Abstract
Context
Little is known about the phenotypic and molecular characteristics associated with changes over time in fatigue and lack of energy in patients with breast cancer.
Objectives
To identify subgroups (i.e., latent classes) of women with distinct fatigue and energy trajectories; evaluate for differences in phenotypic characteristics between the latent classes for fatigue and energy; and evaluate for associations between polymorphisms in genes for pro- and anti-inflammatory cytokines, their receptors, and their transcriptional regulators and latent class membership.
Methods
Patients were enrolled prior to and followed for six months after breast cancer surgery. Latent class analyses were done to identify subgroups of patients with distinct fatigue and energy trajectories. Candidate gene analyses were done to identify cytokine genes associated with these two symptoms.
Results
For both fatigue and lack of energy, two distinct latent classes were identified. Phenotypic characteristics associated with the higher fatigue class were: younger age, higher education, lower KPS score, higher comorbidity, higher number of lymph nodes removed, and receipt of chemotherapy (CTX). Polymorphisms in interleukin (IL) 1 beta and IL10 were associated with membership in the higher fatigue class. Phenotypic characteristics associated with the lower energy class included: a lower KPS score and a higher comorbidity score. A polymorphism in IL1R1 was associated with membership in the lower energy class.
Conclusion
Within each latent class, the severity of fatigue and decrements in energy were relatively stable over the first six months following breast cancer surgery. Distinct phenotypic characteristics and genetic polymorphisms were associated with membership in the higher fatigue and lower energy classes.
Keywords: fatigue, energy, breast cancer, cytokine genes, growth mixture modeling, symptom trajectories
INTRODUCTION
Fatigue is the most common symptom reported by patients who undergo treatment for breast cancer.(1) While over 90% of patients diagnosed with breast cancer will undergo surgery, only a limited number of longitudinal studies have evaluated for preoperative levels of fatigue and changes in fatigue following surgery.(2–5) Across these studies, a variety of demographic (e.g., younger age (3)) and clinical (e.g., partial mastectomy (6), poorer functional status (4, 6)) characteristics, as well as psychological factors (e.g., pre-surgical expectancies for fatigue (3), introversion (5)) were associated with higher levels of fatigue following surgery. In these studies, the length of post-surgical follow-up ranged from one week (3) to one year (6). Only one study was identified that used a type of growth mixture modeling (GMM) to identify subgroups of breast cancer patients (n=290) with distinct fatigue trajectories.(2) Using fatigue assessments done prior to and at 4 and 5 months after surgery, groups of patients with persistently high (21%) and persistently low (79%) levels of fatigue were identified. Patients in the high group were more likely to report lower levels of physical activity and higher levels of anxiety. No demographic or clinical characteristics were associated with membership in the higher fatigue group. While this study provides important information on persistent fatigue, only three assessments were done (i.e., 4 weeks prior to surgery and 4 and 8 months after surgery) and the confidence intervals (CI) were wide which suggests that a larger sample could result in more reliable estimates.
In oncology, fatigue is defined as a distressing, persistent sense of physical, emotional, and/or cognitive tiredness for exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.(7) In contrast, energy can be defined as an individual’s potential to perform physical and mental activity.(8) As noted in our previous publication,(9) an increasing body of evidence suggests that fatigue and energy are distinct but related constructs.(8, 10, 11) For example, instruments like the Profile of Mood States (POMS)(12) have separate scales for fatigue-inertia and energy-vigor. The energy subscale of the POMS evaluates the intensity of energy using a variety of descriptors (e.g., energetic, full of pep). Like the POMS, the Lee Fatigue Scale (LFS) has two subscales (i.e., a fatigue subscale with 13 items and an energy subscale with five items). The LFS asks participants to rate their level of energy using a 0 to 10 numeric rating scale (NRS) on five descriptors (i.e., energetic, active, vigorous, efficient, lively). The original psychometric evaluation of the LFS identified these two distinct subscales.(13) In addition, a recent Rasch analysis of the LFS found that fatigue and energy represented different symptoms.(14) Only one study was identified that evaluated decrements in energy in patients prior to breast cancer surgery.(15) In this study, while 32% of the women reported clinically meaningful levels of fatigue, nearly 50% of the patients reported clinically meaningful decrements in energy levels prior to surgery. No studies were identified that evaluated for subgroups of patients with distinct energy trajectories from prior to through six months following breast cancer surgery.
While some progress has been made in evaluating the role of inflammatory mediators in the development and maintenance of fatigue, recent reviews of fatigue in patients with cancer recommend that additional research be done to establish the underlying mechanisms for fatigue.(1, 16, 17) In all of these reviews, emphasis is placed on understanding the molecular mechanisms that underlie the development of persistent fatigue in oncology patients.
Cytokines, their receptors and transcriptional regulators are one class of polypeptides that mediate inflammatory processes (for reviews see (18–20)). In several studies,(21–24) genetic and epigenetic mechanisms involved in inflammation were associated with fatigue in oncology patients. Only one study was found that reported on associations between polymorphisms in cytokine genes and decrements in energy in oncology patients undergoing radiation therapy and their family caregivers.(9) This preliminary evidence provides support for the role of molecular mechanisms involved in inflammatory processes, particularly cytokines, in the etiology of fatigue and decrements in energy in oncology patients.
Given the paucity of longitudinal studies that aimed to determine distinct phenotypes for fatigue and energy following breast cancer surgery and the need for more molecular-based studies, the purposes of this study, in a sample of women (n=398) who were assessed prior to and monthly for six months following surgery, were to: identify subgroups (i.e., latent classes) of women with distinct fatigue and energy trajectories; evaluate for differences in phenotypic characteristics between the latent classes for fatigue and energy; and evaluate for associations between polymorphisms in genes for pro- and anti-inflammatory cytokines, their receptors, and their transcriptional regulators and latent class membership. We hypothesized that at least two latent classes would be identified for each symptom using GMM and distinct phenotypic characteristics and genetic polymorphisms would be associated with higher fatigue and lower energy latent class membership.
MATERIALS AND METHODS
Patients and Settings
This analysis is part of a larger, longitudinal study that evaluated neuropathic pain and lymphedema in women who underwent breast cancer surgery. The study methods are described in detail elsewhere.(25–28) In brief, patients were recruited from breast care centers located in a Comprehensive Cancer Center, two public hospitals, and four community practices.
Patients were eligible to participate if they: were adult women (≥18 years) who were scheduled to undergo unilateral breast cancer surgery; were able to read, write, and understand English; agreed to participate; and gave written informed consent. Patients were excluded if they were having bilateral breast cancer surgery or had distant metastasis at the time of diagnosis. A total of 516 patients were approached, 410 were enrolled (response rate 79.5%), and 398 completed the enrollment assessment. The most common reasons for refusal were: too busy, overwhelmed with the cancer diagnosis, or insufficient time available to do the enrollment assessment prior to surgery.
Instruments
The demographic questionnaire obtained information on age, marital status, education, ethnicity, employment status, and living situation. Patients rated their functional status using the Karnofsky Performance Status (KPS) scale that ranged from 30 (I feel severely disabled and need to be hospitalized) to 100 (I feel normal; I have no complaints or symptoms).(29) The Self-Administered Comorbidity Questionnaire (SCQ) was used to evaluate comorbidity.(30) Patients were asked to indicate if they had one of 13 common medical conditions; if they received treatment for it (proxy for disease severity); and did it limit their activities (indication of functional limitations). For each condition, a patient can receive a maximum of 3 points. The total SCQ score can range from 0 to 39 points. The SCQ has well-established validity and reliability.(31, 32)
The Lee Fatigue Scale (LFS) consists of 18 items designed to assess physical fatigue and energy.(13) Each item was rated on a 0 to 10 NRS. Total fatigue and energy scores were calculated as the mean of the 13 fatigue items and the 5 energy items, with higher scores indicating greater fatigue severity and higher levels of energy. Patients were asked to rate each item based on how they felt “right now”. The LFS has been used with healthy individuals (13, 33) and in patients with cancer and HIV.(34–37) Cutoff scores of ≥4.4 and ≤4.8 indicate clinically meaningful levels of fatigue severity and low levels of energy, respectively.(38) The LFS has well established validity and reliability. (13, 33) In the current study, Cronbach’s alphas for the fatigue and energy scales were .96 and .93, respectively.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco and by the Institutional Review Boards at each of the study sites. During the preoperative visit, a clinician explained the study; determined the patient’s willingness to participate; and introduced her to the research nurse. The research nurse met with the woman, determined eligibility, and obtained written informed consent prior to surgery. After obtaining consent, patients completed the enrollment questionnaires an average of 4 days prior to surgery. Patients completed the LFS at enrollment and monthly for 6 months (i.e., 7 assessments). Medical records were reviewed for disease and treatment information.
