Abstract
Stage specific embryonic antigen-1 (SSEA-1), also known as CD15, is a member of cluster of differentiation antigens that have been identified in various normal tissues and in different types of cancers including papillary and medullary thyroid carcinoma. SSEA-1 may be expressed in normal stem cells and cancer stem-like cells. To evaluate the potential diagnostic and prognostic utility of SSEA-1 in thyroid tumors, we analyzed the expression of SSEA-1 in normal and neoplastic thyroid tissues by immunohistochemistry (IHC) using a tissue microarray with 158 different tissue cores. To evaluate the potential utility of SSEA-1 as a surface marker, we also assessed the expression of SSEA-1 in thyroid cell lines by flow cytometric analysis. SSEA-1 immunoreactivity was identified in malignant thyroid follicular epithelial cancers but not in the benign thyroid tissues. Anaplastic thyroid (ATC) (80%) and conventional papillary thyroid carcinoma (PTC) (60.7%) showed significantly higher percentage of cases that were SSEA-1 immunoreactive than follicular variant of papillary thyroid carcinoma (FVPTC) (20.6%) and follicular carcinoma (FCA) (32.1%). Flow cytometric analysis of cultured thyroid cell lines showed that a small subpopulation of ATC and PTC thyroid tumor cells had SSEA-1 immunoreactivity which may represent thyroid cancer stem-like cells. The ATC cells expressed more SSEA-1 immunoreactive cells than the PTC cell lines.
Our findings suggest that expression of SSEA-1 immunoreactivity in thyroid neoplasms was associated with more aggressive thyroid carcinomas. SSEA-1 is a marker that detects malignant thyroid neoplasms in formalin fixed-paraffin embedded thyroid tissue sections and may be a useful marker for thyroid cancer stem-like cells.
Keywords: Thyroid carcinoma, SSEA-1, CD15, Flow cytometry
Introduction
Stage specific embryonic antigen 1 (SSEA-1) or CD15 is a cluster of carbohydrate epitopes expressed on normal granulocytes Hodgkin's lymphoma cells and cells from a variety of solid cancers (1-5). ISSEA-1 contains a sugar sequence similar to that of Lewis × blood group antigen and is associated with cancer invasion and metastasis (6).
SSEA-1 is categorized as a type of oncofetal antigen in thyroid tissues (7, 8). In a relatively large-scale study of SSEA-1 expression in thyroid tissues, Miettinen and Karkkainen found strong immunoreactivity for SSEA-1 in fetal thyroid tissue and in most papillary carcinomas(PTCs), in approximately two third of follicular carcinomas(FCAs), and in a few benign lesions, but no immunoreactivity for SSEA-1 was found in anaplastic thyroid carcinomas (ATCs) (7). The immunolocalization of SSEA-, which is commonly referred to as CD15 in the pathology literature, has been somewhat variable. Two studies showed similar immunoreactivity for SSEA-1 in papillary carcinomas and follicular adenoma, while one group found no SSEA-1 immunoreactivity in conventional and diffuse sclerosing variant of papillary carcinomas (9-11). A recent study showed similar SSEA-1 immunoreactivity in conventional PTC as in previous studies, but none of the benign lesions showed SSEA-1 immunoreactivity (12). This latter study also reported that there was also less SSEA-1 immunoreactivity observed in follicular variant of papillary thyroid carcinomas (FVPTCs) (12). The presence of SSEA-1 reactivity has been suggested as an unfavorable prognostic sign in thyroid medullary and papillary carcinoma (13-16).
SSEA-1 has been identified as a biomarker for embryonic stem cells and for thyroid cancer stem cells (CSCs). (17-19). In many studies flow cytometric analysis is a common approach used to characterize stem cells and CSCs and to study the expression of SSEA-1 in various tumors (20, 21).
