Abstract
Background
In addition to cognitive deficits, people with mild cognitive impairment (MCI) can experience motor dysfunction, including deficits in gait and balance. Objective, instrumented motor-performance assessment may allow detection of subtle MCI-related motor deficits, allowing early diagnosis and intervention. Motor assessment under dual-task conditions may increase diagnostic accuracy; however, the sensitivity of different cognitive tasks is unclear.
Objective
To systematically review the extant literature focusing on instrumented assessment of gait and balance parameters for discriminating MCI patients from cognitively intact peers.
Methods
Database searches were conducted in PubMed, EMBASE, Cochrane Library, PsycINFO and Web of Science. Inclusion criteria were: 1) clinically confirmed MCI; 2) instrumented measurement of gait and/or balance; and 3) English language. 4) Reporting gait or balance parameters which could be included in a meta-analysis for discriminating between MCI patients and cognitively intact based on weighted effect size (d).
Results
Fourteen studies met inclusion criteria for and reported quantitative gait (n= 11) or postural balance (n=4) parameters to be included in meta-analysis.. Meta-analysis revealed that several gait parameters including velocity (d=−0.74, p<0.01), stride length (d=−0.65, p< 0.01), stride time (mean: d=0.56, p=0.02; coefficient of variation: d=0.50, p<0.01) discriminated best between MCI and healthy controls under single task conditions. Importantly, dual task assessment increased discriminative power of gait variables wherein gait variables with counting tasks appeared to be more sensitive (range d=0.84–1.35) compared to verbal fluency tasks such as animal naming (range d=0.65–0.94). Balance parameters identified as significant discriminators were anterior-posterior (d=0.49, p<0.01) and medio-lateral (d=−0.34, p=0.04) sway position in the eyes open condition but not eyes closed.
Conclusion
Existing studies provide evidence that MCI affects specific gait parameters. MCI-related gait changes were most pronounced when subjects are challenged cognitively (i.e., dual-task), suggesting that gait assessment with an additional cognitive tasks is useful for diagnosis and outcome analysis in the target population. Static balance seems to also be affected by MCI, although limited evidence exists. Instrumented motor assessment could provide a critical opportunity for MCI diagnosis and tailored intervention targeting specific deficits and potentially slowing progression to dementia. Further studies are required to confirm our findings.
Keywords: Mild Cognitive Impairment, gait, balance, technology, older adults, assessment, analysis
INTRODUCTION
Along with research on dementia, there is an increased interest in mild cognitive impairment (MCI), a transitional cognitive state with a 10–15% yearly progression to dementia[1]. Precise diagnosis of MCI may allow early intervention and prevention of further cognitive and functional decline[2]. To date, sixteen percent of individuals above the age of 70 years have been diagnosed with MCI[3]. By 2050, it is estimated that 1 in 85 persons will be diagnosed with Alzheimer’s disease[4] and MCI has become of focus of studies for early diagnosis and potential intervention.
MCI is characterized by: (1) preserved general cognitive function, (2) objective memory impairment beyond age, (3) lack of dementia and (4) little or no impairment of activities of daily living (ADL) [5–7]. Despite relatively preserved ADL function, studies have reported subtle changes in functional performances such as gait and balance in people with MCI. Although these changes do not cause a drastic decline in everyday function[8], they may be clinically relevant and lead to motor errors in mobility tasks falls. Thus, early identification of subtle MCI-related changes in gait and balance might be relevant for targeting specific interventions aiming to prevent further decline [9,10]. Conventional gait and balance tests may, however, not be sufficiently accurate for detection of subtle MCI-associated motor impairments [11]. Recent advances in electronic gait analysis and wearable technology may allow more precise estimation of MCI-related changes in motor performance. Many spatio-temporal gait variables can be extracted and several seem to be associated with cognitive decline [12]. Identification of gait parameters which are strongly associated with MCI could be relevant for early diagnosis and intervention. However, to our knowledge, a systematic review and meta-analysis comparing instrumented gait variables in people with MCI and healthy controls has not been performed.
Further, dual-task gait assessment may be more helpful to detect cognition-related gait changes as compared to single task assessment [10,13]. Similarly, to our knowledge it has not been systematically investigated whether a gait assessment under dual-task conditions has an added valued in detecting gait dysfunction in MCI patients.
As opposed to dynamic balance assessed during walking, static postural balance during standing is another motor function that is critical to quality of life and seems to have direct association with cognitive function [14]. However, it has not been systematically investigated which specific balance parameters derived from a instrumented static balance assessment (e.g. posturography) are linked to MCI.
Our objective was to systematically review the extant literature focusing on instrumented assessment of gait and balance parameters for discriminating clinically confirmed MCI patients from cognitively intact older adults.
METHODS
This review was performed to be consistent with the PRISMA statement[15]. Searches were conducted in July 2015 in the following databases: PubMed (1946–2015); Thomson Reuters Web of Science (Science Citation Index Expanded 1900–2015) (Conference Proceedings Citation Index- Science 1990–2015); Wiley Online Library Cochrane Library (1898–2014); EBSCO PsycINFO (1597-Present); and Embase.com EMBASE (1947–2015). The search strategy for PubMed can be found in Appendix A and was adapted for all other databases. The reference lists of related reviews in cognition, balance, and gait were also searched for eligible papers.
Inclusion criteria consisted of: (a) population: individuals with confirmed MCI diagnosis according to established definitions (e.g., Petersen’s et al[5], Winblad et al[16]); (b) type of outcome measures: gait variables obtained by instrumented analysis (e.g., electronic walkways, wearable sensors, camera systems) or static postural balance variables obtained by instrumented analysis (e.g., stabilometry); (c) original article; and (d) English language. Articles that only used a stopwatch were excluded, as were articles that did not provide data which could be used in meta-analysis (i.e. mean and standard deviation) or which included a population with comorbid gait disorders (e.g. Parkinson’s disease).
Two reviewers (LB and TP) independently screened the titles and abstracts from the initial search to identify potentially relevant records. If the reviewers were unable to determine a study’s eligibility based on title and abstract, the full text was retrieved. A third reviewer (MS) resolved disagreements between the two screenings. Selected full texts were then reviewed for inclusion, per PRISMA protocol.
Data extraction of the study characteristics and findings was performed by a single reviewer (LB). Study characteristics of interest were: (1) main goal of study; (2) type of MCI definition; (3) participant characteristics; and (4) key results of the study with respect to gait and balance. In two papers where the p-value was not reported [17,18] but the sample size was sufficient to be approximated as normal distributions, the p-value was calculated using independent t-tests between the cognitively healthy and MCI groups. Assessment of the methodological quality of each study was performed using Cochrane Collaboration’s tool for assessing the risk of bias (Appendix B).
Meta-analysis
In order to estimate the discriminative power (i.e. MCI vs. healthy control) of specific gait and balance variables, a meta-analysis was conducted for each variable reported in two or more studies. The outcome of each meta-analysis was the overall effect size (Cohens’d), representing the standardized mean difference between a study group of cognitively healthy individuals (CHI) and a study group with persons with MCI. The Cohen criteria were used for interpretation (d > 0.2 small, > 0.5 medium, > 0.8 large effect)[19].
