Abstract
Most studies that enroll individuals with dementia require a study partner for each participant. Study partners—usually family members—perform several key roles: accompanying the participant to visits, providing information about the participant, and assisting with procedures such as taking medication. Little is known, however, about their experiences when performing these roles. Dementia researchers and institutional review boards (IRBs) need to know these experiences because the study partner role is one key factor in a study’s success. This prospective qualitative study, using up to three semi-structured interviews with 62 study partners involved in a range of dementia studies, documented their subjective experiences. Content analysis demonstrates that study partners perform a range of tasks—often within the context of being a caregiver—that enable cognitively impaired individuals to participate in dementia research. These tasks present study partners with unique burdens and benefits, some of which dementia researchers and IRBs can address.
Keywords: caregiver, dementia research, informant, study partner
Introduction
The pressing need to discover the causes of and effective treatments for Alzheimer disease (AD) and other types of neurodegenerative diseases has led the U.S. to commit to a national plan to prevent and effectively treat AD by 2025 (U.S. Department of Health and Human Services, 2015) and a multi-million dollar public and private research enterprise to support this plan (National Institutes of Health, 2015; Assistant Secretary for Planning and Evaluation, 2014). Because the progressive decline in cognition and function inherent to the disease can make it difficult, and often impossible, for dementia patients to participate in research on their own (Lingler, Parker, KeKosky, and Schulz, 2006), each participant in AD clinical trials is usually required to have a study partner—someone who knows the participant well and can accompany the individual to study visits. Researchers rely on study partners to participate in enrollment decision-making, serve as the participant’s knowledgeable informant and help manage the logistics that enable the participant to comply with a study’s protocol (Black, Taylor, Rabins, Karlawish, 2014; Knopman, 2008). Lack of a study partner who can perform these duties is one of the common reasons for excluding people with AD from research participation (Rollin-Sillaire et al., 2013). Without an available and capable study partner, cognitively impaired individuals may have difficulty accurately reporting their dementia-related symptoms and history of memory impairment, may not fully comprehend issues related to consent, may be unable to adhere to a study’s protocol, or may experience distress during study procedures without the support and guidance of a loved one during the process. The current study examined the perspectives of individuals serving as study partners. It sought to describe their contributions to the research process, identify and describe experiences of any burdens or benefits in this critical role and identify how future recruitment and retention in dementia research might be improved by addressing the interests of study partners.
Study partners have a critical role in dementia research, and there is increasing interest in understanding who are these individuals and how they might impact the research process. Typically, a study partner is a family member or close friend who may also be the participant’s primary caregiver. While most caregivers for people with dementia are adult children (Alzheimer’s Association, 2015), the majority of study partners in dementia research are spouses or domestic partners of the participants (Grill, Raman, Ersntrom, Aisen and Karlawish, 2013). The paradox that most caregivers for people with dementia are adult children while most study partners are a spouse or partner may have a number of explanations. Dementia patients who have adult child study partners in natural history studies were less likely to qualify for AD clinical trials because of factors such as older age, residence in nursing homes or scoring too low on measures of cognitive function (Grill, Monsell, and Karlawish, 2012). In addition, spousal caregivers are reported to be more willing to participate in and have a more positive attitude toward research than adult children (Cary, Rubright, Grill, and Karlawish, 2015).
Investigators have also examined relationships between types of study partners in dementia research and study events or outcomes, including participant dropout rates, adverse events, replacement of study partners, and the impact on outcome measure change scores that replacing study partners may have on study analyses (Grill et al., 2013; Grill, Zhou, Karlawish, Elashoff, 2015; Grill, Zhou, and Karlawish, 2014). For example, participants with spouse study partners had lower dropout rates in AD trials than others, those with adult child study partners had fewer adverse events, and those with a study partner who was neither a spouse nor an adult child had more serious adverse events (Grill et al., 2013). Spouse study partners are also less likely to be replaced than other types of study partners in longitudinal studies, and new replacement study partners are typically adult children (Grill et al., 2014). While these findings based on retrospective data are helpful in understanding the influence that type of study partner may have on the research process, they do not explain what impact the research process may have on study partners.
One way to better understand why certain kinds of people are more likely than others to serve as a study partner and why study partner type is associated with differences in outcomes is to examine the experiences and perspectives of study partners at the time they are involved in research. The better we understand how involvement in dementia research impacts study partners, the more researchers will be able to consider their interests in clinical trial protocols. For example, it is already known that caregivers may experience substantial burden (Etters, Goodall, and Harrison, 2008), and that making a proxy decision for enrollment in dementia clinical trials may add to this burden (Sugarman, Cain, Wallace, and Welsh-Bohmer, 2001). Little else is known about the experiences of and any challenges faced by study partners who are needed for the successful conduct of dementia research.
The aims of this prospective qualitative study were to examine the experiences and perspectives of individuals involved in a range of dementia studies in order to understand their study-related motivations, responsibilities, burdens and perceived benefits of serving as study partners. It was also hoped that determining why and how study partners enable dementia research participation and identifying the impact of that experience on them would inform participant recruitment and retention strategies and help explain the paradox in the kinds of caregivers who participate in dementia research. Finally, we hoped that investigators and institutional review boards would benefit from learning whether dementia research protocols should be designed to better address the interests of study partners who perform tasks that are essential to the process of data collection.
Method
This article reports on the second stage of a two-staged research project to better understand and describe the role of study partners in dementia research. Our approach to the research project as well as our approach to data analysis is informed by grounded theory (Corbin & Strauss, 2008). A grounded theory approach can lead to both the description of a process as well as hypotheses about how the concepts described fit together (Corbin & Strauss, 2008). As the ultimate goal of the research project is to inform the relevant stakeholders about the instrumental role of the study partner in the research enterprise, we adopted a grounded theory informed approach. In the first stage we sought the perspectives and opinions of researchers engaged in dementia research (Black, et al., 2014). The findings from the first stage shaped and informed data collection activities from a sample of study partners in the second stage of this research. In both stages, our qualitative approaches to data collection and analysis were most closely aligned with focused ethnography, which is a specific type of sociological ethnography aimed at small elements of one’s own society that benefits from prior familiarity with the setting (Knoblauch, 2005).
Our goal was to include a convenience sample (Corbin & Strauss, 2008) of study partners who had different types of relationships (i.e., spouses/partners, adult children, other family members or friends) with study participants enrolled in a variety of dementia research projects that ranged from natural history studies posing minimal risk to intervention trials that exposed participants to more than minimal risk. To help ensure that a range of potential cooperating dementia studies were available, this study was conducted at two academic research sites. The Institutional Review Boards (IRBs) of the Johns Hopkins University and the University of Pennsylvania approved this research. Each participant provided written informed consent.
