Abstract
Objectives
Clinically-feasible predictors of opioid analgesic responses for use in precision pain medicine protocols are needed. This study evaluated whether resting plasma beta-endorphin (BE) levels predicted responses to an opioid analgesic, and whether chronic pain status or gender moderated these effects.
Methods
Participants included 73 individuals with chronic low back pain (CLBP) and 88 healthy controls, all using no daily opioid analgesics. Participants attended two identical laboratory sessions during which they received either intravenous morphine (0.08 mg/kg) or saline placebo, with blood samples obtained before drug administration to assay resting plasma BE levels. Once peak drug activity was achieved in each session, participants engaged in an ischemic forearm pain task (ISC) and a heat pain task. Morphine analgesic effects were derived reflecting the difference in pain outcomes between placebo and morphine conditions.
Results
In hierarchical regressions, significant Type (CLBP vs. Control) X BE interactions (p’s<.05) were noted for morphine effects on ISC tolerance, ISC intra-task pain ratings, and thermal VAS unpleasantness ratings. These interactions derived primarily from associations between higher BE levels and smaller morphine effects restricted to the CLBP subgroup. All other BE-related effects, including gender interactions, for predicting morphine analgesia failed to reach statistical significance.
Discussion
BE was a predictor of morphine analgesia for only 3 out of 9 outcomes examined, with these effects moderated by chronic pain status but not gender. On the whole, results do not suggest that resting plasma BE levels are likely to be a clinically useful predictor of opioid analgesic responses.
Keywords: Endogenous Opioid, Beta-Endorphin, Pain, Opioid Analgesic, Precision Medicine, Personalized Medicine, Chronic Pain
Introduction
The concept of precision medicine has been receiving increasing emphasis in healthcare1. Precision medicine refers to treatment strategies that take individual variability into account to optimize outcomes1. Application of this approach to pain management has been suggested2. For example, there would be obvious clinical utility for a set of biomarkers (e.g., genetic variants, phenotypic characteristics, biological assays) known to predict relative risks versus benefits with opioid analgesic therapy for chronic pain management. While a precision pain medicine approach appears feasible in principle, it requires a strong research base to guide selection of predictive biomarkers, and at present this research base is lacking2.
The existing literature suggests several possible predictors of opioid analgesic responses3–13, although many of the available studies have small sample sizes or are clinical studies employing few experimental controls (e.g., no placebo condition). One potential predictor is gender. A meta-analysis suggests that females obtain moderately greater analgesia than do males with comparable doses of morphine, although these findings do not extend to other opioid agents3. A number of other potential predictors have been suggested to date as well, including various genetic polymorphisms (e.g., A118G single nucleotide polymorphism of the mu opioid receptor gene)4,5, negative affect (depression, anxiety)6,7, evoked pain sensitivity8–10, and extent of widespread pain or “fibromyalgia-ness”11,12.
Given that opioid analgesics act by binding to opioid receptors, biomarkers reflecting status within a given individual’s opioid system would appear to be obvious candidate predictors as well. We have been systematically exploring links between individual differences in functioning of the opioid system and responses to opioid analgesics. Using a laboratory-based index of endogenous opioid system function (differences between evoked and clinical pain responses in placebo versus opioid antagonist conditions), we have demonstrated strong and consistent associations between lower endogenous opioid function and greater analgesia with morphine10,13. Although results using this opioid blockade methodology in the laboratory are compelling, these methods are unfortunately not practical for routine use in a clinical setting. Circulating plasma levels of beta-endorphin (BE), an endogenous agonist of the mu opioid receptor, provide a clinically feasible, although unproven, potential indicator of opioid system status that might have relevance to precision medicine. BE, along with a number of other peptides including adrenocorticotrophic hormone, is cleaved from the large precursor molecule proopiomelanocortin14,15,16. BE, in addition to having direct analgesic effects via binding to opioid receptors in pain modulatory brain regions17, is also involved in modulation of stress responses18,19 and the immune system20. BE in plasma originates primarily from the pituitary and immune cells rather than the central nervous system (arcuate nucleus of the hypothalamus) 14,21–23. Plasma BE levels therefore do not necessarily indicate opioid status in the central nervous system, where the primary analgesic effects of BE occur23–25. Nonetheless, a number of studies report associations between plasma BE levels and pain responses, although not always in a consistent direction26–34.
More relevant to the goal of precision pain medicine, a few studies suggest that BE levels might influence, and therefore, predict opioid analgesic responses35–38. However, direct tests of this hypothesis are rare, with only one placebo-controlled and adequately powered study to date37. This latter study did not evaluate the impact of chronic pain status or gender on findings, both of which are variables suspected to influence opioid analgesic responses3,8, and it reported arbitrarily dichotomized analgesic responses (“responder” versus “non-responder”) that grouped together spontaneous back pain intensity and evoked back pain response outcomes in an unspecified fashion. These study limitations reduce the interpretability of its findings.
The current study builds on the work of Rhodin et al.37. The primary aim was to determine in an adequately powered sample using highly controlled experimental evoked pain stimuli whether circulating plasma BE levels at rest predict subsequent responses to an opioid analgesic (morphine). Furthermore, we directly tested for the first time whether gender and chronic pain status moderated the effects of BE on opioid analgesic responses. The secondary aim of this study was to examine whether plasma BE levels predict subsequent evoked pain responses (placebo condition) in one of the largest samples studied to date. Finally, to enhance the chronic pain relevance of the study, we also evaluated whether resting plasma BE levels predicted the acute analgesic effects of morphine on low back pain intensity in the subsample with chronic pain. This study was based on a secondary analysis of the work presented in Bruehl et al.10, but reflects a greatly expanded sample from that which was originally reported.
Materials and Methods
Design
A double-blind, placebo-controlled crossover design with administration of morphine (in randomized, counterbalanced order) was employed to evaluate whether resting BE levels are associated with subsequent analgesic effects of morphine. The study was conducted at two sites (Vanderbilt University Medical Center and Rush University Medical Center), using identical data collection procedures and equipment. The primary study also included a third arm in which the opioid antagonist naloxone was administered (for details, see Bruehl et al.10); however, this arm is not described further because it is not directly related to the current hypotheses.
