Abstract
The diagnosis of central airway tumors is usually challenging because of the vague presentations. Advances in visualization technology in bronchoscopy aid early detection of bronchial lesion. Cryotechnology has great impact on endobronchial lesion sampling and provides better diagnostic yield. Airway tumor involvements result in significant alteration in life quality and lead to poor life expectancy. Timely and efficiently use ablation techniques by heat or cold energy provide symptoms relief for central airway obstruction. Prostheses implantation is effective in maintaining airway patency after ablative procedure or external compression. Combined interventional bronchoscopy modalities and other adjunctive therapies have improvement in quality of life and further benefit in survival. This review aims to provide a diagnostic approach to central airway tumors and an overview of currently available techniques of interventional bronchoscopy in managing symptomatic central airway obstruction.
Keywords: Central airway obstruction, bronchoscopy, intervention, ablative technologies, stents
Introduction
Patients with central airway tumors usually have nonspecific clinical manifestation and lead to delayed diagnosis. Computed tomography (CT) scan is the best noninvasive method for airway lesion evaluation (1). Bronchoscopy is still the mainstay to approaching endobronchial lesion. The conventional methods to obtained specimen include forceps biopsy, brushing or washing the lesion under direct vision. However, the diagnostic yield of conventional forceps biopsy is limited because of the small size of tissue sample and crush artifacts (2,3). Newly developed cryotechnology provide larger sample than that of conventional forceps biopsy and have better diagnostic yield (2).
Dyspnea is the major symptoms in patients with central airway obstruction which limit the daily activity of patients. Unfortunately, not only 20% to 30% patients with primary lung cancer experience this suffering symptoms, 2% patients with solid tumors also have endobronchial metastasis which may postpone their anti-cancer therapy because of severe dyspnea (4,5). In recent decades, interventional bronchoscopy has being an effective treatment modality for central airway obstruction (6-8). In patients with limited performance status due to central airway obstruction, the functional status will improved after interventional bronchoscopy and have the chance to receive chemotherapy (9,10). The exercise capacity, lung function and quality of life are improved after therapeutic bronchoscopy (11). Moreover, the survival may also been prolonged after airway stenting in selective situation (12,13).
Here, we provide a clinical review of interventional bronchoscopy in diagnoses and management of central airway tumors.
Diagnostic modalities
Computed tomography
Only less than 30% patients with tracheal tumors have been diagnosed via chest radiography (14). Traditional CT scan is more sensitive and can provide information on the extent of airway lesions. Newly developed multidetector CT (MDCT) can accurately detect airway tumor locations, natures, quantities (1). The extra luminal anatomy is also clearly depicted. Thus, MDCT is a rapid and non-invasive method to provide comprehensive information about the extent of disease process before surgery or interventional bronchoscopy (1).
Visualization of bronchoscopy
Bronchoscopy is the mainstay to approaching endobronchial lesion under direct vision. In addition to conventionally white light bronchoscopy, autofluorescence (AFB) and narrow band imaging (NBI) are new visualization techniques of bronchoscopy which can aid detection of bronchial mucosa lesions (15). Although the specificity of AFB is low and similar to white light bronchoscopy, there is no doubt in usefulness of detecting early bronchial mucosal lesion and evaluate the margin of mucosal involvement (16,17). NBI is designed to detect angiogenesis and neovascular lesion. The diagnostic accuracy is increased under NBI bronchoscopy (15). Both of them have superior sensitivity comparing with white light bronchoscopy [3-5]. Endobronchial ultrasonography (EBUS) is also helpful in evaluating the extent of airway lesion involvement (14,18).
Tissue sampling: forceps versus cryotechnology
Forceps biopsy under direct bronchoscopic vision is the most common method to obtain tissue sample from endobronchial lesion. However, the size of tissue sample is limited by the forceps size. Small samples with crush artifacts are probably insufficient for accurate diagnosis. The diagnostic yield is only 85% of conventional forceps biopsy and patients usually have to repeat bronchoscopy (2,3).
Cryotechnology has been used as a therapy for central airway obstruction and hemoptysis (19). The samples from cryotechnology are larger than conventional forceps biopsy and have better diagnostic yield (95% to 100%) (3,20,21) (Figure 1). Besides, the tissue architecture will not be damaged by cryotechnology and thus facilitate histological analysis (22). Tumor bleeding is a major concern after biopsy. Theoretically, vasoconstriction and capillary microthrombosis after cryotechnology may ameliorate bleeding (20,23). Hetzel et al. (3) reported a greater incidence of mild bleeding after cryobiopsy. However, the rate of severe bleeding is comparable of forceps biopsy and cryobiopsy. In recently published, prospective study, two cryo biopsies was found to be optimal for diagnosis and greater than three bryobiopsies will increase the risk of bleeding (24). Cryotechnology has been a considerable choice for endobronchial lesion biopsy with better diagnostic yield and minimal complication.