Genomic analyses
Gene selection
Cytokines, their receptors, and their transcriptional regulators are classes of polypeptides that mediate pro- and anti-inflammatory processes. Cytokine dysregulation is associated with fatigue (for reviews see (1, 17, 39)). Pro-inflammatory genes promote systemic inflammation and include: chemokine (C-C-C motif) ligand 8 (CXCL8, previous gene symbol interleukin 8 (IL8)), interferon gamma (IFNG), IFNG receptor 1 (IFNGR1), IL1 receptor 1 (IL1R1), IL2, IL17A, and members of the tumor necrosis factor (TNF) family (i.e., lymphotoxin alpha (LTA), TNF). Anti-inflammatory genes suppress the activity of pro-inflammatory cytokines and include: IL1R2, IL4, IL10, and IL13. Of note, IFNG1, IL1B, and IL6 possess pro- and anti-inflammatory functions. Nuclear factor kappa beta 1 (NFKB1) and NFKB2 are transcriptional regulators of these cytokine genes.(40) All genes were identified according to the approved symbol stored in the Human Genome Organization (HUGO) Gene Nomenclature Committee (HGNC) database (http://www.genenames.org).
Blood collection and genotyping
Of the 398 patients who completed the enrollment assessment, 310 provided a blood sample from which deoxyribonucleic acid (DNA) could be isolated from peripheral blood mononuclear cells (PBMCs). Genomic DNA was extracted from PBMCs using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). DNA was quantitated with a Nanodrop Spectrophotometer (ND-1000) and normalized to a concentration of 50 nanograms/microliter (ng/μL). Genotyping was performed blinded to clinical status and positive and negative controls were included. Samples were genotyped using a custom array on the Golden Gate genotyping platform (Illumina, San Diego, CA) and processed according to the standard protocol using GenomeStudio (Illumina, San Diego, CA).
SNP selection
A combination of tagging single nucleotide polymorphism (SNPs) and literature driven SNPs were selected for analysis. Tagging SNPs were required to be common (i.e., a minor allele frequency ≥0.05) in public databases. SNPs with call rates of <95% or Hardy-Weinberg p-values of <.001 were excluded. As shown in Supplementary Table 1, 83 SNPs from a total of 104 SNPs among 16 candidate genes passed all of the quality control filters and were included in the genetic association analyses. Localization of SNPs on the human genome was performed using the GRCh38 human reference assembly. Regional annotations were identified using the University of California Santa Cruz (UCSC) Human Genome Browser GRCh38/hg38 (http://genome.ucsc.edu/cgi-bin/hgTracks?db=hg38). Potential regulatory involvement of SNPs was investigated using a number of Encode data tracks.(41–44) Linkage disequilibrium was calculated with Plink v1.90_b39(45) using 1000 Genomes “phase1_release_v2.201001123” variants called from all populations.(46)
Statistical Analyses for the Phenotypic Data
Data were analyzed using SPSS version 23 (47) and STATA Version 13.(48) The fatigue and energy data were analyzed separately. Descriptive statistics and frequency distributions were generated for sample characteristics. Independent sample t-tests, Mann-Whitney U tests, Chi square analyses, and Fisher’s Exact tests were used to evaluate for differences in demographic and clinical characteristics between the two latent classes. A p-value of <0.05 was considered statistically significant.
Unconditional GMM with robust maximum likelihood estimation was carried out to identify latent classes with distinct fatigue and energy trajectories using Mplus Version 5.21. These methods are described in detail elsewhere.(28) In brief, a single growth curve that represented the “average” change trajectory was estimated for the whole sample. Then, the number of latent growth classes that best fit the data was identified using guidelines recommended in the literature.(49–51)
Statistical Analyses for the Genetic Data
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by Chi-square or Fisher Exact tests. Measures of linkage disequilibrium ((LD), i.e., D′ and r2) were computed from the patients’ genotypes with Haploview 4.2. The LD-based haplotype block definition was based on D′ confidence interval.(52)
For SNPs that were members of the same haploblock, haplotype analyses were conducted in order to localize the association signal within each gene and to determine if haplotypes improved the strength of the association with the phenotype. Haplotypes were constructed using the program PHASE version 2.1.(53) To improve the stability of haplotype inference, the haplotype construction procedure was repeated 5 times using different seed numbers with each cycle. Only haplotypes that were inferred with probability estimates of ≥.85, across the five iterations, were retained for downstream analyses. Only inferred haplotypes that occurred with a frequency estimate of ≥15% were included in the association analyses, assuming a dosage model (i.e., analogous to the additive model).
Ancestry informative markers (AIMs) were used to minimize confounding due to population stratification.(54–56) Homogeneity in ancestry among patients was verified by principal component analysis,(57) using HelixTree (GoldenHelix, Bozeman, MT). Briefly, the number of principal components (PCs) was sought which distinguished the major racial/ethnic groups in the sample by visual inspection of scatter plots of orthogonal PCs (i.e., PC 1 versus PC2, PC2 versus PC3). This procedure was repeated until no discernable clustering of patients by their self-reported race/ethnicity was possible (data not shown). The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including them in all of the logistic regression models. One hundred and six AIMs were included in the analysis.
For association tests, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta <10%), the genetic model that best fit the data, by maximizing the significance of the p-value was selected for each SNP. Logistic regression analysis, that controlled for significant covariates, as well as genomic estimates of and self-reported race/ethnicity, was used to evaluate the associations between genotype and fatigue and energy class membership. Only those genetic associations identified as significant from the bivariate analyses were evaluated in the multivariate analyses. A backwards stepwise approach was used to create a parsimonious model. Except for genomic estimates of and self-reported race/ethnicity, only predictors with a p-value of <.05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using STATA version 13.(48)
As was done in our previous studies,(9, 23, 25, 27, 58–69) based on the recommendations in the literature,(70, 71) as well as the implementation of rigorous quality controls for genomic data, the non-independence of SNPs/haplotypes in LD, and the exploratory nature of the analyses, adjustments were not made for multiple testing. In addition, significant SNPs identified in the bivariate analyses were evaluated further using logistic regression analyses that controlled for differences in phenotypic characteristics, potential confounding due to population stratification, and variations in other SNPs/haplotypes within the same gene. Only those SNPs that remained significant were included in the final presentation of the results. Therefore, the significant independent associations reported are unlikely to be due solely to chance. Unadjusted (bivariate) associations are reported for all of the SNPs that passed quality control criteria in Supplementary Table 1, to allow for subsequent comparisons and meta-analyses.
RESULTS
GMM Analysis for Fatigue
Two distinct latent classes of fatigue trajectories were identified using GMM (Figure 1A). A two class model was selected because its Bayesian Information Criterion (BIC) was smaller than the one-class and three-class models (Table 1). As shown in Table 2, the majority of the patients were classified into the Higher Fatigue class (n = 244, 61.5%). These patients had estimated fatigue scores that were high prior to surgery (3.90) and remained high over the 6 months of the study. Patients in the Lower Fatigue class (n = 153, 38.5%) had estimated fatigue scores that were lower at enrollment (1.60) and that gradually decreased over time.
Figure 1.