Participants
Participants included 73 individuals with chronic low back pain (CLBP) and 88 healthy controls (Healthy). All participants were recruited through an on-line e-mail recruitment system, local pain clinics, advertisements in local print media, or posted flyers. Inclusion criteria were: age between 18–55; no self-reported history of cardiovascular disease, hypertension, liver or kidney disorders, posttraumatic stress disorder, bipolar disorder, psychotic disorder, diabetes, seizure disorder, or alcohol or drug dependence; no use of anti-hypertensive medications; and no daily use of opioid analgesics. Urine screens for opiates were carried out prior to each laboratory session to confirm the absence of recent use. Additional inclusion criteria for the CLBP group were chronic daily low back pain of at least 3 months’ duration with an average past month severity of at least 3 on a 0–10 verbal numeric pain intensity scale. Individuals with chronic pain related to malignancy, autoimmune disorders, or fibromyalgia were excluded. Potential participants who were pregnant (determined by urine pregnancy screens) were also excluded. No participants in the Healthy group were taking as-needed opioid analgesics, and only 1 (1.1%) reported taking antidepressants. In the CLBP group, 11 participants (15.1%) reported occasional use of opioid analgesics (but none in the preceding 3 days or more), and 3 (4.1%) reported use of antidepressant medications. Back pain was associated with a radicular pattern suggestive of a possible neuropathic component in 58.9% of participants in the CLBP group.
The total sample size available (n=161) had 80% power to detect an association as small as r = 0.22 using a two-tailed p<.05 criterion for significance, and comparable power to detect associations within study subgroups as small as r = 0.32 (CLBP), r = 0.30 (Controls), r = 0.30 (females), and r = 0.32 (males). BE would likely need to exhibit predictive effects of at least a moderate effect size (r = 0.30 – 0.49)39 to prove clinically useful. This study therefore had adequate power to detect clinically meaningful predictive effects of BE even within subgroups for primary outcomes (evoked pain morphine effects). The study was not specifically powered to detect BE associations with the secondary outcomes (placebo pain responses, morphine effects on low back pain).
Study Drug
The opioid analgesic used in this study was morphine sulfate, the prototypic mu opioid receptor agonist, at a dosage of 0.08 mg/kg in 20ml normal saline40. This dosage (approximately 7mg for an average sized male) was selected because it was judged to be sufficient to produce analgesia, but low enough to avoid ceiling effects that might obscure key individual differences in morphine responding. Peak morphine activity is achieved within approximately 15 min41.
Laboratory Evoked Pain Tasks
After peak drug activity was reached, participants engaged in two laboratory evoked pain tasks. First, participants underwent an ischemic pain task based on procedures described by Maurset et al.42. To begin, participants engaged in two minutes of dominant forearm muscle exercise using a hand dynamometer at 50% of his or her maximal grip strength as determined prior to beginning the laboratory procedures. Then they were asked to raise their dominant forearm over their head for 15 sec. A manual blood pressure cuff was then inflated over their dominant biceps to 200 mmHg, the arm was lowered, and the cuff remained inflated until tolerance was reached, up to a maximum of 8 min. Participants were instructed to indicate when they first experienced this task as “painful” (ischemic pain threshold, in sec from task onset). Time elapsed between ischemic task onset and participants’ expressed desire to terminate the task (set at a maximum of 8 min) was used to define ischemic pain tolerance.
The second laboratory evoked pain stimulus was a heat pain task administered using a Medoc TSAII NeuroSensory Analyzer (Medoc US., Minneapolis, MN). Heat pain threshold and tolerance were assessed using an ascending method of limits protocol as in several previous studies40,43,44. Four trials (with 30 sec intervals between each) were conducted for heat pain threshold and tolerance, with each trial conducted sequentially at one of four different non-overlapping sites on the non-dominant ventral forearm. For pain threshold trials, the probe started at an adaptation temperature of 32°C, with the temperature increasing at a ramp rate of 0.5°C/sec until the participant indicated that the stimulus had begun to feel “painful” by depressing a button on a computer mouse. For each tolerance trial, the probe started at an adaptation temperature of 40°C, with the temperature increasing at a ramp rate of 0.5°C/sec until the participant indicated maximum tolerance had been reached. Means of the four thermal pain threshold and tolerance trials were separately derived for use in analyses. To ensure participant safety, a pre-determined maximum temperature was set at 51°C. Prior to beginning the first laboratory session, all participants underwent standardized training to familiarize them with the thermal stimulation device and the concepts of pain threshold and tolerance.
Laboratory Evoked Pain Outcomes
In addition to pain threshold and tolerance measures for both pain tasks, participants were asked to describe the level of evoked pain experienced during each laboratory pain task using 100mm visual analog scales (VAS) of overall pain intensity (anchored with “No Pain” and “Worst Possible Pain”) and pain unpleasantness (anchored with “Not at All Unpleasant” and “The Most Unpleasant Possible”). For the longer duration ischemic pain task only, participants were also asked to rate their current pain using a 0–100 verbal numeric rating scale (NRS; anchored with 0 = “no pain” and 100 = “worst possible pain”) at 30 sec intervals throughout the task, with the mean value representing the overall NRS intra-task pain intensity.
Beta-Endorphin Assays
Blood samples (in purple-top Vacutainer tubes with EDTA) collected prior to drug administration in each of the study sessions were immediately stored on ice. Within 30 min of collection, samples were processed in a cool centrifuge (0–4°C) at 3000rpm for 15 min. Plasma was then extracted and stored at −70°C until assays were conducted. Samples from the Rush study site were shipped (frozen) to Vanderbilt, and all assays were conducted by a single Vanderbilt lab. Plasma BE levels were determined using commercially-available enzyme immunoassay kits following standard published procedures (Phoenix Pharmaceuticals, Burlingame, CA). The detection limit was 0.1 ng/ml, with 0% crossreactivity with met-enkephalin, alpha-MSH, or ACTH. All BE assays were run in duplicate. Means across duplicate assays for each study session were derived, and these values were used to calculate mean resting plasma BE levels (in ng/mL) across laboratory sessions for use in analyses. Resting plasma BE levels were stable across sessions (intraclass correlation = 0.91). Observed mean resting BE values (Table 1) were quite similar to those observed in our prior work using similar selection criteria and assays26,27.
Table 1.
Participant characteristics by participant type.
| Measure | Healthy Controls (n=88) | CLBP (n=73) |
|---|---|---|
| Gender (% female) | 48.9 | 57.5 |
| Race: | ||
| Caucasian | 63.6 | 60.3 |
| African-American | 28.4 | 31.5 |
| Ethnicity: | ||
| Non-Hispanic | 96.6 | 94.4 |
| Age (years)* | 32.1±8.56 | 36.4±11.84 |
| Resting Plasma BE Levels (ng/mL) | 1.3±0.55 | 1.4±0.59 |
| VAS Chronic Pain Intensity (0–100) | 54.9±20.41 | |
| Pain Duration (median, in months) | 82.5 | |
p<.05
Note: Summary statistics are presented as percentages or means (±SD). CLBP = Chronic Low Back Pain. VAS Chronic Pain Intensity was a retrospective visual analog scale measure of overall past month chronic pain intensity.