We used several approaches to characterize the distribution of SSEA-1 in thyroid tissues including immunohistochemical staining of thyroid tissues to detect expression of SSEA-1 in formalin-fixed paraffin-embedded tissue sections in benign and malignant lesions. Flow cytometric analysis with well characterized thyroid cell lines was also used to analyze the expression of SSEA-1 (20, 21).
Materials and Methods
A tissue microarray (TMA) was constructed with sections of normal thyroid (NT, n=10), Hashimoto thyroiditis (HT, n=11), follicular adenoma (FA, n=32), follicular carcinoma (FCA, n=28), papillary thyroid carcinoma (PTC, n=28), follicular variant of papillary thyroid carcinoma (FVPTC, n=29), and anaplastic thyroid carcinoma (ATC, n=10), and stained by immunohistochemistry (IHC) with an antibody to SSEA-1 at a 1:100 dilution (BD Biosciences, Clone MC480). Construction and immunostaining of the TMA was done as previously reported (22). Positive control tissues consisted of cell lines shown to express SSEA-1 by flow cytometric analysis (22). Negative control for immunostaining consisted of substituting normal rabbit serum for the primary antibody. After immunostaining, the individual sections were graded by intensity of staining from 0 (negative) to 3 (strongly positive). The percentage of tumor cells staining positive was also noted (rare <5%, focal 5-25%, and diffuse >25%). Flow cytometric analyses with ATC and PTC cell lines were done to evaluate the percentage of SSEA-1 positive cells as previously reported (20, 21).
Cell Culture
The cell lines TPC-1 BCPAP, THJ-16T and THJ-21T were obtained and cultures as previously reported (20 21).
Flow Cytometric Analysis
Flow cytometric analysis using two papillary thyroid carcinoma (TPC1 and BAP) and two anaplastic thyroid carcinoma cell lines (THJ-16T and THJ-21T) was performed as previously reported (20, 21).
Statistical Analysis
Statistical analyses were performed using the Chi-square test and Student t test. A P-value of <0.05 was considered to be statistically significant.
Results
Immunohistochemical staining for SSEA-1 showed predominantly membranous staining and some cytoplasmic staining (Fig 1). The percentage positive staining of each TMA core was usually less than 5% of the cells (focal staining) although a few cases showed more than 25% of the cells staining positive for SSEA-1 (diffuse staining)
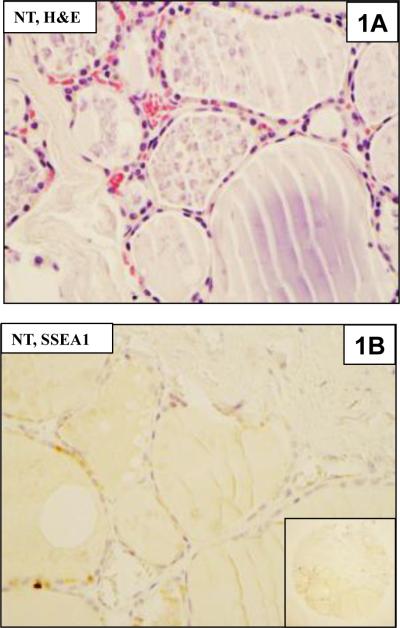
Figure 1.
Hematoxylin and eosin (H & E) and immunohistochemical staining for SSEA-1 in thyroid tissues.
1A. Normal thyroid (NT) tissues from the TMA.
1B. Immunostaining of normal thyroid for SSEA-1 does not show any positive cells. The inset shows the entire TMA core.