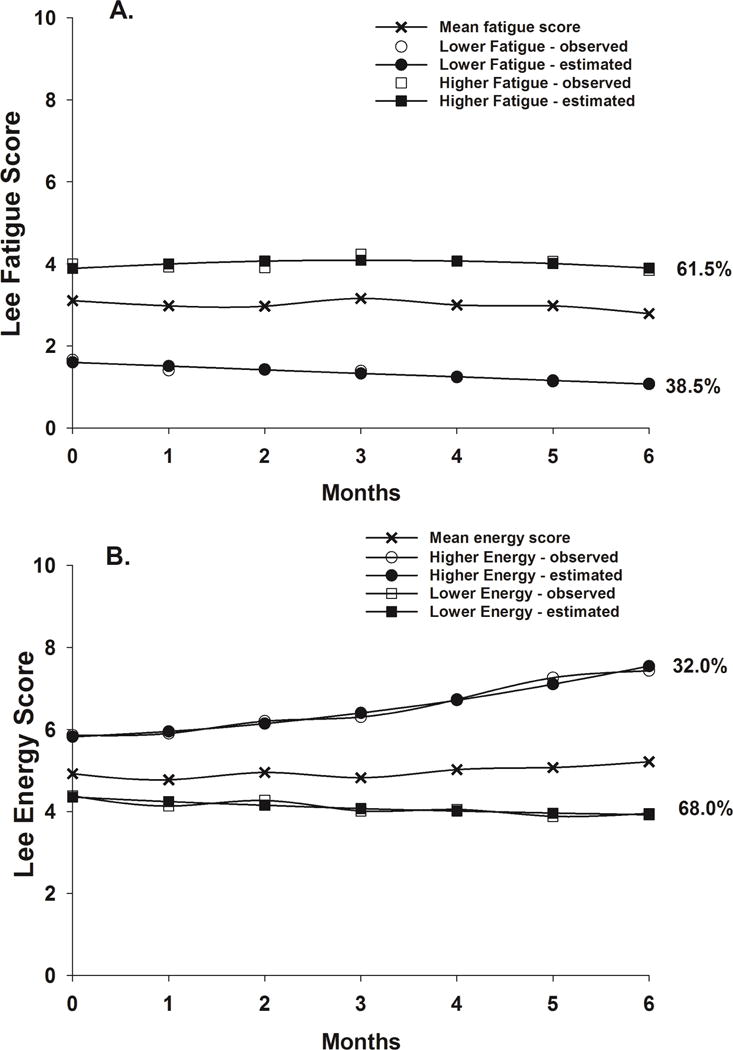
Observed and estimated fatigue (Figure 1A) and energy (Figure 1B) trajectories for patients in each of the latent classes, as well as the mean fatigue and energy scores for the total sample.
Table 1.
Fit Indices for the Lee Fatigue Scale and Lee Energy Scale GMM Class Solutions
| Fatigue | ||||||
|---|---|---|---|---|---|---|
| GMM | LL | AIC | BIC | Entropy | BLRT | VLMR |
| 1-Classa | −5068.45 | 10168.90 | 10232.65 | n/a | n/a | n/a |
| 2-Classb | −5035.46 | 10106.93 | 10178.64 | 0.61 | 68.73* | 68.73** |
| 3-Class | −5026.22 | 10096.44 | 10184.09 | 0.71 | 18.48ns | 18.48ns |
| Energy | ||||||
|---|---|---|---|---|---|---|
| GMM | LL | AIC | BIC | Entropy | BLRT | VLMR |
| 1-Classa | −5454.43 | 10930.87 | 10974.69 | n/a | n/a | n/a |
| 2-Classb | −5430.78 | 10893.56 | 10957.30 | 0.53 | 47.31* | 47.31ns |
| 3-Class | −5422.49 | 10886.98 | 10970.64 | 0.53 | 16.58ns | 16.58* |
p < .05;
p < .01
Latent growth curve with linear and quadratic components; Chi2=34.60, 19 df, p < .02, CFI = .99, RMSEA = .045
2-class model was selected. The BIC was smaller than for the 1-class and 3-class models, and the BLRT indicated that the 2-class solution fit the data better than the 1-class solution.
p< .05
Latent growth curve with linear and quadratic components; Chi2 =50.99, 24 df, p = .001, CFI = .964, RMSEA = .053
2-class model was selected. The BIC was smaller than for the 1-class and 3-class models, and the BLRT indicated that the 2-class solution fit the data better than the 1-class solution.
Abbreviations: AIC = Akaike Information Criterion, BIC = Bayesian Information Criterion, BLRT = parametric bootstrapped likelihood ratio test for K-1 (H0) vs K classes, CFI = Comparative Fit Index, GMM = growth mixture model, LL = loglikelihood, ns = not significant, RMSEA = Root Mean Squared Error of Approximattion, VLMR = Vuong-Lo-Mendell-Rubin likelihood ratio test for K-1 (H0) vs K classes
Table 2.
Parameter Estimates for the Lee Fatigue Scale and Lee Energy Scale GMM Latent Classes
| Fatigue | Lower Fatigue Class (na = 153) |
Higher Fatigue Class (na = 244) |
|---|---|---|
| Parameter Estimates | Means (SE) | |
| Intercept | 1.60*** (0.36) | 3.90*** (0.22) |
| Linear slope | −0.09 (0.12) | 0.13 (0.141 |
| Quadratic slope | 0.00 (0.02) | −0.02 (0.02) |
| Variances | ||
| Intercept | 0.26 (0.20) | 2.53*** (0.36) |
| Linear slope | 0b | 0.09*** (0.02) |
| Quadratic slope | 0b | 0b |
| Energy | Higher Energy Class (na = 127) |
Lower Energy Class (na = 270) |
| Parameter Estimates | Means (SE) | |
| Intercept | 5.82*** (0.76) | 4.35*** (0.16) |
| Linear slope | 0.10 (0.37) | −0.11 (0.14) |
| Quadratic slope | 0.03 (0.06) | 0.01 (0.04) |
| Variances | ||
| Intercept | 1.72 (1.60) | 1.07*** (0.21) |
| Linear slope | 0b | 0b |
| Quadratic slope | 0b | 0b |
p < .001
Predicted class sizes based on their most likely class membership
Random intercepts model only. Random slopes were fixed at zero to assist in estimation
Abbreviations: GMM = growth mixture model, SE = standard error
Differences in Demographic and Clinical Characteristics Between the Fatigue Classes
As summarized in Table 3, patients in the Higher Fatigue class were significantly younger, as well as had more education, a lower KPS score, a higher SCQ score, and a higher number of lymph nodes removed. In addition, a higher percentage of patients in the Higher Fatigue class had received neoadjuvant CTX prior to surgery and adjuvant CTX during the first 6 months following breast cancer surgery.
Table 3.
Differences in Demographic and Clinical Characteristics Between the Lower Fatigue (n= 153) and Higher Fatigue (n= 244) Classes
| Characteristic | Lower Fatigue Class n=153 (38.4%) Mean (SD) |
Higher Fatigue Class n=244 (61.3%) Mean (SD) |
Statistic and p-value |
|---|---|---|---|
| Age (years) | 57.8 (11.9) | 53.1 (11.0) | t=4.09, p<.0001 |
| Education (years) | 15.3 (2.5) | 15.9 (2.8) | t=−2.02, p=.04 |
| Karnofsky Performance Status score | 96.6 (7.0) | 91.1 (11.4) | t=5.86, p<.0001 |
| Self-administered Comorbidity Questionnaire score | 3.8 (2.6) | 4.6 (3.0) | t=−2.64, p=.009 |
| Fatigue severity score at enrollment | 1.6 (1.6) | 4.1 (2.2) | t=−12.55, p<.0001 |
| Number of breast biopsies in past year | 1.5 (0.8) | 1.5 (0.8) | U, p=.47 |
| Number of positive lymph nodes | 0.8 (1.9) | 1.0 (2.4) | t=−0.88, p=.38 |
| Number of lymph nodes removed | 4.8 (5.1) | 6.4 (7.5) | t=−2.43, p=.016 |
| n (%) | n (%) | ||
| Ethnicity White Black Asian/Pacific Islander Hispanic/Mixed ethnic background/Other |
100 (65.8) 19 (12.5) 17 (11.2) 16 (10.5) |
155 (63.8) 21 (8.6) 32 (13.2) 35 (14.4) |
Χ2=2.82, p=.42 |
| Married/partnered (% yes) | 64 (42.1) | 100 (41.5) | FE, p=.92 |
| Work for pay (% yes) | 71 (46.4) | 118 (49.0) | FE, p=.68 |
| Lives alone (% yes) | 40 (26.5) | 54 (22.4) | FE, p=.40 |
| Gone through menopause (% yes) | 96 (63.6) | 151 (64.3) | FE, p=.91 |
| Stage of disease 0 I IIA and IIB IIIA, IIIB, IIIC, and IV |
29 (19.0) 66 (43.1) 48 (31.4) 10 (6.5) |
44 (18.0) 85 (34.8) 92 (37.7) 23 (9.4) |
U, p=.13 |
| Surgical treatment Breast conservation Mastectomy |
123 (80.4) 30 (19.6) |
195 (79.9) 49 (20.1) |
FE, p=1.00 |
| Sentinel node biopsy (% yes) | 130 (85.0) | 197 (80.7) | FE, p=.34 |
| Axillary lymph node dissection (% yes) | 50 (32.7) | 98 (40.3) | FE, p=.14 |
| Breast reconstruction at the time of surgery (% yes) | 33 (21.7) | 53 (21.7) | FE, p=1.00 |
| Neoadjuvant chemotherapy (% yes) | 21 (13.7) | 58 (23.9) | FE, p=.014 |
| Radiation therapy during the first 6 months (% yes) | 87 (56.9) | 137 (56.1) | FE, p=.92 |
| Chemotherapy during the first 6 months (% yes) | 36 (23.5) | 97 (39.8) | FE, p=.001 |
Abbreviations: FE = Fisher Exact test, SD = standard deviation, U = Mann Whitney U test
Candidate Gene Analyses for Fatigue
As summarized in Supplementary Table 1, no associations with fatigue latent class membership were observed for SNPs in CXCL8, INFGR1, IL1R2, IL2, IL4, IL6, IL13, 1L17A, NFKB1, NFKB2, or members of the TNF family (i.e. LTA, TNF). However, genotype frequencies were significantly different between the two latent classes for 8 SNPs spanning three genes: IFNG rs2069718, IL1B rs1143629, IL1B rs1143627, IL1B rs16944, IL1B rs1143623, IL10 rs3024496, IL10 rs1878672, and IL10 rs3024491.