Procedure
All procedures were conducted at the Vanderbilt General Clinical Research Center or a dedicated research room at the Rush University Pain Center. All procedures were approved by the respective Institutional Review Board. After providing informed consent, participants completed a packet of questionnaires, including information regarding demographics and chronic pain. Individuals then participated in two separate but identical experimental sessions (placebo vs. morphine) scheduled at the same time of day to control for variance due to circadian rhythms.
Participants remained seated upright in a comfortable chair throughout all laboratory procedures. During each session, participants initially completed a 10-min seated rest period, after which an indwelling venous cannula was inserted into the dominant arm by a trained research nurse under physician supervision. After a 30-min rest period to allow BE levels to stabilize, a 2mL blood sample was obtained through the cannula for assessment of pre-drug resting BE levels. Next, participants used the VAS Intensity and VAS Unpleasantness measures described above to rate their current level of low back pain, and then received (via the cannula) the assigned study drug. The counterbalanced order of the study drugs was determined based on a list of randomized drug orders prepared (using the Proc Plan procedure in SAS version 9.2, SAS Institute, Cary, NC) at study initiation by an individual having no contact with study participants. The appropriate study drug was provided in blinded fashion to study staff for use in each session by the investigational pharmacy at each institution. The study participants, research nurses, and research assistants were not aware of which drug was being given during each session.
After a 15-min rest period to allow peak drug activity to be achieved, subjects again described their current level of low back pain using the VAS Intensity and VAS Unpleasantness measures. Participants next engaged in the ischemic task using procedures described above, after which the VAS Intensity and VAS Unpleasantness measures were immediately completed to describe responses to this evoked pain stimulus. Then, participants engaged in the thermal pain task to assess heat pain threshold and tolerance, with the VAS Intensity and VAS Unpleasantness measures again immediately completed to describe the pain experienced during the heat pain tolerance trials. Following the final pain task, participants completed the Opioid Adjective Rating Scale45 to describe extent of drug-related side effects being experienced, using a 0 (“Not at All”) to 4 (“Extremely”) Scale. All participants remained in the lab under observation for 2 hours after peak drug activity had been achieved to allow drug effects to remit, after which they were released to a responsible adult.
Statistical Analysis
All analyses were conducted using IBM SPSS for Windows Version 23 (SPSS Inc., Chicago, IL). Analyses of participant characteristics across participant types (Healthy versus CLBP) used independent samples t-tests (for continuous measures) and Chi Square tests (for categorical measures).
Primary analyses used hierarchical regressions to evaluate the impact of resting plasma BE levels on subsequent analgesic responses to morphine. Primary analyses included main effects of BE, gender, and chronic pain status (i.e., participant type), as well as all 2- and 3-way multiplicative interactions of these variables. Significant main effects were only interpreted in the absence of significant interaction effects for relevant variables in interaction models. The source of significant interactions was pursued via simple effects analyses.
All BE analyses entered study site (because only the Rush study site samples were shipped) and assay batch (to address inter-plate variability) into the first step of the regression to address potential influence of these variables on BE levels. Preliminary analyses controlling for these two variables were also conducted to evaluate several other potential confounds that might influence BE assay results. These included age, chronic pain duration (for the CLBP group), body mass index, and as needed use of opioid analgesics. Partial correlation analyses indicated no association between resting BE levels and any of the first three potential confounds (r’s = 0.00 to 0.07, all p’s>.57), although a potential influence of as needed use of opioid analgesics on BE levels was suggested in an analysis of covariance [F(1,157) = 3.03, p=.084]. This variable was therefore also entered into the first step of each regression as a control variable.
The primary outcome variables in this study were indexes of morphine analgesic responses derived for each evoked pain outcome as the difference between morphine and placebo condition values, calculated such that higher scores indicated greater morphine analgesia. In total, there were 5 evoked pain outcomes for the ischemic task (pain threshold, pain tolerance, VAS Intensity and Unpleasantness, and intra-task NRS intensity ratings) and four pain outcomes for the thermal pain task (pain threshold, pain tolerance, and VAS Intensity and Unpleasantness). Placebo condition and morphine analgesic response measures were analyzed for each of these measures. For secondary outcomes reflecting acute morphine analgesic effects on low back pain intensity (in the CLBP group only), pre- to post-drug changes in back pain intensity within each drug condition were first derived for VAS Intensity and VAS Unpleasantness measures. Next, in a manner similar to that used for evoked pain outcomes above, morphine effect measures were derived such that larger positive scores indicated greater acute reductions in low back pain with morphine relative to placebo. All analyses used the maximum number of available cases and a two-tailed probability value of p<.05 as the criterion for significance.
Results
Participant Characteristics and Drug Side Effects
Characteristics of both study subgroups are summarized in Table 1. The two subgroups did not differ significantly in gender, race, ethnicity, or resting plasma BE levels (p’s > .10). Although CLBP participants were significantly older [t(158) = 2.62, p=.01], the difference in age between groups was not large in magnitude. Across the two study subgroups, in absolute terms, the mean levels of all side effects were low in both drug conditions. Relative to the placebo condition, participants when receiving morphine reported significantly greater (on a 0–4 scale) levels of flushing [Morphine: 0.5 (0.71), Placebo: 0.1 (0.33); t (158) = 7.27, p<0.001], itching [Morphine: 0.2 (0.58), Placebo: 0.1 (0.30); t (158) = 3.86, p <0.001], “turning stomach” [Morphine: 0.5 (0.72), Placebo: 0.1 (0.28); t (158) = 6.65, p <0.001], “dry mouth” [Morphine: 0.6 (0.92), Placebo: 0.1 (0.42); t (158) = 6.08, p <0.001], and “nodding” [Morphine: 0.3 (0.68), Placebo: 0.1 (0.45); t (158) = 2.61, p =0.01]. Differences between drug conditions in sweating [Morphine: 0.1 (0.42), Placebo: 0.1 (0.32); t (158) = 1.82, p =0.07], headache [Morphine: 0.2 (0.55), Placebo: 0.2 (0.55); t (158) = 1.29, p =0.20], and vomiting [Morphine: 0.0 (0.19), Placebo: 0.0 (0.16); t (158) = 0.63, p =0.53] were not significantly different. Despite the low overall levels of side effects, participants were significantly accurate in their guess as to the session in which they received morphine (p<.001). However, magnitude of morphine analgesic effects did not differ significantly for any of the outcomes between participants who did versus did not accurately guess the session in which they received morphine (all p’s>.10).