Participants
Study partners who participated in this study were recruited from 12 dementia-related (primarily AD) studies whose principal investigators (PIs) agreed to refer study partners involved in their studies to our project. The cooperating studies included two longitudinal natural history studies of AD, one imaging study involving participants with mild cognitive impairment and nine clinical trials that included seven drug and two non-drug intervention studies that involved in-patient procedures. The coordinator for each study briefly described our project to study partners and asked them for permission to be contacted by our study team to learn more about this study. For study partners who gave permission, the PI (BB) of this study called them, described the study in detail, answered questions and asked if they were willing to participate. Five of the 67 study partners referred to our study were not enrolled; three individuals did not respond to our recruitment calls and two were later determined to be ineligible for the primary dementia study. For study partners who agreed to participate in our study, an initial interview was scheduled and a copy of the consent form was mailed to them in advance and reviewed prior to enrollment.
Data Gathering
Our general approach to interviewing study partners was designed to examine their experiences and perspectives over time during their involvement in dementia research. Study partners were interviewed from 1 to 3 times during their participation in this study. The number of interviews and the time periods between interviews varied based on a number of factors, including the type and duration of the dementia study in which the research participant was enrolled, when during their involvement in a dementia study the study partner was enrolled in our study, and whether the research participant’s—and therefore the study partner’s—involvement in a dementia study ended sooner than expected. For example, for study partners involved in lengthy clinical trials (e.g., an 18-month protocol), our goal was to interview them three times, near the beginning, middle and end of their involvement in the dementia study. For those involved in a relatively short study (e.g., 4–6 weeks), the study partner was interviewed twice—near the beginning and near the end of their involvement in that study. Only one interview was conducted with those whose involvement in a dementia study had just ended or was nearly complete when enrolled in our study.
A total of 133 semi-structured interviews were conducted, each by one of two investigators (BB, HT). Most interviews were conducted in-person when the study partner was on site during one of the study participant’s routine study visits. Most interviews took one hour to complete. Study partners were interviewed by telephone if an in-person interview could not be scheduled at a convenient time or if the individual was involved in a dementia study that enrolled people who lived outside of our study’s geographic area. (Less than a third (29%) of the interviews were conducted by phone.) All interviews were audio recorded and transcribed verbatim. Interview transcripts were verified and all personal information was redacted prior to analysis. Identifying information collected to schedule initial and subsequent interviews was kept separate from other study materials. Each participant completed a brief questionnaire of basic personal demographic characteristics (e.g., gender, education), basic information about the primary participant (e.g., onset of symptoms) and prior research experience of either.
This report focuses on the study partners’ perspectives related to four key themes: (1) their motivation to serve as a study partner, (2) their responsibilities as a study partner, (3) whether their role was challenging or burdensome, and (4) whether they derived any benefits from their involvement in research. These issues were discussed in the initial interviews with study partners and often discussed in any subsequent (second or third) interviews. In addition to providing descriptive findings related to these four themes, this report proposes a framework to consider the relationship between the role of caregivers for a person with dementia and the role of study partner. This report also proposes a relationship between study partner burden and caregiving responsibilities.
Data Analyses
Our approach to data analysis was informed by grounded theory and utilized basic methods of qualitative description (Corbin and Strauss, 2008; Sandelowski 1995; Sandelowski 2000). All transcripts were open coded (Corbin & Strauss, 2008). An initial list of codes (e.g., study partner (SP) motivations, SP burdens) was developed based on domains that were introduced to respondents by the interview guide; other codes were added (e.g., SP support, SP advice) as needed based on topics and issues raised by participants. Codes were defined and applied to relevant text segments. When new codes were added, previously coded interviews were reviewed to link new codes with relevant text. Each transcript was coded independently by two study team members; coded text segments were compared for reliability; and any discrepancies were discussed and resolved by consensus. The coded text was then analyzed independently by two investigators to identify sub-themes (e.g., hope, inconvenience/time commitment) in the data. Matrices (Miles, 1994) displaying key findings within a theme by participant were used to identify patterns in the data and compare findings across interviews (e.g., 1st, 2nd or 3rd interviews) and across participants recruited from different dementia studies. The investigators met to review their analyses, verify patterns identified in the data, and discuss implications of their findings. Tables were used to summarize findings, and figures were constructed to illustrate relationships between concepts. Data analysis was aided by Nvivo 9 (QSR International, Australia) qualitative computer software.
Results
Sixty-two study partners participated in this study; 45 (73%) were recruited from nine clinical trials, 12 (19%) from two natural history studies, and 5 (8%) from a single imaging study. Most (82%) of these study partners were interviewed at least twice, and almost a third (32%) were interviewed three times. The majority was female (61%), Caucasian (92%), and lived with the study participant (86%). While most (81%) of these study partners were either the spouse or domestic partner of the study participant, the sample included eight adult children, two siblings, a mother and a friend. The mean age of study partners who were spouses/partners was 67.2 (± 9.4, range 45–86), and that of non-spouses was 55.0 (±14.7, range 26–80). Study partners’ mean years of education was 16.6 (± 3.3). Employment was lower among spouses/partners (40%) than among the other study partners (75%).
Study partners reported that half (50%) of the study participants were female, a slight majority (53%) had some type of behavioral symptoms (e.g., anxiety, depression), and 31% had participated previously in dementia research. The mean age of the study participants was 69.0 (± 9.0; range 47–85), and they had symptoms of memory problems for an average of 5 (± 3.4) years.
Study Partners’ Reasons for Involvement in Dementia Research
The reasons for involvement fit into two categories: primary motivators and influencing factors (see Table 1). Primary motivators are those mentioned most often by study partners and/or qualified by study partners as the main or sole reason for their involvement as a study partner in dementia research. Study partners reported five primary motivations. First, many study partners reported seeking a direct benefit for the primary participant. For those involved in clinical trials, the desired benefit was often to slow or halt the disease progression, as one spouse explained.
“My expectation is that it would improve his memory. I don’t think there is a cure for Alzheimer’s, I really don’t, but I would love to see an improvement in some of the things that he’s exhibiting right now, memory and confusion and even his depression. If I could even just see a 50%, a 20%--I’d be thrilled with 20%. I’d love a 50% improvement, and just prolong that so that he has a better quality of life. That’s really it, just hoping for a better quality of life for him and for me.”
Table 1.
Study Partners’ Reasons for Involvement in Dementia Research
Primary Motivators
|
Influencing Factors
|
This comment about quality of life also illustrates how study partners may expect that benefits to the patient would also benefit themselves. Study partners involved in natural history studies frequently reported their desire to obtain valuable information such as a diagnosis or an assessment of the participant’s status over time or to have someone else (i.e., study investigators) paying attention to or monitoring the participant for changes.