Regression Analyses for IFNG, IL1B, and IL10 Genotypes and Lower versus Higher Fatigue classes
In order to better estimate the magnitude (i.e., odds ratio, OR) and precision (95% CI) of genotype on the odds of belonging to the Higher as compared to the Lower Fatigue class, multivariate logistic regression models were fit. In these regression analyses, that included genomic estimates of and self-reported race/ethnicity, the only phenotypic characteristics that remained significant in the multivariate model were age (in 5 year increments), KPS score (in 10 unit increments), SCQ score, and receipt of CTX in the 6 months following surgery.
Two SNPs spanning two different genes remained significant in the multivariate logistic regression analyses (Table 4, Figures 2A and 2B). For IL1B rs16944 and IL10 rs3024496, a recessive model fit the data best (p=.002). In the regression analysis for IL1B rs16944, carrying two doses of the rare A allele (i.e., GG+GA versus AA) was associated with a 2.98-fold higher odds of belonging to the Higher Fatigue class. In the regression analysis for IL10 rs3024496, carrying two doses of the rare C allele (i.e., TT+TC versus CC) was associated with a 66% decrease in the odds of belonging to the Higher Fatigue Class.
Table 4.
Multiple Logistic Regression Analyses for Cytokine Genes and Lower Fatigue Versus Higher Fatigue Classes
| Predictor | Odds Ratio | Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|
| IL1B rs16944 | 2.98 | 1.215 | 1.336, 6.626 | 2.67 | .008 |
| Age | 0.96 | 0.012 | 0.936, 0.984 | −3.17 | .002 |
| KPS score | 0.94 | 0.017 | 0.911, 0.978 | −3.21 | .001 |
| SCQ score | 1.10 | 0.062 | 0.998, 1.243 | 1.73 | .083 |
| Any chemotherapy | 2.26 | 0.655 | 1.284, 3.992 | 2.83 | .005 |
| Overall model fit: χ2 = 56.98, p <.0001 | |||||
| IL10 rs3024496 | 0.34 | 0.120 | 0.172, 0.682 | −3.05 | .002 |
| Age | 0.95 | 0.013 | 0.930, 0.979 | −3.58 | <.001 |
| KPS score | 0.95 | 0.017 | 0.916, 0.980 | −3.09 | .002 |
| SCQ score | 1.12 | 0.063 | 1.007, 1.255 | 2.08 | .037 |
| Any chemotherapy | 2.32 | 0.675 | 1.315, 4.106 | 2.90 | .004 |
| Overall model fit: χ2 = 60.96, p <.0001 | |||||
Multiple logistic regression analyses of candidate gene associations with Lower Fatigue versus Higher Fatigue classes (n=301). For each model, the first three principal components identified from the analysis of ancestry informative markers, as well as self-reported race/ethnicity, were retained in all models to adjust for potential confounding due to race/ethnicity (data not shown). For the regression analyses, predictors evaluated in each model included genotype (IL1B rs16944: GG+GA versus AA; IL10 rs3024496: TT+TC versus CC, age (5 years increments), functional status (KPS score in 10 unit increments), self-administered comorbidity questionnaire score, and receipt of chemotherapy within six months after surgery.
Abbreviations: any chemotherapy = receipt of chemotherapy within six months after surgery, CI = confidence interval, IL1B = interleukin 1 beta, KPS = Karnofsky Performance Status, SCQ = Self-administered Comorbidity Questionnaire
Figure 2.
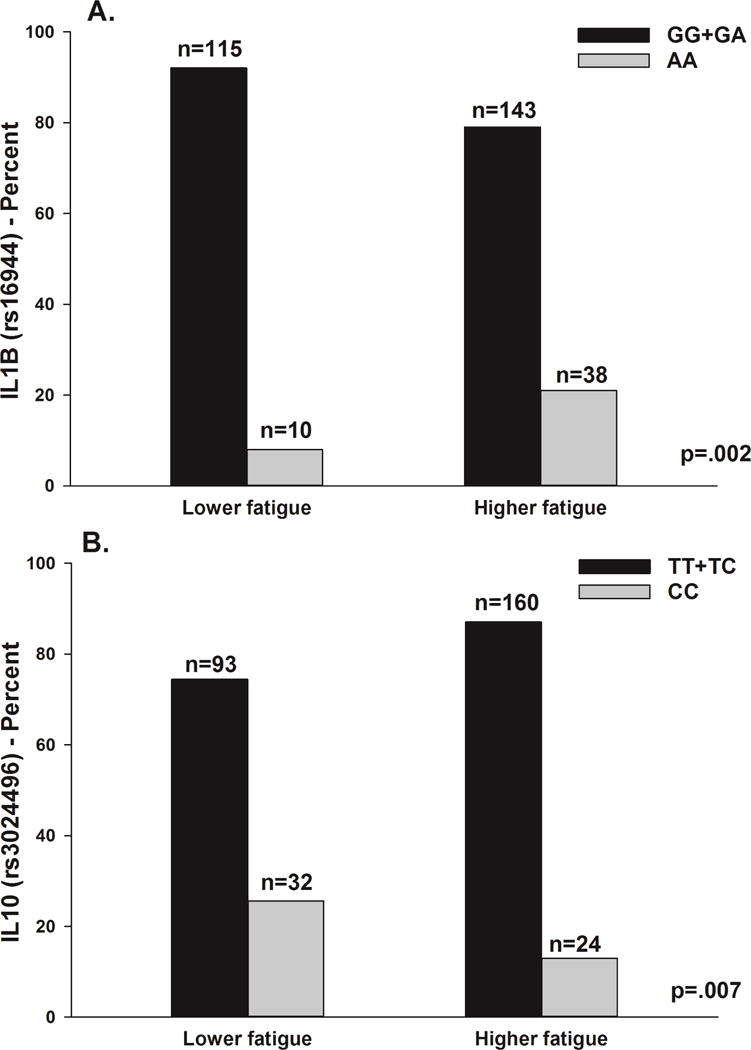
A – Differences between the fatigue latent classes in the percentages of patients who were homozygous or heterozygous for the common allele (GG+GA) or homozygous for the rare allele (AA) for rs16944 in interleukin 1 beta (IL1B). Values are plotted as unadjusted proportions with corresponding p-value.
B – Differences between the fatigue latent classes in the percentages of patients who were homozygous or heterozygous for the common allele (TT+TC) or homozygous for the rare allele (CC) for rs3024496 in interleukin 10 (IL10). Values are plotted as unadjusted proportions with corresponding p-value.