Do Resting Beta-Endorphin Levels Predict Morphine Analgesic Responses?
The primary aim of this study was to determine whether resting plasma BE levels predicted subsequent placebo-controlled analgesic responses to morphine administration. Mean (±SD) evoked pain responses across the placebo and morphine conditions that were used to derive morphine analgesic effects (primary outcomes) are summarized in Table 2. Comparisons of placebo to morphine condition responses indicated that morphine exerted significant analgesic effects for only 2 of the 9 evoked pain outcomes among healthy control participants, but did so for 7 of the 9 outcomes in the CLBP group. Partial correlations (controlling for the BE confounds described above) between plasma BE levels and derived morphine analgesic effect outcomes across participant types and genders are summarized in Table 3. Results revealed no significant associations in the overall sample.
Table 2.
Mean (±S.D.) evoked pain responses by drug condition.
| Participant Type | ||||
|---|---|---|---|---|
|
| ||||
| Healthy Controls (n=88) | CLBP (n=73) | |||
|
| ||||
| Evoked Pain Measure | Placebo | Morphine | Placebo | Morphine |
| ISC Threshold (sec) | 108.6±142.57 | 114.1±143.26 | 80.4±133.77 | 95.2±133.60 |
| ISC Tolerance (sec) | 364.4±158.03 | 382.7±140.97 | 330.3±165.21* | 368.9±136.59 |
| ISC NRS (0–100) | 34.9±24.49 | 32.6±24.83 | 53.2±27.13*** | 46.1±27.41 |
| ISC VAS Intensity (0–100) | 34.4±22.66 | 33.4±22.57 | 53.3±23.77** | 44.8±27.63 |
| ISC VAS Unpleasantness (0–100) | 40.6±24.93* | 35.8±23.96 | 59.7±24.5** | 50.2±28.92 |
| Thermal Threshold (°C) | 44.4±2.92 | 44.7±3.02 | 43.6±3.58*** | 44.6±3.22 |
| Thermal Tolerance (°C) | 48.5±1.39* | 48.7±1.58 | 47.6±1.67** | 47.9±1.68 |
| Thermal VAS Intensity (0–100) | 47.5±25.11 | 45.0±25.88 | 59.8±23.37* | 54.2±27.99 |
| Thermal VAS Unpleasantness (0–100) | 47.7±25.41 | 43.7±26.81 | 59.5±24.56 | 56.9±28.54 |
Note:
p<.05,
p<.01,
p<.001 for comparisons between Placebo and Morphine conditions within each participant type.
CLBP = Chronic Low Back Pain; ISC = Ischemic Task; NRS = Numeric Rating Scale; VAS = Visual Analog Scale.
Table 3.
Partial correlations between resting plasma BE levels (in ng/mL) and evoked pain outcomes across participant types and genders.
| Evoked Pain Outcome | ||
|---|---|---|
|
| ||
| Measure | Placebo Condition | Morphine Analgesic Effect |
| ISC Threshold (sec) | −0.05 | −0.06 |
| ISC Tolerance (sec) | −0.03 | −0.13 |
| ISC NRS (0–100) | 0.11 | 0.07 |
| ISC VAS Intensity (0–100) | 0.06 | 0.09 |
| ISC VAS Unpleasantness (0–100) | 0.07 | 0.12 |
| Thermal Threshold (°C) | −0.11 | 0.07 |
| Thermal Tolerance (°C) | −0.06 | −0.04 |
| Thermal VAS Intensity (0–100) | 0.05 | −0.02 |
| Thermal VAS Unpleasantness (0–100) | −0.05 | −0.05 |
Note: Partial correlations are controlling for BE confounds (as needed opioid analgesic use, assay batch, and study site). CLBP = Chronic Low Back Pain; ISC = Ischemic Task; NRS = Numeric Rating Scale; VAS = Visual Analog Scale.
For ischemic task outcomes, regression analyses indicated a significant Type X BE interaction for morphine analgesic effects on ischemic tolerance [t(151) = −2.05, p =.042]. This interaction was due to a significant inverse association in the CLBP group [beta = −0.28, t(67) = −2.18, p=.033], that was absent in controls [beta = 0.03, t(82) = 0.25, p=.807]. Analyses similarly revealed a significant Type X BE interaction for morphine analgesic effects on ischemic NRS intensity [t(151) = −2.01, p =.047]. In this case, the interaction emerged due to a positive association approaching significance in controls [beta = 0.24, t(81) = 1.78, p=.078] but a nonsignificant inverse association in the CLBP group [beta = −0.11, t(67) = −0.82, p=.418]. All main and interaction effects involving BE for the remaining ischemic task morphine effect outcomes were nonsignificant (p’s>.10).
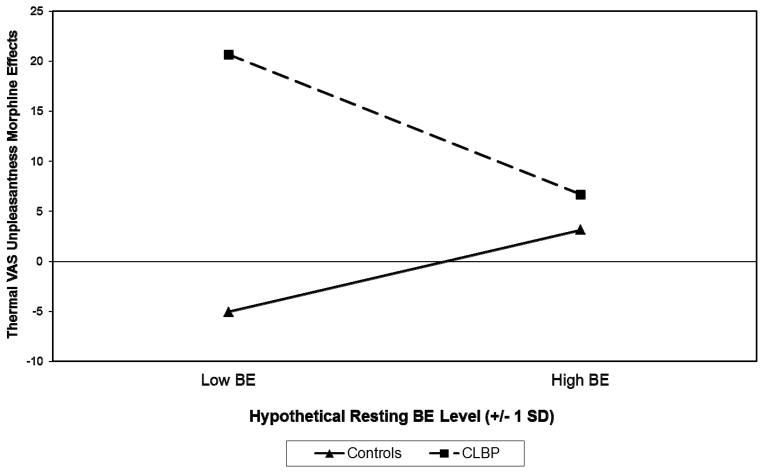
For the thermal task, analyses of thermal pain thresholds revealed only a main effect for Type on morphine analgesic effects [beta = 0.19, t(152) = 2.19, p=.03], indicating greater morphine analgesia in the CLBP group. However, a significant Type X BE interaction was noted for thermal VAS unpleasantness morphine analgesic effects [t(150) = −2.52, p =.013]. In a pattern similar to that observed for morphine effects on ischemic tolerance, this interaction was due to a nonsignificant positive association between BE and morphine effects in controls [beta = 0.18, t(81) = 1.31, p=.193], but a significant inverse association in the CLBP group [beta = −0.27, t(67) = −2.07, p=.042]. To illustrate this latter interaction graphically as recommended by Aiken and West46, the regression equations computed for Healthy Controls and CLBP participants were solved for hypothetical resting plasma BE values (−1 SD and + 1 SD from the observed mean BE value). These values are displayed in Figure 1, which indicates that the greatest morphine analgesia occurred in CLBP participants with low resting BE levels. Also for thermal VAS unpleasantness morphine effects, a trend approaching significance for a Gender X BE interaction was observed [t(150) = −1.77, p =.079]. This interaction was due to no association in females [beta = −0.01, t(79) = −0.09, p=.930] and a nonsignificant inverse association in males [beta = −0.22, t(68) = −1.65, p=.104].