Figure 1.
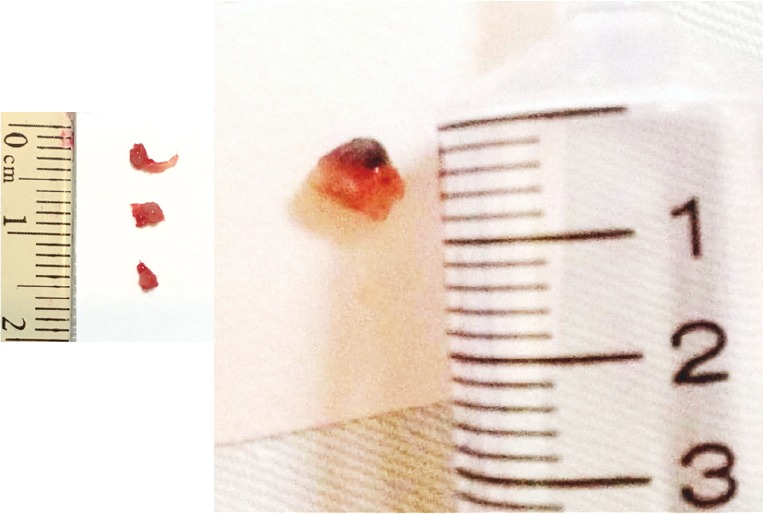
Specimens comparison between traditional forceps biopsy (left) and cryotechnology (right).
Management
There are three types of central airway tumor involvement: endoluminal (tumor within airway), extraluminal (airway narrowing from external compression), and mixed (6) (Figure 2). A variety of techniques in endoluminal tumor debulking has been developed and has been shown to be effective in reliving symptoms (22). In addition to extraluminal and mixed type central tumor involvement, airway stenting is also useful in preventing recurrence and restore the airway patency after intraluminal tumor debulking (25). The choice of techniques depends on the characteristics of stenosis, the condition of patient, available techniques and the operator’s expertise (Table 1).
Figure 2.
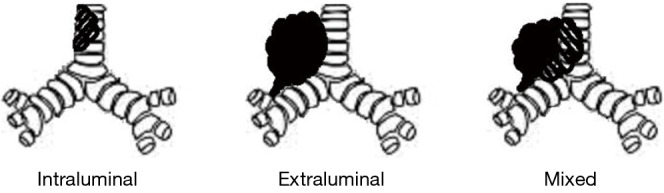
Types of central airway obstruction.
Table 1. Types of central airway obstruction and treatment modalities.
| Intraluminal | Extraluminal | Mixed | |
|---|---|---|---|
| Mechanical debulking | + | − | + |
| Electrocautery | ++ | − | + |
| Argon plasma coagulation | ++ | − | + |
| Laser | ++ | − | ++ |
| Cryotherapy | +++ | − | +++ |
| Stent | +++ | ++ |
–, not recommended; +, weakly recommended; ++, recommended, +++, strongly recommended.
Rigid bronchoscopy and mechanical debulking
Rigid bronchoscopy is a traditional procedure, which have been introduced under general anesthesia (26). Contraindications of rigid bronchoscopy include cervical spine instability, facial trauma, laryngeal obstruction (27). The easy airway security and large working space provide more space for interventional procedure processing. However, in patients with severe airway obstruction and respiratory failure will experience great risk of general anesthesia because of high oxygen demand and illness severity (28).
Recently, Vishwanath et al. (29) reported their 30-cases experience of solely mechanical debulking under rigid bronchoscopy in managing central airway tumors. The successful rate reached 82.6%, however, the complication rate is as high as 32.3% with significant bleeding in 25.8% patients. Although they suggested that mechanical debulking under rigid bronchoscopy was probably quicker than thermal ablative therapy, there was no direct comparison of procedure time between difference methods. Combining mechanical debulking with a complementary thermal ablative procedure may be a safer choice in bleeding control (25).