GMM Analysis for Energy
Two distinct latent classes of energy trajectories were identified using GMM (Figure 1B). A two class model was selected because its BIC was smaller than the one-class and three-class models (Table 1). As shown in Table 2, the majority of the patients were classified into the Lower Energy class (n = 270, 68.0%). These patients had estimated energy scores that were low prior to surgery (4.35) and remained low over the 6 months of the study. Patients in the Higher Energy class (n = 127, 32.0%) had estimated energy scores that were higher at enrollment (5.82) and that gradually increased over time.
Differences in Demographic and Clinical Characteristics Between the Energy Classes
As summarized in Table 5, patients in the Lower Energy class had a lower KPS score and a higher SCQ score. In addition, the percentage of patients based on their stage of disease differed between the two energy classes. However, post hoc contrasts failed to identify the subgroups that differed between the classes.
Table 5.
Differences in Demographic and Clinical Characteristics Between the Higher Energy (n=127) and Lower Energy (n=270) Classes
| Characteristic | Higher Energy Class n=127 (31.9%) Mean (SD) |
Lower Energy Class n=270 (67.8%) Mean (SD) |
Statistic and p-value |
|---|---|---|---|
| Age (years) | 56.5 (10.8) | 54.2 (11.8) | t=1.88, p=.061 |
| Education (years) | 15.7 (2.2) | 15.7 (2.8) | t=0.01, p=.994 |
| Karnofsky Performance Status score | 95.4 (9.4) | 92.2 (10.6) | t=3.06, p=.002 |
| Self-administered Comorbidity Questionnaire score | 3.6 (2.3) | 4.6 (3.0) | t=−3.47, p=.001 |
| Mean energy score at enrollment | 6.1 (2.7) | 4.4 (2.2) | t=−6.26, p<.0001 |
| Number of breast biopsies in past year | 1.5 (0.8) | 1.5 (0.8) | U, p=.604 |
| Number of positive lymph nodes | 0.8 (2.0) | 1.0 (2.3) | t=0.76, p=.450 |
| Number of lymph nodes removed | 5.0 (6.3) | 6.1 (6.9) | t=−1.51, p=.132 |
| n (%) | n (%) | ||
| Ethnicity White Black Asian/Pacific Islander Hispanic/Mixed ethnic background/Other |
86 (68.3) 10 (7.9) 16 (12.7) 14 (11.1) |
169 (62.8) 30 (11.2) 33 (12.3) 37 (13.8) |
Χ2=1.75, p=.627 |
| Married/partnered (% yes) | 50 (39.7) | 114 (42.7) | FE, p=.5 86 |
| Work for pay (% yes) | 66 (52.4) | 123 (45.9) | FE, p=.236 |
| Lives alone (% yes) | 29 (23.0) | 65 (24.4) | FE, p=.801 |
| Gone through menopause (% yes) | 84 (68.3) | 163 (62.0) | FE, p=.2 56 |
| Stage of disease 0 I IIA and IIB IIIA, IIIB, IIIC, and IV |
29 (22.8) 51 (40.2) 39 (30.7) 8 (6.3) |
44 (16.3) 100 (37.0) 101 (37.4) 25 (9.3) |
U, p=.040a |
| Surgical treatment Breast conservation Mastectomy |
100 (78.7) 27 (21.3) |
218 (80.7) 52 (19.3) |
FE, p=.686 |
| Sentinel node biopsy (% yes) | 103 (81.1) | 224 (83.0) | FE, p=.673 |
| Axillary lymph node dissection (% yes) | 40 (31.7) | 108 (40.0) | FE, p= .12 0 |
| Breast reconstruction at the time of surgery (% yes) | 28 (22.2) | 58 (21.5) | FE, p=.896 |
| Neoadjuvant chemotherapy (% yes) | 22 (17.5) | 57 (21.1) | FE, p=.421 |
| Radiation therapy during the first 6 months (% yes) | 75 (59.1) | 149 (55.2) | FE, p=.515 |
| Chemotherapy during the first 6 months (% yes) | 34 (26.8) | 99 (36.7) | FE, p=.054 |
Abbreviations: FE = Fisher Exact test, SD = standard deviation, U=Mann Whitney U test
Post-hoc contrasts of the difference in stage of disease between the Higher Energy and Lower Energy classes failed to identify the sub-groups who differed between the classes (p<.0083).
Candidate Gene Analyses for Energy
As summarized in Supplementary Table 1, no associations with energy class membership were found for SNPs in any gene except IL1R1. The genotype frequency was significantly different between the two latent classes for 1 SNP: IL1R1 rs2110726.
Regression Analyses for IL1R1 Genotype and Higher versus Lower Energy Classes
In order to better estimate the magnitude (i.e., odds ratio, OR) and precision (95% confidence interval, CI) of genotype on the odds of belonging to the Higher as compared to the Lower Energy class, a multivariate logistic regression model was fit. In this regression analysis that included genomic estimates of and self-reported race/ethnicity, the only phenotypic characteristics that remained significant in the multivariate model were KPS score (in 10 unit increments) and receipt of CTX in the 6 months following surgery.
In the multivariate logistic regression analysis, the association between IL1R1 rs2110726 and the energy phenotype remained significant (Table 6, Figures 3). For this SNP, a dominant model fit the data best (p=.021). In the regression analysis, carrying one or two doses of the rare T allele (i.e., CC versus CT+TT) was associated with a 50% decrease in the odds of belonging to the Lower Energy class.
Table 6.
Multiple Logistic Regression Analysis for Cytokine Genes and Higher Energy Versus Lower Energy Classes
| Predictor | Odds Ratio | Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|
| IL1R1 rs2110726 | 0.50 | 0.137 | 0.296, 0.859 | −2.52 | .012 |
| KPS score | 0.96 | 0.015 | 0.931, 0.989 | −2.70 | .007 |
| Any chemotherapy | 1.83 | 0.510 | 1.059, 3.159 | 2.17 | .030 |
| Overall model fit: χ2 = 24.11, p=0.004 | |||||
Multiple logistic regression analyses of candidate gene associations with Higher Energy versus Lower Energy classes (n=301). For each model, the first three principal components identified from the analysis of ancestry informative markers, as well as self-reported race/ethnicity, were retained in all models to adjust for potential confounding due to race/ethnicity (data not shown). For the regression analysis, predictors evaluated in the model included genotype (IL1R1 rs2110726: CC versus CT+TT, functional status (KPS score in 10 unit increments), and receipt of chemotherapy within six months after surgery.
Abbreviations: any chemotherapy = receipt of chemotherapy within six months after surgery, CI = confidence interval, IL1R1 = interleukin 1 receptor 1, KPS = Karnofsky Performance Status, SCQ = Self-administered Comorbidity Questionnaire
Figure 3.
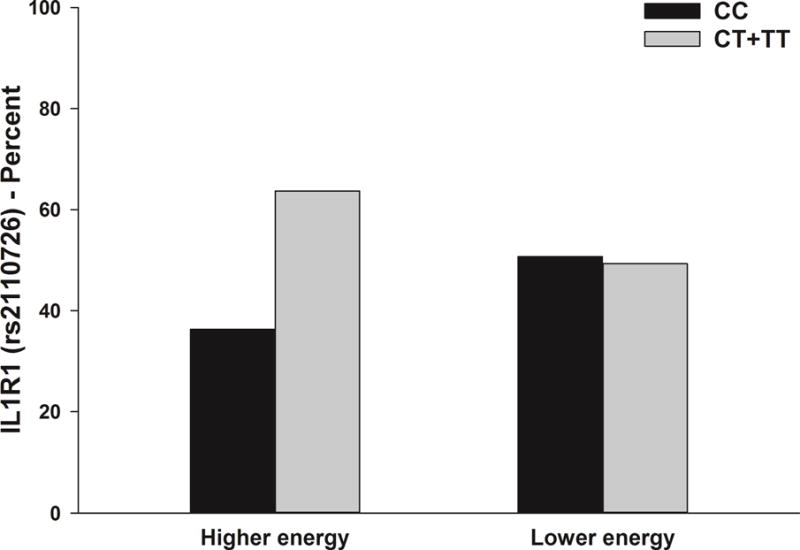
Differences between the energy latent classes in the percentages of patients who were homozygous for the common allele (CC) or heterozygous or homozygous for the rare allele (CT+TT) for rss110726 in interleukin 1 receptor 1 (IL1R1). Values are plotted as unadjusted proportions with corresponding p-value.