Figure 1.
Influence of resting plasma beta-endorphin (BE) levels on morphine analgesic effects for thermal task VAS unpleasantness outcomes by participant type. BE values plotted are hypothetical values representing one standard deviation (SD) below and above the observed sample mean. Larger positive morphine effects indicate greater analgesia with morphine administration. Chronic low back pain (CLBP) participants with low BE levels reported the greatest analgesia with morphine.
Finally, for morphine analgesic effects on thermal VAS intensity, a trend approaching significance was noted for the Gender X Type X BE interaction [t(149) = −1.85, p =.066]. Dissection of this interaction via simple effects analyses indicted a significant Type X BE interaction in males [t(67) = −2.35, p=.022], but no significant Type X BE interaction in females [t(78) = −0.35, p=.725]. Further exploration of the significant two-way interaction in males indicated that it arose from a nonsignificant inverse association in male controls [beta = −0.09, t(39) = −0.52, p=.607] but a much larger and significant inverse association in male CLBP participants [beta = −0.45, t(26) = −2.49, p=.019]. This is similar in direction to the effects observed in CLBP participants across genders for ischemic morphine effect outcomes. All other main and interaction effects involving BE for morphine analgesic effect outcomes on the thermal pain task were nonsignificant (p’s>.10). Conservative adjustment for multiple comparison using the Bonferroni correction (for 9 evoked pain outcomes) would have resulted in all of the BE-related effects reported above being nonsignificant.
Secondary analyses of acute morphine analgesic effects on low back pain outcomes were also conducted. Pre-post drug changes in VAS Intensity ratings of back pain in the morphine condition [Pre-drug: 35.0 (28.33), Post-Drug: 19.1 (23.47)] were significantly larger than those observed in the placebo condition [Pre-drug: 30.4 (25.81), Post-Drug: 24.9 (25.82); t(69) = 4.23, p<.001]. Findings were similar for pre-post drug changes in VAS Unpleasantness ratings of back pain, again with significantly larger changes in the morphine condition [Pre-drug: 37.3 (28.29), Post-Drug: 18.8 (22.01)] than in the placebo condition [Pre-drug: 30.9 (25.12), Post-Drug: 25.8 (25.71); t(69) = 4.67, p<.001]. The magnitude of low back pain morphine effects as derived above (i.e., the difference between morphine and placebo condition changes) was correlated positively and significantly with comparable evoked pain morphine effect outcomes for both VAS Intensity (r’s = 0.37 – 0.39, p’s <.001) and VAS Unpleasantness (r’s = 0.32 – 0.34, p’s <.007). However, regression analyses for morphine effects on low back pain paralleling those in the primary analyses above did not reveal any significant main effects of BE (p’s > .10) or any Gender X BE interactions (p’s > .10).
Do Resting Beta-Endorphin Levels Predict Placebo Condition Evoked Pain Responses?
The secondary aim of this study was to examine whether resting plasma BE levels predict subsequent responses to controlled evoked pain stimuli in the absence of any drug manipulation. Partial correlations between resting BE levels (in ng/mL) and placebo condition evoked pain outcomes across participant types and genders are summarized in Table 3. These results indicated that there were no significant associations between resting plasma BE levels and placebo condition evoked pain responses in the overall sample.
For the placebo condition ischemic task outcomes, regression analyses indicated significant main effects of Type on NRS ratings [beta = 0.29, t(154) = 3.64, p=.000], VAS intensity [beta = 0.31, t(154) = 4.05, p=.000], and VAS unpleasantness [beta = 0.31, t(154) = 3.97, p=.000]. All three indicated greater evoked pain responsiveness in the CLBP group. No other main effects or interactions achieved statistical significance for the ischemic task data, although nonsignificant trends were noted for Gender X BE interactions on VAS intensity [t(152) = −1.90, p =.059] and VAS unpleasantness [t(152) = −1.79, p =.075]. Both interactions were due to a positive association in females that approached significance [VAS Unpleasantness: beta = 0.20, t(86) = 1.81, p=.07; VAS Intensity: beta = 0.17, t(86) = 1.61, p=.11] and a nonsignificant negative association in males [VAS Unpleasantness: beta = −0.09, t(73) = −0.72, p=.48; VAS Intensity: beta = −0.08, t(73) = −0.62, p=.54]. In other words, higher resting BE levels were associated with modestly higher ischemic pain task responses, but only in females.