Positive effect sizes were indicative of an increase in the gait/balance parameter value in persons with MCI when compared to CHI. Likewise, negative effect sizes indicated a decrease in gait/balance parameter value. Heterogeneity was assessed using Cochran’s Q and I2. When studies were homogeneous (Cochran’s Q<0.05, I2>0.75), the effect sizes were calculated using inverse variance analysis; when studies were heterogeneous, the effect sizes were calculated using random effects analysis. The mean effect sizes, 95% confidence intervals (CI), Cochran’s Q, and I2 were calculated for each parameter and used to create forest plots for visualization of the meta-analysis using the MetaXL software (version 2.2, EpiGear, Wilston, Australia). Assessment of publication bias was performed by generating a funnel plot for the most frequently reported gait variable (i.e. single task gait velocity) (Appendix C). Other gait/balance parameters were reported only in a limited number of studies; therefore assessment of publication bias via funnel plots was not possible.
RESULTS
The database searches yielded 3072 papers, with an additional 56 papers found through searching reference lists. After removal of duplicates and title/abstract screening, 213 papers remained for full text screening. Of these, 14 met the inclusion criteria (Fig. 1). The majority of the studies (n=11, 78.6%) focused on the interaction of MCI and gait, while a smaller percentage (n=4, 28.6%) focused on balance. One study included both gait and balance analysis [11]. Motor parameters were obtained by wearable sensors, force plates, and electronic walkways such as the GAITRite (Table 1).
Figure 1.
Flowchart of the process of initial literature search and extraction of studies meeting the inclusion criteria
Table 1.
Instruments used in assessment of balance and gait
The most frequently used definitions of MCI were Winblad et al.’s criteria (n=6) and Petersen et al.’s criteria (n=4), and some papers using these criteria additionally identified amnestic or non-amnestic MCI subtypes (a-MCI, na-MCI) (n=3). Miscellaneous cognitive criteria (n=3) that adhered to the MCI standard were also included in the analysis.
Gait Parameters Reported in Studies
Participants
Of the eleven studies that focused on gait, ten studies compared MCI subjects with healthy age-matched controls[11,17,18,20–26]. Five papers additionally examined differences between persons with MCI and dementia [11,18,20,23,26]. In three papers, subtype differences between a-MCI and na-MCI were additionally examined [21,24,25]. (Table 3).
Table 3.
Summary of included studies involving gait in MCI versus CHI groups
| Study | Study Characteristics (number, mean age %female) |
Instrumented Assessment |
Instrument | Significant gait results in MCI group* |
|---|---|---|---|---|
| Beauchet et al,[21] 2011 |
Criteria: Winblad et al. (2004) CHI: n=21, 70.3 years a-MCI: n=15, 73.3 years, 42.9% na-MCI: n=21, 70.6 years, 26.7% |
Walking at usual pace | GAITRite Gold Walkway (length: 9.72 meters) |
↑ gait velocity variability in a-MCI No change in gait velocity variability for na-MCI |
| Beauchet et al,[20] 2013 |
Criteria: Winblad et al (2004) CHI: n=44, 74.5 years, 63.6% MCI: n=39, 73.6 years, 38.5% AD: n=33, 79.2 years, 63.6% |
Walking at usual pace Walking at fast pace |
GAITRite Gold Walkway (length: 9.72 meters) |
No change in STV at normal walking velocity ↑STV at fast walking velocity |
| Boripuntakul et al,[22] 2014 |
Criteria: a) Petersen et al (2001), b) MMSE ≥ 24, c) MoCA < 26 CHI: n = 30, 71.0 years, 66.7% MCI: n=30, 70.6 years, 66.7% |
Gait initiation and walking at usual pace Gait initiation and walking during counting dual task (backwards by 7) |
GAITRite system (length not reported) |
↑swing time of 1st/2nd step, both tasks ↑step length variability of 1st/2nd step, both tasks |
| Choi et al,[23] 2011 |
Criteria: CERAD-Korea CHI: n=6, 71.6 years, 33.3% MCI: n=7, 72.9 years, 42.9% AD: n=10, 77.2 years, 60% |
Walking at usual pace (25 meters) |
Tri-axial accelerometer, right foot |
↑ Stride time |
| Gillain et al,[11] 2007 |
Criteria: a)cognitive disorder with no major impact on ADL, b)CDR<0.5, c)MMSE≥24 CHI: n=14, 73.5 years, 21% MCI: n=14, 72.9 years, 21% DEM: n=6, 73.7 years, 9% |
Single-leg balance test Single-leg balance test with dual task (countdown from 50) Pull test TUG test TUG test test with dual task (countdown from 50) |
Locometrix® tri- axial accelerometers |
Single Tasking: ↓ gait symmetry Dual Tasking: ↓ stride frequency, gait velocity positively correlates with MMSE score |
| Montero- Odasso et al,[17] 2012 |
Criteria: Winblad et al (2004) CHI: n=25, 71.5 years, 88% MCI: n=43, 75.1 years, 54% |
Walking at usual speed Walking with dual task (counting backward from 100 by 7) Walking with dual task (naming animals) |
GAITRite System (length: 6 meters) |
All assessments: ↓ gait velocity, ↑gait variability, ↑stride time |
| Montero- Odasso et al[25], 2014 |
Criteria: Petersen (2004) aMCI: n=42, 77.3 years, 42% naMCI: n=22, 74.2 years, 64% CHI: n=35, 70.4 years, 83% |
Walking at usual speed Walking with dual task (counting backward from 100 by 1) Walking with dual task (counting backward from 100 by 7) Walking with dual task (naming animals) |
GAITRite System (length: 6 meters) |
↓ gait velocity |
| Muir et al,[18] 2012 |
Criteria: Winblad et al (2004) CHI: n=22, 71.0 years, 88% MCI: n=29, 73.6 years, 59% DEM: n=23, 77.5 years, 61% |
Walking at usual speed Walking with dual task (counting backward from 100 by 1) Walking with dual task (counting backward from 100 by 7) Walking with dual task (naming animals) |
GAITRite System (length: 6 meters) |
All dual tasking: ↓ gait velocity, ↑stride time, ↑STV |
| Nascimbeni et al[27], 2015 |
Criteria: a)MMSE, b)digit span/Corsi span test, c) short story recall, d) attention and visual search CHI: n=10, 72.0 years, 40% MCI: n=13, 76.0 years, 15% |
Walking at usual speed Walking with dual task (phonemic fluency) Walking with dual task (short story recall) Walking with dual task (Counting backward by 1) |
Gait laboratory (length: 12 meters), STEP 32 Gait analysis system |
Phonemic fluency dual task: ↑double support time, ↓ gait velocity Counting backwards dual task: ↑double support time |
| Tarnanas et al[26], 2015 |
Criteria: Winblad (2004) aMCI: n=65, 72.6 years, 62% CHI: n=76,70.1 years, 65% DEM: n=86, 76.6 years, 63% |
Walking at usual pace Walking with dual task (counting backward from 100 by 1) Walking with dual task (animal naming) |
GAITRite system (length: 10 meters) |
All conditions: ↓velocity, ↑coefficient of variation |
| Verghese et al,[24] 2008 |
Criteria: Petersen et al (2001), Winblad et al (2004) CHI: n=295, 79.3 years, 62.4% a-MCI: n=54, 82.6 years, 48.1% na-MCI: n=62, 81.8 years, 70.9% |
Walking at usual pace | GAITRite system (length: 4.572 meters) |
a-MCI and na- subtypes vs CHI: ↓ gait velocity, ↓stride length, ↑double support time |
Compared to an age-matched cognitively healthy control group, if present in the study
Abbreviations: ↑, increased; ↓, decreased; a-MCI, amnestic mild cognitive impairment; ADL, activities of daily living; \ CDR, clinical dementia rating; CHI, age-matched cognitively healthy individuals; DEM, dementia including Alzheimer’s Disease; MCI, mild cognitive impairment; na-MCI, non-amnestic mild cognitive impairment; STV, stride time variability; TUG, Timed Up and Go;
Parameters
Gait parameters and assessments varied substantially amongst the studies, even when the same instrument was used for evaluation. A summary of the studies that used gait assessment is presented in Table 3. Eleven papers reported quantitative gait data, which included gait velocity (n=10), gait velocity variability (n= 6), stride time variability (n=6), stride time (n=4), stride length (n=2), stride frequency (n=1), swing time (n=1), and step regularity (n=1). This paper focuses on the parameters that were reported in two or more papers (e.g., gait velocity, stride length, stride time, stride time variability). Qualitative results are provided for single papers that could not be included in meta-analysis.