Altruism—the desire to contribute to and advance science—was the second most common motivator reported by study partners for their involvement in research. A sub-set of study partners mentioned kinship benefit as a motivator—that their contribution may in some way result in a benefit for a family member (e.g., children or grandchildren) who may be at increased risk for dementia in the future. For example, the spouse of one participant said,
“…we felt that we really should participate in helping others. We have two children who we would like to help as much as we could with any scientific information that can be gained that might help them or our grandchildren in the future.”
The third most common primary motivator was the desire to take action, to do something meaningful or to be proactive in response to the patient’s life-limiting dementia. A few study partners (n=4) expressed the need to try everything available to avoid regret and have peace of mind in the future. The spouse of a study participant expressed both her altruistic motivation to participate as well as her desire to be proactive:
“For me, I just think it’s a disease that there is so little you can do that the opportunity to take any action, whether it’s going to help him directly or indirectly or just help someone else, just feels like—I don’t know. Maybe it’s the illusion of control or something, but just to me otherwise we’d go home, and we’d wait a year, and we’d come back, and we are on medication, there’s no real sense of urgency or nothing to do except live life, and I think for me it’s something we can do that’s more proactive.”
Fourth among the primary motivators was “hope”—hope for a cure, hope for a benefit, “the glimmer of hope,” or hope for a better quality of life for the participant and the study partner. The fifth primary motivator was the recognition that there are limited treatment options for dementia, which left some people feeling either desperate to try anything, that they had nothing to lose by joining a research study, or that a clinical trial was the best treatment option available to them. As one spouse said, “…the thing is in this particular case, with this particular disease, there’s virtually very few or no alternative. There’s no known cure. There’s nothing for Alzheimer’s. You’re gonna reach for whatever you can get.”
In addition to primary motivators for their involvement in dementia research, study partners frequently mentioned one or more influencing factors (see Table 1). These included being influenced by a trial’s characteristics, such as study procedures that were less invasive, posed less risk or were less disruptive to their lives than those of alternative studies. In one case, the study partner was willing to participate in a Phase III trial but unwilling to join a Phase I or Phase II study with less potential for a direct benefit. Others were influenced by a physician’s suggestion to join a study or by the perceived positive reputation of the PI or the institution where the research was being conducted. Several study partners were influenced by their desire to obtain support (e.g., emotional support, caregiver education/advice) from the study team members, as one study partner said, “…the other thing is when you participate in a study I believe for myself I don’t feel that I’m out in a field somewhere all by myself dealing with this disease.” Less frequently mentioned influences were the participant’s family history of dementia and the study partner’s wish to support the participant’s initiation, request and expressed desire to enroll in research.
Role and Responsibilities of Study Partners
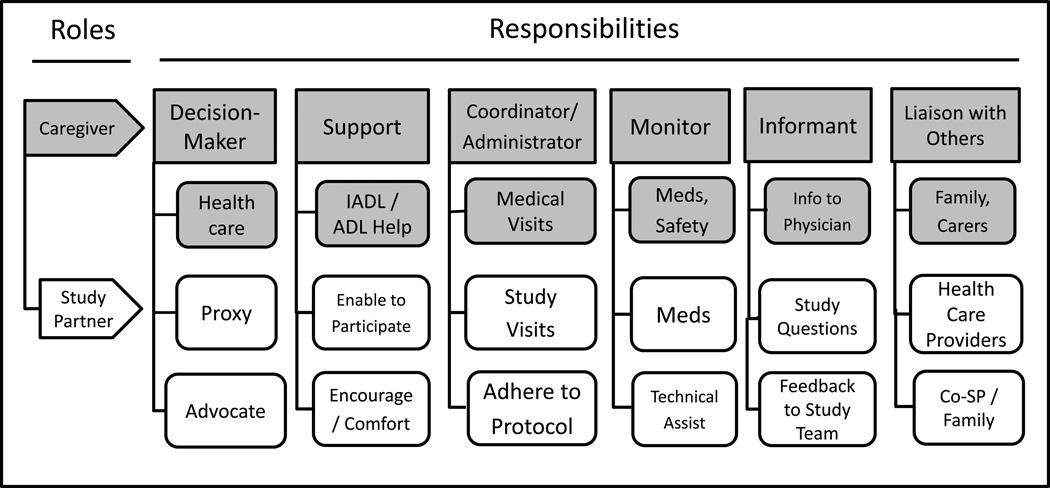
When individuals were asked to describe their role as study partner in the dementia study, they often instead described their role as caregiver for the person with cognitive impairment. For most study partners, this role was clearly within the context of their larger role as a caregiver. One person stated that being a study partner is “synonymous” with being a caregiver. As illustrated in Figure 1, the research responsibilities described by study partners relate directly to their many caregiver responsibilities. In Figure 1, the top row of boxes refer to broad categories of caregiver responsibilities that study partners described. The second row of grey boxes refer to the routine caregiver responsibilities for someone with dementia. The next two rows of white boxes highlight the additional responsibilities that respondents described as directly related to their role as study partners. For example, and not surprisingly, study partners report having long been involved in the navigation of the health care system—with and on behalf of the study participant—as a decision-maker (or co-decision-maker) regarding dementia care as well as other health care needs. Enabling study enrollment as a proxy or co-decision-maker (n=5) or serving as an advocate (n=4) for the study participant is an extension of their existing role as primary caregiver.
Figure 1.
Study Partner Role in Relation to Caregiver Role
At the top of Figure 1 to the right of decision-maker is support. Study partners (n=25) reported serving as a major source of emotional support for the study participant. They serve to encourage study participants to return for study visits when they forget their decision to join a study and/or provide comfort to the participant when undergoing study procedures (e.g., magnetic resonance imaging (MRI)) that may be difficult or distressing for the person with dementia. As one participant’s daughter stated,
“When what is being studied is the erosion of your memory and your brain, it’s hard to keep that. …And so for the study partner, and I would imagine for all the study partners at some point, it’s going to be a continual sort of persuasive enterprise to get the study participant here over and over again when they don’t really feel like it, because they can’t really retain the point of why they’re coming. …And so then that’s really the study partner’s point is not only to be a reliable informant but to continually persuade the participant to come back. Because they don’t, you know, in his case he doesn’t really remember why he’s doing this until you tell him about it again.”
Study partners often serve in other practical ways to help the participant fulfill their research commitments that relate to their caregiver role of coordinator/administrator (see Figure 1, next box along the top). The most commonly mentioned (n=56) responsibility was helping to ensure that the participant completed all required study visits. This included working with study coordinators to schedule the visits, reminding the person with dementia about the visits and ensuring that the individual was dressed properly and ready to go on time, often driving them to the study site and usually accompanying the participant during the visits. In two cases in which a study’s protocol included overnight stays for the participant at the study site, study partners were also required to stay overnight in the room with the participant.