For thermal task placebo condition responses, significant two-way BE interactions were noted. For VAS intensity, significant Type X BE [t(152) = −2.08, p =.039] and Gender X BE interactions [t(152) = −2.63, p =.010] were observed, although both appeared to derive from trivial effects. Simple effects analyses indicated that the former interaction was comprised of a nonsignificant positive association between BE and placebo VAS intensity ratings in healthy controls [beta = 0.10, t(82) = 0.74, p=.46] and no association in the CLBP group [beta = −0.01, t(67) = −0.09, p=.93]. The latter interaction was due to a nonsignificant positive association in females [beta = 0.21, t(79) = 1.66, p=.10] and a nonsignificant negative association in males [beta = −0.18, t(69) = −1.41, p=.16]. For VAS Unpleasantness, similar significant Type X BE [t(152) = −2.40, p =.018] and Gender X BE interactions [t(152) = −2.68, p =.008] were noted. Simple effects analyses revealed that the former interaction was comprised of a nonsignificant positive association between BE and placebo VAS unpleasantness ratings in healthy controls [beta = 0.09, t(89) = 0.69, p=.49] and a slightly larger nonsignificant positive association in the CLBP group [beta = 0.14, t(71) = 1.12, p=.268]. The latter gender interaction was due to a small nonsignificant positive association in females [beta = 0.10, t(86) = 0.80, p=.425] and a trend approaching significance for a negative association between BE and thermal unpleasantness in males [beta = −0.23, t(73) = −1.90, p=.062]. For thermal tolerance, the Gender X BE interaction also approached significance [t(152) = 1.73, p =.086]. This effect was comprised of a nonsignificant negative association in females [beta = −0.14, t(86) = −1.14, p=.256] and an opposing nonsignificant positive association between BE and thermal tolerance in males [beta = 0.20, t(73) = 1.57, p=.121]. Beyond the interactions involving BE described above, analyses of placebo condition thermal pain responses revealed significant main effects for Type [beta = −0.23, t(154) = −3.05, p=.003] and Gender [beta = 0.36, t(154) = 4.97, p=.000] on thermal pain tolerance, indicating greater pain responsiveness among CLBP participants and females, respectively. All other main and interaction effects on placebo condition evoked pain outcomes for both pain tasks were nonsignificant (p’s>.10). With the exception of main effects for Type and Gender on placebo condition evoked pain responses, conservative adjustment for multiple comparison using the Bonferroni correction would have resulted in all other effects reported above being nonsignificant.
Discussion
Optimizing opioid therapy outcomes in the chronic pain setting using a precision pain medicine approach requires a strong research base that identifies reliable predictors of opioid analgesic responses. This study capitalized on a relatively large dataset available that assessed individual differences in responses to placebo-controlled morphine administration. It sought to evaluate the utility of an easily accessible potential biomarker for opioid system status, plasma BE, as a predictor of subsequent analgesic responses to morphine. The possible moderating effects of both gender and chronic pain status on these predictive effects were systematically evaluated. These moderation hypotheses were based on known gender effects on opioid analgesic responses3, possible gender differences in opioid analgesic systems19,47–50, and evidence that chronic pain status might influence opioid systems and analgesic outcomes8,51–53.
Results revealed several significant effects of resting plasma BE on subsequent morphine analgesic effects that, when present, were moderated by chronic pain status and, to a limited degree, by gender. Moderation of BE effects by chronic pain status was noted for morphine effect outcomes on both the ischemic task (pain tolerance and NRS ratings) and the thermal task (VAS Unpleasantness). In addition, a trend towards moderation of BE effects on morphine analgesic responses by both gender and chronic pain status was noted for one morphine effect outcome (thermal VAS Intensity). Overall, BE was found to be a statistically significant predictor of morphine analgesia for only 3 out of the 9 evoked pain outcomes examined. To the extent that conclusions can be drawn from these somewhat sporadic effects, it would appear that higher resting BE levels are associated with reduced morphine analgesia, with this effect limited to individuals experiencing chronic pain (particularly males). Although the acute effects of morphine on back pain outcomes (in the CLBP group) were correlated positively and significantly with comparable evoked pain morphine effects, BE did not demonstrate any predictive ability with regards to analgesic effects of morphine on low back pain.
Mechanisms for the observed moderation effects on morphine analgesia on evoked pain responses cannot be conclusively determined. Presence of associations between BE and morphine analgesic responses only in the CLBP group may have related in part to the fact that morphine produced analgesia (relative to placebo) almost exclusively in the CLBP group. The relatively limited analgesic effects of morphine on evoked pain responses in the control group may have provided too little variability for associations with BE to be detected. Regarding potential mechanisms for associations within the CLBP group, non-human studies suggesting that elevated BE in the CNS is associated with downregulation of opioid receptors54 and therefore the diminished morphine responsiveness in chronic pain patients with elevated BE in the current study might have resulted from BE-related receptor downregulation. This interpretation is weakened by the lack of direct correspondence between BE levels in plasma and the central nervous system24,25. Regarding possible gender moderation observed for one morphine effect outcome, presence of opioid-related effects specific to men are consistent with a few prior studies50,55.
There is little high quality existing research to provide context for the current findings. One small study relevant to the current work reported that higher plasma BE levels were linked to lower postoperative opioid analgesic requirements, a finding possibly consistent with elevated opioid analgesic responsiveness among individuals with higher BE levels35. However, outcomes in this study were uncontrolled and the sample included only 9 patients. The only prior work corresponding somewhat with the methods of the current study examined a comparable size sample of chronic back pain patients as the current study (n=80), and also employed placebo-controlled opioid administration37. This study found that patients classified as opioid “responders” had higher levels of BE than those classified as “non-responders,” with the former category reflecting the somewhat arbitrary cutoff of ≥50% opioid-induced pain relief. Findings using the full range of continuous measures were not reported, and possible gender effects were not examined. Reasons for the different findings between this prior work37 and the current study remain to be determined, but might include differences in how morphine analgesia was operationalized and the specific analgesic agent examined (very short acting remifentanil versus much longer acting morphine).
In the present work, predictive effects for resting plasma BE were not consistently observed across the variety of evoked and clinical back pain opioid analgesic responses examined, despite the highly controlled nature of the study. The current findings suggest that resting plasma BE is unlikely to be an effective predictor variable in precision pain medicine protocols. The sporadic links between BE and morphine analgesic outcomes described above stand in contrast to the consistent and strong associations across multiple evoked and clinical back pain outcomes examined between a functional measure of opioid system status (based on responses to opioid blockade) and morphine responses in our prior work10,13. While this latter method is a more consistent and stronger predictor of opioid analgesic responses, it is also impractical for clinical settings. Therefore, more clinically pragmatic predictors that may indirectly reflect opioid system function, such as baseline evoked pain sensitivity10 or possibly negative affect, may prove to be more useful predictors for inclusion in precision pain medicine algorithms.
The pattern of results examining associations between resting plasma BE levels and subsequent placebo condition evoked pain responses suggested some degree of influence by BE that was moderated by gender, although these effects were quite small in magnitude and likely not clinically meaningful. These findings of weak associations between plasma BE and evoked pain responses despite the large sample size and highly controlled methods employed in this study raise questions as to whether the contradictory literature regarding such associations might reflect a high proportion of spurious findings. The weak associations suggested by the current work do not support further exploration of plasma BE as a biomarker of subsequent acute pain responsiveness.