Effect of MCI on gait parameters
Gait Velocity
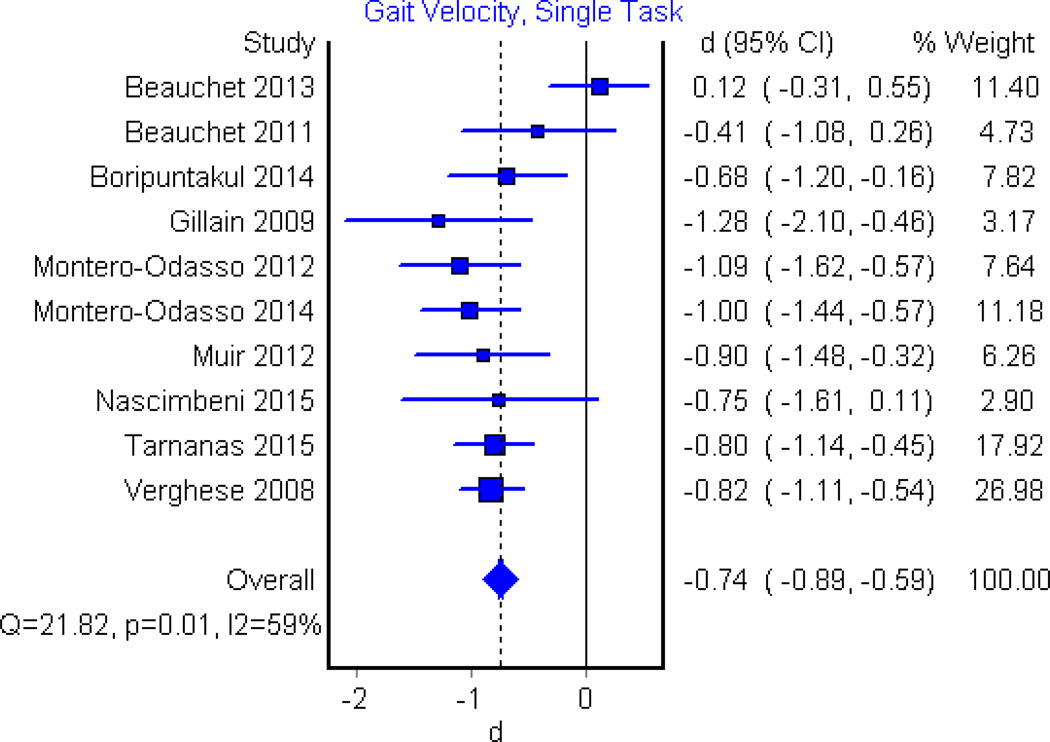
Among articles which reported single task gait velocity, five found significant decrease in persons with MCI in comparison to persons who are cognitively healthy[17,22,24–26], whereas five did not identify significant difference [11,18,20,21,27]. Pooling of data within a meta-analysis of ten eligible studies showed a moderate to large significant effect (d=−0.74, 95%CI, −0.89 to −0.59, p<0.001, Fig. 2). Dual task conditions were examined in five papers. Dual task gait velocity was significantly slower in persons with MCI during backwards counting by 7’s(n=3)[17,18,28], backwards counting by 1’s(n=2)[11,26] and animal naming (n=3)[17,18,26] in comparison to cognitively intact peers. Meta-analysis of these papers revealed significant differences between MCI and healthy controls in all three conditions with largest effect found for counting backwards by 7’s (d=−1.34, 95%CI, −1.74 to −0.93, p<0.01, Fig. 3a) with and counting backwards by 1’s (d=−0.92, 95%CI, −1.19 to −0.66, p<0.01, Fig 3b) and animal naming(d=−0.94, 95% CI, −1.20 to −0.68, p<0.01, Fig. 3c) having similar effect sizes.
Figure 2.
Forest Plot illustrating the effect of MCI on single task gait velocity when compared to cognitively healthy controls. The dotted vertical line corresponds to the overall effect size while the solid vertical line corresponds to no effect.
Figure 3.
Forest Plot illustrating the effect of MCI on dual task gait velocity during backwards (A) counting by 7’s, (B) backwards counting by 1’s, and (C) animal naming when compared to cognitively healthy controls. The dotted vertical line corresponds to the overall effect size.
Stride length
Stride length was examined in three studies for single task[11,24,28], and two studies for dual task conditions[11,28]. In single task conditions, one paper identified a significant decrease in stride length for both aMCI and na-MCI subtypes in comparison to healthy controls[24] while two papers [11,28] identified no significant effect of MCI. Meta-analysis was performed for single task stride length for two papers where mean and standard deviation data was provided[11,24] and revealed a significant medium effect (d=−0.65, 95%CI, −0.88 to −0.41, p< 0.01, Fig.4a). Change in dual task stride length was reported to be insignificant in two papers[11,28], and were excluded from meta-analysis since the studies used the same data set.
Figure 4.
Forest Plot illustrating the effect of MCI on (A) mean stride length during single task, and mean stride time during (B) single task, (C) backwards counting 7’s dual task and (D) animal naming dual task, compared to cognitively healthy controls. The dotted vertical line corresponds to the overall effect size.
Stride time
Under single task conditions, increase in stride time was significant in two studies [17,23], and non-significant in another two [18,27]. Meta-analysis revealed that single task stride time significantly discriminated between both groups with a medium effect size (d=0.56, 95%CI, 0.23 to 0.89, p=0.02, Fig. 4b).
