Abstract
Memory deficits are characteristic of HIV-associated neurocognitive disorders (HAND) and co-occur with hippocampal pathology. The HIV-1 transactivator of transcription (Tat), a regulatory protein, plays a significant role in these events, but the cellular mechanisms involved are poorly understood. Within the hippocampus, diverse populations of interneurons form complex networks; even subtle disruptions can drastically alter synaptic output, resulting in behavioral dysfunction. We hypothesized that HIV-1 Tat would impair cognitive behavior and injure specific hippocampal interneuron subtypes. Male transgenic mice that inducibly expressed HIV-1 Tat (or non-expressing controls) were assessed for cognitive behavior or had hippocampal CA1 subregions evaluated via interneuron subpopulation markers. Tat exposure decreased spatial memory in a Barnes maze and mnemonic performance in a novel object recognition test. Tat reduced the percentage of neurons expressing neuronal nitric oxide synthase (nNOS) without neuropeptide Y immunoreactivity in the stratum pyramidale and the stratum radiatum, parvalbumin in the stratum pyramidale, and somatostatin in the stratum oriens, which are consistent with reductions in interneuron-specific interneuron type 3 (IS3), bistratified, and oriens-lacunosum-moleculare interneurons, respectively. The findings reveal that an interconnected ensemble of CA1 nNOS-expressing interneurons, the IS3 cells, as well as subpopulations of parvalbumin- and somatostatin-expressing interneurons are preferentially vulnerable to HIV-1 Tat. Importantly, the susceptible interneurons form a microcircuit thought to be involved in feedback inhibition of CA1 pyramidal cells and gating of CA1 pyramidal cell inputs. The identification of vulnerable CA1 hippocampal interneurons may provide novel insight into the basic mechanisms underlying key functional and neurobehavioral deficits associated with HAND.
Keywords: NeuroAIDS, Neurodegeneration, Spatial memory, Interneuron specific interneuron type 3 (IS3), Oriens-lacunosum-moleculare cell (O-LM), Bistratified cell
Introduction
Following the discovery of human immunodeficiency virus type 1 (HIV-1) and acquired immunodeficiency syndrome (AIDS), a profile of progressive neurological symptoms came to be associated with HIV-1 infection (Snider et al. 1983; Levy et al. 1985), now collectively referred to as HIV-associated neurocognitive disorders (HAND; Antinori et al. 2007; McArthur et al. 2010). Although there is variation among patients, common symptoms include deficits in attention, learning, memory, and impaired motor function. The neurocognitive aspects of HAND can be severe enough to interfere with independent living (Antinori et al. 2007). Notably, combined antiretroviral therapy (cART) does not fully ameliorate these symptoms (Sacktor et al. 2002; Tozzi et al. 2005; McArthur 2004; Ellis et al. 2007).
HIV may contribute to the progressive neurocognitive impairment in combination antiretroviral therapy (cART)-controlled patients through the continued production of neurotoxic HIV-1 proteins from cellular reservoirs within the central nervous system (CNS) (Johnson et al. 2013). Shortly after infection, HIV-1 can enter the brain and establish central reservoirs (Lane et al. 1996; An et al. 1999), infecting microglia or astrocytes, but not neurons (He et al. 1997; Brack-Werner 1999; Kramer-Hämmerle et al. 2005; Li et al. 2011; Churchill et al. 2009). Uninfected cells can be damaged by viral proteins, as well as by the host neuroinflammatory response to virions and free viral proteins (Nath et al. 1996; Kruman et al. 1998; Nath et al. 1999; Aksenov et al. 2001; Soulas et al. 2009; Zhou and Saksena 2013; Nath and Steiner 2014). Among these neurotoxic factors, the HIV-1 transactivator of transcription (Tat) protein is critical for HIV replication (Zhou and Saksena 2013; Nath and Steiner 2014) and can be secreted by infected cells (Ensoli et al. 1990; Thomas et al. 1994; Rayne et al. 2010; Debaiseux et al. 2012) even in the presence of cART (Johnson et al. 2013). Furthermore, Tat can bind to a large number of extracellular receptors due to its highly basic domain (Philippon et al. 1994; Zhou and Saksena 2013), penetrate and traverse the plasma membrane, and interact with a number of intracellular factors (Debaiseux et al. 2012).
Despite regional differences in CNS vulnerability to HIV-1 (Nath 2015), few studies have examined why some brain regions and neuronal types are preferentially susceptible to the virus. A number of behavioral deficits, including attenuation of spatial learning and memory, which are common features of HAND, can be recapitulated by Tat exposure and may be attributed to cellular and functional deficits in the hippocampus (Li et al. 2004; Fitting et al. 2006; 2013). Long-term potentiation (LTP) is lost when Tat is injected intrahippocampally or expressed endogenously (Li et al. 2004; Behnisch et al. 2004; Fitting et al. 2013). Despite the pronounced loss of LTP and deficits in behavior, CA1 pyramidal cells showed only a modest decrease in the density of apical dendritic spines, but no loss in dendrite length or synaptic integrity at the ultrastructural level, and no alterations in the level of proteins involved in excitatory glutamatergic pre- and post-synaptic function (Li et al. 2004; Fitting et al. 2013). By contrast, there were selective alterations in proteins involved in inhibitory γ-aminobutyric acid-ergic (GABAergic) synaptic function, including a significant decline in synaptotagmin 2 (Syt2) expression (only within stratum radiatum) and marked increases in the expression of gephyrin throughout CA1 (Fitting et al. 2013). These findings concur with human gene array data which show a high degree of correlation between the expression of genes associated with inhibitory GABAergic synaptic transmission, including GAD1 and GABAR1, and the severity of HAND, but showed less robust correlations with genes associated with glutamatergic neurotransmission (Gelman et al. 2012).
GABAergic interneurons are a diverse population of specialized cells responsible for much of the processing that occurs in the hippocampus. With estimates of at least 21 distinct interneuron subtypes in CA1, there are numerous potential targets for the development of hippocampal pathology (McBain and Fisahn 2001; Klausberger and Somogyi 2008; Lovett-Barron and Losonczy 2014). While numerous studies have examined the effects of HIV-1 on glutamatergic transmission in the hippocampus, few studies have assessed whether GABAergic synapses are altered. Pathology within specific GABAergic interneuron subpopulations, and the hippocampal interneuron network as a whole, has been demonstrated in a number of neurological disorders (Korotkova et al. 2010; Tóth et al. 2010; Hazra et al. 2013; Levenga et al. 2013), some of which produce behavioral deficits similar to those observed in experimental models of neuro-acquired immunodeficiency syndrome (neuroAIDS) (Carey et al. 2012; Fitting et al. 2013). In HIV models, reductions in the number of hippocampal neurons expressing parvalbumin (PV) in CA3 and somatostatin (SST) in CA1 have been observed (Masliah et al. 1992; Fox et al. 1997); however, since multiple neuron types in CA1 and CA3 express these markers, the identities of the affected interneuron subtypes remain unclear.
Based on the collective findings above, we hypothesized that specific interneuron subpopulation(s) might be preferentially vulnerable to HIV-1 Tat and that deficits in one or more interneuron subtypes following Tat exposure would coincide with impaired cognitive performance on hippocampus-dependent behavioral tasks. Our data show that HIV-1 Tat exposure was accompanied by memory impairment in mice and resulted in a selective decrease in the proportion of nNOS positive (nNOS+), neuropeptide Y negative (NPY−) neurons in both the stratum pyramidale and stratum radiatum, as well as decreases in somatostatin-positive (SST+) neurons in the stratum oriens and parvalbumin positive (PV+) neurons in the stratum pyramidale.
Materials and methods
Animals and treatments
Male mice were used exclusively throughout these studies. Inducible, glial fibrillary acidic protein-driven transgenic Tat mice (aged 60–80 days) were used to identify the effects of HIV-1 Tat1–86 on hippocampal interneuron subpopulations and associated behavior (Bruce-Keller et al. 2008). In order to induce Tat expression, mice expressing the tat and rtTA transgenes (Tat+) were fed doxycycline-containing chow (DOX; 6 mg/g, Harlan, Indianapolis, IN) for 12–14 days depending on the experiment. Control mice lacking the tat transgene, but expressing the rtTA gene (Tat−), were given the same DOX treatment. Animal procedures were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and are in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Barnes maze
Tat− (n = 13) and Tat+ (n = 8) mice were assessed for spatial learning and motor function in a Barnes maze test (Barnes 1979). Mice consumed DOX chow for 7–8 days prior to 1 day of prehabituation, followed by 4 days of testing and 1 day of probe trials. For prehabituation, mice were placed in the escape hole (positioned in a randomly assigned location) for 2 min, then were placed in the brightly lit center of a Barnes maze (91 cm diameter, 90 cm height, with 20 holes each 5 cm diameter; Stoelting Co., Wood Dale, IL), and guided to the assigned escape hole where they remained for 2 min, then were placed under a glass cylinder next to the escape hole, and allowed up to 3 min to volitionally enter (mice that did not enter the hole were guided in and remained for 2 min). On testing days (four trials per day over 4 days), mice were placed in the brightly lit center of the Barnes maze and were allowed up to 3 min to enter the escape hole. Mice that did not enter the hole were gently guided to the hole and allowed to remain for 2 min. Shorter latencies to find the escape hole, a greater proportion of time spent in the correct quadrant of the maze, and fewer errors were considered indices of greater learning (Camara et al. 2013). On day 5, a reversal probe trial was conducted wherein the maze was turned 180° such that the correct goal box was on the opposite end of the Barnes maze table, and the proportion of time spent in the new target quadrant, latency to find the new escape hole, and number of errors made were assessed. Distances and velocities traveled were used as an index of motor behavior.
