Abstract
Objective(s)
Roux-en-Y Gastric Bypass (RYGB) is well-known to ameliorate type 2 diabetes mellitus (T2DM), and recent work suggests that the pre-operative DiaREM model predicts successful remission up to 1-year post-RYGB. However, no data exist for long-term validity. Therefore, we sought to determine the utility of this score on long-term RYGB effectiveness for T2DM resolution at 2 and 10 years respectively.
Methods
T2DM patients (Age: 48, BMI: 49, HbA1C: 8.1) undergoing RYGB at the University of Virginia between 2004-2006 (n=42) and 2012-2014 (n=59) were evaluated prospectively to assess pre-operative DiaREM score, defined from insulin use, age, HbA1C and type of antidiabetic medication. T2DM partial remission status was based on the American Diabetes Association guidelines (HbA1C <6.5% and fasting glycemia <125 mg/dl, and no anti-diabetic medications). Chi-square test was used to compare patient's T2DM status to their DiaREM probability of remission.
Results
Among RYGB patients with 2-year postoperative data, 2 were lost (n=1 no follow-up and n=1 died) resulting in 57 patients for analysis. For the 10-year postoperative data, 11 were lost (n=6 no follow-up and n=5 died) thereby resulting in only 31 patients for analysis. Patients were distributed by DiaREM score to correlate with the predicted probability of remission as follows: 0-2 (Predicted 94%, 2-year 100% p=0.61, 10-year 100% p=0.72), 3-7 (Predicted 76%, 2-year 94% p=0.08, 10-year 83% p=0.57), 8-12 (Predicted 36%, 2-year 47% p=0.38, 10-year 43% p=0.72), 13-17 (Predicted 22%, 2-year 20% p=0.92, 10-year 33% p=0.64), and 18-22 (Predicted 9%, 2-year 15% p=0.40, 10-year 14% p=0.64).
Conclusions
Pre-operative DiaREM scores are a good tool for predicting both short- and long-term T2DM remission following RYGB. This study highlights the need to identify strategies that improve T2DM remission in those at highest risk.
Introduction and Objective
Morbid obesity has increased exponentially in the United States during recent years, and the associated comorbidities have placed a high burden on healthcare costs, which are estimated to reach 60 billion dollars by 20301-3. Although lifestyle modification remains the first-line therapy to combat obesity, bariatric surgery has become a more frequent treatment for morbid obesity evidenced by an average of over 113,000 patients undergoing weight-loss surgery each year4. Moreover, numerous studies demonstrate the benefits of Roux-en-Y Gastric Bypass (RYGB) for co-morbidity reduction including dyslipidemia, hypertension, and Type 2 Diabetes Mellitus (T2DM)5-8. In fact, a large retrospective analysis reported that 83% of patients undergoing RYGB had improvement or remission of T2DM at 1 year follow-up9. Given the high complication rates of blindness, amputation, cardiovascular, and end stage renal disease in poorly controlled diabetics, there has been wide acceptance of RYGB for management/treatment of metabolic disease in severely obese patients.10 However, recent work suggests that pre-operative clinical (e.g. age, BMI) and biological markers (e.g. inflammation, glucose) predict responsiveness to RYGB for weight loss and diabetes remission 11-14. This suggests that not all people derive equal benefit from bariatric surgery, and better assessment tools are needed prior to surgery to understand who is likely to meet T2DM remission criteria and obtain the best results.
A number of T2DM remission predictors have been developed over the past 10 years to better risk stratify obese patients with T2DM for bariatric surgery15-18. The DiaREM Score developed by Still et al. has been validated with several papers reporting short- and mid-term accuracy and accepted by clinicians as a reasonable prediction tool for the remission of T2DM19,20. However, this tool has not been studied beyond 2 years postoperatively, thereby limiting the long-term understanding of how reliable this tool is for overall glucose management. Recently, our group demonstrated the long-term durability of T2DM resolution following RYGB at 10 years21. However, it is unknown if preoperative DiaREM score would predict long-term T2DM remission following RYGB. Therefore, the purpose of this study was to assess if the DiaREM Score was a valid predictor of the long-term accuracy of this T2DM remission. We hypothesized that the DiaREM Score would successfully predict short-term (2-year) and long-term (10-year) T2DM remission.