Under backwards counting (7’s) and animal naming dual task, two studies [17,18] reported significant differences in stride time between the MCI and CHI groups. Meta-analysis revealed significant differences with a larger effect size for backwards counting (7’s) (d=0.91, 95%CI, 0.53 to 1.30, p<0.01, Fig. 4c) compared to animal naming dual tasks (d=0.84, 95%CI, 0.46 to 1.23, p<0.01, Fig. 4d).
Coefficient of Variation (CoV) of Stride Time
In four papers the increase in single task stride time CoV was significant [17,18,23,26], while two papers did not find that differences were significant [20,27]. Analysis of these six papers revealed a medium positive effect that was significant (d=0.50, 95%CI, 0.29 to 0.71, p<0.01, Fig. 5a).
Figure 5.
Forest Plot illustrating the effect of MCI on coefficient of variation (CoV) during (A) single task, (B) counting backwards by 7’s dual task, (C) counting backwards by 1’s dual task and (D) animal naming dual task when compared to cognitively healthy controls. The dotted vertical line corresponds to the overall effect size.
For dual task stride time CoV, Montero Odasso et al (2012) found a significant increase during both backwards counting by 7’s and animal naming[17], and two papers additionally found a significant increase in backwards counting by 1’s dual task conditions[18,26]. Meta-analysis revealed a significant increase of dual task stride time variability in MCI vs healthy with larger effects for backwards counting tasks (1’s, d=0.86, 95%CI, 0.58 to 1.14, p<0.01, Fig.5b; Fig.7’s, d=0.84, 95%CI, 0.45 to 1.22, p<0.01, Fig 5c), compared to animal naming (d=0.51, 95%CI, 0.26 to 0.76, p<0.01, Fig. 5d).
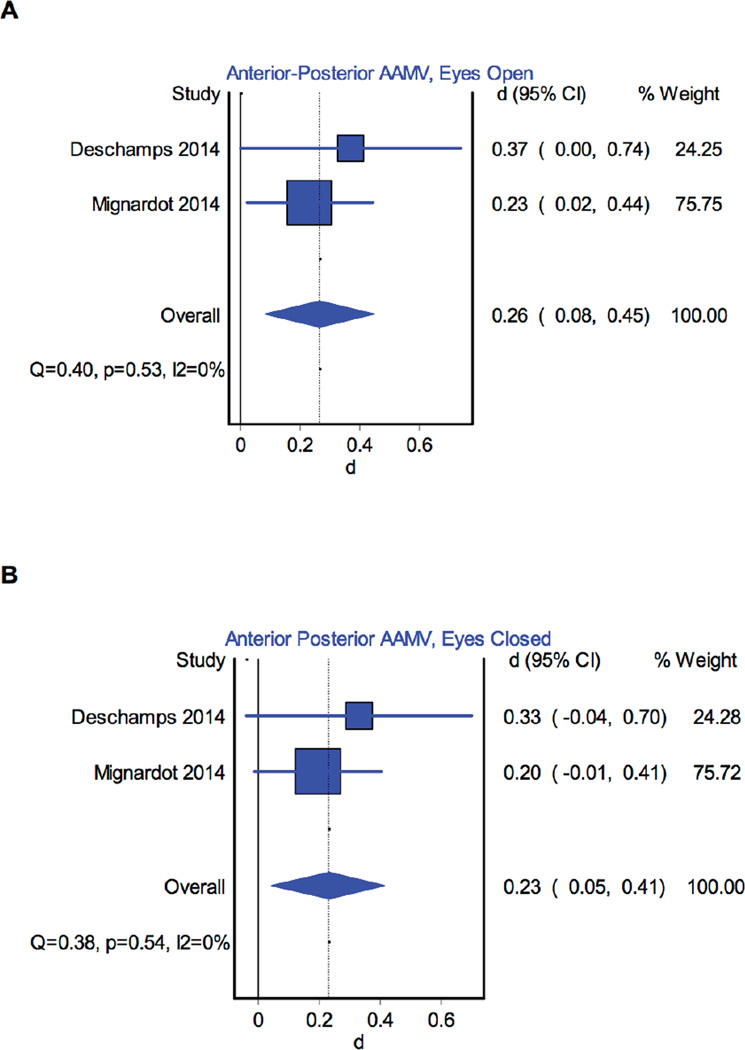
Figure 7.
Forest Plot illustrating the effect of MCI on anterior-posterior absolute average maximum velocity (AAMV) in the (A) eyes open and (B) eyes closed conditions compared to cognitively healthy controls. The dotted vertical line corresponds to the overall effect size.
Qualitative Results
One paper specifically analyzed gait initiation using the GAITrite system[22]. Authors reported a significantly increased step length and step width variability related to walking condition (i.e. single versus dual task) during gait initiation. Although mean spatiotemporal parameters (i.e., swing time, step time, step length, step width) were not significantly different among the first two steps, variability in these parameters was reported to be significant between groups in all but one parameter (step time).
One study examined the effect of walking speed (i.e., habitual vs. fast walking) on outcomes [20]. Authors reported that MCI patients display a high stride time variability during fast pace walking speed which was not seen at slower paces, and thus could be used as a specific biomarker of MCI patients.
Balance Parameters Reported in Studies
Participants
Four papers focused on the interaction of MCI and balance[11,29–31]. All four compared MCI subjects to cognitively healthy controls as well as subjects with mild-to-moderate dementia or dementia[11,29–31]. (Table 4).
Table 4.
Summary of included studies involving balance in MCI versus CHI groups
| Study | Study Characteristics (number, mean age %female) |
Instrumente Assessment |
Instrument | Significant balance results in MCI group* |
|---|---|---|---|---|
| Deschamps et al,[29] 2013 |
Criteria: a) MMSE, b)FAB, c)ADAS-cog, d)TMT parts A/B, f)Free and Cued Selective Reminding Test, g)IADL, h)MRI CHI: n=150, 76.4 years, 30% MCI: n=64, 77.5 years, 39% MMAD: n=61, 78.4 years, 62% |
Stance with EO Stance with EC |
Force platform | ↑ COP mean velocity, EO and EC; ↑ COP ML mean velocity, EO; ↑ COP AP average absolute mean velocity, EO and EC |
| Gillain et al,[11] 2007 |
Criteria: Petersen et al (2001) CHI: n=14, 73.5 years, 21% MCI: n=14, 72.9 years, 21% DEM: n=6, 73.7 years, 9% |
Single-leg balance test Single-leg balance test with dual task (countdown from 50) Pull test TUG test TUG test test with dual task (countdown from 50) |
Locometrix® tri-axial accelerometers |
MCI presents intermediate values, no significant static balance differences |
| Leandri et al,[30] 2009 |
Criteria: Petersen et al (2001) CHI: n=15, 76.0 years, 53.3% aMCI: n=15, 77.6 years, 53.3% MMAD: n=15, 77.6 years, 53.3% |
Stance with EO Stance with EC |
ARGO system (static platform) |
↑AP sway with EC |
| Mignardot et al,[31] 2014 |
Criteria: Winblad et al (2004) CHI: n=228, 72.5 years, 40.3% MCI: n=140, 74.7 years, 34.3% MMAD: n=243, 83 years, 61.7% |
Timed Up and Go Stance with EO Stance with EC |
Biorescue force platform |
↑ COP AP velocity with ↑cognitive impairment |
Compared to an age-matched cognitively healthy control group, if present in the study
Abbreviations: ↑, increased; ↓, decreased; a-MCI, amnestic mild cognitive impairment; ADAS-cog, Alzheimer’s Disease Assessment Scale-Cognitive; ADL, activities of daily living; AP, anteroposterior; CDR, clinical dementia rating; CHI, cognitively healthy individuals; COG, center of gravity; COP, center of pressure; DEM, dementia; EC, eyes closed; EO, eyes open; FAB, Frontal Assessment Battery; IADL, Instrumental Activities of Daily Living scale; MCI, mild cognitive impairment; MMAD, mild-to-moderate Alzheimer’s disease; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; TMT, trail-making test
Parameters
A summary of the seven studies that included balance assessment for an MCI group is presented in Table 4. Twenty-one unique parameters were identified in the included papers, with quantitative balance data presented in five of the seven papers [13,36,40,42,43].