Novel object recognition
Tat− (n = 14) and Tat+ (n = 14) mice were assessed for neurocognitive function in a novel object recognition test (Ennaceur and Delacour 1988; Dere et al. 2007) after 14 days on DOX. Briefly, mice were habituated to a testing room, placed in an open field (35 × 40× 40 cm; Stoelting Co., Wood Dale, IL), and allowed to explore two identical round objects (plastic toys in the shape of kiwis, oranges, potatoes, or tomatoes; each ∼6 × 3 cm) for a 10-min training trial. After a 4-h inter-trial interval, one of the previously explored objects was replaced with a novel cone-shaped object of similar size (a plastic toy in the shape of a half ear of corn or a half-pickle), which mice were allowed to explore for a 10-min retention trial. Across subjects, replacement of the familiar object on the left vs. right side of the apparatus was counterbalanced to control for potential side-preferences. For each trial, the time spent investigating the object in the novel position, as a function of the entire time spent investigating, was calculated: [(novel object time − familiar object time)/(novel object time + familiar object time)]. To assess motor behavior, the overall distance traveled, as well as the time and frequency spent rearing were recorded.
Vision testing
Tat− (n =6) and Tat+ (n=6) mice were assessed for visual function based on prior methods (Wersinger et al. 2002) prior to DOX exposure, and at 7 and 14 days following DOX exposure. In brief, mice were suspended approximately 12 in. above a vertical ring-stand and were gently lowered with the ring-stand approximately 2 in. from the left or right visual field (close enough to allow visual, but not whisker, contact with the ring-stand). The left and right visual fields were assessed consecutively for each mouse. Visual responding was considered positive when mice reached with the forepaws for the rod when presented to both the left and right side (a response to only one visual field would have been considered a negative response). Two observers rated visual responses.
Immunohistochemistry
Brains were fixed in 4 % paraformaldehyde using transcardial perfusion, removed, halved, and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA). Hemisected brains were sectioned in the sagittal plain at a thickness of 16 μm for analysis of interneuron populations or in the coronal plane at a thickness of 40 μm to determine the reference volume using stereology. A Leica CM1850 cryostat was used to cut the sections (Leica Biosystems, Buffalo Grove, IL). Sections were mounted on slides and then stored at −80 °C until use. Tissue sections for interneuron analyses were blocked, then incubated with individual primary antibodies (anti-PV, Synaptic Systems, Cat. No. 195 004, guinea pig polyclonal, 1:1200; anti-nNOS, Abcam, ab1376, goat polyclonal, 1:5000; anti-NPY, Abcam, ab30914, rabbit polyclonal, 1:100; anti-SST, Santa Cruz Biotechnology, sc-13099, rabbit polyclonal, 1:50; anti-NeuN, Chemicon, MAB377, mouse monoclonal, 1:500) for 18 h at 4 °C, and rinsed in phosphate-buffered saline (PBS). Solutions containing appropriate, fluorescently labeled secondary antibodies (goat anti-guinea pig IgG-Cy3, Abcam ab6965-100, 1:500; Abcam ab102370, 1:500; donkey anti-goat IgG-Alexa Fluor® 488, Invitrogen, A11055, 1:500; goat anti-rabbit IgG-Alexa Fluor® 647, Invitrogen A21244, 1:500; donkey anti-mouse IgG-Alexa Fluor® 594, Invitrogen, A21203, 1:500; goat anti-mouse IgG-488 Oregon green, Molecular Probes, 0-6380, 1:500) were placed onto tissue sections for 2 h at room temperature. Slices were then rinsed 3 × 10 min in PBS. All tissue sections were incubated in PBS/Hoechst solution (0.5 μg/ml, Invitrogen, H3570) for 5–10 min, repeatedly rinsed in PBS, and mounted in ProLong Gold Antifade reagent (Invitrogen, P36930).
Reference volume analysis
Hoechst-stained 40-μm serial sections were sampled through the entire hippocampus. From a random starting position, sections at evenly spaced intervals were sampled to obtain an unbiased stereological assessment of hippocampal volume (Gundersen et al. 1988). In short, sections from halved brains were taken from Tat− (n = 6) and Tat+ (n = 5) mice and imaged using a Zeiss Axio-Observer Z1 microscope, MRm digital camera, and a 10× objective (Zeiss, Oberkochen, Germany). Montages of the entire left hemisphere of the brain (5 × 6) were obtained utilizing a motorized stage encoder (Zeiss) and a computerized tile-reconstruction method (Zeiss, Axio-Vision software). Hippocampal volume was then estimated by overlaying a standardized grid over the image of the brain and performing a point count analysis (Gundersen et al 1988; Mouton 2002).
Imaging
Hippocampi were imaged using a Zeiss LSM 700 microscope at 20× magnification (Zeiss, Oberkochen, Germany). Neurons, as noted by the presence of NeuN immunoreactivity within the nucleus, were counted in stratum oriens, stratum pyramidale, stratum radiatum, and stratum lacunosum-moleculare of CA1. Immunoreactivity for each protein was noted independently, and the markings were overlaid to confirm co-expression of proteins without bias. From 4 to 10 sections (typically 7 to 8) were analyzed and averaged per animal, and 6 mice per experimental group were used for all experiments. All cell count data are presented as a percent of the total NeuN+ population in each layer. To examine the potential effects of the Tat transgene on the neuritic processes, densitometric analysis of PV+ dendrites and SST+ axons was performed using ImageJ (NIH, Bethesda, MD). In addition, dendritic varicosities of PV+ interneurons were quantified on the five most pronounced dendrite segments from each image within stratum radiatum from 2 to 3 sections per animal. Dendrites not reaching at least 50 μm in length or discontinuous dendrites were not analyzed.
Statistical analyses
Behavioral endpoints were recorded and digitally encoded by an ANY-maze animal tracking system (Stoelting Co., Wood Dale, IL) and assessed via repeated-measures analyses of variance (ANOVA) with novel object recognition trial (training trial or retention trial) or Barnes maze testing day (days 1–5) and mouse genotype (Tat− or Tat+) as the respective within-, and between-, subjects factors. Tukey’s honestly significant difference post hoc tests were used to assess group differences following main effects. Interactions were delineated via simple main effects and main effect contrasts with alpha corrected for multiple comparisons. Immunohistochemical endpoints were assessed with one-tailed Student’s t tests. Effects were considered significant when p<0.05.
Results
HIV-1 Tat expression impairs mnemonic performance
Inducing HIV-1 Tat expression in the CNS of mice significantly impaired spatial learning in the Barnes maze test. Compared to Tat− controls, Tat+ mice demonstrated significantly longer latencies to find the escape hole [F(1,76)=9.39, p<0.05] and spent a significantly lower proportion of time [F(1,76)=8.17, p<0.05] in the correct quadrant of the Barnes maze (Fig. 1). Irrespective of genotype, all mice took significantly longer to find the escape hole following a 180° swap of the escape location on the probe trial day [F(4,76)=3.42, p<0.05] compared to any previous day’s performance (p = 0.0001–0.01; Fig. 1). No differences were observed in the number of errors made or the distance traveled between Tat− and Tat+ mice (Fig. 1); however, a difference in velocity was observed between Tat− and Tat+ mice [F(1,76)=5.35, p<0.05] with a significant reduction observed in the reversal probe trial [F(4,76)=8.45, p<0.05], compared to day 1 performance (p < 0.0001). All mice demonstrated significantly decreased maze exploration (indicated by distance traveled) [F(4,76)=12.86, p<0.05] and made fewer errors [F(4, 76) = 11.37, p < 0.05] compared to day 1 (p = 0.0001–0.02; Fig. 1).
Fig. 1.
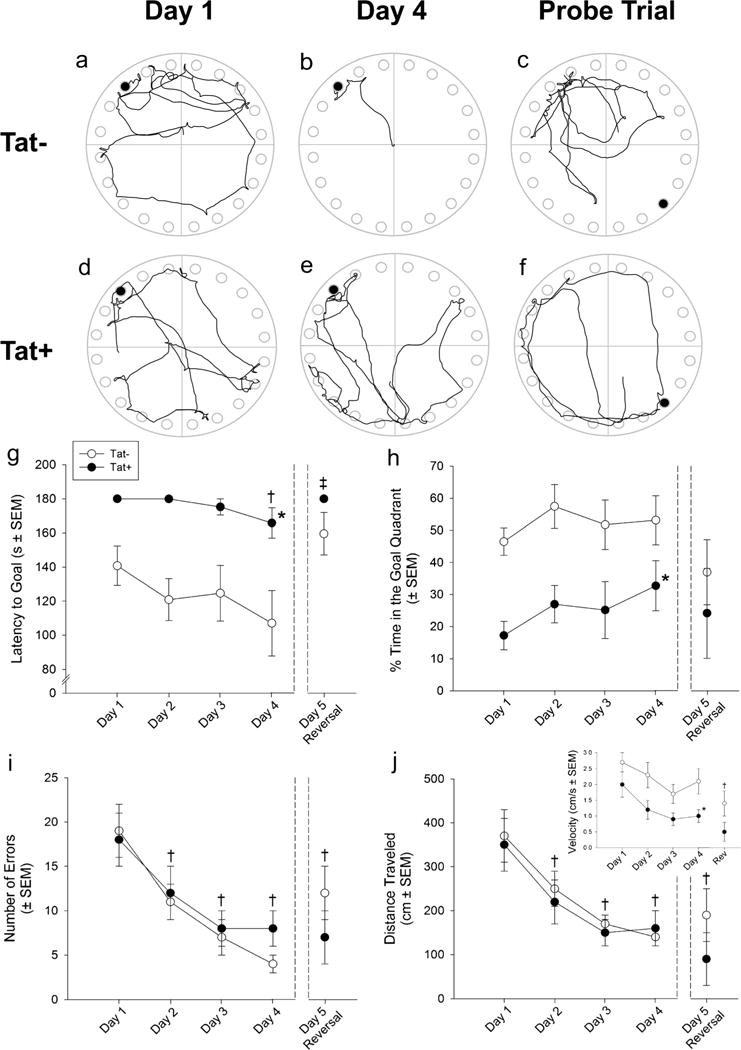
Representative traces of the movements of Tat− (a–c) and Tat+„ (d–f) mice exploring a Barnes maze on testing days 1 (a, d) and 4 (b, e), as well as the reversal probe trial [escape hole reversed 180° (c, f)]. Escape hole is noted as the black circle in each figure. Tat+ mice (n = 8) demonstrate significantly longer latencies to find the escape hole (g) and (h) spend a significantly lower proportion of time in the correct quadrant of the Barnes maze test, indicating impaired learning compared to Tat− control mice (n = 13). Neither the number of errors made (i) nor the distance traveled (j) differs between Tat− and Tat+ mice, but decreases among all animals from day 1 performance. Tat+ mice have a reduced velocity compared to Tat− mice, and all mice show reduced velocity in the reversal probe trail compared to day 1 performance (j, inset). The asterisk indicates a main effect for Tat− and Tat+ mice to significantly differ. The dagger sign indicates performance significantly differs from day 1 performance. The double dagger sign indicates reversal probe trial performance significantly differs from all other testing days, except day 1 (main effects, repeated measures ANOVA, p < 0.05)
Similarly, HIV-1 Tat expression significantly impaired cognitive performance in a novel object recognition task [F(1, 26) = 5.67, p < 0.05]. Compared to performance in the training trial, Tat− control mice spent a significantly greater proportion of time with a novel object in the retention trial (p = 0.0007; Fig. 2) indicating intact mnemonic performance. However, Tat+ mice demonstrated impaired object recognition during the retention phase (Fig. 2), spending significantly less time investigating the novel object compared to Tat− controls (p = 0.04). No significant differences were observed between Tat− or Tat+ mice on the total amount of time spent investigating objects nor on any motor measure investigated (Table 1).