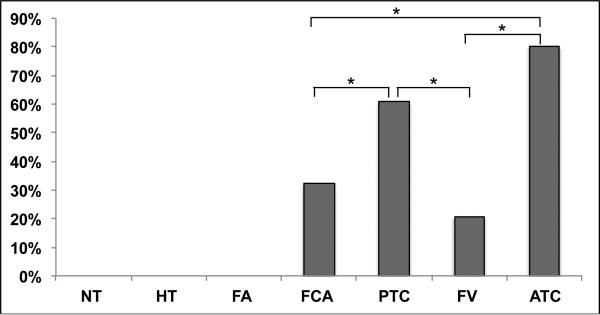
Immunohistochemical staining for SSEA-1 on the TMA is summarized in Table 1. SSEA-1 was negative in all benign thyroid tissues, but there was varying degree of positivity in all of the malignant thyroid neoplasms including conventional PTC, FVPTC, FCA, and ATC (Fig 1-4). Among cases that were positive for SSEA-1, the IHC staining was usually focal. Among different types of tumors, ATC showed the highest SSEA expression levels in most tumors (8/10), which was significantly more frequent than FCA (9/28) (p = 0.023) and FVPTC (6/29) (p = 0.001). PTC (60.7%, 17/28) was more likely to express SSEA-1 than FVPTC (p= 0.01) as well as FCA (p=0.028).There is no significant difference in the percentage of positive cases between PTCs and ATCs (Fig 5).
Table 1.
Distribution of SSEA-1- positive cells in normal and neoplastic thyroid tissues.
| Tumor Type | Total No. (158) | Positive No. (%) |
|---|---|---|
| NT | 10 | 0% (0/10) |
| HT | 11 | 0% (0/11) |
| NG | 10 | 0% (0/10) |
| FA | 32 | 0% (0/32) |
| FCA | 28 | 32.1% (9/28) |
| PTC | 28 | 60.7% (17/28) |
| FV | 29 | 20.6% (6/29) |
| ATC | 10 | 80% (8/10) |
NT-Normal thyroid; HT-Hashimoto's thyroiditis; NG-Nodular goiter; FA-Follicular adenoma; FCA-Follicular carcinoma; PTC-Papillary thyroid carcinoma; FVPTC-Follicular variant of papillary thyroid carcinoma; ATC-Anaplastic thyroid carcinoma.
Staining was rare or focal in most tumors except for two ATCs and one PTC which showed diffuse staining. The percentage of cells positive for SSEA-1 was enumerated as indicated in the Material and Methods.
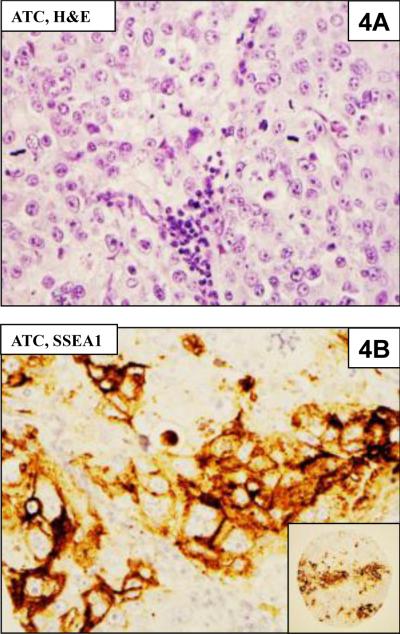
Figure 4.
4A. Anaplastic thyroid carcinoma (ATC) from the TMA
4B. Immunohistochemical staining for SSEA-1 showing positive staining of cells for SSEA-1. The inset shows the entire TMA core.
Figure 5.
Analysis of SSEA-1 expression of thyroid tumors. SSEA-1 in PTC was significantly higher than in FCA and FV (p <0.05), SSEA-1 in ATC was significantly higher than in FCA and FV (p< 0.05).
No statistically significant association was observed between SSEA-1 staining and age, gender, tumor size, vessel invasion or nodal status in PTC, FVPTC, FC and ATC. In univariate analyses, SSEA positivity in ATC was associated with worse survival (p=0.036). Six of the six ATC cases showing SSEA expression died of cancer, while two of two ATC cases without SSEA staining were still alive on follow-up. Two patients with ATCs positive for SSEA-1 were lost to follow up.