Effect of MCI on anterior-posterior static balance parameters
Sway variables
For the eyes open condition, anterior posterior (AP) mean sway position, measured as the distance from the starting point, was found to be insignificant in significant in one paper[29] but not [30] in the eyes open condition. Meta-analysis of two papers[29,30] found a small-medium effect size for AP mean sway position (d=0.49, 95%CI, 0.16 to 0.82, 0.0, Fig. 6a).
Figure 6.
Forest Plot illustrating the effect of MCI on anterior-posterior mean position in the (A) eyes open and (B) eyes closed; and on mediolateral mean position in the (C) eyes open and (D) eyes closed condition compared to cognitively healthy controls. The dotted vertical line corresponds to the overall effect size.
For the eyes closed condition, AP mean position was found to significantly increase in eyes closed condition in one study [30] but not in another[32].Meta-analysis of two papers[29,30] showed a medium but not significant effect of MCI on mean position in the eyes closed condition (d=0.55, 95%CI, −0.55 to 1.65, p=0.33, Fig. 6b).
Sway velocity variables
For AP sway velocity, one paper found MCI lead to a significant increase in trunk velocity for both mean and average absolute maximum values[29], while others found that neither the sway speed[33] nor the average absolute maximum velocity[31] were significantly affected by MCI. Meta-analysis of average absolute maximum velocity for two papers[29,31] identified a small significant effect of MCI in the eyes open condition (d=0.26, 95%CI, 0.08 to 0.45, p<0.01, Fig. 7a).
For the eyes closed condition, AP average absolute maximum velocity was found to significantly increase in eyes closed condition in one study [29], but not in another[31]. Meta-analysis of average absolute maximum velocity for two papers[29,31] identified a small significant effect of MCI in the eyes closed condition (d=0.23, 95%CI, 0.05 to 0.41, p=0.01, Fig. 7b).
Effect of MCI on mediolateral static balance parameters
Sway position variables
For the eyes open condition, one paper reported an insignificant effect on ML sway position[30] and another reported a significant effect[29]. Meta-analysis revealed a small and significant effect was found in the eyes open condition (d=−0.34, 95%CI, −0.67 to −0.01, p=0.04, Fig. 6c). Meta-analysis of two papers[29,30] revealed no significant effect on ML mean position in the eyes closed condition (d=−0.05, 95%CI, −0.86 to 0.75, p=0.48, Fig. 6d).
Sway velocity variables
One paper found sway speed increased significantly compared to healthy controls in the eyes open [32], while two papers reported a non-significant difference[30,33]. For the eyes closed condition, one paper reported that MCI caused significant increase in sway speed and position [32], while another reported a significant difference only with eyes closed that disappeared with post hoc tests[30].
Qualitative Results
Increased cognitive impairment was associated with increased velocity standard deviation[29] and absolute average maximum velocity (AAMV) increase[29] in static balance, supporting our findings from meta-analysis that persons with MCI have increased postural sway during standing.
DISCUSSION
Overall, this systematic review and meta-analysis provides sound evidence that MCI adversely affects gait and balance. To our knowledge, this is the first systematic review that provides a comprehensive overview and meta-analysis of studies using objective instrumented assessment of gait and balance. Using this approach, we were able to extract a variety of parameters in both gait and balance in order to identify the most sensitive parameters related to MCI. Our results show that MCI has a substantial impact on specific gait variables.. Moreover, we found static balance is also affected by MCI, indicating that early cognitive changes have a measurable effect on postural control system and puts patients at increased risk of balance failures and falls.
Changes in Gait Parameters
Single task conditions
This systematic review demonstrates that gait performance is reduced in people with MCI as reflected by changes in a number of spatiotemporal parameters. When gait is assessed under single task conditions, gait velocity showed a large effect size for discriminating between MCI and cognitively intact, indicating that this parameter plays a key role in MCI. This result is in line with a number of studies that have identified reduced gait velocity as a predictor for adverse health events including mortality, frailty, or functional dependence [34–36]. Slow gait is a nonspecific variable, however, which is also linked to aging and many aging-related gait disorders. Assessment of gait velocity alone does not provide insight into the specific gait pattern related to MCI, which in turn may limit the sensitivity and specificity of discrimination between people with MCI and cognitively intact.
Dual task conditions
Use of dual-task paradigm exposes deficits through evaluation of activities which simultaneously demand attention resources[37]. One of the main findings of our systematic summary and meta-analysis is that dual-task gait assessment increases the sensitivity of gait analysis for discriminating between MCI and healthy groups. Effect sizes were substantially higher for spatiotemporal variables as compared to single task. This information is of high relevance when designing a protocol for diagnosing MCI-specific gait changes and for documenting the impact of specific interventions.
Moreover, we performed meta-analysis for analyzing the impact of different cognitive tasks used in dual-task protocols. One interesting finding is that sensitivity of dual-task gait assessment differs depending on the cognitive task used. Arithmetic tasks with a high cognitive demand (−7) have the highest sensitivity, which may have important clinical implications. These findings suggest that a high cognitive load is required in dual-task protocol for making MCI-specific gait changes emerge. The use of the adequate cognitive tasks has been extensively discussed in the literature in cognitively healthy [38,39] and dementia patients [18,40–42]. In dementia, simple cognitive tasks seem to be more appropriate because complex tasks may be too demanding and hamper a reliable dual task assessment [43]. However, in MCI it has been less clear which cognitive task is best for high sensitivity of gait analysis. Based on our results, it seems that increasing cognitive demand are increases sensitivity. Verbal fluency tasks such as animal naming appear to have a lesser demand than arithmetic tasks because it uses semantic memory as opposed to working memory[44]. In contrast, a low demand arithmetic task (−1) had very similar results to single task conditions because it is more rhythmic and can may cue step pattern[18].
Spatiotemporal features of gait
In our meta-analysis we identified several gait parameters beyond velocity, which may help to indicate MCI-related gait changes. Meta-analysis revealed that MCI affects stride time in both single and dual task conditions. Although the effect sizes are smaller when compared to gait velocity, once again the largest effect appears in arithmetic dual task. Stride length data was only available for single task assessments, but also showed that MCI had a significant effect. These two results suggest that the effect of MCI on gait velocity is due to both spatial and temporal modifications in gait.