Fig. 2.
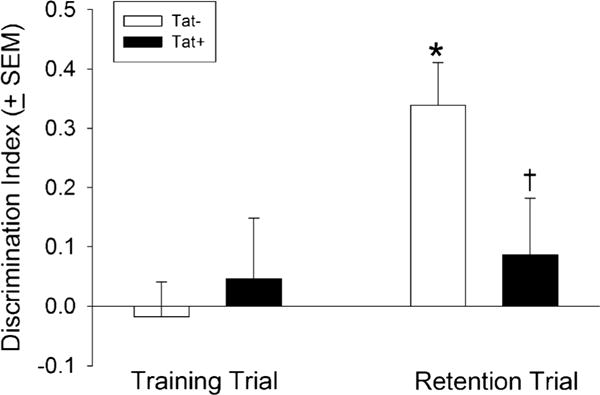
Tat+ mice (n = 14) spend a significantly lower proportion of time investigating a novel (vs. familiar) object in a novel object recognition test, indicating impaired cognitive performance compared to Tat− control mice (n = 14). The asterisk indicates a significant difference from the respective training trial exploration. The dagger sign indicates a significant difference between Tat− and Tat+ mice in the retention trial (interaction, repeated measures ANOVA, p < 0.05)
Table 1.
Raw exploration time and motor behavior among Tat− and Tat+ mice (n = 14/group) that were assessed in a novel object recognition task (mean ± SEM)
| Tat−, n = 14 | Tat+, n = 14 | |
|---|---|---|
| Familiar object exploration (s) | 12 ± 2 | 15 ± 2 |
| Novel object exploration (s) | 24 ± 3 | 20 ± 3 |
| Total distance traveled (m) | 13 ± 1 | 11 ± 1 |
| Rearing events per trial (10 min) | 51 ± 8 | 44 ± 8 |
| Total time rearing (s) | 43 ± 7 | 35 ± 6 |
No significant differences were observed between Tat− and Tat+ mice
Given that a visual component is present in the Barnes maze and novel object recognition tasks, vision was assessed in a separate group of mice. All Tat− and Tat+ mice responded positively to a stimulus presented to the left and right fields of vision prior to DOX exposure and at 7 and 14 days following DOX exposure.
Hippocampal volume is unchanged following Tat exposure
Point count analysis of hippocampal volume revealed no difference between Tat− (16.71 ± 0.39 mm3) and Tat+ (17.62 ± 0.47 mm3) mice. Additionally, the volume of the CA1 subfield was unchanged between Tat− (9.97 ± 0.19 mm3) and Tat+ (10.74 ± 0.69 mm3) mice indicating that there was no difference in the volume of the hippocampus or in hippocampal area CA1 following 12–14 days of Tat induction.
HIV-1 Tat expression is associated with reductions in specific CA1 nNOS-expressing interneuron subpopulations
We investigated Tat effects on a subset of nNOS+/NPY+ cells known as ivy cells. These cells are found primarily in the stratum pyramidale as well as the stratum radiatum of CA1 (Fuentealba et al. 2008). To assess the vulnerability of nNOS+/NPY+ cells to HIV-1 Tat, hippocampal sections were immunolabeled for nNOS, NPY, and NeuN (Figs. 3 and 4). The distribution of nNOS and NPY immunoreactivity within CA1 was consistent with previous research (Morris 1989; Jinno et al 1999; Fuentealba et al. 2008). Significant reductions in the percentage of nNOS-expressing cells were observed in the stratum pyramidale [t(8) = 2.25, p < 0.05] and stratum radiatum [t(8) = 2.43, p < 0.05] of Tat+ mice compared to Tat− mice (Fig. 3). Importantly, the reduction in nNOS+ interneurons was specifically restricted to the subpopulation of interneurons that did not possess NPY antigenicity in both the stratum pyramidale [t(8) = 2.36, p < 0.05] and stratum radiatum [t(8) = 1.92, p < 0.05] after Tat exposure, indicating ivy cells were not selectively vulnerable. No significant differences were observed for NPY+ interneurons lacking nNOS in any layer of CA1. Tat induction did not significantly alter the percentage of NPY+ interneurons or interneurons coexpressing nNOS and NPY in any layer of the hippocampus (Fig. 3).
Fig. 3.
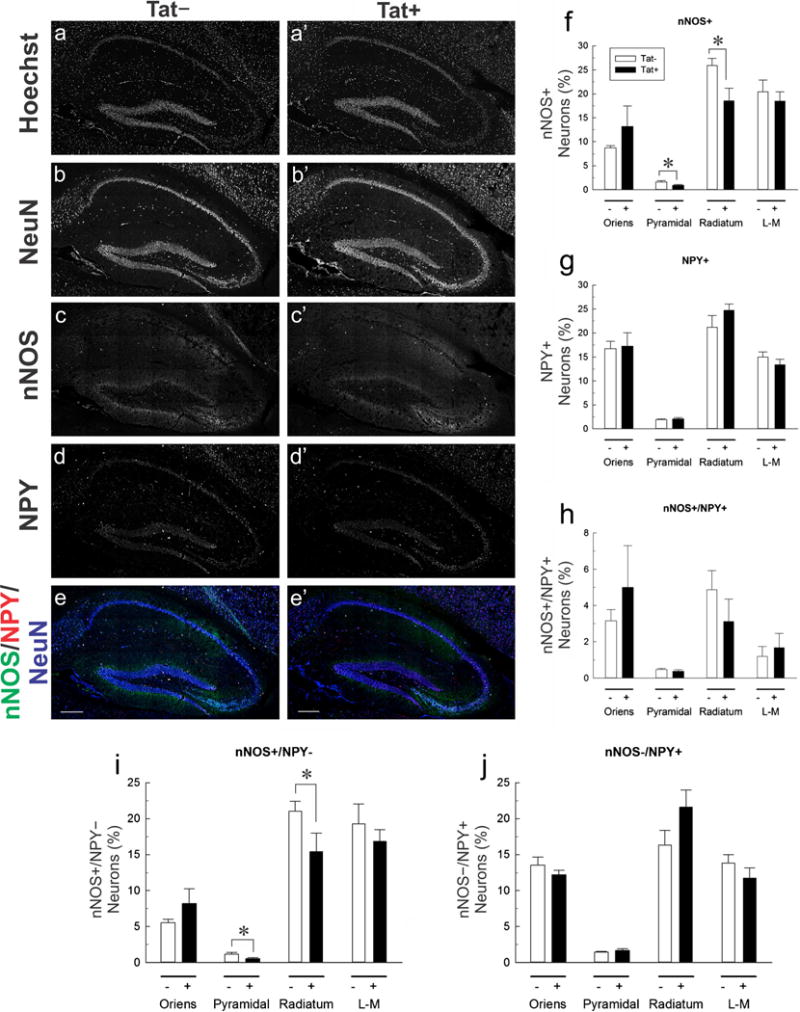
Effects of HIV-1 Tat induction on neuronal nitric oxide synthase (nNOS) and neuropeptide Y (NPY) immunoreactive interneuron subpopulations in hippocampal area CA1. a–e Immunoreactivity for nNOS (c, c′), NPY (d, d′), and the neuronal marker, NeuN (b, b′), was colocalized in sections from Tat+ and Tat− mice counterstained using Hoechst dye (a, a′). e, e′ NeuN (blue), nNOS (green), and NPY (red) merged. f–j The percentage of labeled neurons in hippocampal area CA1 by layer and marker. The proportion of nNOS+ neurons in stratum pyramidale (Pyramidal) and stratum radiatum (Radiatium) is significantly decreased in Tat+ compared to Tat− mice (*p < 0.05, one tailed t test) (f), while the percentage of NPY+ neurons or neurons co-expressing nNOS and NPY in any layer is unaffected by Tat (g, h). The percentage of cells expressing nNOS in the absence of NPY is significantly reduced in stratum pyramidale and in stratum radiatum (*p < 0.05, one tailed t test) (i), while the proportion of NPY cells that lack nNOS was unaffected by Tat (j). Stratum oriens (Oriens), stratum lacunosum-moleculare (L–M). Scale bar = 200 μm
Fig. 4.