Electrocautery
Electrocautery used high-frequency electric current to cause heating which lead to coagulation or tissue vaporization (30). It can be delivered via rigid or flexible bronchoscopy. The effects depend on the power setting, surface area, electrical properties of tissue and the contact time (22). In one large retrospective series of electrocautery for airway obstruction, the luminal improvement was achieved in 78% patients with 6.8% complication rate (31). Electrocautery provided equally effective and constant palliation in patients with airway obstruction as that provided by neodymiumdoped:yttrium-aluminum-garnet (Nd:YAG) laser therapy. However, the cost of electrocautery is significant lower than Nd:YAG laser (32). Thus, electrocautery is a more accessible modality worldwide.
The complications include damage of underlying cartilage which may lead to airway stenosis, bleeding, perforation, pneumothorax, malfunction of pacemaker (22). Besides, airway fire is also a major concern in the presence of high oxygen concentration and flammable substance (such as silicone stent or endotracheal tube). Above complications could be ameliorated after restricting the fractional inspired oxygen less than 40% and the limiting the power output do not exceed 30W (30).
Argon plasma coagulation (APC)
APC is a non-contact method of electrocautery and can be introduced via both rigid and flexible bronchoscopy (33). The noncontact feature of APC allows extent and rapid coagulation, but the depth of penetration is more superficial (2–3 mm) and limited by increased tissue resistance after coagulation and desiccation (33,34). The major applications of APC are hemostasis and tissue destruction and particular effective in vascular lesion (35). Besides, APC is an effective therapy for airway obstruction secondary to endobronchial lesion (36). Not only significant endobronchial tumor reduction, clinical dyspnea score and forced expiratory volume in one second (FEV1) are also improved after procedure (37). Because of the superficial effect and superiority in hemostasis, APC is usually used as a part of multi-modality approach for tumor debulking (33).
The complication rate of APC is approximately 2% (30). The most severe fatal complication is intracardiac gas embolism and cerebral embolism (38,39). Above conditions may be minimized by keeping the flow to less than 0.8 L/min and maintaining a safe distance of 2–5 mm (34).
Laser
Laser therapy is a widely used ablative technique for tumor debulking which can be performed using flexible or rigid bronchoscopy with different gases (22). Neodymium: yttrium-aluminum-garnet (Nd-YAG) laser is the most frequent used (34). The most common indication is relieving central airway obstruction. It is particular effective for intraluminal and mixed type airway obstruction and less suitable for extraluminal compression (40). In additional to constant and immediate effect in tumor shrinkage, the quality of life and overall health are also improved significantly in patients with malignant central airway obstruction due to lung cancer (41). Although laser monotherapy is quite effective in alleviating symptoms from malignant central airway obstruction, survival benefit will be improved when it combined with multimodal adjuvant therapy (including brachytherapy, chemotherapy, radiotherapy, stenting, chemoradiotherapy) (42). However, the high cost of laser therapy is a disadvantage of the usefulness worldwide and can be alternated with electrocautery, APC or cryotherapy (32,40).
The complications of laser therapy is around 8%, including hemorrhage, endobronchial fire, pneumothorax, and air embolism (40). The periprocedural death is 1–3% (30). Because of the inability to visualizing penetration depth, perforation of major vessel will lead to fatal hemoptysis. This can be avoided by limiting the power less than 40W (34,40). Endobronchial fire can be prevented by restricting the fractional inspired oxygen less than 40%. Besides, patients who were over 60 years of age, with arterial hypertension or chronic obstructive pulmonary disease were associated with higher complication rate (43). Thus, in additional to the modification in setting, the patient selection is also important to minimizing complications.
Cryotherapy
Cryotherapy uses extremely cold (−70 °C) to tissue and causes tissue necrosis with repeated freezing and thawing cycle (30). It can be introduced with rigid or flexible bronchoscopy. The effect of cryotherapy is usually delayed but prolonged, with capillary microthrombi formation (25). The efficacy depends on the cooling rate, the minimum temperature reached, the cycle of freezing and thaw (22). Unlike thermal ablative therapy (electrocautery, laser, APC), cryotherapy has no risk in airway fire and will not lead to tissue edema after procedure (23). Because of low water content of cartilage, collagen and poorly vascularized tissue, cryotherapy has very small effects on them. As such, the scarring is minimal and the damage of cartilage is few (23). Cryotherapy is mainly indicated for a palliative treatment for intraluminal airway obstruction and not recommended in submucosal lesion or extraluminal compression (34). The overall success rate of significant recanalization exceeds 80% in one systematic review (44). In additional to improvement in dyspnea, cough, and hemoptysis, the lung function, performance status are also improved after cryotherapy (19). When combined with systemic chemotherapy and/or radiation, the survival benefit is significant (9,45). As the effect of cryotherapy is delayed, it is usually not suitable for patients with impending respiratory failure secondary to airway obstruction. Use the principle of cryoadhesion, cryosurgery has been performed with rapid relief of tumor obstruction. However, additional modalities, such as APC, are required to achieve hemostasis (34). Recently, Boujaoude et al. reported one case with acute central airway obstruction has been managed successfully with cryosurgery alone using “freeze and pull” technique. Although the experience is few, however, it provides the opportunity for patients with emergent airway obstruction under high oxygen requirement to liberating from mechanical ventilation (46).
