for the von Frey test were analyzed using a one-way ANOVA on the post-opioid administration data. Statistical significance was defined as a probability of <0.05.
Results
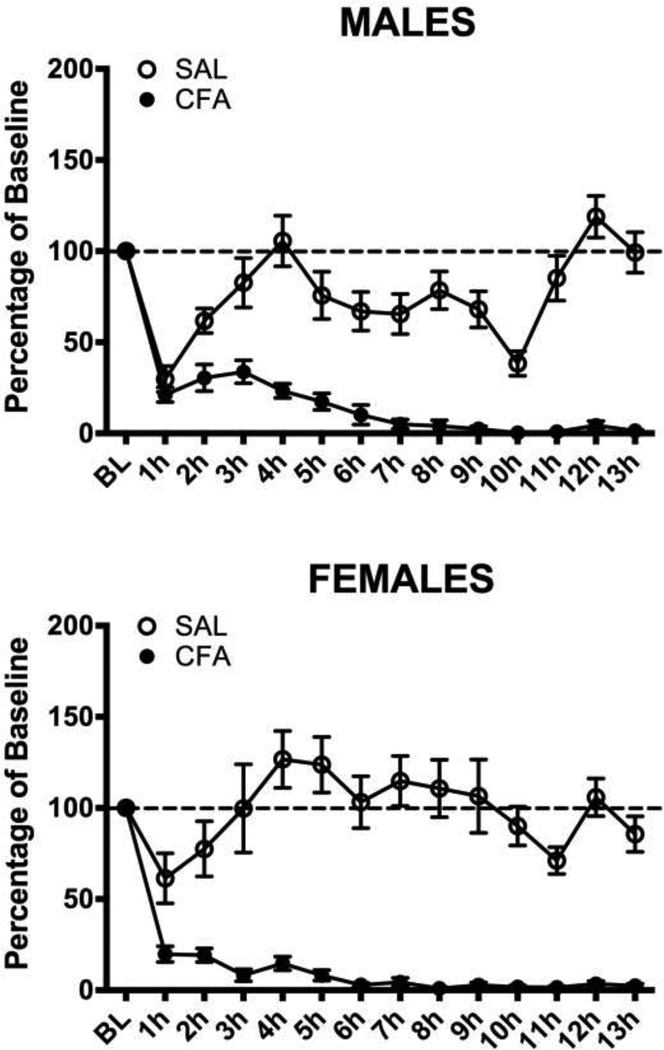
Consistent with our previous paper [16], female rats had significantly higher levels of baseline running than male rats (Mean = 4898 vs. 1317 revolutions/day; t(227)=−9.692, p<.001). Administration of CFA into the right hindpaw caused a significant decrease in wheel running in both male (F(1,110) = 184.294, p<.001) and female (F(1,115) = 79.310, p<.001) rats compared to saline-treated controls. Running dropped dramatically immediately following the injection and continued to decrease until wheel running was almost completely inhibited 6 – 7 hrs after CFA administration (Fig. 1). Injection of saline into the hindpaw of control rats caused a transient depression of wheel running in male and female rats that returned to baseline levels in less than 3 hours.
Figure 1. Hindpaw inflammation decreases wheel running in male and female rats.
CFA or saline was injected into the right hindpaw of male (Top) and female (Bottom) rats. Injection of saline caused a transient decrease in running that returned to baseline levels within 3 hrs. In contrast, injection of CFA caused a complete and prolonged inhibition of wheel running in both male and female rats. Sample sizes ranged from 49 – 68 rats in each condition. The sample sizes are large because these rats were subsequently tested with different opioid doses.