Some study partners (n=12) referred to themselves as an “administrator”, “coordinator” or “chief of staff” for the study participant. In addition to logistical support, study partners reported taking notes during study visits, listening, asking questions and soliciting additional information from the study team. Study partners often expressed a strong commitment to protocol adherence and to be as accurate as possible in carrying out their responsibilities. One participant’s spouse said,
“To make sure that he gets the meds, that is to me primary. …And then making sure he gets here. He must have asked me ten times yesterday, ‘What are we doing tomorrow? What day is it?’ …So I need to make sure that I’ve got this schedule straight, that we get up on time, if he has to fast, I have to make sure I’m in the kitchen before he is so he’s not eating his breakfast, so he’s not having his coffee. I have to make sure that he really follows the program 100%. And one night I forgot to give him his two pills at night and I thought, ‘I’m failing!’ I really feel the stress of making sure he follows this to the letter.”
Moving along the top of Figure 1, study partners (n=26) involved in drug studies often served to monitor the participants’ adherence to the medication regimen. Monitoring pill-taking could involve helping to fill weekly pill boxes, observing whether pills were taken or, if the study partner did not live with the participant, calling to check on whether pills were taken as prescribed. Along with supervising medication use—the study drug and any other non-study medications—study partners usually monitored the participant’s health status in order to alert the study team if any changes occurred. In some cases (n=8), study partners also provided technical assistance by doing in-home finger sticks to monitor blood glucose levels or, as mentioned in the quote above, ensuring that the participant fasted when required ahead of study visits that included a blood draw.
The role of informant (see Figure 1) for the study participant was the second most common research responsibility mentioned by study partners (n=46). One way study partners served as informants was to complete structured instruments used to assess the participant’s cognitive, behavioral, functional and health status. Study partners were also asked to provide feedback to the study team on changes they observed during a study that might reflect adverse events and inform them of the participant’s health services use (e.g., hospitalization).
Several study partners (n=5) described serving as a liaison person when other people outside of the study needed or wanted to know details about the participant’s involvement in a clinical trial as an extension of their role as a caregiver keeping relevant providers and others up to date on the status of the patient. For example, some study partners (n=4) mentioned letting the participant’s health care provider know about the patient’s involvement in a trial and, if an adverse event occurred, providing the link between study team members and the patient’s care provider. In a few situations (n=5), two or more people (e.g., a spouse and an adult child or two adult children) were involved in monitoring and fulfilling study partner responsibilities and coordination was required between/among them. In one case, the study participant lived in an assisted living facility, requiring the study partner to help the residential staff understand and cooperate with the study’s medication regimen that the resident needed to follow for the clinical trial.
Burdens and Challenges for Study Partners
When study partners were asked if they experienced any challenges or burdens associated with their research responsibilities, one-third stated definitively that their research involvement was not a burden or resulted in no additional burden for them. These comments came from study partners involved in all three types of dementia studies (i.e., natural history, imaging and clinical trials) represented in the sample.
Of those who identified burdens associated with the role of study partner (see Table 2), the most common were those related to the inconveniences (n=11) and time commitment (n=9) associated with research participation. Long study visits, frequent visits, waiting between study procedures and delays that occurred during visits seemed particularly challenging for some people (n=14). Changes made by the study team in scheduled visits were disruptive to study partners’ other activities, especially when they occurred at the last minute or interfered with fulltime work schedules that included little employer flexibility for taking personal time off. Time commitment was not a burden identified by those involved in natural history studies that required one annual study visit or studies lasting only a few weeks.
Table 2.
Burdens for Study Partners
Inconvenience & time commitment
|
Travel to study visits
|
Study partner emotional distress
|
Primary participant-related burdens
|
Aspects of the study site
|
Managing research logistics
|
The second most common challenge reported by study partners (n=23) related to traveling to study visits. For example, travel time was a burden when people lived long distances from the study site or if traffic was heavy when their arrival and departure times coincided with rush hours. Travel was particularly stressful for people who were not accustomed to driving in larger metropolitan areas or when weather conditions were bad. For those whose commute was several hours, some were burdened by travel expenses for fuel or lodging if they stayed overnight. Less commonly mentioned (n=2) was anxiety related to driving in the inner cities where these study sites were located.
Serving as a study partner was at times emotionally distressing for some people (n=22), but the sources of their distress varied across individuals. Six different sources of distress were reported. For a few informants (n=3), study questions were distressing if they had difficulty knowing how to answer or if the questions (e.g., about the participant’s function or behavior problems) foreshadowed what the caregiver might face in the future. When studies involved participation over many months or years, some study partners (n=3) were troubled by learning about the participant’s disease progression from assessment results over time or having to acknowledge the subject’s decline when responding to study questions. Sitting in the waiting area at the sites was distressing for a few study partners (n=3) because they encountered other AD patients with different severity levels and a range of symptoms. Study partners (n=3) also noted that when study procedures (e.g., lumbar punctures, MRIs) did not go well or if any part of a study visit (e.g., delays, long wait times) was difficult for the participant then the experience was hard on the study partner as well. A few people (n=2) felt stressed over the need to carefully follow the study’s protocol (as one quote above suggested), and a few (n=3) acknowledged feeling disappointed if the participant derived no obvious benefits from the study. The five study partners involved in one study that was stopped early due to the lack of positive results were particularly disheartened.
Eight study partners reported that helping the cognitively impaired participant follow the protocol and stay involved in a study was a challenge. For example, a few study partners reported struggles getting the study participant to take study pills, return for study visits or cooperate with study procedures. In the cases (n=2) when participants stayed overnight in hotels to attend study visits, coping with an unfamiliar environment could be difficult for the patient and thus challenging for the study partner. These aspects of research participation—including being the participant’s “cheerleader” and “memory keeper”—were often emotion laden tasks for study partners.
Characteristics of the study sites, which were located within large medical complexes, were challenging for some study partners (n=4). Navigating these sites could be frustrating or anxiety producing, especially if various study procedures (e.g., cognitive assessments, imaging, blood draws, eye exams) were done in different locations during a single visit. Environmental factors, including uncomfortable room temperatures during long waits, poor accommodations and lack of attention to study partners’ needs for in-patient stays, occasionally added to study partner burdens. In two cases, participants were mistakenly billed for study procedures, requiring study partners to resolve the mistake through multiple calls to insurance and billing offices.
Finally, a few people (n=4) acknowledged that managing the logistics of study participation was challenging for them. These responsibilities that needed to be incorporated into their daily life included monitoring pill-taking, scheduling and coordinating study visits, and adhering to other protocol tasks such as handling blood glucose tests or ensuring that the participant fasted prior to a study visit.