Overlap Between the Fatigue and Energy Latent Classes
An analysis of membership in the fatigue and energy classes in this study revealed that of the 397 patients evaluated: 16.6% (n=66) were in both the Lower Fatigue and Lower Energy classes; 21.9% (n=87) were in both the Lower Fatigue and Higher Energy classes; 10.1% (n=40) were in both the Higher Fatigue and Higher Energy classes; and 51.4% (n=204) were in both the Higher Fatigue and Lower Energy classes.
DISCUSSION
This study is the first to use GMM to identify subgroups of breast cancer patients with distinct trajectories of fatigue and energy, as well as evaluate for associations between a number of cytokine genes and these distinct phenotypes. Since less is known about decrements in energy following breast cancer surgery, this section begins with a discussion of the fatigue findings. In the section that discusses the energy findings, similarities and differences between the two symptoms in phenotypic characteristics and molecular markers are described.
Fatigue Findings
In the current study, 62% of the patients reported fatigue scores at the clinically meaningful cutoff score that occurred prior to surgery and persisted for six months following surgery. This finding contrasts with the previous study by Bodtcher and colleagues who reported that only 21% of their sample reported high levels of fatigue prior to surgery, and that their fatigue decreased at three months and then returned to pretreatment levels at 8 months after surgery.(2) While the demographic and clinical characteristics of the two samples were relatively similar, differences in the instruments used to assess fatigue as well as in the statistical approaches used to create the latent classes may explain the difference in percentages of patients in the higher fatigue class.
In terms of demographic characteristics, patients who were younger and had more years of education were more likely to be in the higher fatigue class. While our findings are consistent with those of Bodtcher et al.(2) and Montgomery et al.,(3) they contrast with other studies that found no association(4–6) between age and severity of fatigue in women following breast cancer surgery. In the study by Montgomery et al.,(3) mediational analyses demonstrated that preoperative expectations of postoperative levels of fatigue accounted in part for the effects of age on the severity of postoperative fatigue. The authors commented that their findings could be explained by Social Learning Theory which suggests that an individual’s previous experiences with fatigue might shape one’s expectancies for the symptom.(72, 73) They hypothesized that older patients may have had previous experiences with surgery and/or fatigue which lowered their expectations for fatigue. An equally important consideration in all of the studies cited above is that the mean age of the patients ranged from late 40s to late 50s. Therefore, additional studies are needed that evaluate the association between age and changes in fatigue severity following breast cancer surgery in older age groups.
While two studies found no association between education and fatigue severity in breast cancer survivors and women who underwent adjuvant treatment,(74, 75) our findings are consistent with those of Huang et al.(6) who evaluated fatigue trajectories in women who were followed for 12 months after breast cancer surgery. Given these inconsistent findings and the relatively high levels of education in the current and previous studies,(74, 75) additional research is warranted to determine the specific factors associated with educational attainment (e.g., different levels of social responsibility, different employment opportunities) that may contribute to variations in fatigue severity.
Across a series of studies, the evidence is clear that a higher number of comorbidities, poorer performance status, and higher fatigue severity prior to the initiation of cancer treatment are associated with worse fatigue trajectories or membership in the higher fatigue class (for review see Bower and Ganz (1)). While stage of disease and type of surgery were not associated with membership in the Higher Fatigue class, in our study, patients who had a higher number of lymph nodes removed as well as those who received neoadjuvant or adjuvant CTX were more likely to be in the Higher Fatigue class. An examination of the clinical characteristics that predicted higher fatigue severity in the four studies that evaluated patients following breast cancer surgery (2–5) reveal a rather disparate list of risk factors. These inconsistent findings can be partially explained by the number and types of demographic, clinical, symptom, and psychological characteristics that were placed in the various types of multivariate analyses. Future meta-analyses and larger studies with a more comprehensive list of potential predictors would provide insights into the characteristics that clinicians need to assess to identify breast cancer patients who are at higher risk for more severe fatigue.
IL1B is a pro-inflammatory cytokine that is synthesized in a variety of cells including circulating monocytes and tissue macrophages. In our study, patients who were homozygous for the rare A allele in IL1B rs16944 were more likely to be classified in the higher fatigue class. This SNP lies upstream of the IL1B gene. It lies in a region of histone modification that is suggestive of regulatory activity (i.e., H3K27Ac) and to a lesser extent in a region associated with promoters (i.e., H3K4Me1). However, based on available data in ENCODE, since this region does not contain a DNase Hypersensitivity Cluster or evidence of a transcription binding site, the location of any nearby regulatory DNA elements remains inconclusive. This SNP has been evaluated in a number of studies of fatigue in oncology patients. In contrast to our findings, in a study of 33 fatigue and 14 non-fatigued breast cancer survivors,(76) patients who were heterozygous or homozygous for the rare T allele (A allele in our study) were more likely to be in the non-fatigued group that was assessed using the Multidimensional Fatigue Symptom Inventory.(77, 78) In other studies of newly diagnosed breast cancer patients who recently completed treatment,(22) breast cancer survivors,(79) and men with prostate cancer who underwent RT,(80) no associations were found with IL1B rs16944 and the fatigue phenotype. These inconsistent findings may be related to the instruments used to assess fatigue, the methods used to create the “fatigue phenotype” (e.g., dichotomization using a cutpoint versus latent class analysis), and/or the timing of the assessment of fatigue in relationship to the patient’s disease trajectory (e.g., active treatment versus survivorship).
IL10 is an anti-inflammatory cytokine that regulates the growth and differentiation of B cells, natural killer cells, and cytotoxic, helper, and regulatory T cells.(81) In our study, patients who were homozygous for the rare C allele in IL10 rs3024496 were less likely to be in the higher fatigue class. This SNP is in a 3′ untranslated (UTR) region of the fifth exon of IL10. It is upstream from four TargetScan miRNA that are predicted to be putative miRNA regulatory sites and may be involved in miRNA regulatory actions. However, based on ENCODE data, little support exists for transcription factor binding in this region. Consistent with the findings from the current study, this polymorphism was associated with increased production of IL10 in cell culture.(82) In addition, in a recent study,(83) the IL10 pathway was found to be differentially expressed between patients with breast cancer who reported low as compared to high levels of evening fatigue during CTX.
Energy Findings
In the current study, 68.0% of the patients reported energy scores that were below the cutoff score for clinically meaningful decrements in energy levels (i.e., ≤4.8). Of note, these decrements in energy levels persisted in the Lower Energy class over the six months of the study. Comparisons of these findings with our previous study of patients and family caregivers(9) is difficult because diurnal variations in energy levels were not evaluated in the current study.
Similar to the fatigue findings, in the bivariate analyses, a lower KPS score, a higher SCQ score, and receipt of CTX during the six months after breast cancer surgery were associated with membership in the Lower Energy class. In addition, while stage of disease was associated with membership in the Lower Energy class (but not fatigue class membership), due to the relatively small sample sizes for the highest stage of disease, post hoc contrasts failed to identify subgroup differences. Again, while direct comparisons with our previous study are not possible,(9) a lower KPS score was associated with membership in the Low Morning and Moderate Evening energy classes. In both studies, KPS scores were approximately 90 which suggest that even with high functional status scores, some patients can experience significant decrements in energy levels.
Two additional characteristics warrant consideration. First, for both fatigue and energy, the receipt of adjuvant CTX was associated with membership in the class with the poorer outcomes. While higher levels of fatigue are associated with the receipt of CTX,(84–86) this study is the first to document an association between receipt of adjuvant CTX and decrements in energy levels. Second, for both symptoms, clinically meaningful levels of fatigue and decrements in energy levels, prior to surgery, were associated with membership in the Higher Fatigue and Lower Energy classes, respectively. Taken together these findings suggest that clinicians need to assess for both fatigue severity as well as decrements in energy levels prior to and following breast cancer surgery.