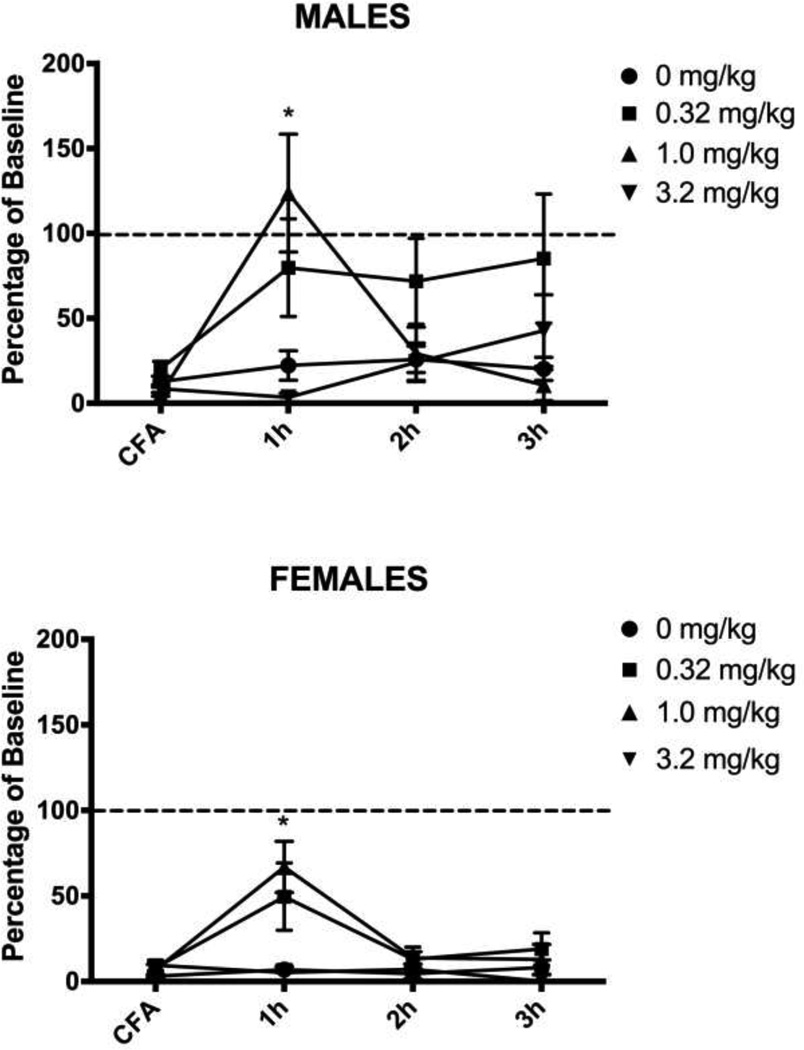
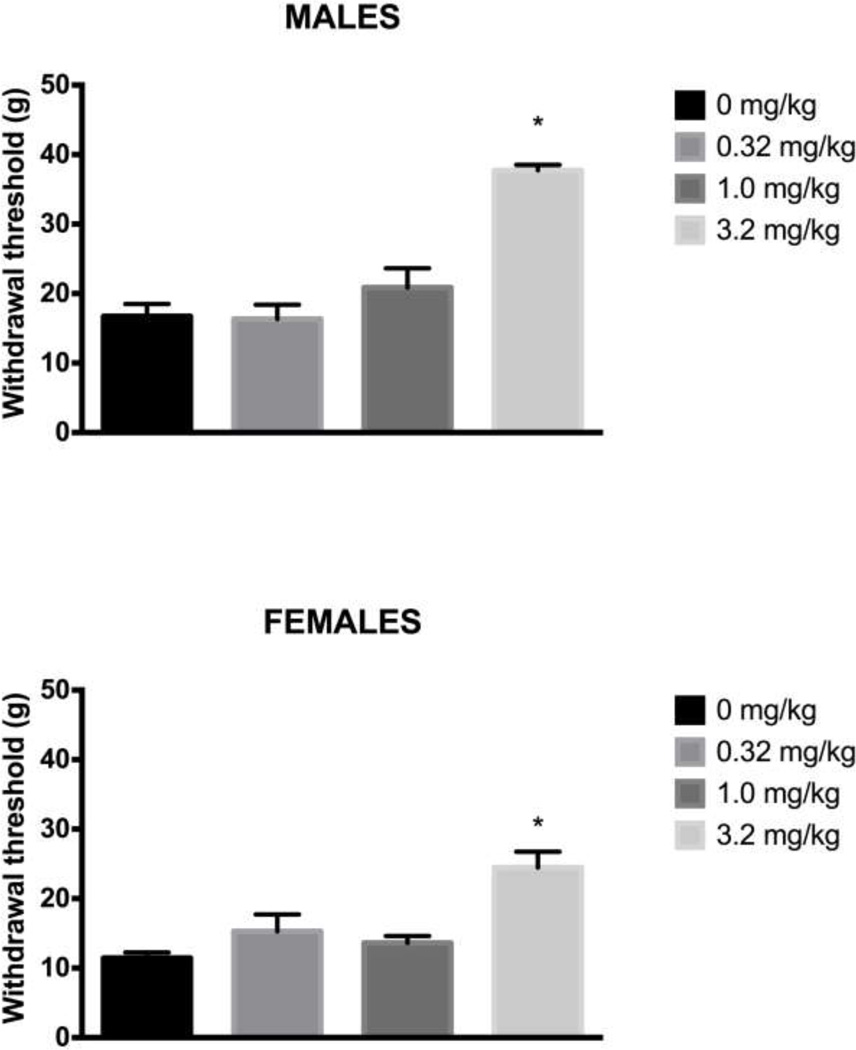
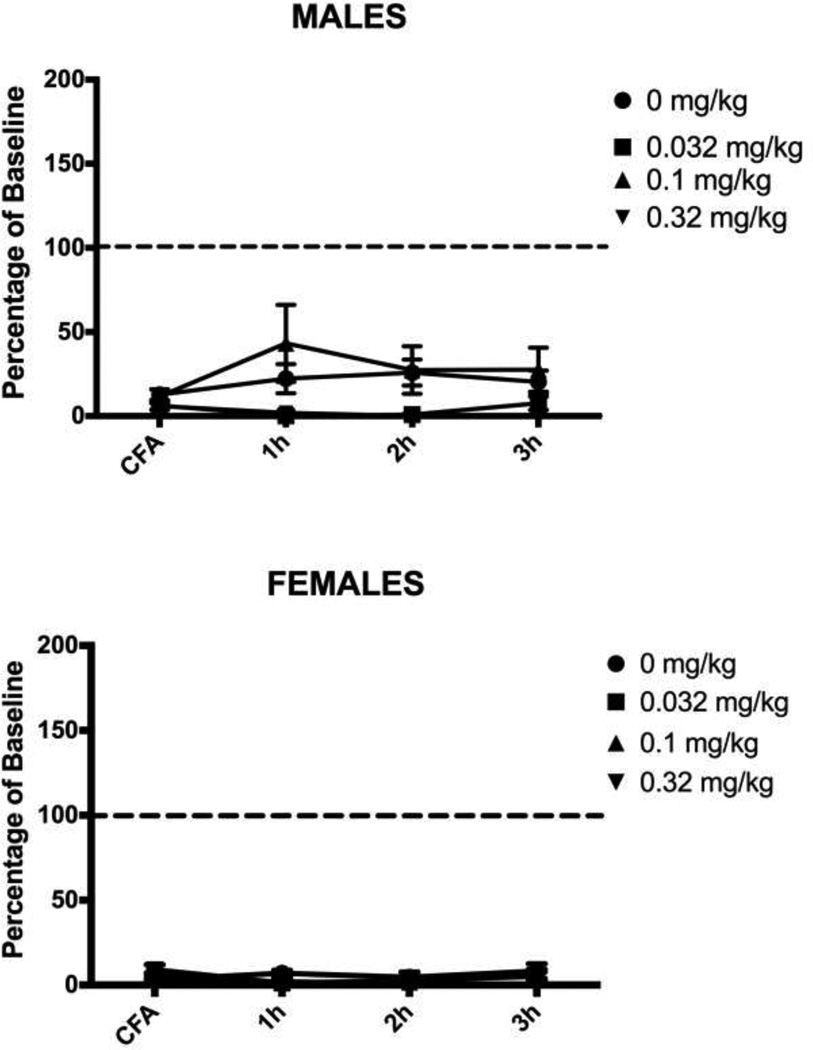
Administration of morphine 24 hrs after induction of hindpaw inflammation caused a significant main effect on wheel running in male (F(3,29) = 5.439, p = .004) and female (F(3,27) = 5.241, p = .006) rats. Very little running occurred in CFA-treated rats injected with saline. Injection of 1.0 mg/kg of morphine transiently reversed inflammation-induced depression of wheel running in both male (Tukey test, p = .024) and female (Tukey test, p = .017) rats. This antinociceptive effect persisted for one hour, after which CFA-induced depression of wheel running was evident again (Fig. 2). Administration of a very low dose of morphine (0.32 mg/kg) had mixed effects. This dose of morphine restored running in 7 of 10 male and 4 of 9 female rats, but inflammation–induced depression of wheel running was unaffected in the remaining rats (i.e., running below 15% of baseline levels). The highest (3.2 mg/kg) dose of morphine did not reverse inflammation-induced depression of wheel running despite blocking mechanical allodynia assessed with the von Frey test (Male: F(3,29) = 27.882, p < .001; Female: F(3,27) = 9.015, p < .001). The two lower doses of morphine had no effect on CFA-induced mechanical allodynia (Fig. 3).
Figure 2. Low doses of morphine briefly restore inflammation-depressed wheel running.
Male and female rats injected with 0.32 or 1.0 mg/kg of morphine 24 hours after CFA administration showed an increase in wheel running during the hour following the injection. Only the 1.0 mg/kg dose of morphine reached statistical significance compared to the vehicle-treated controls, but analysis of individual rats showed that injection of 0.32 mg/kg of morphine restored running in 7 of 10 male rats and 4 of 9 female rats. Rats injected with the highest dose of morphine (3.2 mg/kg) did not differ from rats receiving saline – running was depressed in both groups. Sample sizes ranged from 6 – 10 rats/condition. * indicates p<.05 from saline-treated animals.