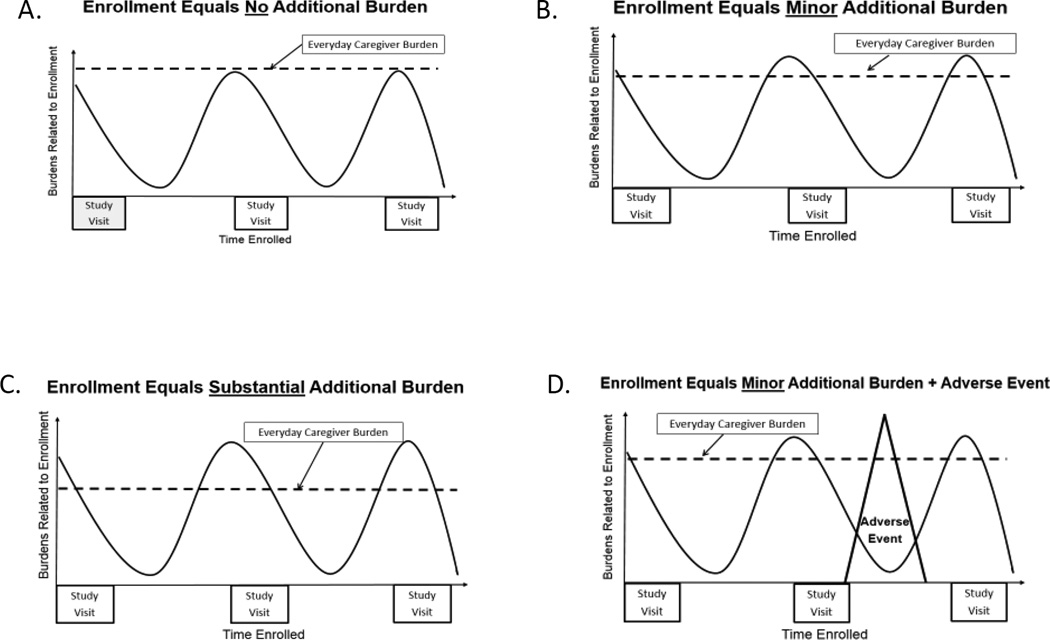
Four general patterns in the relationship between caregiver burden and study partner burden emerged from the data and are illustrated in Figure 2 A–D. In each diagram, the dashed horizontal line represents everyday caregiver burden, and the wavy line represents study participation burdens, which tend to increase just before (e.g., when getting ready for visits, traveling to visits) and during study visits (e.g., navigating the study site’s environment, going to multiple locations for procedures). These figures, which are not based on quantitative data, are presented as models to suggest how over the course of research involvement study-related burden can increase and decrease and how it integrates with a study partner’s ongoing caregiver burden. For some people, study participation posed no additional burden as one spouse’s quote suggests (see Figure 2A); “There’s no burden on me to participate in the study.” In other cases, serving as a study partner was a minor additional burden as this quote from the mother of a person with early onset dementia illustrates (see Figure 2B);
“…it interferes with some of the things that I might do. And, we’re here so often that it does take time away, and we’re tired when we go back…both of us are tired after we leave for the day.”
Figure 2.
Relationships Between Study-Related Burden and Caregiver Burden
For others, study participation was a substantial additional burden, such as the time and expense required for some, as noted by one spouse (see Figure 2C):
“This is a 4-hour drive for us. It’s right at our limit for a day trip. This is our first time coming up in one day and going home in one day. Before we were staying at a hotel so there was some cost involved to us.”
While adverse study events were seldom described, when they occurred there was a spike in study partner burden as suggested by another spouse’s quote (see Figure 2D), “…it’s expensive for the gas, expensive for a hotel room, and it’s very expensive when you go to the emergency room.”
Benefits for Study Partners
When study partners were asked if research participation provided any benefits for them, several people (n=6) stated clearly that there were no benefits for them as study partners. The majority of study partners, however, mentioned one or more types of benefits (see Table 3) that they received serving as a study partner, some of which had also been mentioned as motivating or influencing factors for joining research. The most commonly identified study partner benefit (n=16) was knowledge, particularly information and feedback on the participant’s illness, diagnostic clarity, or disease progression. The spouse of one participant said, “…I think we’ll glean more medical information on him personally which will be useful. I was thrilled that all of the MRI and all the memory tests will then be available to his doctor.” In some cases (n=3), study partners noted the relief they felt from learning that the participant did not need medication or that a study assessment did not reveal anything unusual in the patient’s status. Some people (n=5) gained knowledge about AD and dementia in general, and a few (n=5) acknowledged that they had learned a lot about the research process.
Table 3.
Benefits for Study Partners
Knowledge
|
Support
|
Opportunity to be proactive
|
Opportunity to advance science
|
Hope
|
| Benefit to primary participant is benefit to study partner |
| Close monitoring of primary participant |
The second most commonly mentioned study partner benefit (n=15) was the support they received from study team members. This included emotional support in their role as caregiver and practical support in the form of caregiver education and advice on how to manage the daily challenges of living with or caring for a person with dementia. As one spouse said,
“And the other thing with that too is that as far as caregiving when you get involved in a study, and you have people who are asking you all the questions over and over again, it’s sort of nice because it’s the one time where you get it all off your chest.”
A few study partners (n=3) noted that the development of relationships with study team members over the course of a study was a positive experience for them.
Some study partners (n=11) reported that research involvement gave them the opportunity to be proactive in response to the diagnosis of dementia. Since there are no disease-altering therapies currently available for most causes of dementia, participating in research gave them “something to do” about the illness, and this gave some people a sense of empowerment. Research involvement also gave study partners the opportunity to advance science, which fulfilled the desire for some (n=9) to be altruistic, and their role in the research process gave them a sense of satisfaction through their contribution.
Some study partners (n=7) reported that the opportunity to participate in research gave them hope—hope for an effective treatment and hope for the participant. The spouse of one participant stated, “Oh, there is a sense of potential hope that maybe this would have some positive effect;” and the husband of another person said, “Just the hope. Fingers crossed. Prayers every day. That’s all.”
In several cases (n=6), study partners suggested that when the study provided a benefit to the participant it was, in turn, beneficial to them. For example, when the study drug enabled the participant to sleep more, this gave the caregiver a break. Others (n=3) noted that the close monitoring of the participant across study visits was a benefit since this was done at no cost to the study partner.
Discussion
These findings from study partners involved in a variety of dementia research studies show that this commitment is typically experienced in the context of being a caregiver for the cognitively impaired study participant. In this critical role, they take on a range of study tasks that must be managed along with their other responsibilities and obligations. Below, we summarize this role and its implications for the design and conduct of dementia research.