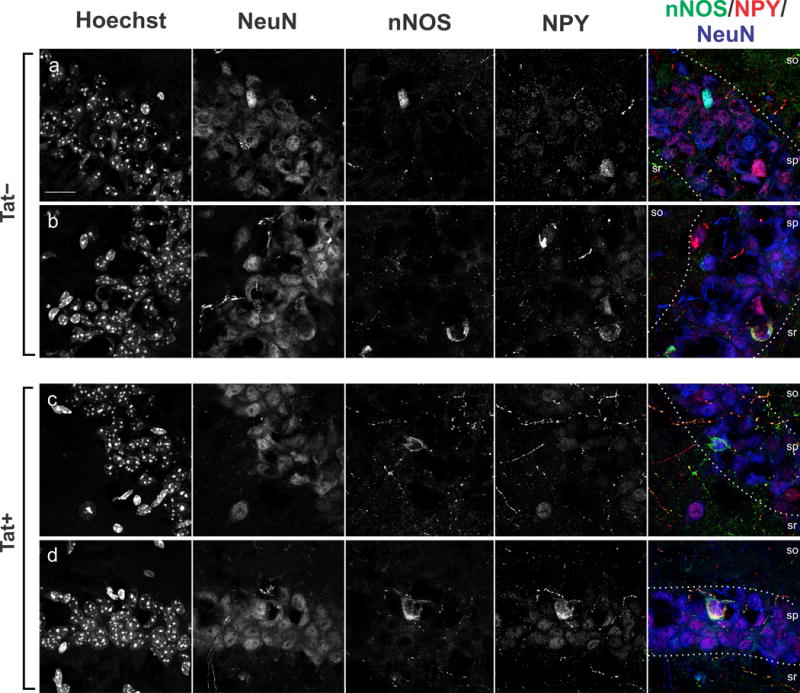
Subcellular localization of neuronal nitric oxide synthase (nNOS) and neuropeptide Y (NPY) immunoreactivity in interneurons in hippocampal area CA1 of Tat+ and Tat− mice. a–d Immunoreactivity for nNOS (green), NPY (red), and the neuronal marker, NeuN (blue), was colocalized in sections from Tat+ and Tat− mice counterstained using Hoechst dye (nuclei). a, c nNOS+/NPY− interneurons located in stratum pyramidale (sp). b, d nNOS+ and NPY+ interneurons of stratum pyramidale. Stratum oriens (so), stratum radiatum (sr). Scale bar = 10 μm
The proportion of SST+ and PV+ neurons is reduced by HIV-1 Tat expression
To assess the vulnerability of PV+ and SST+ interneurons, hippocampal sections were immunolabeled for PV, SST, and NeuN (Figs. 5 and 6). PV immunoreactivity was nearly exclusive to stratum pyramidale and stratum oriens, consistent with previous research (Kosaka et al. 1987). Observations of SST immunoreactivity in stratum oriens, stratum pyramidale, and stratum radiatum also agree with previous findings (Oliva et al. 2000); however, SST+ neurons occurring in the stratum radiatum were not quantified as part of this study (Fig. 5). The percentage of PV+ neurons were significantly diminished in the stratum pyramidale of Tat+ mice compared to Tat− mice [t(10) = 1.839, p < 0.05]. While no significant effect of Tat was observed in PV+/SST− interneurons, there was a trend toward significance in PV+/SST+ interneurons in the stratum pyramidale [t(10) = 1.711, p = 0.059]. No effect of Tat was observed in PV+ interneuron groups in the stratum oriens. In contrast, the proportion of SST+ interneurons was reduced in the stratum oriens [t(10) = 2.664, p < 0.05], but not in the stratum pyramidale of Tat+ mice. While there were no significant differences in stratum oriens to suggest that subpopulations of SST+ neurons were reduced in the layer, there was a trend toward significant decreases in the proportion of PV−/SST+ [t(10) = 1.673, p = 0.063]. These data indicate subregion-specific declines in interneuron populations, supporting the idea of selective vulnerability among hippocampal neuron subpopulations.
Fig. 5.
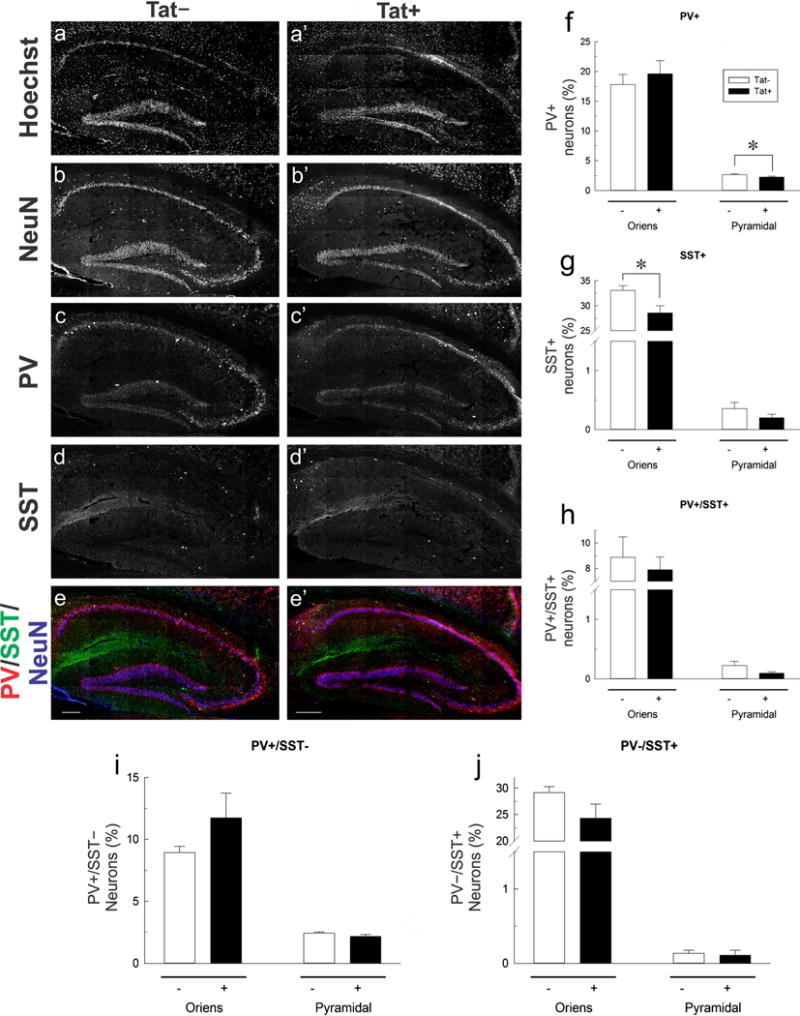
Effects of HIV-1 Tat induction on parvalbumin (PV) and somatostatin (SST) immunoreactivity in interneurons within hippocampal area CA1. a–d Low-magnification images of PV, SST, and NeuN immunoreactivity in sections from Tat+ and Tat− mice additionally counterstained with Hoechst dye. a–e Hippocampal sections from Tat+ and Tat–mice treated with DOX, probed with Hoechst (a, a′), NeuN (b, b′), PV (c, c′), and SST (d, d′). e, e′ NeuN (blue), PV (green), and SST (red) merged. f–j The percentage of labeled neurons in hippocampal area CA1 by layer and marker. A decrease in the percent of PV+ neurons is seen in stratum pyramidale (Pyramidal) (f), and a decrease in the percent of SST+ neurons is observed in stratum oriens (Oriens) (g). No significant decrease is observed for neurons expressing both PV and SST or for neurons that express either marker in the absence of the other (h–j). The asterisk denotes a significant reduction from Tat− control population percentage (one tailed t test, p < 0.05). Scale bar = 200 μm
Fig. 6.
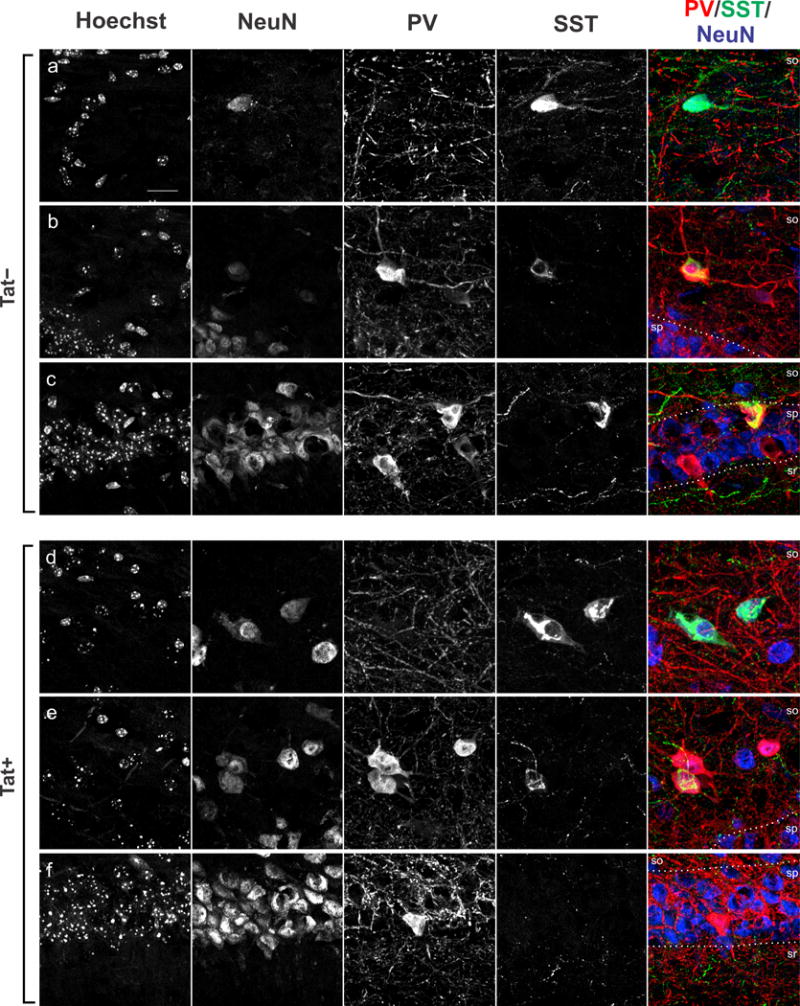
Subcellular localization of parvalbumin (PV) and somatostatin (SST) immunoreactivity in interneurons in hippocampal area CA1 of Tat+ and Tat− mice. a–d High-magnification images of PV (red), SST (green), and NeuN (blue) immunoreactivity in sections from Tat+ and Tat− mice additionally counterstained with Hoechst dye (a–f). a, d PV+/SST− interneurons located in stratum oriens (so). b, e PV+ and SST+ interneurons of the stratum oriens. c, f PV+ neurons of stratum pyramidale (sp). Stratum radiatum (sr). Scale bar = 10 μm
PV+ and SST+ processes in CA1 appear unaffected by Tat
ImageJ analysis of PVexpression in the stratum radiatum and SST expression in the stratum lacunosum-moleculare showed no differences in fiber density between Tat+ and Tat− mice. There were also no differences in dendritic varicosity number or size in PV+ neurons (Fig. 7).