The complication rate is low (0–11%), most of them were minor and easily manageable (44). The risk of perforation and airway fire are non-existent, since the cartilage is cryoresistant. Thus, cryotherapy is safe while the inflammable substance nearby the obstructive lesion (Figure 3).
Figure 3.
Cryotherapy successfully recannulized airway obstruction secondary to silicone stent induced granulation.
Stent
The endobronchial stent is the main choice to alleviate extraluminal airway compression and is also available to relieve intraluminal and mixed type central airway obstruction when combining with other endobronchial therapies (25). Airway stent is usually a palliative treatment for malignancy. It provides a bridge for airway patency in patients undergoing systemic chemotherapy or radiation. Although the overall survival was not changed, the median survival time could be increased after airway stenting if it was combined with adjuvant therapy (47).
Theoretically, the ideal stent should be strength enough, not migrate, easy to insert and remove, flexible enough to mimic airway physiology, not impair mucociliary clearance, not induce granulation tissue formation, and cost-effective (26). However, it has not been developed.
Before stent implantation, the size, diameter and length have to be chosen according to CT scans and bronchoscopy.
Silicone stents
Silicone stent has to be deployed by rigid bronchoscopy under general anesthesia (30). The most commercially available silicone stents are Dumon stents, which have two main types: straight and bifurcated stents (26). The size, length and diameter are chosen preoperatively and can be adjust by cutting. Because it is easy to insert and remove, with few granulomatous reactions, silicone stent has been recommended as first line treatment and is particular suitable for benign lesion for the longer lifespan (25).
The major drawbacks of silicone stent include the necessity of general anesthesia, higher migration rate, mucus plugging, thicker wall and lead to narrow internal diameter. Mucolytic nebulization is essential to prevent dense secretion and asphyxiation (25).
Self-expandable metallic stents (SEMS)
SEMS is mainly used to treat airway obstruction or tracheoesophageal (TE) fistula due to malignancy (Figure 4). Metallic stent is also an alternative choice in benign disease if the patients are poor surgical candidates or the airway is highly tortuous (30,48). SEMS can be introduced via rigid and flexible bronchoscopy. There are partial covered, fully covered and uncovered stent. Covered stent is preferred in malignant lesions and particular suitable for covering TE fistula (49). Uncovered stent is mainly used in highly distorted stenosis. Unlike silicone stent, SEMS provide thinner wall construction, greater airway diameter, better conformation with irregular airway, easier placement and less migration rate. The size of stent should be chosen accurately before procedure. The length of stent has to provide 0.5–1.0 cm overlap at each end of the stenosis. The diameter of the stent should be 1–2 mm greater than the estimated diameter of the airway (30). Fluoroscopy is usually necessary for SEMS placement. However, it is not available in many intensive care units (ICU) and requires special facilities. Lin et al. (28) reported 26 mechanically ventilated patients have been successful inserted metallic stent under flexible bronchoscopy without fluoroscopy guidance. More than half patients have liberated from ventilator successfully.
Figure 4.
Self-expandable metallic stents (SEMS) implanted successfully for covering tracheoesophageal (TE) fistula in patient who was under mechanical ventilation without fluoroscopy guidance.
Metallic stent is effective in reliving symptoms of airway obstruction, facilitating to liberate from ventilator, improving pulmonary function and performance status (50-52). Compared with poor performance status, patients with malignant central airway obstruction under intermediate performance status have survival benefit after airway stenting (12). This suggests that timely airway stenting before morbid complication is quite important.
The complications of SEMS include stent malposition, migration, fracture, mucus impaction, halitosis, bacterial colonization and granulomatous tissue formation. In uncovered and partial covered stent, the re-epithelialization and excessive granulation formation in the edges are the most frequent complications, which cause the difficulty in stent removal (48,53). Benign structure airway obstruction prior to stent implantation is independent factor for granulation tissue formation (54). SMES fracture is of major concern because of the possibility of complete airway obstruction and perforation (55). Pretreatment tortuous airway in 3-dimensional CT scan predicts metallic stent fracture. SEMS implantation in such situation should be cautiously evaluated (55,56). Besides, the complication rate in benign conditions is twice higher than malignant disease (42.2% vs. 21.1%) owing to the longer follow-up period and longer lifespan (51). In one largest series of lung transplant recipients, the 5-year survival was significant lower in patients receiving metallic stent insertion. Re-stenosis and airway bacterial colonization are the major problems (52). Because of severe complication after long term SEMS implantation, the US Food and Drug Administration (FDA) have warned that metallic stent is not advised to treat benign disease.