Flow cytometric analysis in cell lines
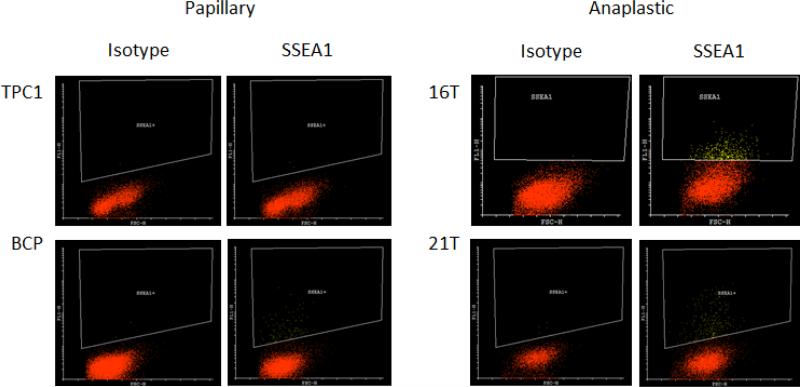
Flow cytometric analysis of SSEA-1 expression in thyroid cell lines was conducted using two anaplastic thyroid carcinoma cell lines and two papillary thyroid carcinoma cell lines as previously reported (Fig 6) (21). There were higher levels of SSEA-1 expression in the THJ-16T and THJ-21T ATCs cell lines (5.4% and 2.8% respectively) compared to the PTC BCP and TPC1 cell lines (0.1% and 1% respectively), as previously reported (21).
Figure 6.
Flow cytometric analysis of SSEA-1 content in two papillary (TPC1 and BCP) and two anaplastic (16T and 21T) thyroid carcinoma cell lines. The TPC and BCP papillary lines had little or no SSEA1 expression (0.1% and 1% respectively), whereas, the THJ-16T and THJ- 21T anaplastic cells lines expressed a significantly higher levels of SSEA1 as indicated by yellow dots (5.4% and 2.8% respectively). The isotypic immunoglobulin was used as the negative control for each cell line.
Discussion
Analysis of SSEA-1 expression in formalin-fixed paraffin-embedded thyroid tissue sections showed variable expression of this marker. The most striking finding was the absence or very low levels of SSEA-1 expression in non-neoplastic and benign thyroid tissues and increasing levels of expression from low to high grade thyroid neoplasms. Several studies of SSEA-1 or CD15 expression in thyroid tissues have produced varying results. This may be related to different antibodies used by various investigators as well as variation in tissue fixation and processing in different laboratories. Both flow cytometric analysis of the thyroid cancer cell lines and immunostaining of malignant neoplasms on the TMA showed only a minority of cells with SSEA-1 expression. Thus the SSEA-1 expression within the tumors may be underrepresented on the TMA due to the small sample size of the tissue cores. Because SSEA-1 is a marker of stem cells (23, 24) as well as CSC (17, 18), one would expect to find a few normal stem cells in non-neoplastic thyroid tissues. Thus the limited sizes of the TMA cores could partly explain the absence of positivity in non-neoplastic and benign thyroid tissues in this study.
The early report of Miettinen et al in which SSEA-1 was detected in PTC but not in ATC cells was surprising, since SSEA-1 positivity was detected in both formalin-fixed paraffin embedded tissues by immunohistochemistry as well as in ATCs cell lines by flow cytometry in our study. This discrepancy is potentially due to different antibodies and a different method of antigen retrieval for the paraffin sections in the present study.
In the ATC group, we found that six of eight ATC patients who showed significant SSEA-1 immunoreactivity died of their disease, while in the absence of significant staining the two patients were alive at the conclusion of the study. The small number of ATCs in the study does not allow any definitive conclusions about the role of SSEA-1 in predicting tumor behavior, but these preliminary observations suggest that more aggressive tumors are more likely to express SSEA-1.