Patients and Methods
Patients
A prospectively collected database of patients undergoing weight-loss surgery was queried. We identified all patients undergoing RYGB performed for morbid obesity (BMI >35) between 1/1/2004 – 12/31/2005 and 7/1/12 – 6/30/13. This database included the patient name, phone number, age, sex, preoperative weight, preoperative T2DM status including medications, insulin dosage, and hemoglobin A1C (HbA1c) values. We obtained data for patients lost to follow-up using Electronic Medical Record (EMR) review for all encounters and telephone survey. This study was approved by the Institutional Review Board (IRB) at the University of Virginia.
Telephone Follow-up
Trained medical personnel followed a standardized IRB approved phone script to call patients lost to follow-up. This was done to assess changes in T2DM status as well as current anti-diabetic medication regimen and last HbA1c. If the phone numbers listed were disconnected, an online people search tool (Intelius People Search, Bellevue, WA) was used to locate patients using publically available records such as census data, property records, telephone, and email records.
DiaREM Calculation
T2DM was defined according to the American Diabetes Association (ADA) guidelines, including fasting glucose > 126 mg/dL or HbA1c > 6.5%22. A DiaREM Score for each patient was calculated based on their preoperative age, HbA1c, and diabetic medications using the method described by Still et al. 10,19. Analogous to this prior work 10,19, we combined “partial remission” and “complete remission” for final analysis. Specifically, “partial” remission of T2DM was defined by HbA1c < 6.5%, fasting blood glucose levels < 125 mg/dL and no use of anti-diabetic medications for a minimum of 12 months. “Complete” remission was defined by HbA1c < 6.0%, fasting glucose < 100 mg/dL, and no use of anti-diabetic medication for least 12 months22.
Statistical Analysis
The primary outcome for this study was actual T2DM remission at 2- and 10-years compared with expected remission based on DiaREM Score. Secondary outcomes include diabetes specific outcomes for patients 2-years and 10-years after RYGB. Data were compared using Chi-square test (χ2 test) and a p<0.05 was used for statistical significance. SAS version 9.4 (SAS Company, Cary NC) was used for statistical analyses.
Results
Preoperative T2DM Incidence
There were 120 patients who underwent RYGB in the 2-year follow-up group and 489 patients in the 10-year follow-up group. Within this population 59 and 42 people with T2DM met criteria for DiaRem modeling in the 2-year and 10-year groups respectively. Among RYGB patients with 2-year postoperative data, 2 were lost (n=1 no follow-up and n=1 died) resulting in 57 (98% follow-up) patients for analysis. For the 10-year postoperative data, 11 were lost (n=6 no follow-up and n=5 died) thereby resulting in only 31 (74% follow-up) patients for analysis (Table 1). The average Age: 45.8 years, BMI: 48.0 kg/m2, HbA1C: 7.7% for the 10-year group and an average Age: 49.2 years, BMI: 52.1 kg/m2, HbA1C: 8.3% for the 2-year group in our population.
Table 1. Pre-Operative Factors.
| 2-Year Cohort | 10 Year Cohort | p-value | |
|---|---|---|---|
| Sample Size (n) | 57 | 31 | |
| Female (%) | 75 | 55 | .01 |
| Age < 40y (%) | 21 | 35 | .04 |
| Age 40-49y (%) | 30 | 26 | .21 |
| Age 50-59y (%) | 26 | 26 | .91 |
| Age 60y+ (%) | 23 | 13 | .06 |
| 2 Pre-surgery BMI (kg/m2), mean (SEMSD) | 52.1 (1.349.6) | 48.0 (6.6) | .07 |
| HbA1C < 6.5 (%) | 172 | 25 | .11 |
| HbA1C 6.5 – 6.9 (%) | 23 | 23 | .94 |
| HbA1C 7.0 – 8.9 (%) | 30 | 29 | .89 |
| HbA1C 9.0+ (%) | 30 | 23 | .23 |
| Insulin use (%) | 32 | 32 | .97 |
Demonstrates the sample size and pre-operative characteristic of the 2-year and 10-year cohorts in this population. BMI = body mass index. HbA1C = Hemoglobin A1C.