Developing stents
Concerning the granulation tissue formation after airway stenting, drug eluting stent with mitomycin C have been observed to reducing granulomatous reaction and less mucus trapping. In animal studies, cisplatin eluting stent have steadily and sustained released drug up to 4 weeks and helpful to against malignant cells (57).
In benign or reversible cases, the airway stent is usually needed temporary. There are 70% patients with post-intubation or post-tracheostomy tracheal stenosis had been successfully treated by silicone stent implantation after 18 months (58). Thus, the removability of stent is quite important. Biodegradable stent has been designed to maintain airway patency for a predetermined duration. It is a temporary support device until the nature tissue regains its strength and can be removed by cellular activity in a biological environment. There are four human studies until now. Only one adult study showed relative good outcome up to 4 years, the outcomes of the other 3 pediatric studies were not satisfactory and associated with repeat stenting after stent absorption. Besides, the cost of biodegradable stent is much higher than others. The safety and toxicity are also the major concerns (59).
Symptoms from central airway tumors result in poor quality of life and disaster outcome. The survival would not exceed 1–2 months if the patient untreated. However, in such situation, patients’ performance status, pulmonary function and comorbidities are usually limited the possibility of surgery (25). Interventional bronchoscopy provides a less invasive choice with high technical success rate (90–98%) and is effective for central airway obstruction to improve quality of life and survival (10,11,13). The complication is relative rare (3.9%) with few mortality (0.5%) (60). Nevertheless, the benefit is difficult to predict in patients under different situation. Guibert et al. (61) conducted a retrospective study involving 204 patients with malignant central airway obstruction. They found that higher American Society of Anesthesiologists (ASA) score, non-squamous histology, metastatic tumors and those who were treatment naïve patients had the worst survival. In one largest, multicenter, prospective study, Ost et al. (10) demonstrated that patients with ASA >3, renal failure, primary lung cancer, left mainstem involvement and TE fistula were associated with failure. They also found that the complication rate was higher in those with ASA >3, re-do bronchoscopy, receiving procedure under emergent or urgent situation. Thirty-day mortality was associated with poor performance status and ASA >3 (60). However, the patients with higher baseline Borg score had the greater improvement in dyspnea and quality of life. Since patients at the highest risk may have the greatest benefit, therapeutic bronchoscopy should not be withheld from patients solely based on risk assessment (10).
Conclusions
Newly developed visualization technologies of bronchoscopy provide more rapid, accurate, precise diagnosis of endobronchial lesions. Cryobiopsy is a safe technique with greater diagnostic yield and provides a better specimen quality than traditional forceps biopsy.
Interventional bronchoscopy enables the restoration of airway patency and plays an important role in the treatment and palliation for malignant airway obstruction. Mechanical debulking, electrocautery, APC and laser provide immediately symptoms relieve under urgent situation. Cryotherapy is preferred to use in the selective situation because of the delayed effects. Airway stenting is particular suitable for extraluminal compression and is also effective for intraluminal involvement. Drug eluting and biodegradable stents are developing to reduce the complication after long term implantation. Since more complication and less benefit have been expected in urgent situation, timely interventional bronchoscopy should be prudently considered before morbidity coming. The selection of modalities depends on the patients’ condition, disease manifestation, availability of equipment and physicians’ expertise.