Several study limitations should be highlighted. The current study only examined plasma BE, and results therefore cannot be generalized to BE sampled from within the central nervous system (i.e., in cerebrospinal fluid; CSF). Although theoretical justifications for examining CSF BE levels as a predictor of opioid analgesic responses are stronger than for plasma BE, clinical utility of such findings would be limited due to the clinical difficulties of routinely obtaining CSF samples for BE assays. A second potential limitation is that participants were significantly accurate in identifying which session they received morphine, although this knowledge did not appreciably influence the magnitude of observed morphine effects. Pragmatically, this lack of effective blinding parallels the typical clinical situation in which patients know they are receiving an analgesic, suggesting that under clinical circumstances the current results might be expected to hold true. Another limitation is that only a single opioid analgesic agent was examined. The possibility that the pattern of results might have been different with a different opioid analgesic agent cannot be ruled out. The current work also only applies to prediction of responses to a single opioid analgesic dose. Whether results might differ in using resting plasma BE to predict responses to daily opioid dosing in chronic pain patients is unknown. Related to this latter issue, the recruitment strategy used and the inclusion criterion that subjects not be taking daily opioid analgesics (for safety reasons; see Bruehl et al.10) may have produced a sample quite different from those observed in the typical pain management setting. Although information regarding past month pain intensity and presence of a radicular pattern of pain complaints was obtained, other detailed medical information (e.g., imaging results) that might be used to inform generalization of study results to pain management settings was unavailable.
In summary, results suggest that resting plasma BE has little ability to predict subsequent analgesic responses to morphine. The limited effects observed were restricted to evoked pain outcomes in individuals with chronic pain (particularly males). When present, the direction of association was between elevated plasma BE and reduced opioid analgesic responsiveness. This pattern fits a hypothetical pathway in which elevated endogenous opioid levels result in downregulated opioid receptors that in turn diminish responsiveness to exogenous opioid analgesics, but the limited magnitude and consistency of these effects suggest they are more a scientific curiosity rather than an effect useable to achieve the goal of precision pain medicine. The impact of gender on observed effects was minimal.
Acknowledgments
This research was supported by NIH Grants R01-DA031726 and R01-DA037891, and CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. Contents of this work are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors would like to express their appreciation to the research nurses of the Vanderbilt General Clinical Research Center and the Department of Anesthesiology at Rush University for their assistance in data collection.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruehl S, Apkarian AV, Ballantyne JC, et al. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. J Pain. 2013;14:103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niesters M, Dahan A, Kest B, et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151:61–68. doi: 10.1016/j.pain.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Hayashida M, Nagashima M, Satoh Y, et al. Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype. Pharmacogenomics. 2008;9:1605–1616. doi: 10.2217/14622416.9.11.1605. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda K, Hayashida M, Ide S, et al. Association between OPRM1 gene polymorphisms and fentanyl sensitivity in patients undergoing painful cosmetic surgery. Pain. 2009;147:194–201. doi: 10.1016/j.pain.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Fillingim RB, Hastie BA, Ness TJ, et al. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RR, Haythornthwaite JA, Tella P, et al. Basal heat pain thresholds predict opioid analgesia in patients with postherpetic neuralgia. Anesthesiology. 2006;104:1243–1248. doi: 10.1097/00000542-200606000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg E, Midbari A, Haddad M, et al. Predicting the analgesic effect to oxycodone by ‘static’ and ‘dynamic’ quantitative sensory testing in healthy subjects. Pain. 2010;151:104–109. doi: 10.1016/j.pain.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Bruehl S, Burns JW, Gupta R, et al. Endogenous opioid function mediates the association between laboratory-evoked pain sensitivity and morphine analgesic responses. Pain. 2013;154:1856–1864. doi: 10.1016/j.pain.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiol. 2013;119:1434–1443. doi: 10.1097/ALN.0b013e3182a8eb1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda AM, As-Sanie S, Rajala B, et al. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology. 2015;122:1103–1111. doi: 10.1097/ALN.0000000000000637. [DOI] [PubMed] [Google Scholar]
- 13.Bruehl S, Burns JW, Gupta R, et al. Endogenous opioid inhibition of chronic low-back pain influences degree of back pain relief after morphine administration. Reg Anesth Pain Med. 2014;39:120–125. doi: 10.1097/AAP.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon S. POMC-derived peptides and their biological action. Ann N Y Acad Sci. 1999;885:22–40. doi: 10.1111/j.1749-6632.1999.tb08663.x. [DOI] [PubMed] [Google Scholar]
- 15.Castro MG, Morrison E. Post-translational processing of proopiomelanocortin in the pituitary and in the brain. Crit Rev Neurobiol. 1997;11:35–57. doi: 10.1615/critrevneurobiol.v11.i1.30. [DOI] [PubMed] [Google Scholar]
- 16.Guillemin R, Vargo T, Rossier J, et al. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977;197:1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- 17.Gramsch C, Höllt V, Mehraein P, et al. Regional distribution of methionine-enkephalin- and beta-endorphin-like immunoreactivity in human brain and pituitary. Brain Res. 1979;171:261–270. doi: 10.1016/0006-8993(79)90332-9. [DOI] [PubMed] [Google Scholar]
- 18.McCubbin JA. Stress and endogenous opioids: behavioral and circulatory interactions. Biol Psychol. 1993;352:91–122. doi: 10.1016/0301-0511(93)90008-v. [DOI] [PubMed] [Google Scholar]
- 19.al’Absi M, France C, Harju A, et al. Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosom Med. 2006;68:292–298. doi: 10.1097/01.psy.0000203240.64965.bd. [DOI] [PubMed] [Google Scholar]
- 20.Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332:1685–1690. doi: 10.1056/NEJM199506223322506. [DOI] [PubMed] [Google Scholar]