2-Year and 10-Year T2DM Remission
In the 2-year group, 65% of people achieved T2DM remission while the 10-year group had 58% with T2DM remission. Using the methods of Still et al. 10,19, we stratified the DiaREM scores into five groups with corresponding remission rates: 0–2 (88%–99%), 3–7 (64%–88%), 8–12 (23%–49%), 13–17 (11%–33%), 18–22 (2%–16%). Each group was listed with the correlating percent remission from the original study. The results in Table 2 displays the percent of patients across the preoperative risk criteria for the cohorts at 2- and 10-years, and highlights that the majority of patients fell within the 3-7 and 8-12 score range. Finally, Table 3 shows the percent remission of T2DM for our two cohorts compared to that reported by Still and colleagues 10,19. There was no difference in percent remission of T2DM in the 2-year or 10-year groups compared to the prediction based on DiaREM Score (all p>0.05; Table 3 and Figure 1).
Table 2. DiaREM Scores by Cohort.
| DiaREM Score | 2-year (%) | 10-year (%) |
|---|---|---|
| 0-2 | 7 | 6 |
| 3-7 | 32 | 39 |
| 8-12 | 30 | 23 |
| 13-17 | 9 | 10 |
| 18-22 | 23 | 22 |
Illustrates the percentage of patients within each DiaREM score range between the 2-year and 10-year groups. High score reflects elevated risk and low propensity for diabetes remission. As demonstrated, a majority of patients in both groups had intermediate scores 3-12.
Table 3. DiaREM Score Validation of T2DM Remission at 2- and 10-years.
| DiaREM Score | Mean Probability of Remission (%) | Actual 2-year Remission (%) | 2-year p-value | Actual 10-year Remission (%) | 10-year p-value |
|---|---|---|---|---|---|
| 0-2 | 94 | 100 | 0.61 | 100 | 0.72 |
| 3-7 | 76 | 94 | 0.08 | 83 | 0.57 |
| 8-12 | 36 | 47 | 0.38 | 43 | 0.72 |
| 13-17 | 22 | 20 | 0.92 | 33 | 0.64 |
| 18-22 | 9 | 15 | 0.40 | 14 | 0.64 |
Demonstrates DiaREM probability of diabetes remission vs. actual remission for each score at both 2 and 10 years.
Figure 1. DiaREM Score Validation of T2DM Remission at 2- and 10-years.
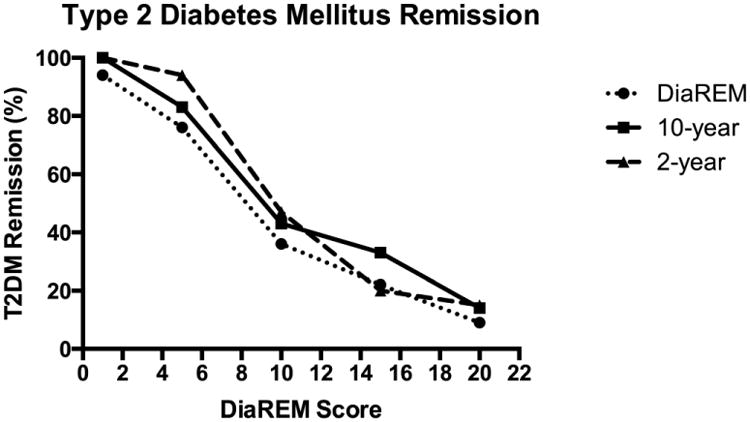
Illustrates the percent reduction of T2DM by DiaREM Score for each cohort including 2-year, 10-year, and the original cohort from Still et al. at 1 year19. There was no statistical difference across score categories among the cohorts.
Discussion
The novel finding from this study is that the DiaREM score provides long-term (10-year) validation predicting T2DM remission following RYGB. This finding is consistent with prior studies reporting short-term validation of DiaREM20. Despite our current understanding of the reasons for RYGB effectiveness on T2DM, reports suggest that nearly half of people may not maintain T2DM remission overtime. As a result, we recognize that RYGB serves to reset the system but it is critical for patients to fundamentally change their lifestyles. In either case, our data suggest that using the DiaREM score to predict improved glycemic control in patients following RYGB is a reasonable way to identify people likely to respond to RYGB for diabetes management9. As seen in Table 3 the percent of patients across the preoperative risk criteria highlights that the majority of patients fell within the 3-7 and 8-12 score range suggesting moderate risk for not meeting T2DM remission as reported for the Still et al. cohort. Ultimately, this prediction tool will allow better risk-stratification and patient selection for RYGB as validated by both our center at 2 and 10 years as well as the Cleveland Clinic group at 2-4 years 20. The standard population undergoing this population has changed over the past 10-15 years as evidenced by the growing majority of patients undergoing RYGB at our institution being females while we are operating on older patients with higher HbA1C (Table 1).