The expression of SSEA-1 in FVPTC was less than in PTC in our study. The expression in this subgroup of PTC has been variable in the literature (12). However the definition of FVPTC has been modified in recent studies, since some tumors with nuclear features of FVPTC may behave as benign follicular lesions that are now designated as noninvasive follicular neoplasms with papillary-like nuclear features or NIFTP (25). Future studies with FVPTC will have to consider encapsulation of the tumors as well as tumor invasion before designating these neoplasms as benign or malignant neoplasms (25). Since this was not done in the present study, it is not possible to classify the tumors designated as FVPTC more rigorously.
Cancer stem cells (CSCs) are malignant cells within a tumor that share some of the characteristics with normal stem cells, including self-renewal and expression of SOX2, OCT4 and other stem cell markers (20, 21). CSCs are thought to be responsible for drug resistance and metastasis (19). SSEA-1 has been linked to CSCs in glioblastoma multiforme, medulloblastomas and in thyroid carcinomas (24-29). We also observed a small percentage of ATC and PTC epithelial cells from cultured cell lines expressing SSEA-1. Our detailed studies of these cells suggested that they are CSCs (22). These findings suggest that SSEA-1 may be a marker for thyroid tumor-initiating cells or CSCs. ATC cell lines contained more than twice the number of SSEA-1 positive cells than PTC cell lines, which is consistent with the histological finding of higher SSEA-1 immunoreactivity in ATC compared to PTCs. Studies of Ma and coworkers (17, 18) found that SSEA-1 positive cells showed enhanced vimentin expression and reduced E-Cadherin expression suggesting that they were most likely derived from the epithelial-to-mesenchymal transition (EMT) program a finding commonly observed during tumor progression (21). Ma et al also noted that the SSEA-1 positive cells were capable of asymmetric and symmetric division in vitro, features common to tumor initiating cells or CSCs (17, 22).
In summary, SSEA-1 positive cells can be detected in a small percentage of malignant tumor cells in formalin fixed-paraffin embedded thyroid tissue sections and these cells are more commonly found in highly aggressive thyroid cancers such as ATCs. Flow cytometric analyses using thyroid cell lines indicate that SSEA-1 labels a small percentage of thyroid tumor cells which are more frequent in ATCs compared to PTCs and may represent thyroid cancer stem-like cells.
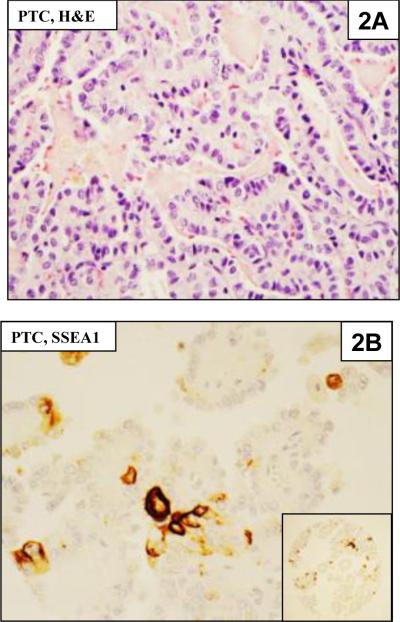
Figure 2.
2A. Papillary thyroid carcinoma (PTC) in H & E section
2B. Immunostaining of PTC showing focal staining of cells in the TMA for SSEA-1. The inset shows the entire TMA core.
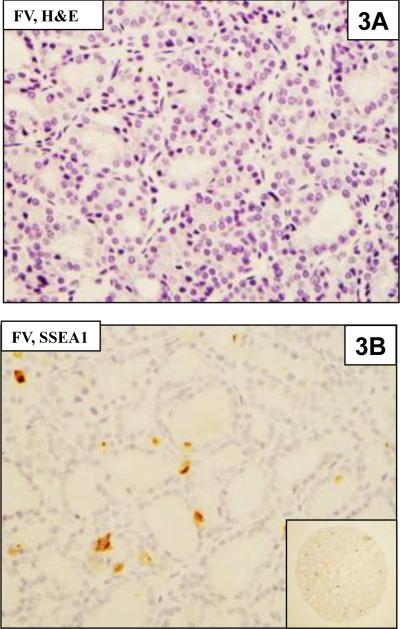
Figure 3.