- 21.Mousa S, Shakibaei M, Sitte N, et al. Subcellular pathways of beta-endorphin synthesis, processing, and release from immunocytes in inflammatory pain. Endocrinology. 2004;145:1331–1341. doi: 10.1210/en.2003-1287. [DOI] [PubMed] [Google Scholar]
- 22.Frederickson RC, Geary LE. Endogenous opioid peptides: review of physiological, pharmacological and clinical aspects. Prog Neurobiol. 1982;19:19–69. doi: 10.1016/0301-0082(82)90020-x. [DOI] [PubMed] [Google Scholar]
- 23.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 24.Bach FW, Langemark M, Secher NH, et al. Plasma and cerebrospinal fluid beta-endorphin in chronic tension-type headache. Pain. 1992;51:163–168. doi: 10.1016/0304-3959(92)90257-C. [DOI] [PubMed] [Google Scholar]
- 25.Baker DG, West SA, Orth DN, et al. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinology. 1997;22:517–529. doi: 10.1016/s0306-4530(97)00053-x. [DOI] [PubMed] [Google Scholar]
- 26.Bruehl S, Chung OY, Burns JW, et al. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2007;130:208–215. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruehl S, Burns JW, Chung OY, et al. What do plasma beta-endorphin levels reveal about endogenous opioid analgesic function? Eur J Pain. 2012;16:370–380. doi: 10.1002/j.1532-2149.2011.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hargreaves KM, Dionne RA, Mueller GP. Plasma beta-endorphin-like immunoreactivity, pain and anxiety following administration of placebo in oral surgery patients. J Dent Res. 1983;62:1170–1173. doi: 10.1177/00220345830620111601. [DOI] [PubMed] [Google Scholar]
- 29.Feldreich A, Ernberg M, Lund B, et al. Increased β-endorphin levels and generalized decreased pain thresholds in patients with limited jaw opening and movement-evoked pain from the temporomandibular joint. J Oral Maxillofac Surg. 2012;70:547–556. doi: 10.1016/j.joms.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Maekawa K, Minakuchi H, et al. Responses of the hypothalamic-pituitary-adrenal axis and pain threshold changes in the orofacial region upon cold pressor stimulation in normal volunteers. Arch Oral Biol. 2007;52:797–802. doi: 10.1016/j.archoralbio.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Matejec R, Ruwoldt R, Bödeker RH, et al. Release of beta-endorphin immunoreactive material under perioperative conditions into blood or cerebrospinal fluid: significance for postoperative pain? Anesth Analg. 2003;96:481–486. doi: 10.1097/00000539-200302000-00034. [DOI] [PubMed] [Google Scholar]
- 32.Misra UK, Kalita J, Tripathi GM, et al. Is β endorphin related to migraine headache and its relief? Cephalalgia. 2013;33:316–322. doi: 10.1177/0333102412473372. [DOI] [PubMed] [Google Scholar]
- 33.Leonard TM, Klem SA, Asher MA, et al. Relationship between pain severity and serum beta-endorphin levels in postoperative patients. Pharmacotherapy. 1993;13:378–381. [PubMed] [Google Scholar]
- 34.Bach FW, Fahrenkrug J, Jensen K, et al. Plasma beta-endorphin during clinical and experimental ischaemic pain. Scand J Clin Lab Invest. 1987;47:751–758. [PubMed] [Google Scholar]
- 35.Cohen MR, Pickar D, Dubois M, et al. Stress-induced plasma beta-endorphin immunoreactivity may predict postoperative morphine usage. Psychiatry Res. 1982;6:7–12. doi: 10.1016/0165-1781(82)90032-4. [DOI] [PubMed] [Google Scholar]
- 36.Wu TT, Wang ZG, Ou WL, et al. Intravenous flurbiprofen axetil enhances analgesic effect of opioids in patients with refractory cancer pain by increasing plasma β-endorphin. Asian Pac J Cancer Prev. 2014;15:10855–10860. doi: 10.7314/apjcp.2014.15.24.10855. [DOI] [PubMed] [Google Scholar]
- 37.Rhodin A, Grönbladh A, Ginya H, et al. Combined analysis of circulating β-endorphin with gene polymorphisms in OPRM1, CACNAD2 and ABCB1 reveals correlation with pain, opioid sensitivity and opioid-related side effects. Mol Brain. 2013;6:8. doi: 10.1186/1756-6606-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dabo F, Nyberg F, Zhou Q, et al. Plasma levels of beta-endorphin during pregnancy and use of labor analgesia. Reprod Sci. 2010;17:742–747. doi: 10.1177/1933719110370059. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum; 1988. pp. 75–108. [Google Scholar]
- 40.Fillingim RB, Ness TJ, Glover TL, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Berkowitz BA, Ngai SH, Hempstead J, et al. Disposition of naloxone: use of a new radioimmunoassay. J Pharmacol Exp Ther. 1975;195:499–504. [PubMed] [Google Scholar]
- 42.Maurset A, Skoglung LA, Hustveit O, et al. A new version of the ischemic tourniquet pain test. Meth Find Exp Clin Pharmacol. 1992;13:643–647. [PubMed] [Google Scholar]
- 43.Bruehl S, Dengler-Crish CM, Smith CA, et al. Hypoalgesia related to elevated resting blood pressure is absent in adolescents and young adults with a history of functional abdominal pain. Pain. 2010;149:57–63. doi: 10.1016/j.pain.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung OY, Bruehl S, Diedrich L, et al. Baroreflex sensitivity associated hypoalgesia in healthy states is altered by chronic pain. Pain. 2008;138:87–97. doi: 10.1016/j.pain.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Zacny JP. A possible link between sensation-seeking status and positive subjective effects of oxycodone in healthy volunteers. Pharmacol Biochem Behav. 2010;95:113–120. doi: 10.1016/j.pbb.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- 47.al’Absi M, Wittmers LE, Ellestad D, et al. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- 48.France CR, al’Absi M, Ring C, et al. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biol Psychol. 2005;70:168–174. doi: 10.1016/j.biopsycho.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 49.Frew AK, Drummond PD. Stress-evoked opioid release inhibits pain in major depressive disorder. Pain. 2008;139:284–292. doi: 10.1016/j.pain.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 50.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 51.Bruehl S, McCubbin JA, Harden RN. Theoretical review: altered pain regulatory systems in chronic pain. Neurosci Biobehav Rev. 1999;23:877–890. doi: 10.1016/s0149-7634(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 52.Bruehl S, Chung OY. Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain. 2006;124:287–294. doi: 10.1016/j.pain.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Bruehl S, Burns JW, Chung OY, et al. Interacting effects of trait anger and acute anger arousal on pain: the role of endogenous opioids. Psychosom Med. 2011;73:612–619. doi: 10.1097/PSY.0b013e318227cb88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petraschka M, Li S, Gilbert TL, et al. The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience. 2007;146:1795–1807. doi: 10.1016/j.neuroscience.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruehl S, Burns JW, Chung OY, et al. Hypoalgesia associated with elevated resting blood pressure: evidence for endogenous opioid involvement. J Behav Med. 2010;33:168–176. doi: 10.1007/s10865-009-9241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]