In our population, diabetes remission was typically higher than predicted, although there was no statistical difference in the proportions predicted versus actual. To be congruent with the original model by Still et al.19, we used a combination of “partial remission” and “complete remission” to validate the long-term durability of RYGB on T2DM remission. While this method captures more patients in the remission group compared with using only “complete remission”, it accurately reflects the improvement in glucose control and provides value to the outcome of the operation. As pointed out by several groups, the partial remission group is likely to proceed to complete remission, resulting in the phenomenon of late remission19,20,23. The difference in timing of diabetes remission likely represents gastrointestinal signaling pathways versus weight loss induced remission of T2DM9,24. Indeed, since Pories et al. first described the rapid amelioration of T2DM after RYGB a vast amount of research has gone into elucidating the pathophysiology and identifying potential drug targets25. We did not assess the mechanism by which RYGB contributed to long-term T2DM remission as this is beyond the scope of the current study. However, changes in insulin sensitivity and beta cell function are likely key determinants. In either case, our results suggest that diabetes remission up to 10 years is highly probable, and preoperative factors including HbA1c, age, insulin usage, and antidiabetic oral medications provide a good tool for predicting the resolution of diabetes.
There are limitations of this study that may affect our interpretations. Our sample size is modest and we have incomplete clinical outcomes to understand the mechanism by which RYGB induces and maintains T2DM remission. Next, this study design includes retrospective analysis without the same cohort of people measured serially overtime, thereby limiting our ability to make causal inference on the ability of RYGB to have durable effects on glucose control. However, our database has been prospectively collected for the past 30 years and is regularly updated when patients are seen at follow-up. There have been efforts to maintain yearly follow-up beyond 2 years but due to the large referral base, the geographic distance of many patients from our medical center, the role of primary care physicians in following these patients on a regular basis, and the cost and time of visits for the patient who feel well and in good health, achieving complete follow-up in person has been challenging. Nevertheless, this study represents the first evaluation of the DiaREM model in T2DM patients up to 10 years after bariatric surgery and our results suggest it is a valid approach for predicting those likely to meet diabetes remission, Lastly, telephone follow-up was used to characterize diabetes remission and some clinical related outcomes for the DiaREM score. While telephone screening likely improved the yield at 10 year follow up, it is limited by lack of objectivity and subject bias for willingness to answer questions. However, telephone follow-up has been validated in the bariatric population by Harper et al.26, and our approach provides novel insight to the capability of the DiaREM Score on understanding the durability of RYGB induced diabetes remission.
Conclusion
In conclusion, the major finding in the present study reports that the preoperative DiaREM scores is a good tool for predicting both short- and long-term T2DM remission following RYGB. The current report of 10-year results suggests that many people meet diabetes remission. However, these data point towards a critical shift in focus toward long-term care of patients after RYGB. Subsequently, the DiaREM score appears to be a valid long-term predictor of T2DM remission after RYGB, and represents a potential strategy to identify individuals who are likely to reach T2DM remission. Further work is required to determine optimal medical strategies based on preoperative health for optimizing RYGB-induced diabetes remission in those people at highest risk.