Fig. 7.
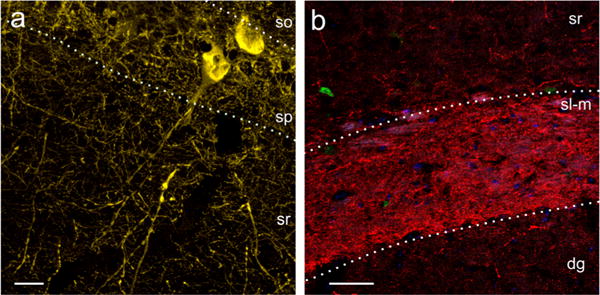
No evidence of changes to parvalbumin-immunoreactive (PV+) dendrites or somatostatin-immunoreactive (SST+) axons is observed following Tat induction. a PV+ neurons and neurites in Tat− mice. No differences in the density of PV+ fibers or increases in the number of dendritic varicosities was seen in the stratum radiatum (sr). Scale bar = 20 μm. b Tat− SST+ axons in stratum lacunosum-moleculare (sl-m). No difference in fiber density of SST+ axons in the SL-M was observed. Stratum oriens (so), stratum pyramidale (sp), dentate gyrus (dg). Scale bar = 50 μm
Discussion
Given the great diversity in function, morphology, and localization of hippocampal interneurons, the highly localized reduction of Syt2+ fibers described by Fitting et al (2013) suggests selective vulnerability of distinct subpopulations of interneurons to HIV-1 Tat. We thus hypothesized that a subpopulation of hippocampal interneurons would be selectively vulnerable to HIV-1 Tat and used an immunohistochemical/morphological approach to identify neurodegenerative changes in cells within defined regions of CA1. We found that Tat+ animals exhibited aberrant learning behavior in both the Barnes maze and novel object recognition tests. Tat expression reduced the population of nNOS+/NPY− interneurons in both the stratum radiatum and stratum pyramidale, SST+ cells in the stratum oriens, and PV+ cells in the stratum pyramidale. nNOS+/NPY+ or nNOS−/NPY+ neurons remained unchanged.
The pathology of specific interneuron subpopulations, and the hippocampal interneuron network as a whole, have been demonstrated in a number of neurological disorders (Korotkova et al. 2010; Tóth et al. 2010; Antonucci et al. 2012; Hazra et al. 2013; Levenga et al. 2013), some of which produce behavioral deficits in learning similar to the findings of Fitting et al (2013). Due to neuronal heterogeneity in the hippocampus (Bouilleret et al. 2000; Moga et al. 2002; Avignone et al. 2005), differential responses to Tat-induced excitotoxicity and neuroinflammation may be expected. In a kainate-induced model of excitotoxicity, a loss of GAT-1, a GABA reuptake protein, was greater in the stratum pyramidale than in the stratum oriens and the stratum radiatum, suggesting regional variability in selective vulnerability of hippocampal interneurons (Bouilleret et al. 2000). In HIV models, reductions in the number of cells expressing PV in CA3, and SST in CA1 were observed, although identification of the affected subtypes was incomplete (Masliah et al. 1992; Fox et al. 1997). The results of the present study indicate that there is at least one subtype of nNOS+ interneuron within CA1 that is selectively vulnerable to HIV-1 Tat. nNOS+ interneurons appear in all layers of the hippocampus, a majority of which are subpopulations of neurogliaform cells (NGFCs; nNOS+/NPY−; Jinno et al. 1999; Tricoire et al. 2010). In addition to NGFCs, there are ivy (nNOS+/NPY+) and interneuron-specific interneuron type III (IS3; nNOS+/NPY−) cells. Ivy cells exist primarily in the stratum pyramidale, as well as the stratum radiatum (Fuentealba et al. 2008; Lapray et al. 2012). nNOS+/NPY− cells have been found in the stratum pyramidale (IS3s) and stratum radiatum (NGFCs; Acsády et al. 1996a, b; Porter et al. 1998; Jinno et al. 1999; Jinno and Kosaka 2002, 2004; Tricoire et al. 2010; Armstrong et al. 2012; Somogyi et al. 2012).
Our findings suggest that the ivy cells (i.e., nNOS+/NPY+ neurons residing in the stratum pyramidale) are not vulnerable to HIV-1 Tat as previously hypothesized (Fuentealba et al. 2008). Rather, a significant decline was observed in the number of nNOS+/NPY− interneurons in both the stratum pyramidale and stratum radiatum. In the stratum pyramidale, these vulnerable neurons are thought to be IS3s (given the lack of NPY). Additionally, these neurons are positive for vasoactive intestinal polypeptide (VIP), and calretinin (CR) (Acsády et al. 1996a, b; Porter et al. 1998; Jinno et al. 1999; Jinno and Kosaka 2002; Tricoire et al 2010) and the expression of nNOS differentiates them as IS3s versus an IS1 subtype (Tricoire et al. 2010). Functionally, IS3s support feedback inhibition within CA1 (Gulyás et al. 1996; Chamberland et al. 2010; Chamberland and Topolnik 2012; Tyan et al. 2014). Of particular interest, the selective vulnerability of multiple subsets of CR+ cells in the hippocampus may occur in a temporal-lobe epilepsy model, a good predictor of neuronal vulnerability to excitotoxicity. Those cells that remain exhibit pathologic dendritic varicosities and a loss of dendritic complexity and synapses, as a result of excitotoxic injury (Tóth et al. 2010; Tóth and Maglóczky 2014). Patients with epilepsy can have fewer nNOS+ interneurons within CA1 (Leite et al. 2002).
The population of nNOS+/NPY− cells affected by Tat in stratum radiatum likely consists of multiple nNOS+ interneuron subtypes, including IS3s (Acsády et al. 1996a, b; Porter et al. 1998; Jinno et al. 1999; Jinno and Kosaka 2002; Tricoire et al. 2010) and NGFCs (Tricoire et al. 2010; Armstrong et al. 2012; Tricoire and Vitalis 2012; Somogyi et al. 2012). Since IS3s represent a small proportion of nNOS+ cells in the stratum radiatum, it is probable that NGFCs constitute a majority of nNOS+/NPY− cells lost (Jinno et al. 1999; Jinno and Kosaka 2002; Tricoire et al. 2010; Armstrong et al. 2012). The presence or absence of CR, which is expressed in IS3s, but not NGFCs, might be used to further differentiate these interneuron subtypes in the future (Jinno and Kosaka 2002; Armstrong et al. 2012; Somogyi et al. 2012). Considering the lack of cell death in the hippocampus observed at early time points following Tat induction (Fitting et al. 2013), we speculate that the affected interneuron subtypes are downregulating nNOS in response to excitotoxic injury in order to restore homeostasis (Hu et al. 2008; Di et al. 2012).
nNOS is required for the normal functioning of the hippocampal interneuron network and maintenance of related behavioral endpoints. In complete nNOS knockout mice, there is an attenuation of contextual fear conditioning and spatial memory formation similar to what is observed in Tat transgenic mice (Kirchner et al. 2004; Weitzdoerfer et al. 2004; Kelley et al. 2009; Fitting et al. 2013). While nNOS is important for normal hippocampal function (Kirchner et al. 2004; Zanelli et al. 2009), research indicates that nNOS-induced neuronal damage occurs in pathological states (Cui et al. 2007; Eugenin et al. 2007; Steinert et al. 2010; Wang et al. 2010; Drury et al. 2014; Hsu et al. 2014; Wu et al. 2014). The role of nNOS in excitotoxicity, including HIV-1 Tat excitotoxicity, is reportedly linked to its activation of PSD-95 and NMDA receptor complexes (Christopherson et al. 1999; Eugenin et al. 2007; Fan et al. 2010; King et al. 2010; Steinert et al. 2010). Exposure to excitotoxic levels of glutamate causes temporal dysregulation of nNOS activation-inactivation processes (Rameau et al. 2007). Thus, Tat-induced increases in the activation of nNOS may enhance NMDA receptor phosphorylation, the recruitment of additional AMPA receptors, and the generation of peroxynitrite resulting in greater susceptibility to excitotoxic damage in nNOS-expressing interneurons (Yu et al. 1997; Grima et al. 2001; Rameau et al. 2007; Hossain et al. 2012). Lastly, we speculate that the proposed compensatory downregulation of nNOS may be absent or may be less prevalent in nNOS+/NPY+ neurons due to the neuroprotective effects of NPY, which can regulate neuronal excitability via paracrine/autocrine signaling (Xapelli et al. 2006; Smialowska et al. 2009).
The present findings stand in contrast to prior studies that did not demonstrate a loss of PV+ interneurons in CA1 of HIV-infected patients or rodent models (Masliah et al. 1992; Guo et al. 2012). Herein, we differentiate the percentages of CA1 PV-expressing cells by CA1 layer and by SSTco-expression, allowing for delineation of CA1 cell types (PV+ interneurons including bistratified and oriens-lacunosum-moleculare (O-LM) cells; Kosaka et al. 1987; Buhl et al. 1994; Masliah et al. 1992; Freund and Buzsáki 1996; Jinno and Kosaka 2000, 2002a; Klausberger et al. 2004; Klausberger and Somogyi 2008; Müller and Remy 2014; Yamada and Jinno 2015). Bistratified cells were discerned from O-LM interneurons via the relative intensity of PV (high in bistratified) and SST (minimal in bistratified) immunoreactivity. In contrast, O-LMs exert an opposing PV/SST profile, with a subset of O-LMs being entirely PV− (Chittajallu et al. 2013; Müller and Remy 2014). Our data show a modest effect of Tat on the total PV+ population of the stratum pyramidale; however, this was not restricted to a particular PV+ cell type. Although we believe Tat may be selectively affecting bistratified cells based on a trend toward reduced PV+/SST+ interneuron numbers, additional information is needed for confirmation. Interestingly, while the loss of PV+ cells in CA1 is not reported in patients with neuroAIDS, many of the PV+ interneurons were reportedly unhealthy with damaged neurites being the chief descriptor (Masliah et al. 1992). Despite limitations in generalizing findings from Tat transgenic mice to HIV-infected individuals, the collective findings suggest PV+ interneuron subsets are selectively vulnerable to HIV—perhaps through synaptodendritic injury and pruning.