Most study partners described research responsibilities that related directly to multiple roles (e.g., decision-maker, coordinator, monitor and supporter) that they were already fulfilling as caregivers to cognitively impaired loved ones. Their research responsibilities were specific to a study’s protocol that required adherence to detailed instructions (e.g., pill-taking, blood-glucose monitoring, fasting) and that usually involved a learning curve for those who had never participated previously in research. This is a novel caregiving role, and the researchers who conducted the studies in which these study partners were participating (Black, et al. 2014) also noted that the study partner role sometimes involves a learning process for the individual. In clinical trials, study partner responsibilities often included more than simply serving as an informant and accompanying the participant to study visits. The researchers we interviewed (Black, et al. 2014) identified a range of study partner responsibilities that included decision-maker, informant, manager of study logistics and comforter for the study participant. This suggests that study partners with no prior experience managing research-related tasks would benefit from educational materials that attempt to increase their research literacy, shorten their learning curve, and avoid some of the stress associated with being a new study partner.
The impact of this role can be beneficial or burdensome or both. If it is burdensome, then it adds additional caregiving challenges. This is especially true for caregivers who are employed fulltime. This finding resonates with previous research (Grill et al., 2013) that found study partners are less likely to be adult children, who are more likely to be employed and/or have childcare responsibilities. A substantial body of research has examined the concept of “caregiver burden” and means to address it. Considerably less research has examined “study partner burden,” that is, whether it exists, and, if it does, what are its features, determinants, and ways to address it. This research shows that study partners who experienced challenges and burdens during their involvement in research described a wide range of issues that seemed most pronounced just prior to and during study visits. Any distress that study partners experienced during that window of time could be due to multiple factors related to the participant’s dementia symptoms and how that individual coped with study requirements, travel-related circumstances, characteristics of the study site, length of and delays in study visits or how study procedures were managed. Any aspect of the study that was difficult for the study participants often led to challenges for the study partners in their role as supporter, encourager, memory keeper and advocate for the participant. Some study partners were distressed by questions on outcome measures or assessment results that could signal progression of the patient’s illness. This is consistent with our findings from researchers (Black, et al. 2014) who reported that acknowledging the patient’s decline appeared to be a burden for some study partners. Researchers should be sensitive to this possibility and be prepared to provide support to study partners as they learn of and adjust to changes in the participant’s status. Less commonly mentioned as challenges by study partners was managing the logistics of adhering to the study protocol between study visits. It is important for both researchers and potential study partners to recognize how research involvement may be burdensome to those who serve as study partners.
Notably, informed consent forms typically describe the study partner duties of informant and accompanying the participant to study visits. It is important to specify in the consent form all study-related duties and any potential burdens for study partners. How well they learn and are able to carry out the range of research tasks in light of their caregiving and other daily responsibilities may influence whether they view the role of study partner as challenging or burdensome and whether they choose to continue or withdraw from participation if the process becomes too difficult to manage.
While AD dementia trials are not designed to benefit study partners, many of our participants identified practical or emotional gains that they derived from their research involvement. Increased knowledge was the most commonly identified benefit of research participation by study partners. Thus, for many, it was a learning experience—learning about the patient’s illness and symptoms, learning about dementia and AD, or simply learning about how research is conducted and their role in that process. Researchers need to be aware of their role in educating study partners and be prepared to provide information about dementia and the research process in lay language. Other identified benefits of participating in research include caregiver support, opportunities to be proactive in facing dementia (empowerment), opportunities to advance science (satisfaction), and hope for the future. A less commonly mentioned gain was that a direct benefit of research for the participant was a benefit for the study partner. This mirrors the relationship noted above in which challenges for the participant are often challenges for the study partner. This interdependence between patient and caregiver and the risks and benefits of study enrollment was also identified in a study by Karlawish and colleagues (2001) as a factor that caregivers consider when deciding whether to enroll in research. Researchers need to recognize these relationships and identify ways to minimize risks and optimize benefits for both the participants and study partners.
Some study partners were very single-minded about why they agreed to and often sought out research involvement, even if it meant traveling for hours to reach the study site. Their primary motivation was clearly to seek a research intervention that would slow the progression of or even cure their loved one’s dementia or at least improve the patient’s symptoms and quality of life. Often these individuals reported a sense of desperation over the lack of effective therapies. Other investigators (Karlawish, Casarett, Klocinski, and Sankar, 2001) have also found that feelings of desperation could lead caregivers to join dementia research. Clinical trials represent an opportunity for hope and enable people to take action against a devastating illness even if the chances of a direct benefit are slim. This phenomenon has been studied most in the context of Phase I cancer trials (Agrawal and Emanuel 2003; Kass et al 2008; Weinfurt et al 2008; Kass et al 2009; Sulmasy et al 2010). While some commentators note concern that hope for benefit may indicate a misunderstanding of the intent of an early phase trial, others note that it is possible for a severely ill patient to simultaneously understand the purpose of the trial and hope for direct benefit. While their hope for benefit may be higher than the true likelihood of expected benefit, such a hope ought not to exclude a subject from consideration from enrollment under the assumption that holding on to such a hope indicates they don’t understand the trial well enough to provide informed consent. The former is referred to as therapeutic misconception, therapeutic optimism or unrealistic optimism (Agrawal and Emanuel 2003; Sulmasy et al 2010; Crites and Kodish 2013). The important point for this population is that either phenomenon may exist for the dementia patient as well as the study partner. We agree with Sulmasy et al (2010) and Weinfurt et al (2008) that investigators should consider adopting approaches to assure themselves that prospective subjects and their study partners understand the likelihood of direct benefit and acknowledge their hopes for benefit.
More commonly, study partners identified multiple reasons for participating in research, with altruism often being among them, as others (Karlawish et al., 2001; Lawrence, Pickett, Ballard, and Murray, 2014; Black, Wechler, and Fogarty, 2013) have also reported. Some study partners took solace in knowing that, even if a study did not benefit the participant directly, it would help to advance science and perhaps indirectly benefit their children or grandchildren. Another clear influence for some study partners was their desire for caregiver support from study team members. Through research involvement they sought emotional support, information and practical advice to help them cope and feel less alone in their caregiving role. In the first phase of our project (Black, et al. 2014), researchers identified altruism, hope for a direct benefit for participants, desire for dementia-related education and caregiver support as motivations for study partners to agree to research involvement that are consistent with our current findings. By explicitly asking study partners what they hope to achieve through study participation, researchers could more directly determine whether and how they might be supportive of these caregivers who are making such important contributions to the advancement of science.