Variability of stride time provides a measure of gait stability from stride-to-stride[45]. Calculated effect size from the reviewed studies suggests that increased stride-time variability has moderate to high power to discriminate between MCI and healthy groups, depending on the condition (i.e. single task vs dual task) and thus may serve as an additional parameter for early diagnosis of MCI-related gait deficits. Stride time variability in dual task has been repeatedly reported as a sensitive indicator of cognitive change [17,46].
It has been identified that participant walking strategy changes with distance traveled, resulting in a significant effect on gait variability [47]. Finding of our review support the influence of walking distance on measuring MCI-related changes in gait variability. For example, in a paper using a 6 meter GAITrite, single task, dual task backwards counting (7’s) and animal naming coefficient of variation were not significant[18]. In contrast, in a paper using 10 meter GAITrite, all three of these values were reported to be significant[26]. These results suggest that a sufficient walking distance is highly relevant in order to measure gait variability as a marker for MCI.
Additionally, we found some evidence that fast pace walking increases sensitivity for diagnosis MCI related gait changes. Further, we found that MCI-specific gait changes may particularly emerge during gait initiation. While we could not perform a meta-analysis because only a single study was available, findings may indicate that MCI-related gait changes emerge during more demanding gait situations (i.e., fast walking) and more demanding gait phases (i.e., gait initiation). Similar to dual-task walking (i.e., cognitive stress test), a fast walking (i.e. motor stress test) might be helpful in order to identify gait changes in MCI.
Changes in Balance Parameters
This systematic review shows that MCI has significant effects on static postural balance. Meta-analysis of both AP and ML sway position identified small-medium effect sizes that were significant in the eyes open but not the eyes closed condition. Although these subtle changes in postural sway may not have a severe impact on activities of daily living, they may indicate a progression toward more severe impairment.
During eyes open balance testing, visual information is processed for maintaining balance. Research suggests that people with MCI have deficits in processing of visual information [48] that results in increased postural sway during balance testing, as discussed previously [49]. Our results support this theory and suggest that MCI-related balance deficits are related impaired central processing of visual information that is critical for balance control.
Limited effects observed during eyes closed condition might be related to lack of reliability of static balance testing in this specific condition. It was identified in a paper by Helbostad et al[50] that eyes closed balance assessments seem to be less reliable than the same assessments in eyes open condition.
Another interesting finding was that AP sway speed and mean position was found to have greater changes with MCI than ML in both qualitative and quantitative analysis. In a past study, Franssen et al[51] identified that persons with MCI had poorer performance in tests of equilibrium and limb coordination. Our results support this, and reveal that AP sway position may be the most sensitive balance parameter for early discrimination of MCI and CHI. AP sway in static balance is more frequently involved in body stability than ML due to the range of motion available for the body [30]; this natural range could explain the larger effect size of AP mean position as compared to ML mean position in the eyes open condition.
Implications for Clinical Intervention
One major strength of this review is that we performed a meta-analysis using studies only which provided a clinically established MCI definition. Our results show that, overall, dual task assessment is the most sensitive tool for gait based MCI screening. This is an important step forward in developing a clinically validated approach for measuring MCI related motor deficits, although further studies are required in order to validate the findings of this review.
Information of this review could be useful for promoting specific interventions aiming reverse early motor changes associated with MCI. It has been shown that multicomponent exercise (e.g., aerobic exercise, muscle strength training, gait training) improves gait velocity and stride length in MCI participants [52], Progressive resistance and functional training has been shown to be effective for improving fast walking speed in cognitively impaired. . However, there is still room for improvement in current interventions, including specific tailoring to the motor deficits found in this review. For instance, there is limited evidence on intervention effects on stride time variability[9] although this parameter seems to play a critical role in MCI syndrome. New gait training paradigms have shown that gait variability can be influenced in cognitively intact, but studies have not yet been performed in the target population of MCI. It remains to be determined if specific motor learning exercise programs for walking (e.g., overground and treadmill) designed to reinforce rhythmic stepping[53] are effective for reducing gait variability.
Additionally, we found some evidence that MCI-related gait disturbances appear specifically under demanding situations, such as fast walking[20]. This suggests that exercise training in MCI patients should include challenging gait tasks focusing on improvement of gait control in situations with both increased motor (i.e. fast walking) and cognitive (i.e. dual tasking) demand. There is some evidence that gait velocity can be improved in the cognitively impaired under both motor and cognitively challenging conditions[54], using a combination of dual-task training, and progressive strength and functional training[9,55]. However, further studies are required in larger populations in order to investigate the effect of this training on important clinical outcomes such as progression of MCI or fall risk.
Importantly, we identified that MCI significantly impacts ML and AP balance control during eyes open condition. This opens opportunities for novel interventions paradigms aiming to retrain visual processing of information relevant for postural balance. For instance, it was identified that both MCI patients and age-matched controls use similar compensation strategies for maintaining static balance when provided visual feedback, indicating that compensation systems are intact and may be a target for balance training[56,57].
An interesting study demonstrated that “non-motor cognitive dual task training” resulted in motor performance benefits for healthy older adults[58]. This suggests that cognitive training may be an excellent addition to existing training paradigms, particularly for persons with limited mobility.
LIMITATIONS
A lack of uniformity among the study design (e.g. walking distance, variables measured, instrument) may have affected the validity of analysis for the statistical measurements. The number of parameters included in each meta-analysis varied, depending on the number of studies which reported a specific parameter. This may have biased our findings. For parameters which were more frequently reported (e.g., gait velocity), the meta-analysis results are more precise. Furthermore, funnel plot analysis suggests presence of a publication which may have affected the validity of our analysis. In performing meta-analysis, our pragmatic approach was to include the maximum number of studies reporting each parameter in order to accurately evaluate the evidence that is currently available.
Speed dependency of gait variables was not discussed in this paper since only one paper contained data at a fast walking speed[20]. Time-to-boundary measures, or nonlinear measures of postural sway were not examined in these papers but may provide information on more subtle changes in motor control in the MCI population. We acknowledge that more studies using a standardized instrumented assessment procedure are required to verify the validity of our results
CONCLUSION AND CLINICAL IMPLICATIONS
Use of motor-performance measures, particularly under cognitively challenging conditions (i.e. dual task), may provide a sensitive, early, and non-invasive means for screening of clinically relevant MCI-specific motor disturbances. Identification of early gait and MCI deficits could provide a critical opportunity for early intervention before gait and balance changes have a major impact on ADLs, fall risk, and overall independence. This review provides sound evidence on which parameters should be used in gait and balance assessment, and provides a basis for future studies aiming to further develop, verify, and refine a standardized clinical motor assessment protocol for people with MCI.
Table 2.