Reductions in SST gene expression in the brains of HIV patients are linked to the development of depression (Everall et al. 2006). Fox et al. (1997) showed a reduction in SST+ interneurons in HIV patients, but did not differentiate between the layers of CA1. We observed a strong net reduction of SST+ interneurons in the stratum oriens, but not in the stratum pyramidale. The stratum radiatum was not quantified due to the relative rarity of SST+ cells in this region. Interestingly, there was a trend toward reductions in SST+/PV− interneurons in the stratum oriens following Tat induction. The loss of SST has been shown to have profound effects on LTP within CA1, with differential effects in the apical and basilar dendrites of pyramidal cells based on receptor subtypes and response to specific oscillation patterns (Fan and Fu 2014). Considering the decline in SST+ neurons observed in this study and previous findings of reduced SST expression in HIV patients (Everall et al. 2006), it will be important to examine whether region-specific SST losses contribute to impairments in LTP and related behavior following Tat-exposure (Behnisch et al. 2004; Fitting et al. 2013). In support of this notion, ablating SST+ interneurons, focal to distal apical dendrites of pyramidal cells in SLM (likely O-LMs), causes a loss of cue-based fear-learning, while removal of PV+ neurons, focal to basal dendrites and proximal portions of apical dendrites in the stratum radiatum, does not result in loss of fear learning (Leão et al. 2012; Lovett-Barron et al. 2014; Müller and Remy 2014). While it is not possible to say with certainty which SST+ population is affected in the present study, the net loss in SST in the stratum oriens is most likely due to O-LM cell vulnerability.
We and others have found that HIV-1 Tat can impair learning and memory, and the present study begins to advance our understanding of the limbic neuronal types that may underlie such effects. In the present study, Tat+ mice exhibited deficient performance on two hippocampally dependent tasks, the Barnes maze and novel object recognition tests, compared to their Tat− counterparts. Consistent with these results, stereotaxic injections of Tat directly to dorsal hippocampus have recently been demonstrated to increase the latency to find a hidden platform in a Morris water maze and to impair novel object recognition among Sprague-Dawley rats (Harricharan et al. 2015). Similar intracerebroventricular injections of Tat impaired radial arm maze performance concurrent with disrupted LTP in the hippocampus of C57BL/6J mice (Li et al. 2004). Additionally, similar impairment of Barnes maze performance and object recognition has been observed in a separate GFAP-driven Tat-inducible mouse model (Carey et al. 2012). Considering that behavioral deficits similar to those seen in neuroAIDS can be traced back to the dysfunction of one or more of the interneuron types discussed here, it is reasonable to assume that imbalance in this complex circuit may contribute to the attenuation of spatial memory observed in HAND (Masliah et al. 1992; Buhl et al. 1994; Sik et al. 1995; Acsády et al. 1996b; Fox et al. 1997; Ali et al. 1998; Kirchner et al. 2004; Klausberger et al. 2004; Weitzdoerfer et al. 2004; Carey et al. 2012; Leão et al. 2012; Fitting et al. 2013; Lovett-Barron et al. 2014; Sun et al. 2014; Tyan et al. 2014; Müller and Remy 2014).
Hippocampal interneurons interact as part of a functional network and it is clear that the removal of even one cell type from the network can have drastic effects on information processing, pyramidal cell excitation, and consequent behavioral outcomes (Moga et al. 2002; Dugladze et al. 2007; Goldin et al. 2007; Tóth et al. 2010; Peng et al. 2013; Long et al. 2014; Lovett-Barron et al. 2014; Lovett-Barron and Losonczy 2014; Tóth and Maglóczky 2014; Orbán-Kris et al. 2015). Importantly, the susceptible nNOS+/NPY− interneurons of the stratum pyramidale and the stratum radiatum, PV+ cells of the stratum pyramidale, and SST+ interneurons of the stratum oriens form a microcircuit known to be involved in a complex feedback loop/input gating mechanism that regulates network synchronization within CA1 (Fig. 8; Buhl et al. 1994; Sik et al. 1995; Acsády et al. 1996b; Ali et al. 1998; Klausberger et al. 2004; Chamberland et al. 2010; Chamberland and Topolnik 2012; Leão et al. 2012; Sun et al. 2014; Tyan et al. 2014; Müller and Remy 2014; Milstein et al. 2015). Herein, we propose this microcircuit to be selectively vulnerable to HIV-1 Tat. Given clinical observations that implicate HAND- and psychostimulant-related neurocognitive deficits to be associated with interneuron losses in other brain regions (Chana et al. 2006), the present findings suggest that a proportionally small, interconnected ensemble of hippocampal CA1 interneurons may contribute to key functional and neurobehavioral deficits observed in HAND.
Fig. 8.
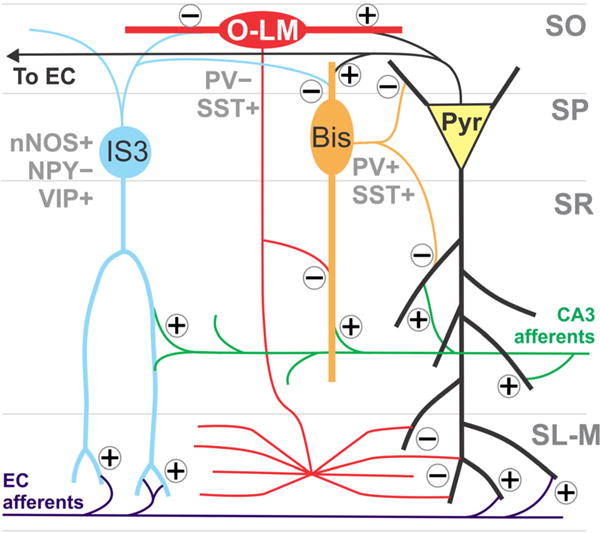
The interneurons affected by Tat form a microcircuit within the CA1 area of the hippocampus. CA1 pyramidal cells receive inputs from both hippocampal area CA3 and the entorhinal cortex. Oriens-lacunosum-moleculare (O-LM) cells gate inputs from the entorhinal cortex onto pyramidal cell distal apical dendrites. CA3 inputs are disinhibited by bistratified cells and Shaffer collateral-associated interneurons in the stratum radiatum (Sik et al. 1995; Klausberger et al. 2004; Leão et al. 2012). O-LMs receive excitatory inputs from CA1 pyramidal cells and the septum, and inhibitory innervation from interneuron-specific interneuron type 3 (IS3) cells (Acsády et al. 1996b; Sun et al. 2014; Tyan et al. 2014; Müller and Remy 2014). Bistratified cells receive excitatory input from Shaffer collaterals, CA1 pyramidal cells, and the septum (Ali et al. 1998; Klausberger et al. 2004; Müller and Remy 2014) and innervate pyramidal cells (Buhl et al. 1994; Sik et al. 1995; Klausberger et al. 2004). In addition to being inhibited by O-LMs, bistratified cells receive GABAergic inputs from the neuronal nitric oxide (nNOS)+/neuropeptide Y (NPY)− IS3s (Leão et al. 2012; Tyan et al 2014). IS3s innervate O-LMs preferentially, as well as bistratified cells, in the stratum oriens (Acsády et al. 1996b; Tyan et al. 2014). Disruption of this circuit by Tat may disrupt the gating of inputs from CA3 and the entorhinal cortex and therefore produce aberrant pyramidal cell outputs, which could account for the behavioral phenotype observed following Tat exposure (Sik et al. 1995; Klausberger et al. 2004; Leão et al. 2012). CA1 pyramidal cell (yellow), bistratified cell (Bis, orange), O-LM (red), IS3 (light blue), Shaffer collateral input from CA3 (green), perforant path input from the entorhinal cortex (EC, purple). Glutamatergic synapses are noted with a plus (+) and GABAergic synapses are noted with a minus (−)
Acknowledgments
This work was supported by grants K99 DA033878 (SF), K99 DA039791 (JJP), F32 DA039039 (CJS), T32 DA007027 (WDM and CJS), R01 DA018633 (KFH), R01 DA024461 (PEK), R01 DA034231 (PEK and KFH), R01 DA033200 (KFH), R01 MH094626 (ARM), R21 MH103695 (ARM), and K02 DA027374 (KFH) from the National Institute on Drug Abuse. The authors thank Dr. Marie Bonhomme for the assistance with the immunohistochemical analysis, Mr. Phu Vo for the help in maintaining the mice, Ms. Yun Ji for laboratory support, and Dr. Thomas Reeves for proofreading the manuscript.
Footnotes
Compliance with ethical standards Animal procedures were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee and are in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines.
Conflict of interest The authors declare that they have no conflicts of interest.