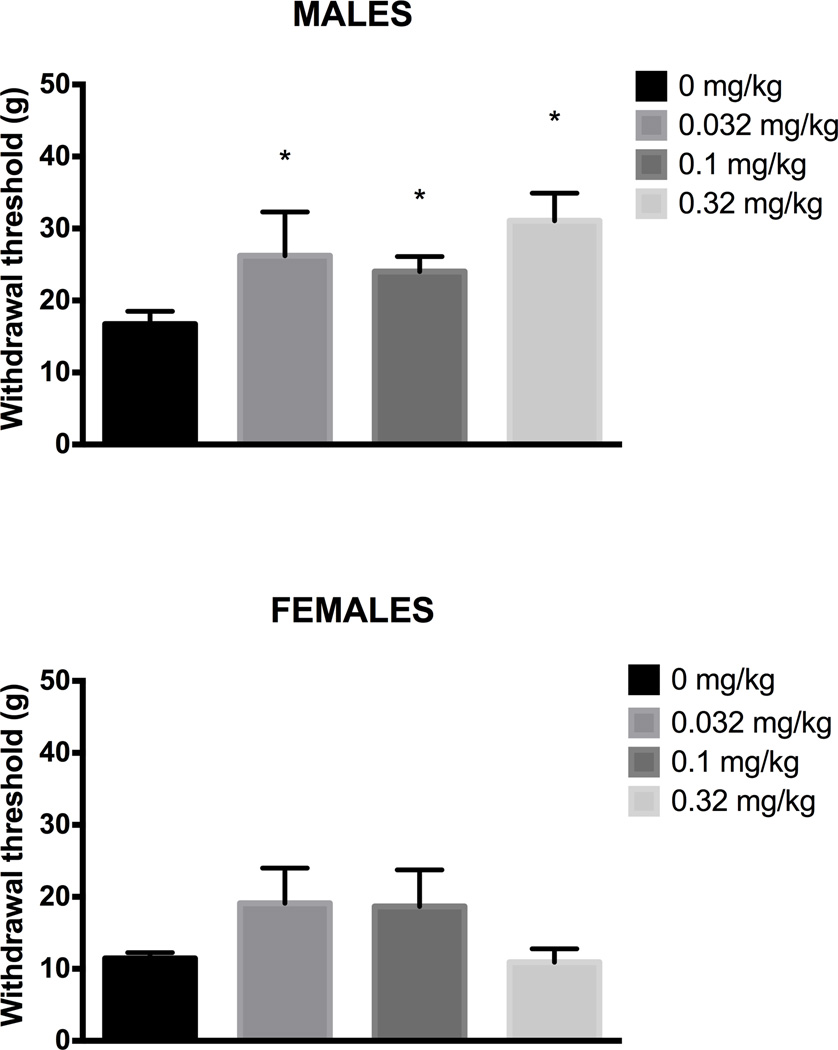
Figure 3. Only the highest dose of morphine reverses CFA-induced mechanical allodynia.
Mechanical allodynia measured with the von Frey test was assessed 30 min after morphine administration. Administration of 3.2 mg/kg, but not lower doses of morphine reduced CFA-induced mechanical allodynia in male (Top) and female (Bottom) rats. The two lowest doses of morphine had no effect on CFA-induced mechanical allodynia compared to saline-treated animals. The rats and sample sizes are the same as in Figure 2. * indicates p<.05 from saline-treated animals.