Acknowledgements
Funding: This study was supported in part by grants from Chang Gung Memorial Hospital (CMRPG3B0981-3, and CMRPG3B0381-3 by Dr. Fu-Tsai Chung). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper. No additional external funding was received for this study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Luo M, Duan C, Qiu J, et al. Diagnostic Value of Multidetector CT and Its Multiplanar Reformation, Volume Rendering and Virtual Bronchoscopy Postprocessing Techniques for Primary Trachea and Main Bronchus Tumors. PloS One 2015;10:e0137329. 10.1371/journal.pone.0137329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hetzel J, Hetzel M, Hasel C, et al. Old meets modern: the use of traditional cryoprobes in the age of molecular biology. Respiration 2008;76:193-7. 10.1159/000135934 [DOI] [PubMed] [Google Scholar]
- 3.Hetzel J, Eberhardt R, Herth FJ, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J 2012;39:685-90. 10.1183/09031936.00033011 [DOI] [PubMed] [Google Scholar]
- 4.Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. 10.1164/rccm.200210-1181SO [DOI] [PubMed] [Google Scholar]
- 5.Coriat R, Diaz O, de la Fouchardiere C, et al. Endobronchial metastases from colorectal adenocarcinomas: clinical and endoscopic characteristics and patient prognosis. Oncology 2007;73:395-400. 10.1159/000136794 [DOI] [PubMed] [Google Scholar]
- 6.Boyd M, Rubio E. The utility of interventional pulmonary procedures in liberating patients with malignancy-associated central airway obstruction from mechanical ventilation. Lung 2012;190:471-6. 10.1007/s00408-012-9394-8 [DOI] [PubMed] [Google Scholar]
- 7.Scarlata S, Graziano P, Lucantoni G, et al. Endoscopic treatment of primary benign central airway tumors: Results from a large consecutive case series and decision making flow chart to address bronchoscopic excision. Eur J Surg Oncol 2015;41:1437-42. 10.1016/j.ejso.2015.08.157 [DOI] [PubMed] [Google Scholar]
- 8.Neyman K, Sundset A, Espinoza A, et al. Survival and complications after interventional bronchoscopy in malignant central airway obstruction: a single-center experience. J Bronchology Interv Pulmonol 2011;18:233-8. 10.1097/LBR.0b013e318222a7da [DOI] [PubMed] [Google Scholar]
- 9.Fang YF, Hsieh MH, Wang TY, et al. Removal of endobronchial malignant mass by cryotherapy improved performance status to receive chemotherapy. ScientificWorldJournal 2014;2014:369739. [DOI] [PMC free article] [PubMed]
- 10.Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. 10.1378/chest.14-1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oviatt PL, Stather DR, Michaud G, et al. Exercise capacity, lung function, and quality of life after interventional bronchoscopy. J Thorac Oncol 2011;6:38-42. 10.1097/JTO.0b013e3181f8a298 [DOI] [PubMed] [Google Scholar]
- 12.Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. 10.1016/j.athoracsur.2010.06.093 [DOI] [PubMed] [Google Scholar]
- 13.Stratakos G, Gerovasili V, Dimitropoulos C, et al. Survival and Quality of Life Benefit after Endoscopic Management of Malignant Central Airway Obstruction. Journal of Cancer 2016;7:794-802. 10.7150/jca.15097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherani K, Vakil A, Dodhia C, et al. Malignant tracheal tumors: a review of current diagnostic and management strategies. Curr Opin Pulm Med 2015;21:322-6. 10.1097/MCP.0000000000000181 [DOI] [PubMed] [Google Scholar]
- 15.Zaric B, Kovacevic T, Stojsic V, et al. New technologies in diagnostic bronchoscopy - an age of meta-analyses. Expert Rev Med Devices 2016;13:789-91. 10.1080/17434440.2016.1218276 [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Gao X, Tian Q, et al. A comparison of autofluorescence bronchoscopy and white light bronchoscopy in detection of lung cancer and preneoplastic lesions: a meta-analysis. Lung Cancer 2011;73:183-8. 10.1016/j.lungcan.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Sun J, Garfield DH, Lam B, et al. The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer: a meta-analysis. J Thorac Oncol 2011;6:1336-44. 10.1097/JTO.0b013e318220c984 [DOI] [PubMed] [Google Scholar]
- 18.Haas AR, Vachani A, Sterman DH. Advances in diagnostic bronchoscopy. Am J Respir Crit Care Med 2010;182:589-97. 10.1164/rccm.201002-0186CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14. 10.1378/chest.127.6.2007 [DOI] [PubMed] [Google Scholar]