Criteria for MCI reported in studies
| Criteria | Papers n, % |
Citations |
|---|---|---|
| Petersen et al. [5,59] | 4, 28.5% | [22,24,25,30] |
| Winblad et al. [16] | 6, 42.8% | [17,18,20,21,26,31] |
| CERAD [60] | 1, 7.1% | [23] |
| Miscellaneous | 3, 21.42% | [11,27,29] |
CERAD = Consortium to Establish a Registry for Alzheimer’s Disease
Acknowledgments
This research was performed in collaboration with the interdisciplinary Consortium on Advanced Motion Performance (iCAMP) at the University of Arizona, Banner Sun Health Research Institute, the Arizona Center on Aging, and Arizona State University. It was funded in part by the Flinn Foundation: Arizona Aging and Cognitive Collaborative grant(award number 1907), the Undergraduate Biology Research Program (HHMI 52006942) at the University of Arizona, and by the National Institute on Aging (award number 2R42AG032748). We thank Charles Huang and Tulcy Patel who assisted with the screening process, and Qianzi Zhang for her support in the meta-analysis
Sponsor’s Role: The sponsors had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest: All authors report no conflict of interest or any financial support received.
Author Contribution: Bahureksa: preparation of manuscript, interpretation and analysis of data, literature search, study selection; Najafi: development of study concept and design, critical revision of manuscript; Saleh: literature search; Sabbagh: critical revision of manuscript; Coon: critical revision of manuscript; Mohler: development of study concept and design, critical revision of manuscript; Schwenk: development of study concept and design, study selection, critical revision of manuscript. All authors contributed to final approval of the published version.
REFERENCES
- 1.Huang C, Mattis P, Julin P. Identifying functional imaging markers of mild cognitive impairment in early alzheimer’s and parkinson’s disease using multivariate analysis. Clinical Neuroscience Research. 2007;6:367–373. [Google Scholar]
- 2.Dierckx E, Engelborghs S, De Raedt R, De Deyn PP, Ponjaert-Kristoffersen I. Mild cognitive impairment: What’s in a name? Gerontology. 2007;53:28–35. doi: 10.1159/000095763. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA. Prevalence of mild cognitive impairment is higher in men The mayo clinic study of aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 6.Dubois B, Albert ML. Amnestic mci or prodromal alzheimer’s disease? The Lancet Neurology. 2004;3:246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 7.Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, Vellas B, Touchon J. Mild cognitive impairment (mci) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the mci working group of the european consortium on alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55:570–581. doi: 10.1159/000228918. [DOI] [PubMed] [Google Scholar]
- 9.Schwenk M, Zieschang T, Englert S, Grewal G, Najafi B, Hauer K. Improvements in gait characteristics after intensive resistance and functional training in people with dementia: A randomised controlled trial. BMC geriatrics. 2014;14:73. doi: 10.1186/1471-2318-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer S, Schumacher V. The interplay between cognitive and motor functioning in healthy older adults: Findings from dual-task studies and suggestions for intervention. Gerontology. 2011;57:239–246. doi: 10.1159/000322197. [DOI] [PubMed] [Google Scholar]
- 11.Gillain S, Warzee E, Lekeu F, Wojtasik V, Maquet D, Croisier JL, Salmon E, Petermans J. The value of instrumental gait analysis in elderly healthy, mci or alzheimer’s disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Annals of physical and rehabilitation medicine. 2009;52:453–474. doi: 10.1016/j.rehab.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-alzheimer’s dementia. The New England journal of medicine. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 13.Granacher U, Bridenbaugh SA, Muehlbauer T, Wehrle A, Kressig RW. Age-related effects on postural control under multi-task conditions. Gerontology. 2011;57:247–255. doi: 10.1159/000322196. [DOI] [PubMed] [Google Scholar]
- 14.Tell GS, Lefkowitz DS, Diehr P, Elster AD. Relationship between balance and abnormalities in cerebral magnetic resonance imaging in older adults. JAMA Neurology. 1998:55. doi: 10.1001/archneur.55.1.73. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: Report of the international working group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 17.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: The interplay between gait variability, dual tasking, and risk of falls. Archives of physical medicine and rehabilitation. 2012;93:293–299. doi: 10.1016/j.apmr.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait & posture. 2012;35:96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavior sciences, ed Second Edition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 20.Beauchet O, Allali G, Launay C, Herrmann FR, Annweiler C. Gait variability at fast-pace walking speed: A biomarker of mild cognitive impairment? The journal of nutrition, health & aging. 2013;17:235–239. doi: 10.1007/s12603-012-0394-4. [DOI] [PubMed] [Google Scholar]
- 21.Beauchet O, Allali G, Thiery S, Gautier J, Fantino B, Annweiler C. Association between high variability of gait speed and mild cognitive impairment: A cross-sectional pilot study. Journal of the American Geriatrics Society. 2011;59:1973–1974. doi: 10.1111/j.1532-5415.2011.03610_9.x. [DOI] [PubMed] [Google Scholar]
- 22.Boripuntakul S, Lord SR, Brodie MA, Smith ST, Methapatara P, Wongpakaran N, Sungkarat S. Spatial variability during gait initiation while dual tasking is increased in individuals with mild cognitive impairment. The journal of nutrition, health & aging. 2014;18:307–312. doi: 10.1007/s12603-013-0390-3. [DOI] [PubMed] [Google Scholar]
- 23.Choi JS, Oh HS, Kang DW, Mun KR, Choi MH, Lee SJ, Yang JW, Chung SC, Mun SW, Tack GR. Comparison of gait and cognitive function among the elderly with alzheimer’s disease, mild cognitive impairment and healthy. Int J Precis Eng Manuf. 2011;12:169–173. [Google Scholar]
- 24.Verghese J, Robbins M, Holtzer R, Zimmerman M, Wang C, Xue X, Lipton RB. Gait dysfunction in mild cognitive impairment syndromes. Journal of the American Geriatrics Society. 2008;56:1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, Muir-Hunter SW. The motor signature of mild cognitive impairment: Results from the gait and brain study. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69:1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarnanas I, Papagiannopoulos S, Kazis D, Wiederhold M, Widerhold B, Tsolaki M. Reliability of a novel serious game using dual-task gait profiles to early characterize amci. Frontiers in Aging Neuroscience. 2015:7. doi: 10.3389/fnagi.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nascimbeni A, Caruso S, Salatino A, Carenza M, Rigano M, Raviolo A, Ricci R. Dual task-related gait changes in patients with mild cognitive impairment. Functional neurology. 2015:1–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Maquet D, Lekeu F, Warzee E, Gillain S, Wojtasik V, Salmon E, Petermans J, Croisier JL. Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild alzheimer’s disease: Simple versus dual task: A preliminary report. Clinical physiology and functional imaging. 2010;30:51–56. doi: 10.1111/j.1475-097X.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- 29.Deschamps T, Beauchet O, Annweiler C, Cornu C, Mignardot JB. Postural control and cognitive decline in older adults: Position versus velocity implicit motor strategy. Gait & posture. 2014;39:628–630. doi: 10.1016/j.gaitpost.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Leandri M, Cammisuli S, Cammarata S, Baratto L, Campbell J, Simonini M, Tabaton M. Balance features in alzheimer’s disease and amnestic mild cognitive impairment. Journal of Alzheimer’s disease : JAD. 2009;16:113–120. doi: 10.3233/JAD-2009-0928. [DOI] [PubMed] [Google Scholar]