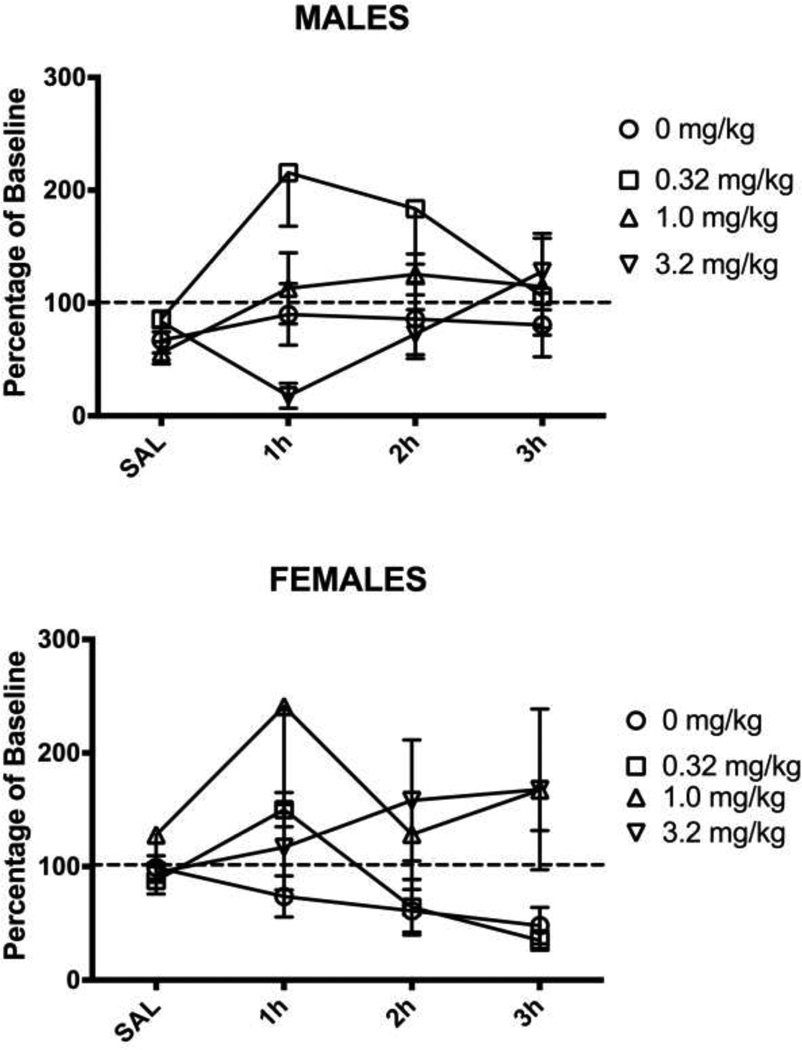
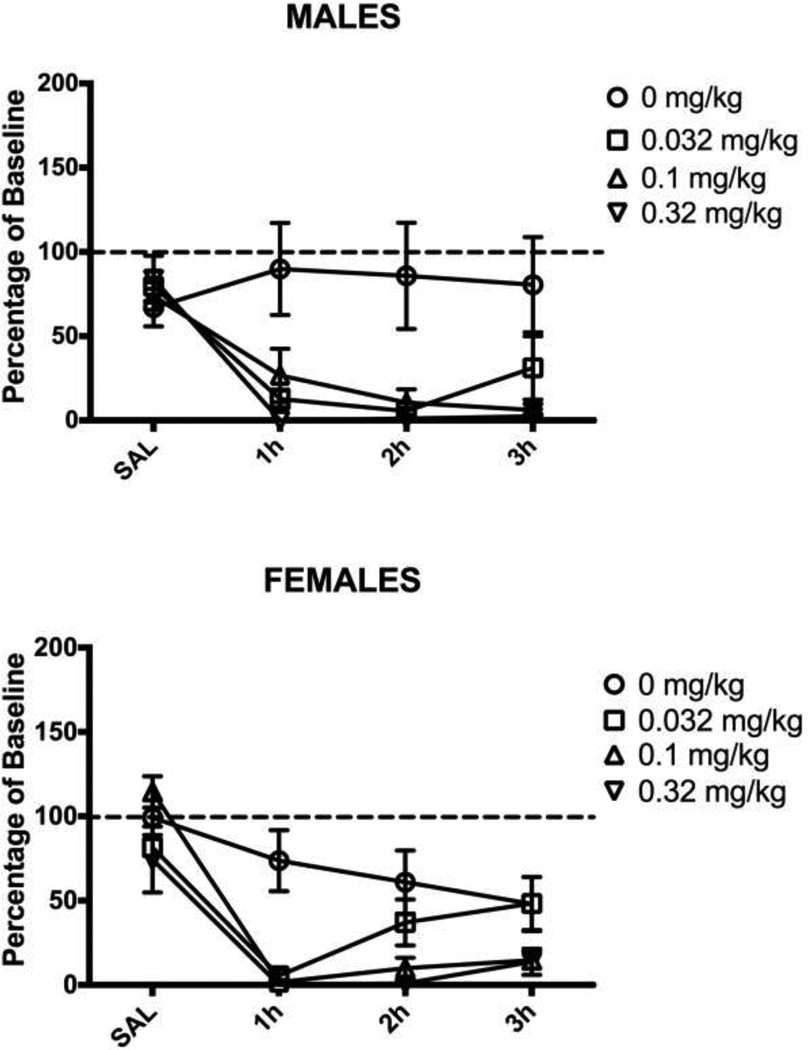
The finding that 3.2 mg/kg of morphine had no effect on inflammation-induced wheel running, but did reverse mechanical allodynia indicates that disruptive side effects prevent wheel running. This hypothesis was tested by administering the same doses of morphine to male and female rats treated with saline instead of CFA into the hindpaw. Morphine had a dose-dependent effect on wheel running during the first hour following administration in these normal male rats (F(3,30) = 4.949, p = .007; Fig. 4). In contrast, administration of morphine had no consistent effect on wheel running in pain-free female rats regardless of dose (F(3,42) = 1.585, n.s.; Fig. 4).