Acknowledgments
Conflicts of Interest and Source of Funding: NIH T32HL007849
Disclosures: Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL007849. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Sturm R. Increases in clinically severe obesity in the United States, 1986-2000. Arch Intern Med. 2003;163(18):2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 3.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 4.Livingston EH. The incidence of bariatric surgery has plateaued in the U.S. Am J Surg. 2010;200(3):378–385. doi: 10.1016/j.amjsurg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci C, Gaeta M, Rausa E, Macchitella Y, Bonavina L. Early impact of bariatric surgery on type II diabetes, hypertension, and hyperlipidemia: a systematic review, meta-analysis and meta-regression on 6,587 patients. Obes Surg. 2014;24(4):522–528. doi: 10.1007/s11695-013-1121-x. [DOI] [PubMed] [Google Scholar]
- 7.Sjostrom L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA. 2014;311(22):2297–2304. doi: 10.1001/jama.2014.5988. [DOI] [PubMed] [Google Scholar]
- 8.Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36(8):2175–2182. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pories WJ, Mehaffey JH, Staton KM. The surgical treatment of type two diabetes mellitus. Surg Clin North Am. 2011;91(4):821–836. viii. doi: 10.1016/j.suc.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Milone M, Di Minno MN, Leongito M, et al. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol. 2013;19(39):6590–6597. doi: 10.3748/wjg.v19.i39.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panunzi S, De Gaetano A, Carnicelli A, Mingrone G. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. Ann Surg. 2015;261(3):459–467. doi: 10.1097/SLA.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 12.Khanna V, Malin SK, Bena J, et al. Adults with long-duration type 2 diabetes have blunted glycemic and beta-cell function improvements after bariatric surgery. Obesity (Silver Spring) 2015;23(3):523–526. doi: 10.1002/oby.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malin SK, Bena J, Abood B, et al. Attenuated improvements in adiponectin and fat loss characterize type 2 diabetes non-remission status after bariatric surgery. Diabetes Obes Metab. 2014;16(12):1230–1238. doi: 10.1111/dom.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatoum IJ, Blackstone R, Hunter TD, et al. Clinical Factors Associated With Remission of Obesity-Related Comorbidities After Bariatric Surgery. JAMA Surg. 2015:1–8. doi: 10.1001/jamasurg.2015.3231. [DOI] [PubMed] [Google Scholar]
- 15.Palmisano S, Silvestri M, Giuricin M, et al. Preoperative Predictive Factors of Successful Weight Loss and Glycaemic Control 1 Year After Gastric Bypass for Morbid Obesity. Obes Surg. 2015 doi: 10.1007/s11695-015-1662-2. [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Kim YJ. Prediction of Diabetes Remission in Morbidly Obese Patients After Roux-en-Y Gastric Bypass. Obes Surg. 2015 doi: 10.1007/s11695-015-1823-3. [DOI] [PubMed] [Google Scholar]
- 17.Lee WJ, Almulaifi A, Chong K, et al. The Effect and Predictive Score of Gastric Bypass and Sleeve Gastrectomy on Type 2 Diabetes Mellitus Patients with BMI < 30 kg/m(2) Obes Surg. 2015;25(10):1772–1778. doi: 10.1007/s11695-015-1603-0. [DOI] [PubMed] [Google Scholar]
- 18.Cotillard A, Poitou C, Duchateau-Nguyen G, et al. Type 2 Diabetes Remission After Gastric Bypass: What Is the Best Prediction Tool for Clinicians? Obes Surg. 2015;25(7):1128–1132. doi: 10.1007/s11695-014-1511-8. [DOI] [PubMed] [Google Scholar]
- 19.Still CD, Wood GC, Benotti P, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014;2(1):38–45. doi: 10.1016/S2213-8587(13)70070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aminian A, Brethauer SA, Kashyap SR, Kirwan JP, Schauer PR. DiaRem score: external validation. Lancet Diabetes Endocrinol. 2014;2(1):12–13. doi: 10.1016/S2213-8587(13)70202-X. [DOI] [PubMed] [Google Scholar]
- 21.Mehaffey JH, LaPar DJ, Clement KC, et al. 10-Year Outcomes After Roux-en-Y Gastric Bypass. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes A. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argyropoulos G. Bariatric surgery: prevalence, predictors, and mechanisms of diabetes remission. Curr Diab Rep. 2015;15(4):15. doi: 10.1007/s11892-015-0590-9. [DOI] [PubMed] [Google Scholar]
- 24.Ponsky TA, Brody F, Pucci E. Alterations in gastrointestinal physiology after Roux-en-Y gastric bypass. J Am Coll Surg. 2005;201(1):125–131. doi: 10.1016/j.jamcollsurg.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Pories WJ, Caro JF, Flickinger EG, Meelheim HD, Swanson MS. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg. 1987;206(3):316–323. doi: 10.1097/00000658-198709000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper J, Madan AK, Ternovits CA, Tichansky DS. What happens to patients who do not follow-up after bariatric surgery? Am Surg. 2007;73(2):181–184. [PubMed] [Google Scholar]