3A. Follicular variant of PTC (FVPTC) in H & E section
3B. Immunohistochemical staining of a FVPTC showing rare cells positive for SSEA-1 on the TMA. The inset shows the entire TMA core.
Acknowledgements
We kindly thank Dr. John A. Copland III (Mayo Clinic, Jacksonville, FL) for the THJ-16T and THJ-21T cell lines, Dr. Rebecca E. Schweppe (University of Colorado, Denver, CO) for the BCPAP cell line, Dr. Daniel T. Ruan (Brigham and Women's Hospital, Boston, MA) for the TPC-1 cell line, the staffs of the Translational Research in Pathology (TRIP), Flow Cytometry, 3P, and Experimental Pathology laboratories (University of Wisconsin Carbone Cancer Center Cancer Center Support Grant P30 CA014520) for their services.
Footnotes
Presented as a Poster Session at the 105th Meeting of the United States and Canadian Academy of Pathology in Seattle WA, 2016.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- 1.Hanjan SNS, Kearney JF, Cooper MD. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982;23:172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- 2.Hsu SM, Jaffe ES. Leu-M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984;82:29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- 3.Jang TJ, Park JB, Lee JI. The Expression of CD10 and CD15 Is Progressively Increased during Colorectal Cancer Development. Korean J Pathol. 2003;47:340–347. doi: 10.4132/KoreanJPathol.2013.47.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadota A, Masutani M, Takei M, Horie T. Evaluation of expression of CD15 and sCD15 in non-small cell lung cancer. Int J Oncol. 1999;15:1081–1089. doi: 10.3892/ijo.15.6.1081. [DOI] [PubMed] [Google Scholar]
- 5.Brooks SA, Leathem AJ. Expression of the CD15 antigen (Lewis x) in breast cancer. Histochem J. 1995;27:689–693. [PubMed] [Google Scholar]
- 6.Shirahama T, Ikoma M, Muramatsu T, Ohi Y. Expression of SSEA-1 carbohydrate antigen correlates with stage, grade and metastatic potential of transitional cell carcinoma of the bladder. J Urol. 1992;148:1319–1322. doi: 10.1016/s0022-5347(17)36900-8. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M, Kärkkäinen P. Differential reactivity of HBME-1 and CD15 antibodies in benign and malignant thyroid tumours. Preferential reactivity with malignant tumours. Virchows Arch. 1996;429:213–219. doi: 10.1007/BF00198336. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca E, Castanhas S. Sobrinho-Simões M. Carbohydrate antigens as oncofetal antigens in papillary carcinoma of the thyroid gland. Endocr Pathol. 1997;8:301–303. doi: 10.1007/BF02739932. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca E, Castanhas S, Sobrinho-Simões M. Expression of simple mucin type antigens and Lewis type 1 and type 2 chain antigens in the thyroid gland. An immunohistochemical study of normal thyroid tissues, benign lesions and malignant tumors. Endocr Pathol. 1996;7:291–301. doi: 10.1007/BF02739836. [DOI] [PubMed] [Google Scholar]
- 10.Van Hoeven KH, Kovatich AJ, Miettinen M. Immunocytochemical evaluation of HBME-1, CA 19-9, and CD-15 (Leu-M1) in fine-needle aspirates of thyroid nodules. Diagn Cytopathol. 1998;18:93–97. doi: 10.1002/(sici)1097-0339(199802)18:2<93::aid-dc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Koo JS, Shin E, Hong SW. Immunohistochemical characteristics of diffuse sclerosing variant of papillary carcinoma: comparison with conventional papillary carcinoma. APMIS. 2010;118:744–752. doi: 10.1111/j.1600-0463.2010.02653.x. [DOI] [PubMed] [Google Scholar]