Figure 4. Administration of morphine depresses running in uninjured male rats.
Administration of 3.2 mg/kg of morphine depressed running for about one hour in uninjured male rats. Administration of 0.32 mg/kg increased running. Administration of the same doses of morphine had no effect on wheel running in female rats. There was no significant effect of injecting 1.0 mg/kg of morphine on running in either male or female rats. Sample sizes ranged from 6 – 14 rats/condition.
Administration of buprenorphine had no effect on pain-depressed wheel running in male or female rats (Fig. 5). Wheel running was depressed 24 hrs after CFA administration whether male (F(3,28) = 1.817, n.s.) or female rats were injected with buprenorphine or not. The lack of variability in female rats prevented parametric analysis of these data, but the magnitude of the depression of wheel running is evident in all four groups (Fig. 5). All three doses of buprenorphine attenuated mechanical allodynia measured with the von Frey test in the male rats (F(3,28) = 3.313, p = 0.034), but had no effect in female rats (Fig. 6; F(3,24) = 1.965, n.s.). Administration of the same doses of buprenorphine produced prolonged depression of wheel running in normal male (F(3,27) = 7.868, p = .001) and female rats (Fig. 7; F(3,32) = 5.564, p = .003).
Figure 5. Buprenorphine does not restore inflammation-induced wheel running.
Male and female rats were injected with buprenorphine 24 hours after induction of inflammation. There was no effect of buprenorphine in male or female rats regardless of dose. Sample sizes ranged from 6 – 11 rats/condition.
Figure 6. Buprenorphine attenuates mechanical allodynia in male rats.
Mechanical allodynia was assessed using the von Frey test 30 min after buprenorphine administration. Administration of buprenorphine blocked mechanical allodynia in male rats compared to saline treated control rats. There was no significant effect of injecting buprenorphine on mechanical allodynia in female rats. The same rats were tested here and in Figure 4. * indicates p<.05 from saline-treated animals.
Figure 7. Buprenorphine depresses wheel running in uninjured rats.
Uninjured male and female rats were injected with buprenorphine. All doses of buprenorphine depressed running in normal healthy male and female rats. Sample sizes ranged from 6 – 8 rats/condition.
Discussion
The present data show that neither morphine nor buprenorphine, when given at analgesic doses, were effective in restoring pain-depressed wheel running. Low doses of morphine (0.32 and 1.0 mg/kg) restored wheel running, but these doses did not inhibit mechanical allodynia measured with the von Frey test. This discrepancy between morphine doses that restore wheel running and those that inhibit mechanical allodynia indicate that the lack of restoration of wheel running is not because these opioids lack antinociceptive efficacy, but because of disruptive side effects. The inhibition of wheel running caused by opioid administration in normal uninjured male and female rats demonstrates that the side effects caused by opioids can be as disruptive as the pain it is supposed to treat.
Morphine and buprenorphine are among the most commonly used opioids to treat pain [26]. Comparative studies in humans indicate that buprenorphine produces greater pain relief than morphine with a longer duration of action [15], but both drugs have an extensive side effect profile that limits their use [15]. The antinociceptive effects of these drugs in rodents have been well characterized. Both morphine and buprenorphine produce antinociception following systemic administration with the duration of antinociception longer following buprenorphine than morphine administration [21]. Our findings indicate that while both opioids have anti-allodynic effects at 30 min post-administration (Fig. 3 and 6), the doses that inhibit allodynia are not effective at restoring pain-depressed function. These differences in opioid efficacy when nociception is assessed using pain-evoked vs. pain-depressed behavioral measures raise important questions about the clinical relevance of most drug development studies in animals.
There are many advantages to home cage wheel running to assess nociception, but it is susceptible to other disabling health conditions (e.g., illness and movement disorders) and can only be used if animals demonstrate sufficient baseline levels of running. The lack of restoration of wheel running following higher doses of morphine and buprenorphine administration can be explained by adverse side effects. Opioids are known to cause a wide range of side effects (e.g., sedation, dysphoria, nausea), any of which could interfere with restoration of wheel running. Consistent with our data, low but not high doses of morphine restores pain-depressed intracranial self-stimulation [18]. Adverse side effects of opioids are a well-known clinical problem. The use of home cage wheel running provides a method to evaluate treatments for both analgesic efficacy and adverse side effects. The use of peripherally restricted opioid agonists is one approach to inhibit nociception with minimal central nervous system-mediated side effects [28].