- 31.Mignardot JB, Beauchet O, Annweiler C, Cornu C, Deschamps T. Postural sway, falls, and cognitive status: A cross-sectional study among older adults. Journal of Alzheimer’s disease : JAD. 2014;41:431–439. doi: 10.3233/JAD-132657. [DOI] [PubMed] [Google Scholar]
- 32.Shin BM, Han SJ, Jung JH, Kim JE, Fregni F. Effect of mild cognitive impairment on balance. Journal of the neurological sciences. 2011;305:121–125. doi: 10.1016/j.jns.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Jeon SY, Han SJ, Jeong JH, Fregni F. Effect of exercise on balance in persons with mild cognitive impairment. NeuroRehabilitation. 2014;35:271–278. doi: 10.3233/NRE-141120. [DOI] [PubMed] [Google Scholar]
- 34.Schwenk M, Howe C, Saleh A, Mohler J, Grewal G, Armstrong D, Najafi B. Frailty and technology: A systematic review of gait analysis in those with frailty. Gerontology. 2014;60:79–89. doi: 10.1159/000354211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: The role of physical performance. Journal of the American Geriatrics Society. 1995;43:603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 36.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the health, aging and body composition study. Journal of the American Geriatrics Society. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 37.Beauchet O, Berrut G. [gait and dual-task: Definition, interest, and perspectives in the elderly] Psychologie & neuropsychiatrie du vieillissement. 2006;4:215–225. [PubMed] [Google Scholar]
- 38.Beauchet O, Dubost V, Aminian K, Gonthier R, Kressig RW. Dual-task-related gait changes in the elderly: Does the type of cognitive task matter? Journal of motor behavior. 2005;37:259–264. [PubMed] [Google Scholar]
- 39.Melzer I, Oddsson LI. The effect of a cognitive task on voluntary step execution in healthy elderly and young individuals. Journal of the American Geriatrics Society. 2004;52:1255–1262. doi: 10.1111/j.1532-5415.2004.52353.x. [DOI] [PubMed] [Google Scholar]
- 40.Barberger-Gateau P, Fabrigoule C. Disability and cognitive impairment in the elderly. Disability and rehabilitation. 1997;19:175–193. doi: 10.3109/09638289709166525. [DOI] [PubMed] [Google Scholar]
- 41.Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: The effect of a dual task in aging and alzheimer’s disease. Neurology. 1997;48:955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- 42.Redfern MS, Muller ML, Jennings JR, Furman JM. Attentional dynamics in postural control during perturbations in young and older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2002;57:B298–B303. doi: 10.1093/gerona/57.8.b298. [DOI] [PubMed] [Google Scholar]
- 43.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: Results from the einstein aging study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 44.Beauchet O, Dubost V, Gonthier R, Kressig RW. Dual-task-related gait changes in transitionally frail older adults The type of the walking-associated cognitive task matters. Gerontology. 2005;51:48–52. doi: 10.1159/000081435. [DOI] [PubMed] [Google Scholar]
- 45.Beauchet O, Annweiler C, Lecordroch Y, Allali G, Dubost V, Herrmann Fç R, Kressig RW. Walking speed-related changes in stride time variability: Effects of decreased speed. Journal of neuroengineering and rehabilitation. 2009;6:32. doi: 10.1186/1743-0003-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. Journal of neuroengineering and rehabilitation. 2011;8:2–2. doi: 10.1186/1743-0003-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait & posture. 2009;29:261–266. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Perrochon A, Kemoun G, Dugue B, Berthoz A. Cognitive impairment assessment through visuospatial memory can be performed with a modified walking corsi test using the ‘magic carpet’. Dementia and geriatric cognitive disorders extra. 2014;4:1–13. doi: 10.1159/000356727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Redfern MS, Jennings JR, Mendelson D, Nebes RD. Perceptual inhibition is associated with sensory integration in standing postural control among older adults. The journals of gerontology Series B, Psychological sciences and social sciences. 2009;64:569–576. doi: 10.1093/geronb/gbp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helbostad JL, Askim T, Moe-Nilssen R. Short-term repeatability of body sway during quiet standing in people with hemiparesis and in frail older adults. Archives of physical medicine and rehabilitation. 2004;85:993–999. doi: 10.1016/j.apmr.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Franssen EH, Souren LE, Torossian CL, Reisberg B. Equilibrium and limb coordination in mild cognitive impairment and mild alzheimer’s disease. Journal of the American Geriatrics Society. 1999;47:463–469. doi: 10.1111/j.1532-5415.1999.tb07240.x. [DOI] [PubMed] [Google Scholar]
- 52.Doi T, Makizako H, Shimada H, Yoshida D, Tsutsumimoto K, Sawa R, Misu S, Suzuki T. Effects of multicomponent exercise on spatial-temporal gait parameters among the elderly with amnestic mild cognitive impairment (amci): Preliminary results from a randomized controlled trial (rct) Archives of gerontology and geriatrics. 2013;56:104–108. doi: 10.1016/j.archger.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Brach JS, Van Swearingen JM, Perera S, Wert DM, Studenski S. Motor learning versus standard walking exercise in older adults with subclinical gait dysfunction: A randomized clinical trial. Journal of the American Geriatrics Society. 2013;61:1879–1886. doi: 10.1111/jgs.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwenk M, Zieschang T, Oster P, Hauer K. Dual-task performances can be improved in patients with dementia: A randomized controlled trial. Neurology. 2010;74:1961–1968. doi: 10.1212/WNL.0b013e3181e39696. [DOI] [PubMed] [Google Scholar]
- 55.Silsupadol P, Lugade V, Shumway-Cook A, van Donkelaar P, Chou LS, Mayr U, Woollacott MH. Training-related changes in dual-task walking performance of elderly persons with balance impairment: A double-blind, randomized controlled trial. Gait & posture. 2009;29:634–639. doi: 10.1016/j.gaitpost.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grewal GS, Schwenk M, Lee-Eng J, Parvaneh S, Bharara M, Menzies RA, Talal TK, Armstrong DG, Najafi B. Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: A randomized controlled trial. Gerontology. 2015;61:567–574. doi: 10.1159/000371846. [DOI] [PubMed] [Google Scholar]
- 57.Schwenk M, Grewal GS, Honarvar B, Schwenk S, Mohler J, Khalsa DS, Najafi B. Interactive balance training integrating sensor-based visual feedback of movement performance: A pilot study in older adults. Journal of neuroengineering and rehabilitation. 2014;11:164. doi: 10.1186/1743-0003-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li KZ, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley PA. Benefits of cognitive dual-task training on balance performance in healthy older adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2010;65:1344–1352. doi: 10.1093/gerona/glq151. [DOI] [PubMed] [Google Scholar]
- 59.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Archives of neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 60.Seo EH, Lee DY, Lee JH, Choo IH, Kim JW, Kim SG, Park SY, Shin JH, Do YJ, Yoon JC, Jhoo JH, Kim KW, Woo JI. Total scores of the cerad neuropsychological assessment battery: Validation for mild cognitive impairment and dementia patients with diverse etiologies. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2010;18:801–809. doi: 10.1097/JGP.0b013e3181cab764. [DOI] [PubMed] [Google Scholar]