- 12.Ohta M, Ookoshi T, Naiki H, Imamura Y. HBME-1 and CD15 immunocytochemistry in the follicular variant of thyroid papillary carcinoma. Pathol Int. 2015;65:119–125. doi: 10.1111/pin.12252. [DOI] [PubMed] [Google Scholar]
- 13.Schr/Sder S, Schwarz W, Rehpenning W, Loning T, Becker W. Prognostic significance of Leu-M1 immunostaining in papillary carcinomas of the thyroid gland. Virchows Arch. 1998;411:435–439. doi: 10.1007/BF00735224. [DOI] [PubMed] [Google Scholar]
- 14.Willgeroth C, Floegel R, Rosler B. The importance of S-100 protein positive Langerhans cells and Leu-M1 positive tumor cells for prognosis of papillary thyroid cancer. Zentralbl Chir. 1992;117:603–606. [PubMed] [Google Scholar]
- 15.Neuhold N, Langle F, Gnant M, Hollenstein U, Niederie B. Relationship of CD15 immunoreactivity and prognosis in sporadic medullary thyroid carcinoma. J Cancer Res Clin Oncol. 1992;118:629–634. doi: 10.1007/BF01211810. [DOI] [PubMed] [Google Scholar]
- 16.Langel F, Soliman T, Neuhold N, Widhalm G, Niederie B, Roka S, Kaserer K, Blauensteiner W, Dam K, Clodi M, et al. CD15 (LeuM1) immunoreactivity: prognostic factor for sporadic and hereditary medullary thyroid cancer? Study Group on Multiple Endocrine Neoplasia of Austria. World J Surg. 1994;18:583–587. doi: 10.1007/BF00353771. [DOI] [PubMed] [Google Scholar]
- 17.Ma R, Bonnefond S, Morshed SA, Latif R, Davies TF. Stemness is derived from thyroid cancer cells. Front Endocrinol (Lausanne) 2014;5:114. doi: 10.3389/fendo.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma R, Minsky N, Morshed SA, Davies TF. Stemness in human thyroid cancers and derived cell lines: the role of asymmetrically dividing cancer stem cells resistant to chemotherapy. J Clin Endocrinol Metab. 2014;99:E400–9. doi: 10.1210/jc.2013-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi P, Misteli T. In vitro generation of human cells with cancer stem cell properties. Nat Cell Biol. 2011;13:1051–1061. doi: 10.1038/ncb2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardin H Guo Z, Shan W, Montemayor-Garcia C, Asioli S, Yu X-M, Harrison AD, Chen H, Lloyd RV. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. 2014;184:2342–2354. doi: 10.1016/j.ajpath.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardin H, Yu X-M, Harrison AD, et al. Generation of Novel thyroid cancer stem-like cell clones: Effects of resveratrol and valproic acid. Am J Pathol. 2016;186:1662–1673. doi: 10.1016/j.ajpath.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buehler D, Hardin H, Shan W, Montemayor-Garcia C, Rush PS, Asioli S, Chen H, Lloyd RV. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2013;26:54–61. doi: 10.1038/modpathol.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–442. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim y, Jeong J, Kang H, Lim J, Heo J, Ratajczak J, Ratajczak MZ, Shin DM. The molecular nature of very small embryonic-like stem cells in adult tissues. J Stem Cells. 2014;7:55–62. doi: 10.15283/ijsc.2014.7.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0386. doi:10.1001/jamaoncol.2016.0386 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanagisawa M. Stem cell glycolipids. Neurochem Res. 2011;36:1623–1635. doi: 10.1007/s11064-010-0358-1. [DOI] [PubMed] [Google Scholar]
- 27.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 28.Son MJ, Woolard K, Nam DH, et al. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read TA, Fogarty MP, Markant SL, et al. Identification of CD15 as a marker for tumor- propagating cells cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–147. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]