- 20.Rubio ER, le SR, Whatley RE, et al. Cryobiopsy: should this be used in place of endobronchial forceps biopsies? Biomed Res Int 2013;2013:730574. [DOI] [PMC free article] [PubMed]
- 21.Chou CL, Wang CW, Lin SM, et al. Role of flexible bronchoscopic cryotechnology in diagnosing endobronchial masses. Ann Thorac Surg 2013;95:982-6. 10.1016/j.athoracsur.2012.11.044 [DOI] [PubMed] [Google Scholar]
- 22.Hardavella G, George J. Interventional bronchoscopy in the management of thoracic malignancy. Breathe (Sheff) 2015;11:202-12. 10.1183/20734735.008415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Q, Shi B, Tian Y, et al. Fibrobronchoscopic cryosurgery for secondary malignant tumors of the trachea and main bronchi. Thorac Cancer 2016;7:459-66. 10.1111/1759-7714.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segmen F, Aktaş Z, Öztürk A, et al. How many samples would be optimal for endobronchial cryobiopsy? Surg Endosc 2016. [Epub ahead of print]. 10.1007/s00464-016-5095-3 [DOI] [PubMed] [Google Scholar]
- 25.Guibert N, Mazieres J, Marquette CH, et al. Integration of interventional bronchoscopy in the management of lung cancer. Eur Respir Rev 2015;24:378-91. 10.1183/16000617.00010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semaan R, Yarmus L. Rigid bronchoscopy and silicone stents in the management of central airway obstruction. J Thorac Dis 2015;7:S352-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst A, Silvestri GA, Johnstone D, American College of Chest P. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. 10.1378/chest.123.5.1693 [DOI] [PubMed] [Google Scholar]
- 28.Lin SM, Lin TY, Chou CL, et al. Metallic stent and flexible bronchoscopy without fluoroscopy for acute respiratory failure. Eur Respir J 2008;31:1019-23. 10.1183/09031936.00099507 [DOI] [PubMed] [Google Scholar]
- 29.Vishwanath G, Madan K, Bal A, et al. Rigid bronchoscopy and mechanical debulking in the management of central airway tumors: an Indian experience. J Bronchology Interv Pulmonol 2013;20:127-33. 10.1097/LBR.0b013e318290b8de [DOI] [PubMed] [Google Scholar]
- 30.Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66 Suppl 3:iii1-21. [DOI] [PubMed] [Google Scholar]
- 31.Wahidi MM, Unroe MA, Adlakha N, et al. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol 2011;6:1516-20. 10.1097/JTO.0b013e3182242142 [DOI] [PubMed] [Google Scholar]
- 32.Boxem T, Muller M, Venmans B, et al. Nd-YAG laser vs bronchoscopic electrocautery for palliation of symptomatic airway obstruction: a cost-effectiveness study. Chest 1999;116:1108-12. 10.1378/chest.116.4.1108 [DOI] [PubMed] [Google Scholar]
- 33.Miller SM, Bellinger CR, Chatterjee A. Argon plasma coagulation and electrosurgery for benign endobronchial tumors. J Bronchology Interv Pulmonol 2013;20:38-40. 10.1097/LBR.0b013e318282d3ca [DOI] [PubMed] [Google Scholar]
- 34.Sachdeva A, Pickering EM, Lee HJ. From electrocautery, balloon dilatation, neodymium-doped:yttrium-aluminum-garnet (Nd:YAG) laser to argon plasma coagulation and cryotherapy. J Thorac Dis 2015;7:S363-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalar L, Sökücü SN, Özdemir C, et al. Endobronchial argon plasma coagulation for treatment of Dieulafoy disease. Respir Care 2015;60:e11-3. 10.4187/respcare.03307 [DOI] [PubMed] [Google Scholar]
- 36.Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. 10.1378/chest.119.3.781 [DOI] [PubMed] [Google Scholar]
- 37.Lee BR, Oh IJ, Lee HS, et al. Usefulness of Rigid Bronchoscopic Intervention Using Argon Plasma Coagulation for Central Airway Tumors. Clin Exp Otorhinolaryngol 2015;8:396-401. 10.3342/ceo.2015.8.4.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest 2008;134:1066-9. 10.1378/chest.08-0474 [DOI] [PubMed] [Google Scholar]
- 39.Shaw Y, Yoneda KY, Chan AL. Cerebral gas embolism from bronchoscopic argon plasma coagulation: a case report. Respiration 2012;83:267-70. 10.1159/000328939 [DOI] [PubMed] [Google Scholar]
- 40.Khemasuwan D, Mehta AC, Wang KP. Past, present, and future of endobronchial laser photoresection. J Thorac Dis 2015;7:S380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaric B, Kovacevic T, Stojsic V, et al. Neodymium yttrium-aluminium-garnet laser resection significantly improves quality of life in patients with malignant central airway obstruction due to lung cancer. Eur J Cancer Care (Engl) 2015;24:560-6. 10.1111/ecc.12256 [DOI] [PubMed] [Google Scholar]