Most animal research focuses exclusively on the antinociceptive effects of treatments without regard to the side effects that may co-occur. The limited opioid efficacy in restoring pain-depressed wheel running is consistent with the large side effect profile of opioids in humans [4]. Testing the effects of opioids in uninjured male rats showed that increasing the dose of morphine caused a decrease in wheel running. A decrease in wheel running was especially pronounced following buprenorphine administration. These data demonstrate the difficulty in finding a dose that blocks abnormal pain without producing disruptive side effects. The ideal morphine dose to restore function was one that did not reverse mechanical allodynia nor depress wheel running when administered to normal rats. This finding seems to be consistent with clinical observations. That is, although opioids are effective in reducing chronic pain, the effects on function [14,32] or overall quality of life [10,25] are surprisingly limited.
As has been reported previously, both the antinociceptive and side effects of morphine tended to be greater in male compared to female rats [8,19]. This sex difference is apparent in the present study as low doses of morphine restored pain-depressed wheel running to a greater extent in male than female rats. Male and female rats differ considerably in baseline running rates as has been reported by others [11,16]. Thus, assessing restoration of wheel running as a percent of baseline masks the fact that the actual amount of running following administration of low doses of morphine was greater in female compared to male rats. Of course, comparing the antinociceptive efficacy of morphine in male and female rats is of limited value because so many factors (e.g., metabolism, hormone levels, opioid receptor distribution) influence antinociceptive efficacy [7,19]. Although changes in hormone levels across the female cycle have been shown to influence wheel running [29,33], the near complete inhibition of wheel running caused by hindpaw inflammation likely overwhelms any estrous cycle-related effects. The more important question is whether opioids restore function in each sex. Our results indicate that the antinociceptive and side effects of opioids are greater in male compared to female rats. For example, buprenorphine only reversed mechanical allodynia in male rats.
In sum, these results indicate that restoration of pain-depressed function in rodents and humans requires maximizing the antinociceptive efficacy and minimizing disruptive side effects. Furthermore, these data indicate that pain-depressed wheel running may be an especially useful tool for analgesic drug discovery and development because a treatment that is effective in restoring pain-depressed wheel running must produce antinociception without disabling side effects such as dysphoria or sedation. Although the present study does not indicate which component (e.g., sensory-discriminative or affective) causes depression of function, home cage wheel running could be used to determine whether blockade of the affective dimension of pain is sufficient to restore wheel running as has been shown with studies of pain using conditioned place preference [3,22]. Most preclinical studies use mechanical and/or thermal hypersensitivity to assess chronic pain in rodents [20], but this approach is limited in that the functional consequences of the treatment is not assessed. The side effects that disrupt normal activity have surely limited translation of potential pain treatments from the laboratory to the clinic. Pain-depressed home cage wheel running overcomes this problem by simultaneously assessing antinociception and side effects.
Highlights.
Home cage wheel running can be used to assess pain and opioid analgesia
Low doses of morphine reversed pain-depressed wheel running only
High doses of morphine reversed evoked hypersensitivity only
All buprenorphine doses depressed wheel running in all rats
Wheel running reveals limitations of opioids to restore normal activity
Acknowledgments
The authors thank Courtney Miskell, Andrea Lee, and Shauna Schoo for technical assistance. This investigation was supported in part by funds provided by medical and biological research by the State of Washington Initiative Measure No. 171 to RK and NIH grant NS095097 to MMM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors declare a conflict of interest.