Limitations of this study include its design as a qualitative study with a convenience sample of participants. While qualitative methods are appropriate for this relatively unexamined topic that seeks to obtain the subjective views of individuals who are engaged in a complex process, this approach does not capture the opinions of a larger, more representative sample from which statistical inferences can be made. Our sample includes a slightly higher percentage of study partners who were spouses/partners and females and who were somewhat younger than typically reported in AD clinical trials (Grill et al., 2013). AD dementia clinical trials in general and this research in particular are limited by under-representation of non-spousal study partners and minority ethnic groups (Grill et al., 2013; Cooper, Tandy, Balamurali, and Livingston, 2010). The inclusion of more individuals from these groups could result in different findings and may serve to better inform recruitment and retention efforts for dementia research. This project was conducted at only two academic research sites in the USA, and its findings may not reflect the perspectives of study partners involved in research conducted in non-academic sites or in dementia research settings outside of the USA. This report did not explicitly examine differences/similarities in findings between study partners involved in specific types of studies because of the imbalance in number of participants from across the 12 cooperating dementia studies. Future research should examine issues such as whether motivators differ for those involved, for example, in intervention trials where hope for a direct benefit may be paramount versus natural history studies where altruism may be the predominant factor.
Strengths of this research include its prospective design; this provided the opportunity to include individuals who were actively serving as study partners and to conduct multiple interviews with the majority of participants. In addition, multiple procedures were used to help verify the quality and trustworthiness of our data and data analyses (Creswell, 1998). First, we obtained data from multiple and different sources. Findings from the first stage of our research informed the design and conduct of the second stage and served as a source of comparison with findings from our interviews with study partners. Including study partners representing different relationships with research participants in a range of dementia studies shed light on themes from multiple perspectives. Second, interviews with study partners were conducted over a prolonged period of 27 months. This enabled us to recruit study partners from multiple ongoing studies and allowed for prolonged engagement with study partners involved in lengthy clinical trials. Multiple interviews with single individuals provided opportunities to review and follow-up on previously discussed issues, check for additional clarification if needed and learn of new events that might have influenced an individual’s perspective on their research experience. Finally, peer review and debriefing (Flick, 2006) occurred periodically over the course of this study by co-investigators who did not participate in data collection or data analysis and by a panel of advisors who represented dementia researchers, IRB members and caregivers who previously served as study partners. Their engagement and reflections provided opportunities to ensure appropriate methods, clarify meanings derived from the data, and confirm interpretations of study findings. In addition, preliminary findings of this study were presented at scientific meetings that provided opportunities for questions and feedback from other researchers, IRB members and bioethicists who were unaffiliated with this study.
Implications for IRBs
IRBs that review research involving cognitively impaired participants must ensure that protocols include the availability and engagement of a capable study partner to support each subject and the study’s success. Review boards need to ensure that investigators have identified details of the protocol that have implications for and potential burdens on study partners and informed them of these. Measures to minimize burdens on study partners should be included in protocols. IRBs should also ensure that recruitment and consent materials provide information that is relevant to the study partner’s role responsibilities, including logistical requirements and potential emotional burdens. If responsibilities and time commitments are substantial, IRBs ought to consider whether and, if so, to what extent study partners should receive financial compensation for their time or reimbursement for study related expenses (e.g., gas and lodging).
Implications for Researchers
Given the key role that study partners play in dementia research, it is important for researchers to understand and consider the interests of these individuals as caregivers in the design and conduct of dementia research. This should include identifying ways to accommodate the needs of study partners, particularly those who have other primary responsibilities (e.g., jobs, other family members), regarding the time commitments required for research participation. It is also critical to recognize that the challenges/burdens that study partners face may be both practical (e.g., managing logistics, adhering to a protocol) and emotional (e.g., acknowledging the participant’s illness or decline, anxiety coming to or navigating the study site, struggling with the participant’s reactions to study procedures). Obtaining feedback from study partners on how they are managing the research process and identifying practical ways to reduce study partner burden and shorten their learning curve may impact retention rates and their willingness to enroll in future studies. Since caregiver support and education may be both a motivator for and benefit of research involvement, researchers should be prepared to provide, if possible, dementia-related information, caregiver support and/or referrals to resources if that is not part of a study’s protocol since it represents the standard of care. In addition, researchers should consider whether study team members are adequately trained and prepared to provide dementia education and caregiver support. Study staff should take responsibility when a research procedure is incorrectly billed as a clinical procedure.
Implications for Potential Study Partners
Individuals who serve as study partners in dementia research have a range of duties that vary by study protocol and require a commitment of time and energy. They need to clearly understand all the role responsibilities and potential burdens (logistical, emotional and financial) when making an informed enrollment decision. Study partners should be informed that any potential research burdens vary by other factors, including characteristics of the study and its procedures, of the primary participant, and of the caregiver. The provision of comfort, support and encouragement to the participant by study partners may be an emotion laden task and their ability to comfort, console and reassure the participant should be identified as an element of their willingness to participate in and help complete the study.
Conclusions
Study partners are essential to conducting dementia research involving cognitively impaired participants. Their reasons for research involvement vary, but they typically have a strong desire to help others—particularly their loved one with dementia. While research involvement seems to add little or no additional burden on some study partners, others experience a range of study-related challenges and burdens. At the same time, most study partners derive some type of benefit from the experience that helps to make their commitment to research worthwhile. These potential benefits and burdens should be explicitly described to prospective study partners at the time of enrollment. Caregivers serving as study partners should be recognized as legitimate stakeholders in the assessment of risks, benefits and burdens and in the development of recruitment and retention strategies. These observations warrant further study in other dementia research settings and with more diverse samples to determine whether other issues of interest to study partners should be acknowledged and addressed by the research community.
Acknowledgments
The authors wish to acknowledge and thank all of the study partners who volunteered to participate in this study and who shared their experiences and views on the role of dementia research study partners.
Funding
This research was funded by the National Institute on Aging, grant # R01 AG038440.
Abbreviations
- AD
Alzheimer disease
- IRB
Institutional Review Board
- PI
Principal investigator
- US/USA
United States of America
Footnotes
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest.
Betty Black and Holly Taylor designed the study, participated in data collection and analysis, and wrote the paper. Peter Rabins and Jason Karlawish designed the study, assisted in identifying eligible dementia studies, and participated in writing the paper. All approved the final version of the paper.