- 42.Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser is associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol 2007;2:59-64. 10.1097/JTO.0b013e31802bff2d [DOI] [PubMed] [Google Scholar]
- 43.Perin B, Zaric B, Jovanovic S, et al. Patient-related independent clinical risk factors for early complications following Nd: YAG laser resection of lung cancer. Ann Thorac Med 2012;7:233-7. 10.4103/1817-1737.102184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SH, Choi WJ, Sung SW, et al. Endoscopic cryotherapy of lung and bronchial tumors: a systematic review. Korean J Intern Med 2011;26:137-44. 10.3904/kjim.2011.26.2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhikai Z, Lizhi N, Liang Z, et al. Treatment of central type lung cancer by combined cryotherapy: experiences of 47 patients. Cryobiology 2013;67:225-9. 10.1016/j.cryobiol.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 46.Boujaoude Z, Young D, Lotano R, et al. Cryosurgery for the immediate treatment of acute central airway obstruction. J Bronchology Interv Pulmonol 2013;20:45-7. 10.1097/LBR.0b013e31827cdb8b [DOI] [PubMed] [Google Scholar]
- 47.Saji H, Furukawa K, Tsutsui H, et al. Outcomes of airway stenting for advanced lung cancer with central airway obstruction. Interact Cardiovasc Thorac Surg 2010;11:425-8. 10.1510/icvts.2010.238196 [DOI] [PubMed] [Google Scholar]
- 48.Fortin M, MacEachern P, Hergott CA, et al. Self-expandable metallic stents in nonmalignant large airway disease. Can Respir J 2015;22:235-6. 10.1155/2015/246509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung FT, Lin HC, Chou CL, et al. Airway ultraflex stenting in esophageal cancer with esophagorespiratory fistula. Am J Med Sci 2012;344:105-9. 10.1097/MAJ.0b013e3182367b6a [DOI] [PubMed] [Google Scholar]
- 50.Serrano C, Laborda A, Lozano JM, et al. Metallic stents for tracheobronchial pathology treatment. Cardiovasc Intervent Radiol 2013;36:1614-23. 10.1007/s00270-013-0602-6 [DOI] [PubMed] [Google Scholar]
- 51.Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg 2011;6:46. 10.1186/1749-8090-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottlieb J, Fuehner T, Dierich M, et al. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 2009;34:1417-22. 10.1183/09031936.00041909 [DOI] [PubMed] [Google Scholar]
- 53.Chung FT, Chen GY, Chou CL, et al. Remove airway ultraflex stents by flexible bronchoscope. Am J Med Sci 2012;343:267-72. 10.1097/MAJ.0b013e31822a6bc3 [DOI] [PubMed] [Google Scholar]
- 54.Chung FT, Lin SM, Chou CL, et al. Factors leading to obstructive granulation tissue formation after ultraflex stenting in benign tracheal narrowing. Thorac Cardiovasc Surg 2010;58:102-7. 10.1055/s-0029-1186266 [DOI] [PubMed] [Google Scholar]
- 55.Chung FT, Lin SM, Chen HC, et al. Factors leading to tracheobronchial self-expandable metallic stent fracture. J Thorac Cardiovasc Surg 2008;136:1328-35. 10.1016/j.jtcvs.2008.05.039 [DOI] [PubMed] [Google Scholar]
- 56.Yu CT, Chou CL, Chung FT, et al. Tracheal torsion assessed by a computer-generated 3-dimensional image analysis predicts tracheal self-expandable metallic stent fracture. J Thorac Cardiovasc Surg 2010;140:769-76. 10.1016/j.jtcvs.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 57.Hohenforst-Schmidt W, Zarogoulidis P, Pitsiou G, et al. Drug Eluting Stents for Malignant Airway Obstruction: A Critical Review of the Literature. J Cancer 2016;7:377-90. 10.7150/jca.13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 2009;35:429-33; discussion 933-4. 10.1016/j.ejcts.2008.10.041 [DOI] [PubMed] [Google Scholar]
- 59.Dutau H, Musani AI, Laroumagne S, et al. Biodegradable Airway Stents - Bench to Bedside: A Comprehensive Review. Respiration 2015;90:512-21. 10.1159/000442054 [DOI] [PubMed] [Google Scholar]
- 60.Ost DE, Ernst A, Grosu HB, et al. Complications Following Therapeutic Bronchoscopy for Malignant Central Airway Obstruction: Results of the AQuIRE Registry. Chest 2015;148:450-71. 10.1378/chest.14-1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guibert N, Mazieres J, Lepage B, et al. Prognostic factors associated with interventional bronchoscopy in lung cancer. Ann Thorac Surg 2014;97:253-9. 10.1016/j.athoracsur.2013.07.118 [DOI] [PubMed] [Google Scholar]