References
- 1.Altarifi AA, Negus SS. Differential tolerance to morphine antinociception in assays of pain-stimulated vs. pain-depressed behavior in rats. European Journal of Pharmacology. 2015;748:76–82. doi: 10.1016/j.ejphar.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altarifi AA, Rice KC, Negus SS. Effects of μ-opioid receptor agonists in assays of acute pain-stimulated and pain-depressed behavior in male rats: role of μ-agonist efficacy and noxious stimulus intensity. Journal of Pharmacology and Experimental Therapeutics. 2015;352:208–217. doi: 10.1124/jpet.114.219873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auvray M, Myin E, Spence C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci Biobehav Rev. 2010;34:214–223. doi: 10.1016/j.neubiorev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 5.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Craft RM, Clark JL, Hart SP, Pinckney MK. Sex differences in locomotor effects of morphine in the rat. Pharmacology, Biochemistry and Behavior. 2006;85:850–858. doi: 10.1016/j.pbb.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currow DC, Phillips J, Clark K. Using opioids in general practice for chronic non-cancer pain: an overview of current evidence. Med. J. Aust. 2016;204:305–309. doi: 10.5694/mja16.00066. [DOI] [PubMed] [Google Scholar]
- 10.Daitch J, Frey ME, Silver D, Mitnick C, Daitch D, Pergolizzi J. Conversion of chronic pain patients from full-opioid agonists to sublingual buprenorphine. Pain Physician. 2012;15:ES59–ES66. [PubMed] [Google Scholar]
- 11.Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol. Behav. 1988;43:625–630. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- 12.Freitas KC, Hillhouse TM, Leitl MD, Negus SS. Effects of acute and sustained pain manipulations on performance in a visual-signal detection task of attention in rats. Drug Dev. Res. 2015;76:194–203. doi: 10.1002/ddr.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatti A, Dauri M, Leonardis F, Longo G, Marinangeli F, Mammucari M, Sabato AF. Transdermal buprenorphine in non-oncological moderate-to-severe chronic pain. Clin Drug Investig. 2010;30(Suppl 2):31–38. doi: 10.2165/1158409-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Hook MA, Moreno G, Woller S, Puga D, Hoy K, Balden R, Grau JW. Intrathecal morphine attenuates recovery of function after a spinal cord injury. J. Neurotrauma. 2009;26:741–752. doi: 10.1089/neu.2008.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovell BC, Ward AE. Pain relief in the post-operative period: a comparative trial of morphine and a new analgesic buprenorphine. J. Int. Med. Res. 1977;5:417–421. doi: 10.1177/030006057300100206. [DOI] [PubMed] [Google Scholar]
- 16.Kandasamy R, Calsbeek JJ, Morgan MM. Home cage wheel running is an objective and clinically relevant method to assess inflammatory pain in male and female rats. J. Neurosci. Methods. 2016;263:115–122. doi: 10.1016/j.jneumeth.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwilasz AJ, Negus SS. Dissociable effects of the cannabinoid receptor agonists Δ9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. Journal of Pharmacology and Experimental Therapeutics. 2012;343:389–400. doi: 10.1124/jpet.112.197780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitl MD, Negus SS. Pharmacological modulation of neuropathic pain-related depression of behavior: effects of morphine, ketoprofen, bupropion and [INCREMENT]9-tetrahydrocannabinol on formalin-induced depression of intracranial self-stimulation in rats. Behavioural Pharmacology. 2015:1. doi: 10.1097/FBP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J. Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 21.Morgan MM, Reid RA, Stormann TM, Lautermilch NJ. Opioid selective antinociception following microinjection into the periaqueductal gray of the rat. J Pain. 2014;15:1102–1109. doi: 10.1016/j.jpain.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J. Neurosci. 2015;35:7264–7271. doi: 10.1523/JNEUROSCI.3862-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negus SS. Expression and treatment of pain-related behavioral depression. Lab Anim (NY) 2013;42:292–300. doi: 10.1038/laban.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, Schoelles KM. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006605.pub2. CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pergolizzi J, Böger RH, Budd K, Dahan A, Erdine S, Hans G, Kress H-G, Langford R, Likar R, Raffa RB, Sacerdote P. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone) Pain Pract. 2008;8:287–313. doi: 10.1111/j.1533-2500.2008.00204.x. [DOI] [PubMed] [Google Scholar]
- 27.Rabbitts JA, Holley AL, Groenewald CB, Palermo TM. Association Between Widespread Pain Scores and Functional Impairment and Health-Related Quality of Life in Clinical Samples of Children. J Pain. 2016;17:678–684. doi: 10.1016/j.jpain.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Physician. 2011;14:249–258. [PubMed] [Google Scholar]
- 29.Steiner M, Katz RJ, Carroll BJ. Detailed analysis of estrous-related changes in wheel running and self-stimulation. Physiol. Behav. 1982;28:201–204. doi: 10.1016/0031-9384(82)90127-5. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson GW, Bilsky EJ, Negus SS. Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. The Journal of Pain. 2006;7:408–416. doi: 10.1016/j.jpain.2006.01.447. [DOI] [PubMed] [Google Scholar]
- 31.Tierney M, Fraser A, Kennedy N. Physical activity in rheumatoid arthritis: a systematic review. J Phys Act Health. 2012;9:1036–1048. doi: 10.1123/jpah.9.7.1036. [DOI] [PubMed] [Google Scholar]
- 32.Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L. Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain Physician. 2008;11:S181–S200. [PubMed] [Google Scholar]
- 33.Wollnik F, Turek FW. Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats. Physiol. Behav. 1988;43:389–396. doi: 10.1016/0031-9384(88)90204-1. [DOI] [PubMed] [Google Scholar]