Only one SNP in the IL1R1 gene was associated with membership in the Lower Energy class. In this study, patients who were heterozygous or homozygous for the rare T allele in rs2110726 were less likely to be in the Lower Energy class. This SNP is located in the 3′ UTR of the IL1R1 gene. It is 35 bases upstream from a polymorphic CpG methylation site (i.e., rs200426703). While population data are not available for rs200426703 directly, rs2110726 is in high LD (R2 = 0.0232341, D′ = 1.0) with a SNP (i.e., rs3024496) that is 30 bases downstream from the methylated site (i.e., 65 bases downstream from rs2110726 and flanking rs200426703; see Supplementary Figure 1). Methylated sites may be involved in gene regulation(87) and methylation may be allelic specific.(88) The strong LD observed between the SNPs flanking this region including the methylated polymorphic site suggests that rs2110726 may be a proxy for the genotype at the methylated site (i.e., rs200426703). Therefore, polymorphisms in rs2110726 may be related to any gene regulation activity that may occur at the polymorphic methylated site. In a previous study with the same sample,(25) patients who were heterozygous or homozygous for the rare T allele had a lower odds of reporting preoperative breast pain. Taken together, these findings are consistent with studies of IL1 function in mice, in which removal of IL1R function or blockade of IL1A led to a decrease in inflammation and pain behaviors.(89) Additional functional studies are needed to determine if the minor allele of rs2110726 is associated with a decrease in IL1R1 function and therefore a decrease in the pro-inflammatory effects of IL1A.
Limitations
Several study limitations need to be acknowledged. While a growing body of evidence suggests that diurnal variations in both fatigue and energy warrant consideration in future studies,(9, 36, 38, 85, 86, 90, 91) in the current study, patients were asked to evaluate their levels of fatigue and energy at the time they completed the questionnaire. In addition, since most patients had early stage breast cancer, differences in fatigue and energy class memberships associated with stage of disease could not be evaluated. Third, no direct measurements of systemic levels of inflammatory markers were obtained to provide additional information on the underlying mechanisms for fatigue severity and decrements in energy. Finally, the genetic associations identified in this study warrant confirmation in future studies and functional studies are needed to confirm the impact of these polymorphisms on inflammatory mediators.
Conclusions
Findings from this study suggest that within each latent class, the severity of fatigue and decrements in energy were relatively stable from prior to through six months following breast cancer surgery. In addition, distinct phenotypic characteristics and genetic polymorphisms were associated with membership in the higher fatigue and lower energy classes. While these findings warrant confirmation in future studies, the phenotypic and genetic findings from this study support the growing body of literature that suggests that fatigue and energy are distinct but related symptoms.(8–11) Additional research is warranted to evaluate for differences in the underlying mechanisms for both symptoms. Future studies can focus on an evaluation of additional immune pathways as well as molecular markers of metabolic and neuroendocrine function.(17) A better understanding of the molecular mechanisms that underlie these two symptoms could lead to the earlier identification of high risk patients and the development and testing of novel mechanistically-based interventions.
Supplementary Material
Acknowledgments
This study was funded by grants from the National Cancer Institute (NCI, CA107091 and CA118658) and the Oncology Nursing Foundation. Dr. Christine Miaskowski is an American Cancer Society Clinical Research Professor and is supported by a K05 award from the NCI (CA168960). This project is supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bower JE, Ganz PA. Symptoms: Fatigue and Cognitive Dysfunction. Adv Exp Med Biol. 2015;862:53–75. doi: 10.1007/978-3-319-16366-6_5. [DOI] [PubMed] [Google Scholar]
- 2.Bodtcher H, Bidstrup PE, Andersen I, et al. Fatigue trajectories during the first 8 months after breast cancer diagnosis. Qual Life Res. 2015;24:2671–9. doi: 10.1007/s11136-015-1000-0. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery GH, Schnur JB, Erblich J, Diefenbach MA, Bovbjerg DH. Presurgery psychological factors predict pain, nausea, and fatigue one week after breast cancer surgery. J Pain Symptom Manage. 2010;39:1043–1052. doi: 10.1016/j.jpainsymman.2009.11.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotonda C, Guillemin F, Bonnetain F, Velten M, Conroy T. Factors associated with fatigue after surgery in women with early-stage invasive breast cancer. Oncologist. 2013;18:467–75. doi: 10.1634/theoncologist.2012-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vries J, Van der Steeg AF, Roukema JA. Determinants of fatigue 6 and 12 months after surgery in women with early-stage breast cancer: a comparison with women with benign breast problems. J Psychosom Res. 2009;66:495–502. doi: 10.1016/j.jpsychores.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Huang HP, Chen ML, Liang J, Miaskowski C. Changes in and predictors of severity of fatigue in women with breast cancer: A longitudinal study. Int J Nurs Stud. 2014;51:582–92. doi: 10.1016/j.ijnurstu.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012–39. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerdal A. A theoretical extension of the concept of energy through an empirical study. Scand J Caring Sci. 2002;16:197–206. doi: 10.1046/j.1471-6712.2002.00079.x. [DOI] [PubMed] [Google Scholar]
- 9.Aouizerat BE, Dhruva A, Paul SM, et al. Phenotypic and molecular evidence suggests that decrements in morning and evening energy are distinct but related symptoms. J Pain Symptom Manage. 2015;50:599–614 e3. doi: 10.1016/j.jpainsymman.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerdal A. A concept analysis of energy. Its meaning in the lives of three individuals with chronic illness. Scand J Caring Sci. 1998;12:3–10. doi: 10.1080/02839319850163075. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor PJ. Mental energy: Assessing the mood dimension. Nutr Rev. 2006;64:S7–9. doi: 10.1111/j.1753-4887.2006.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 12.McNair DM, Lorr M, Droppleman LF. EDITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 13.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–8. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 14.Lerdal A, Kottorp A, Gay CL, Lee KA. Lee Fatigue And Energy Scales: exploring aspects of validity in a sample of women with HIV using an application of a Rasch model. Psychiatry Res. 2013;205:241–6. doi: 10.1016/j.psychres.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Onselen C, Aouizerat BE, Dunn LB, et al. Differences in sleep disturbance, fatigue and energy levels between women with and without breast pain prior to breast cancer surgery. Breast. 2013;22:273–6. doi: 10.1016/j.breast.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barsevick AM, Irwin MR, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432–40. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saligan LN, Olson K, Filler K, et al. The biology of cancer-related fatigue: a review of the literature. Support Care Cancer. 2015;23:2461–78. doi: 10.1007/s00520-015-2763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagundes C, LeRoy A, Karuga M. Behavioral Symptoms after Breast Cancer Treatment: A Biobehavioral Approach. J Pers Med. 2015;5:280–95. doi: 10.3390/jpm5030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. doi: 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon DC, Ho YS, Chiu K, Wong HL, Chang RC. Sickness: From the focus on cytokines, prostaglandins, and complement factors to the perspectives of neurons. Neurosci Biobehav Rev. 2015;57:30–45. doi: 10.1016/j.neubiorev.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Smith AK, Conneely KN, Pace TW, et al. Epigenetic changes associated with inflammation in breast cancer patients treated with chemotherapy. Brain Behav Immun. 2014;38:227–36. doi: 10.1016/j.bbi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bower JE, Ganz PA, Irwin MR, et al. Cytokine genetic variations and fatigue among patients with breast cancer. J Clin Oncol. 2013;31:1656–61. doi: 10.1200/JCO.2012.46.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Illi J, Miaskowski C, Cooper B, et al. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–47. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aouizerat BE, Dodd M, Lee K, et al. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biol Res Nurs. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- 25.McCann B, Miaskowski C, Koetters T, et al. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. J Pain. 2012;13:425–37. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–87. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miaskowski C, Dodd M, Paul SM, et al. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PLoS One. 2013;8:e60164. doi: 10.1371/journal.pone.0060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunn LB, Cooper BA, Neuhaus J, et al. Identification of distinct depressive symptom trajectories in women following surgery for breast cancer. Health Psychol. 2011;30:683–92. doi: 10.1037/a0024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948;1:634–56. [Google Scholar]