References
- Acsády L, Arabadzisz D, Freund TF. Correlated morphological and neurochemical features identify different subsets of vasoactive intestinal polypeptide-immunoreactive interneurons in rat hippocampus. Neuroscience. 1996a;73:299–315. doi: 10.1016/0306-4522(95)00610-9. [DOI] [PubMed] [Google Scholar]
- Acsády L, Görcs TJ, Freund TF. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996b;73:317–334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Ali AB, Deuchars J, Pawelzik H, Tomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol. 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SF, Groves M, Gray F, Scaravilli F. Early entry of widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci F, Alpár A, Kacza J, Caleo M, Verderio C, Giani A, Martens H, Chaudhry FA, Allegra M, Grosche J, Michalski D, Erck C, Hoffman A, Harkany T, Matteoli M, Härtig W. Cracking down on inhibition: elective removal of GABAergic interneurons from hippocampal networks. J Neurosci. 2012;32:1989–2001. doi: 10.1523/JNEUROSCI.2720-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C, Krook-Magnuson E, Soltesz I. Neurogliaform and ivy cells: a major family of nNOS expressing GABAergic neurons. Front Neural Circuits. 2012;6:23. doi: 10.3389/fncir.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avignone E, Frenquelli BG, Irving AJ. Differential responses to NMDA receptor activation in rat hippocampal interneurons and pyramidal cells may underlie enhanced pyramidal cell vulnerability. Eur J Neurosci. 2005;22:3077–3090. doi: 10.1111/j.1460-9568.2005.04497.x. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Schwaller B, Schurmans S, Celio MR, Fritschy JM. Neurodegenerative and morphogenic changes in a mouse model of temporal lobe epilepsy do not depend on the expression of the calcium-binding proteins parvalbumin, calbindin, or calretinin. Neuroscience. 2000;97:47–58. doi: 10.1016/s0306-4522(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Koerner H, Baune BT. TNF-α and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology. 2013;38:3102–3114. doi: 10.1016/j.psyneuen.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Carey AN, Sypek EI, Singh HD, Kaufman MJ, McLaughlin JP. Expression of HIV-Tat protein is associated with learning and memory deficits in the mouse. Behav Brain Res. 2012;229:48–56. doi: 10.1016/j.bbr.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S, Topolnik L. Inhibitory control of hippocampal inhibitory neurons. Front Neurosci. 2012;6:165. doi: 10.3389/fnins.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S, Salesse C, Topolnik D, Topolnik L. Synapse-specific inhibitory control of hippocampal feedback inhibitory circuit. Front Cell Neurosci. 2010;4:130. doi: 10.3389/fncel.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E, HNRC Group Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology. 2006;67:1486–1489. doi: 10.1212/01.wnl.0000240066.02404.e6. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Craig MT, McFarland A, Yuan X, Gerfen S, Tricoire L, Erkkila B, Barron SC, Lopez CM, Liang BJ, Jeffries BW, Pelkey KA, McBain CJ. Dual origins of functionally distinct OLM interneurons revealed by differential 5HT3AR expression. Nat Neurosci. 2013;16:1598–1607. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:7467–7473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Cui H, Hayashi A, Sun HS, Belmares MP, Cobey C, Phan T, Schweizer J, Salter MW, Wang YT, Tasker RA, Garman D, Rabinowitz J, Lu PS, Tymianski M. PDZ protein interactions underlying NMDA receptor-mediated excitotoxicity and neuroprotection by PSD-95 inhibitors. J Neurosci. 2007;27:9901–9915. doi: 10.1523/JNEUROSCI.1464-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaiseux S, Rayne F, Yezid H, Beaumelle B. The ins and outs of HIV-1 Tat. Traffic. 2012;13:355–363. doi: 10.1111/j.1600-0854.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Di JH, Li C, Yu HM, Zheng JN, Zhang GY. nNOS downregulation attenuates neuronal apoptosis by inhibiting nNOS-GluR6 interaction and GluR6 nitrosylation in cerebral ischemic reperfusion. Biochem Biophys Res Commun. 2012;420:594–599. doi: 10.1016/j.bbrc.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Drury PP, Davidson JO, Mathai S, van den Heuij JG, Bennett L, Tan S, Silverman RB, Gunn AJ. nNOS inhibition during profound asphyxia reduces seizure burden and improves survival of striatal phenotypic neurons in preterm fetal sheep. Neuropharmacology. 2014;83:62–70. doi: 10.1016/j.neuropharm.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugladze T, Vida I, Tort AB, Gross A, Otahal J, Heinemann U, Kopell NJ, Gloveli T. Impaired hippocampal rythmogenesis in a mouse model of mesial temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2007;104:17530–17535. doi: 10.1073/pnas.0708301104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ensoli B, Barillari G, Salahuddin SZ, Gallo RC, Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi’s sarcoma lesions of AIDS patients. Nature. 1990;345:84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MVL, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Salaria S, Atkinson JH, Young C, Corbeil J, Grant I, Masliah E. Diminished somatostatin gene expression in individuals with HIV and major depressive disorder. Neurology. 2006;67:1867–1869. doi: 10.1212/01.wnl.0000244436.04036.a2. [DOI] [PubMed] [Google Scholar]
- Fan W, Fu T. Somatostatin modulates LTP in hippocampal CA1 pyramidal neurons: differential activation conditions in apical and basal dendrites. Neurosci Lett. 2014;561:1–6. doi: 10.1016/j.neulet.2013.12.025. [DOI] [PubMed] [Google Scholar]
- Fan J, Vasuta OC, Zhang LY, Wang L, George A, Raymond LA. N-Methyl-D-aspartate receptor subunit- and neuronal-type dependence of excitotoxic signaling through post-synaptic density 95. J Neurochem. 2010;115:104–156. doi: 10.1111/j.1471-4159.2010.06994.x. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of Tat: effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav. 2006;84:189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fitting S, Ignatowska-Jankowska BM, Bull C, Skoff RP, Lichtman AH, Wise LE, Fox MA, Su J, Medina AE, Krahe TE, Knapp PE, Guido W, Hauser KF. Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice. Biol Psychiatry. 2013;73:443–453. doi: 10.1016/j.biopsych.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L, Alford M, Achim C, Mallory M, Masliah E. Neurodegeneration of somatostatin immunoreactive neurons in HIV-encephalitis. J Neuropathol Exp Neurol. 1997;207:42–51. doi: 10.1097/00005072-199704000-00004. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Márton LF, Csicsvari J, Thonsom A, Somogyi P, Klausberger T. Ivy cells: a population of nitric-oxide-producing, slow spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57:917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BJ, Chen T, Lisiniccia JG, Soukup VM, Carmicam JR, Starkey JM, Masliah E, Commins DL, Brandt D, Grant I, Singer EJ, Levin AJ, Miller J, Winkler JM, Fox HS, Luxon BA, Morgello S. The national neuroAIDS tissue consortium brain gene array: two types of HIV-associated neurocognitive impairment. PLoS One. 2012;7:e46178. doi: 10.1371/journal.pone.0046178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin M, Epsztein J, Jorquera I, Represa A, Ben-Ari Y, Crépel V, Cossart R. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci. 2007;27:9560–9572. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima G, Benz B, Ho KQ. Glial-derived arginine, the nitric oxide precursor, protects neurons from NMDA-induced excitotoxicity. Eur J Neurosci. 2001;14:1762–1770. doi: 10.1046/j.0953-816x.2001.01799.x. [DOI] [PubMed] [Google Scholar]
- Gulyás AI, Hájos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. AMPIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Guo M, Bryant M, Sultana S, Jones O, Royall W., 3rd Effects of vitamin A deficiency and opioids on parvalbumin+ interneurons of the HIV-1 transgenic rat. Curr HIV Res. 2012;10:463–468. doi: 10.2174/157016212802138715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan R, Thaver V, Russell VA, Daniels WM. Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats. Behav Brain Funct. 2015;11:3. doi: 10.1186/s12993-014-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra A, Gu F, Aulakh A, Berridge C, Eriksen JL, Ziburkus J. Inhibitory neuron and hippocampal circuit dysfunction in an aged mouse model of Alzheimer’s disease. PLoS One. 2013;8:e64318. doi: 10.1371/journal.pone.0064318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hossain MI, Kamaruddin MA, Cheng HC. Aberrant regulation and function of SRc family tyrosine kinases: their potential contributions to glutamate-induced neurotoxicity. Clin Exp Parmacol Physiol. 2012;39:684–691. doi: 10.1111/j.1440-1681.2011.05621.x. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Chang YC, Lin YC, Sze CI, Huang CC, Ho CJ. Cerebral microvascular damage occurs early after hypoxia-ischemia via nNOS activation in the neonatal brain. J Cereb Blood Flow Metab. 2014;34:668–676. doi: 10.1038/jcbfm.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Sun YJ, Zhou QG, Chen L, Hu Y, Luo CX, Wu JY, Xu JS, Li LX, Zhu DY. Negative regulation of neurogenesis and spatial memory by NR2B-containing NMDA receptors. J Neurochem. 2008;106:1900–1913. doi: 10.1111/j.1471-4159.2008.05554.x. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Colocalization of parvalbumin and somatostatin-like immunoreactivity in the mouse hippocampus: quantitative analysis with optical dissector. J Comp Neurol. 2000;428:377–388. [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of expression of calcium binding proteins and neuronal nitric oxide synthase in different populations of hippocampal GABAergic neurons in mice. J Comp Neurol. 2002;449:1–25. doi: 10.1002/cne.10251. [DOI] [PubMed] [Google Scholar]
- Jinno S, Kosaka T. Patterns of colocalization of neuronal nitric oxide synthase and somatostatin like immunoreactivity in the mouse hippocampus: quantitative analysis with optical dissector. Neuroscience. 2004;124:797–808. doi: 10.1016/j.neuroscience.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Jinno S, Aika Y, Fukuda T, Kosaka T. Quantitative analysis of neuronal nitric oxide synthase immunoreactive neurons in the mouse hippocampus with optical dissector. J Comp Neurol. 1999;410:398–412. [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Nath A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. PNAS USA. 2013;110:155888–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JB, Balda MA, Anderson KL, Itzhak Y. Impairments in fear conditioning in mice lacking the nNOS gene. Learn Mem. 2009;16:371–378. doi: 10.1101/lm.1329209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Hazleton JE, Morgello S, Berman JW. Mechanisms of HIV-tat-induced phosphorylation of N-methyl-D-aspartate receptor subunit 2A in human primary neurons. Am J Pathol. 2010;176:2819–2830. doi: 10.2353/ajpath.2010.090642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner L, Weitzdoerfer R, Hoeger H, Url A, Schmidt P, Engelmann M, Villar SR, Fountoulakis M, Lubec G, Lubec B. Impaired cognitive performance in neuronal nitric oxide synthase knockout mice is associated with hippocampal protein derangements. Nitric Oxide. 2004;11:316–330. doi: 10.1016/j.niox.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fushc EC, Ponomarenko A, von Engelhardt J, Moyner H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Katsumaru H, Hama K, Wu Y, Heizmann CW. GABAergic neurons containing the Ca2+ binding protein parvalbumin in the rat hippocampus and dentate gyrus. Brain Res. 1987;419:119–130. doi: 10.1016/0006-8993(87)90575-0. [DOI] [PubMed] [Google Scholar]
- Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;6:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- Lapray D, Lasztoczi B, Lagler M, Vinet TJ, Katona L, Valenti O, Hartwich K, Borhegyi Z, Somogyi P, Klausberger T. Behavior-dependent specialization of identified hippocampal interneurons. Nat Neurosci. 2012;15:1265–1271. doi: 10.1038/nn.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RN, Mikulovic S, Leão KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort ABL, Kullander K. O-LM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;11:1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite JP, Shimelli L, Terra-Bustamante VC, Costa ET, Assirati JA, de Nucci G, Martins AR. Loss and sprouting of nitric oxide synthase neurons in the human epileptic hippocampus. Epilepsia. 2002;43:235–242. doi: 10.1046/j.1528-1157.43.s.5.29.x. [DOI] [PubMed] [Google Scholar]
- Levenga J, Krishnamurthy P, Rajamohamedsait H, Wong H, Frank TF, Cain P, Sigurdsson EM, Hoeffer CA. Tau pathology induces loss of GABAergic interneurons leading to altered synaptic plasticity and behavioral impairments. Acta Neuropathol Commun. 2013;1:34. doi: 10.1186/2051-5960-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Shimabukuro J, Hollander H, Mills J, Kaminsky L. Isolation of AIDS-associated retroviruses from cerebrospinal fluid and brain of patients with neurological symptoms. Lancet. 1985;2:586–588. [PubMed] [Google Scholar]
- Li ST, Matsushita M, Moriwakai A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H. HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning [corrected] Ann Neurol. 2004;55:362–371. doi: 10.1002/ana.10844. [DOI] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L. IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway DKK1 in a STAT 3-dependent manner. J Immunol. 2011;186:6771–6778. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LL, Song YM, Xu L, Yi F, Long HY, Zhou L, Qin XH, Feng L, Xiao B. Aberrant neuronal synaptic connectivity in CA1 area of the hippocampus from pilocarpine-induced epileptic rats observed by fluorogold. Int J Clin Exp Med. 2014;7:2687–2695. [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M, Losonczy A. Behavioral consequences of GABAergic neuronal diversity. Curr Opin Neurobiol. 2014;26:27–33. doi: 10.1016/j.conb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Kaifosh P, Kheirbek MA, Damielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV, Losonczy A. Dendritic inhibition in the hippocampus supports fear learning. Science. 2014;343:857–863. doi: 10.1126/science.1247485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, Hansen LA, Wiley CA. Selective neuronal vulnerability in HIV encephalitis. J Neuropathol Exp Neurol. 1992;51:585–593. doi: 10.1097/00005072-199211000-00003. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Bloss EB, Apostolides PF, Vaidya SP, Dilly GA, Zemelman BV, Magee JC. Inhibitory gating of input comparison in the CA1 microcircuit. Neuron. 2015;87:1274–1289. doi: 10.1016/j.neuron.2015.08.025. [DOI] [PubMed] [Google Scholar]
- Moga D, Hof PR, Vissavajjhala P, Moran TH, Morison JH. Parvalbumin-containing interneurons in rat hippocampus have an AMPA receptor profile suggestive of vulnerability to excitotoxicity. J Chem Neuroanat. 2002;23:249–253. doi: 10.1016/s0891-0618(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Morris BJ. Neuronal localization of neuropeptide Y gene expression in rat brain. J Comp Neurol. 1989;290:358–368. doi: 10.1002/cne.902900305. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principals and practices of unbiased stereology: an introduction for bioscientists. The Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- Müller C, Remy S. Dendritic inhibition mediated by O-LM and bistratified interneurons in the hippocampus. Front Synaptic Neurosci. 2014;6:23. doi: 10.3389/fnsyn.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Eradication of human immunodeficiency virus from brain reservoirs. J Neurovirol. 2015;21:227–234. doi: 10.1007/s13365-014-0291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Steiner J. Synaptodendritic injury with the HIV-Tat protein: what is the therapeutic target? Exp Neurol. 2014;251:112–114. doi: 10.1016/j.expneurol.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martic C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–3366. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbán-Kris K, Szabadi T, Szilágyi T. The loss of Ivy cells and the hippocampal input modulatory O-LM cells contribute to the emergence of hyperexcitability in the hippocampus. Rom J Morphol Embryol. 2015;56:155–161. [PubMed] [Google Scholar]
- Peng Z, Zhang N, Wei W, Huang CS, Cetina Y, Otis TS, Houser CR. A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons. J Neurosci. 2013;33:14392–14405. doi: 10.1523/JNEUROSCI.2045-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon V, Velluntini C, Gambarelli D, Harkiss G, Arbuthnott G, Metzger D, Roubin R, Filippi P. The basic domain of the lentiviral Tat protein is responsible for damages in mouse brain: involvement of cytokines. Virology. 1994;205:519–529. doi: 10.1006/viro.1994.1673. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci. 1998;10:3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- Rameau GA, Tukey DA, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, Getzoff ED, Ziff EB. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayne F, Debaisieux S, Yezid H, Lin YL, Mettling C, Konate K, Chazal N, Arold ST, Pugniere M, Sanchez F, Bonhoure A, Briant L, Loret E, Roy C, Beaumelle B. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010;29:1348–1362. doi: 10.1038/emboj.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A, Buzsáki G. Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci. 1995;15:6651–6665. doi: 10.1523/JNEUROSCI.15-10-06651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowska M, Domin H, Zieba B, KoŸniewska E, Michalik R, Piotrowski P, Katja M. Neuroprotective effects of neuropeptide Y-Y2 and Y5 receptor agonists in vitro and in vivo. Neuropeptides. 2009;43:235–249. doi: 10.1016/j.npep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14:403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Somogyi J, Szabo A, Somogyi P, Lamsa K. Molecular analysis of ivy cells of the hippocampal CA1 stratum radiatum using spectral identification of immunofluorophores. Front Neural Circuits. 2012;6:35. doi: 10.3389/fncir.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulas C, Donahue RE, Dunbar CE, Persons DA, Alvarez X, Williams KC. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in long-term repopulated primates. Am J Pathol. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X. Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Rep. 2014;7:269–280. doi: 10.1016/j.celrep.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CA, Dobkin J, Weinberger OK. TAT-mediated transcellular activation of HIV-1 long terminal repeat directed gene expression by HIV-1-infected peripheral blood mononuclear cells. J Immunol. 1994;153:3831–3839. [PubMed] [Google Scholar]
- Tóth K, Maglóczky Z. The vulnerability of calretinin-containing hippocampal interneurons to temporal lobe epilepsy. Front Neuroanat. 2014 doi: 10.3389/fnana.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, Eross L, Vajda K, Halász P, Freund TF, Maglóczky Z. Loss and reorganization of calretinin-containing interneurons in the epileptic human hippocampus. Brain. 2010;133:2763–2777. doi: 10.1093/brain/awq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, Lorenzini P, Visco-Comandini U, Vlassi C, Quartuccio ME, Giulianelli M, Noto P, Galgani S, Ippolito G, Antinori A, Narciso P. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005;21:706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- Tricoire L, Vitalis T. Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Front Neural Circuits. 2012;6:82. doi: 10.3389/fncir.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire L, Pelkey KA, Daw MI, Sousa VH, Miyoshi G, Jeffries B, Cauli B, Fishell G, McBain CJ. Common origins of hippocampal ivy and nitric oxide synthase expressing neurogliaform cells. J Neurosci. 2010;30:2165–2176. doi: 10.1523/JNEUROSCI.5123-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyan L, Chamberland S, Magnin E, Camiré O, Francavilla R, David LS, Deisseroth K, Topolnik L. Dendritic inhibition provided by interneuron-specific cells controls the firing rate and timing of the hippocampal feedback inhibitory circuitry. J Neurosci. 2014;34:4534–4547. doi: 10.1523/JNEUROSCI.3813-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WW, Hu SQ, Li C, Zhiu C, Qi SH, Zhang GY. Transduced PDZ1 domain of PSD-95 decreases Src phosphorylation and increases nNOS (Ser847) phosphorylation contributing to neuroprotection after cerebral ischemia. Brain Res. 2010;1328:162–170. doi: 10.1016/j.brainres.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Weitzdoerfer R, Hoeger H, Engidawork E, Engelmann M, Singewald N, Lubec G, Lubec B. Neuronal nitric oxide synthase knock-out mice show impaired cognitive performance. Nitric Oxide. 2004;10:130–140. doi: 10.1016/j.niox.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., 3rd Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wu S, Yue Y, Tian H, Lao L, Wang Y, Xiang J, Wang S, Ding H. Tamiprosate protects neurons against ischemic stroke by disrupting the interaction between PSD95 and nNOS. Neuropharmacology. 2014;83:107–117. doi: 10.1016/j.neuropharm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Xapelli S, Fabienne A, Ferreira R, Silva AP, Malva JO. Neuropeptide Y as an endogenous antiepileptic, neuroprotective and pro-neurogenic peptide. Recent Pat CNS Drug Discov. 2006;1:315–324. doi: 10.2174/157488906778773689. [DOI] [PubMed] [Google Scholar]
- Yamada J, Jinno S. Subclass-specific formation of perineuronal nets around parvalbumin-expressing GABAergic neurons in Ammon’s horn of the mouse hippocampus. J Comp Neurol. 2015;523:790–804. doi: 10.1002/cne.23712. [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, 2nd, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:647–648. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- Zanelli S, Naylor M, Kapur J. Nitric oxide alters GABAergic synaptic transmission in cultured hippocampal neurons. Brain Res. 2009;1297:23–31. doi: 10.1016/j.brainres.2009.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Saksena NK. HIV associated neurocognitive disorders. Infect Dis Rep. 2013;5:38–50. doi: 10.4081/idr.2013.s1.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