References
- Agrawal M, Emanuel EJ. Ethics of phase 1 oncology studies: reexamining the arguments and data. JAMA. 2003;290:1075–1082. doi: 10.1001/jama.290.8.1075. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia 2015. 2015 doi: 10.1016/j.jalz.2015.02.003. Retrieved from https://www.alz.org/facts/downloads/facts_figures_2015.pdf. [DOI] [PubMed]
- Assistant Secretary for Planning and Evaluation. National Plan to Address Alzheimer’s Disease 2014 Update. US Department of Health and Human Services; 2014. Retrieved from http://aspe.hhs.gov/daltcp/napa/NatlPlan2015.shtml. [Google Scholar]
- Black BS, Taylor H, Rabins PV, Karlawish J. Researchers’ perspectives on the role of study partners in dementia research. International Psychogeriatrics. 2014;26:1649–1657. doi: 10.1017/S1041610214001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BS, Wechler M, Fogarty L. Decision-making for participation in dementia research. American Journal of Geriatric Psychiatry. 2013;21:355–363. doi: 10.1016/j.jagp.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary MS, Rubright JD, Griall JD, Karlawish J. Why are spousal caregivers more prevalent than non-spousal caregivers as study partners in AD dementia clinical trials? Alzheimer Disease and Associated Disorders. 2015;29:70–74. doi: 10.1097/WAD.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Tandy AR, Balamurali TBS, Livingston G. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care and research. American Journal of Geriatric Psychiatry. 2010;18:193–203. doi: 10.1097/JGP.0b013e3181bf9caf. [DOI] [PubMed] [Google Scholar]
- Corbin J, Strauss A. Basics of Qualitative Research. 3rd. Thousand Oaks, CA: Sage Publications; 2008. [Google Scholar]
- Crites J, Kodish E. Unrealistic optimism and the ethics of phase I cancer research. Journal of Medical Ethics. 2013;39:403–406. doi: 10.1136/medethics-2012-100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JW. Qualitative Inquiry & Research Design: Choosing Among Five Traditions. Thousand Oaks, CA: Sage Publications; 1998. [Google Scholar]
- Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: A review of the literature. Journal of the American Academy of Nurse Practitioners. 2008;20:423–428. doi: 10.1111/j.1745-7599.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Flick U. An Introduction to Qualitative Research. Thousand Oaks, CA: Sage Publications; 2006. [Google Scholar]
- Grill JD, Monsell D, Karlawish J. Are patients whose study partners are spouses more likely to be eligible for Alzheimer’s disease clinical trials? Dementia and Geriatric Cognitive Disorders. 2012;33:334–340. doi: 10.1159/000339361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Raman R, Ernstrom K, Aisen P, Karlawish J. Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology. 2013;20:282–288. doi: 10.1212/WNL.0b013e31827debfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Zhou Y, Karlawish J. Does study partner type impact the rate of Alzheimer’s disease progression? Journal of Alzheimers Disease. 2014;38:507–514. doi: 10.3233/JAD-131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill JD, Zhou Y, Karlawish J, Elashoff D. Frequency and impact of informant replacement in Alzheimer disease research. Alzheimer Disease and Associated Disorders. 2015;29:242–248. doi: 10.1097/WAD.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlawish J, Casarett D, Klocinski J, Sankar P. How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology. 2001;56:789–792. doi: 10.1212/wnl.56.6.789. [DOI] [PubMed] [Google Scholar]
- Kass NE, Sugarman J, Medley AM, Fogarty LA, Taylor HA, Daugherty CK, Emerson MR, Goodman SN, Hlubocky FJ, Hurwitz HI, Carducci M, Goodwin-Landher A. An intervention to improve cancer patients' understanding of early-phase clinical trials. IRB: Ethics & Human Research. 2009;31:1–10. [PMC free article] [PubMed] [Google Scholar]
- Kass N, Taylor H, Fogarty L, Sugarman J, Goodman SN, Goodwin-Landher A, Carducci M, Hurwitz H. Purpose and benefits of early phase cancer trials: what do oncologists say? What do patients hear? Journal of Empirical Research on Human Research Ethics. 2008;3:57–68. doi: 10.1525/jer.2008.3.3.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblauch H. Focused ethnography. Forum Qualitative Sozialforschung/Research. 2005;6 Art 44. [Google Scholar]
- Knopman DS. Clinical trial design issues in mild to moderate Alzheimer disease. Cognitive and Behavioral Neurology. 2008;21:197–201. doi: 10.1097/WNN.0b013e318190cf75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence V, Pickett J, Ballard C, Murray J. Patient and carer views on participating in clinical trials for prodromal Alzheimer’s disease and mild cognitive impairment. International Journal of Geriatric Psychiatry. 2014;29:22–31. doi: 10.1002/gps.3958. [DOI] [PubMed] [Google Scholar]
- Lingler JH, Parker LS, KeKosky ST, Schulz R. Caregivers as subjects of clinical trials: a review of human subjects protection practices in published studies of Alzheimer disease pharmacotherapies. IRB: Ethics & Human Research. 2006;28:11–18. [PubMed] [Google Scholar]
- Miles MB, Huberman AM. Qualitative Data Analysis. 2nd. Thousand Oaks, CA: Sage Publications; 1994. [Google Scholar]
- National Institutes of Health. Estimates of funding for various research, condition and disease categories (RCDC) US Department of Health & Human Services; 2015. Retrieved from http://report.nih.gov/categorical_spending.aspx. [Google Scholar]
- Rollin-Sillaire A, Breuilh L, Salleron J, Bombois S, Cassagnaud P, Deramecourt V, Mackowiak AM, Pasquier F. Reasons that prevent the inclusion of Alzheimer’s disease patients in clinical trials. British Journal of Clinical Pharmacology. 2013;74:1089–1097. doi: 10.1111/j.1365-2125.2012.04423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelowski M. Qualitative analysis: What is it and how to begin? Research in Nursing & Health. 1995;18:371–375. doi: 10.1002/nur.4770180411. [DOI] [PubMed] [Google Scholar]
- Sandelowski M. Whatever happened to qualitative description? Research in Nursing & Health. 2000;23:334–340. doi: 10.1002/1098-240x(200008)23:4<334::aid-nur9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Sulmasy DP, Astrow AB, He MK, Seils DM, Meropol NJ, Micco E, Weinfurt KP. The culture of faith and hope: patients' justifications for their high estimations of expected therapeutic benefit when enrolling in early phase oncology trials. Cancer. 2010;116:3702–3711. doi: 10.1002/cncr.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman J, Cain C, Wallace R, Welsh-Bohmer KA. How proxies make decisions about research for patients with Alzheimer’s disease. Journal of the American Geriatric Society. 2001;49:1110–1119. doi: 10.1046/j.1532-5415.2001.49218.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. National Plan to Address Alzheimer’s Disease: 2015 Update. 2015 Retrieved from: http://aspe.hhs.gov/national-plan-address-alzheimer%E2%80%99s-disease-2015-update.
- Weinfurt KP, Seils DM, Tzeng JP, Compton KL, Sulmasy DP, Astrow AB, Solarino NA, Schulman KA, Meropol NJ. Expectations of benefit in early-phase clinical trials: implications for assessing the adequacy of informed consent. Medical Decision Making. 2008;28:575–581. doi: 10.1177/0272989X08315242. [DOI] [PMC free article] [PubMed] [Google Scholar]