- 30.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–63. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 31.Brunner F, Bachmann LM, Weber U, et al. Complex regional pain syndrome 1–the Swiss cohort study. BMC Musculoskelet Disord. 2008;9:92. doi: 10.1186/1471-2474-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cieza A, Geyh S, Chatterji S, et al. Identification of candidate categories of the International Classification of Functioning Disability and Health (ICF) for a Generic ICF Core Set based on regression modelling. BMC Med Res Methodol. 2006;6:36. doi: 10.1186/1471-2288-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gay CL, Lee KA, Lee SY. Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs. 2004;5:311–8. doi: 10.1177/1099800403262142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KA, Portillo CJ, Miramontes H. The fatigue experience for women with human immunodeficiency virus. J Obstet Gynecol Neonatal Nurs. 1999;28:193–200. doi: 10.1111/j.1552-6909.1999.tb01984.x. [DOI] [PubMed] [Google Scholar]
- 35.Miaskowski C, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–32. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 36.Miaskowski C, Paul SM, Cooper BA, et al. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J Pain Symptom Manage. 2008;35:632–43. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–89. doi: 10.1188/06.ONF.E79-E89. [DOI] [PubMed] [Google Scholar]
- 38.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010;33:201–12. doi: 10.1097/NCC.0b013e3181c75f2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barsevick A, Frost M, Zwinderman A, et al. I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19:1419–27. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–99. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 41.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimson A, Farh KK, Johnston WK, et al. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 44.Consortium EP, Dunham I, Kundaje A, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang CC, Chow CC, Tellier LC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genomes Project, C. Abecasis GR, Altshuler D, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SPSS IBM SPSS for Windows (Version 23) Chicago, Illinois: SPSS, Inc; 2015. [Google Scholar]
- 48.StataCorp Stata Statistical Software: Release 14. College Station, Texas: Stata Corporation; 2015. [Google Scholar]
- 49.Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2:302–317. [Google Scholar]
- 50.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–569. [Google Scholar]
- 51.Tofighi D, Enders CK. Identifying the correct number of classes in growth mixture models. Charlotte, NC: Information Age Publishing; 2008. [Google Scholar]
- 52.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 53.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Human Mutation. 2008;29:648–58. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- 55.Hoggart CJ, Parra EJ, Shriver MD, et al. Control of confounding of genetic associations in stratified populations. Am J Hum Genet. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: population substructure and genome-wide association studies. Hum Mol Genet. 2008;17:R143–50. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price AL, Patterson NJ, Plenge RM, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 58.Dhruva A, Aouizerat BE, Cooper B, et al. Cytokine gene associations with self-report ratings of morning and evening fatigue in oncology patients and their family caregivers. Biol Res Nurs. 2015;17:175–84. doi: 10.1177/1099800414534313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miaskowski C, Cataldo JK, Baggott CR, et al. Cytokine gene variations associated with trait and state anxiety in oncology patients and their family caregivers. Support Care Cancer. 2015;23:953–65. doi: 10.1007/s00520-014-2443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doong SH, Dhruva A, Dunn LB, et al. Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biol Res Nurs. 2015;17:237–47. doi: 10.1177/1099800414550394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Langford DJ, Schmidt B, Levine JD, et al. Preoperative Breast Pain Predicts Persistent Breast Pain and Disability After Breast Cancer Surgery. J Pain Symptom Manage. 2015;49:981–94. doi: 10.1016/j.jpainsymman.2014.11.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alfaro E, Dhruva A, Langford DJ, et al. Associations between cytokine gene variations and self-reported sleep disturbance in women following breast cancer surgery. Eur J Oncol Nurs. 2014;18:85–93. doi: 10.1016/j.ejon.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephens K, Cooper BA, West C, et al. Associations between cytokine gene variations and severe persistent breast pain in women following breast cancer surgery. J Pain. 2014;15:169–80. doi: 10.1016/j.jpain.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Merriman JD, Aouizerat BE, Cataldo JK, et al. Association between an interleukin 1 receptor, type I promoter polymorphism and self-reported attentional function in women with breast cancer. Cytokine. 2014;65:192–201. doi: 10.1016/j.cyto.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merriman JD, Aouizerat BE, Langford DJ, et al. Preliminary evidence of an association between an interleukin 6 promoter polymorphism and self-reported attentional function in oncology patients and their family caregivers. Biol Res Nurs. 2014;16:152–9. doi: 10.1177/1099800413479441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saad S, Dunn LB, Koetters T, et al. Cytokine gene variations associated with subsyndromal depressive symptoms in patients with breast cancer. Eur J Oncol Nurs. 2014;18:397–404. doi: 10.1016/j.ejon.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Langford DJ, West C, Elboim C, et al. Variations in potassium channel genes are associated with breast pain in women prior to breast cancer surgery. J Neurogenet. 2014;28:122–35. doi: 10.3109/01677063.2013.856430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunn LB, Aouizerat BE, Langford DJ, et al. Cytokine gene variation is associated with depressive symptom trajectories in oncology patients and family caregivers. Eur J Oncol Nurs. 2013;17:346–53. doi: 10.1016/j.ejon.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miaskowski C, Cooper BA, Dhruva A, et al. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PLoS One. 2012;7:e40560. doi: 10.1371/journal.pone.0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 71.Bakan D. The test of significance in psychological research. Psychol Bull. 1966;66:423–37. doi: 10.1037/h0020412. [DOI] [PubMed] [Google Scholar]
- 72.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 73.Bandura A, Adams NE, Beyer J. Cognitive processes mediating behavioral change. J Pers Soc Psychol. 1977;35:125–39. doi: 10.1037//0022-3514.35.3.125. [DOI] [PubMed] [Google Scholar]
- 74.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Von Ah DM, Kang DH, Carpenter JS. Predictors of cancer-related fatigue in women with breast cancer before, during, and after adjuvant therapy. Cancer Nurs. 2008;31:134–44. doi: 10.1097/01.NCC.0000305704.84164.54. [DOI] [PubMed] [Google Scholar]
- 76.Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav Immun. 2008;22:1197–200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–52. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 79.Reinertsen KV, Grenaker Alnaes GI, Landmark-Hoyvik H, et al. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behav Immun. 2011;25:1376–83. doi: 10.1016/j.bbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Jim HS, Park JY, Permuth-Wey J, et al. Genetic predictors of fatigue in prostate cancer patients treated with androgen deprivation therapy: preliminary findings. Brain Behav Immun. 2012;26:1030–6. doi: 10.1016/j.bbi.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 82.Assis S, Marques CR, Silva TM, et al. IL10 single nucleotide polymorphisms are related to upregulation of constitutive IL-10 production and susceptibility to Helicobacter pylori infection. Helicobacter. 2014;19:168–73. doi: 10.1111/hel.12119. [DOI] [PubMed] [Google Scholar]
- 83.Kober KM, Dunn L, Mastick J, et al. Gene expression profiling of evening fatigue in women undergoing chemotherapy for breast cancer. Biological Research for Nursing. doi: 10.1177/1099800416629209. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 85.Wright F, D’Eramo Melkus G, Hammer M, et al. Trajectories of Evening Fatigue in Oncology Outpatients Receiving Chemotherapy. J Pain Symptom Manage. 2015;50:163–75. doi: 10.1016/j.jpainsymman.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright F, D’Eramo Melkus G, Hammer M, et al. Predictors and Trajectories of Morning Fatigue Are Distinct From Evening Fatigue. J Pain Symptom Manage. 2015;50:176–89. doi: 10.1016/j.jpainsymman.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Illingworth RS, Bird AP. CpG islands–‘a rough guide’. FEBS Lett. 2009;583:1713–20. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 88.Shoemaker R, Deng J, Wang W, Zhang K. Allele-specific methylation is prevalent and is contributed by CpG-SNPs in the human genome. Genome Res. 2010;20:883–9. doi: 10.1101/gr.104695.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torres R, Macdonald L, Croll SD, et al. Hyperalgesia, synovitis and multiple biomarkers of inflammation are suppressed by interleukin 1 inhibition in a novel animal model of gouty arthritis. Annals of the Rheumatic Diseases. 2009;68:1602–8. doi: 10.1136/ard.2009.109355. [DOI] [PubMed] [Google Scholar]
- 90.Dimsdale JE, Ancoli-Israel S, Ayalon L, Elsmore TF, Gruen W. Taking fatigue seriously, II: variability in fatigue levels in cancer patients. Psychosomatics. 2007;48:247–52. doi: 10.1176/appi.psy.48.3.247. [DOI] [PubMed] [Google Scholar]
- 91.Jim HS, Small B, Faul LA, et al. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42:321–33. doi: 10.1007/s12160-011-9294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


