Abstract
Background
To analyze the clinicopathological features and prognosis of younger patients with esophageal adenocarcinoma (EAC).
Methods
A total of 2,601 patients diagnosed with EAC between 1988 and 2011 were selected from the Surveillance, Epidemiology, and End Results (SEER) database. All patients underwent primary tumor resection and regional lymphadenectomy without preoperative radiotherapy. The patients were into four age groups (<45, 45–59, 60–74, ≥75), with 94, 813, 1,272 and 422 patients in each group respectively.
Results
Patients in the age <45 group were more likely to have lymph node (LN) metastasis (P=0.002), postoperative radiotherapy (P<0.001) and advanced T and N stage (P=0.003, 0.014) compared to the other three groups. We then conducted two Cox proportional hazards model adjusted for the sex, race, number of LNs examined, histological grade, postoperative radiation. The hazard ratio (HR) was higher in patients <45 y and the survival rate were paradoxically lower compared to the patients between 45–60 years old (P=0.046, 0.039).
Conclusions
The patients <45 y had the most aggressive clinicopathological features of EAC and poorer survival rate after radical esophagectomy.
Keywords: Esophageal adenocarcinoma (EAC); young patients; the Surveillance, Epidemiology, and End Results (SEER); prognosis
Introduction
Esophageal carcinoma is one of the most lethal malignant tumor throughout the world (1). The two main histological types are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Although the incidence of EAC is significant lower than ESCC in East Asia, it has increased rapidly in the western world within last 30 years, and become the predominant type in America and European countries (2,3).
Ageing is one of the risk factors for EAC (4). However, the incidence of EAC in each age group, particularly in young people has risen significantly according to epidemiological studies (5-7). Despite some reports about EAC of young patients have been published, there are still controversial on whether young patients have more advanced stage at presentation and worse prognosis than the older patients (8-11).
In this retrospective study, we intended to analyze the clinicopathologic features and the prognosis of young patients with EAC using a population based cohort.
Methods
The Surveillance, Epidemiology, and End Results (SEER) of the National Cancer Institute are the largest population-based cancer registry in the United States, including approximately 26% of the US population (12). The study cohort was created from the available data in the SEER-Medicare 2011 release. To identify the population of interest, SEER data from 1988 to 2011 were collected and reported using codes as documented by the North American Association of Central Cancer Registries (NAACCR). Only those who underwent esophagectomy and lymphadenectomy with or without gastrectomy, and histologically confirmed EAC (ICD-O-3 codes 8140, 8141, 8144, 8210, 8211, 8255, 8260, 8261, 8263, 8310, 8323, 8480, 8481, 8574, 8576) were included in our study. Patients who had preoperative radiation therapy or unknown radiation-surgery sequence, and those who underwent local tumor excision or unknown surgery method were excluded; Patients who did not have complete clinicopathological data were also ruled out. In total, 2,601 patients whose medical data met the selection criteria were included in our study. According to the American Joint Commission on Cancer stage (7th edition), pT and pN were restaged.
Statistical analysis
The continuous variables were reported as mean ± SD whereas the categorical variables as percentages. Comparisons between groups were performed using t-test for continuous variables and chi-square test for categorical variables. Generalized smoothing splines with knot locations generated automatically in generalized additive models (13) were used to explore the shape of the dose-response relation between N status and age by R package mgcv and ggplot2. Disease specific survival were estimated using the Kaplan-Meier method, and a Cox proportion hazard model were used to calculate hazard ratios (HR) and 95% confidence interval (95% CI). A two-sided statistical significance was defined as P<0.05. All analyses were performed with R Project software version 3.1.2 (http://www.R-project.org).
Results
Age grouping method
In order to find the threshold for the “young” patients, we used the Kaplan-Meier to compare the survival rate using different grouping methods. We found the prognosis of patients with age <45 y was worse than patients of other age groups (Figure 1). All the 2,601 patients were dividing into four groups based on age: 94 (3.6%) patients were younger than 45 y, 813 (31.3%) patients were between the age of 45–59 y, 1,272 (48.9%) patients were between the age of 60–74 y, and 422 (16.2%) patients were older than 75 y. The average age of younger patients was 39.1±5.2 years old.
Figure 1.
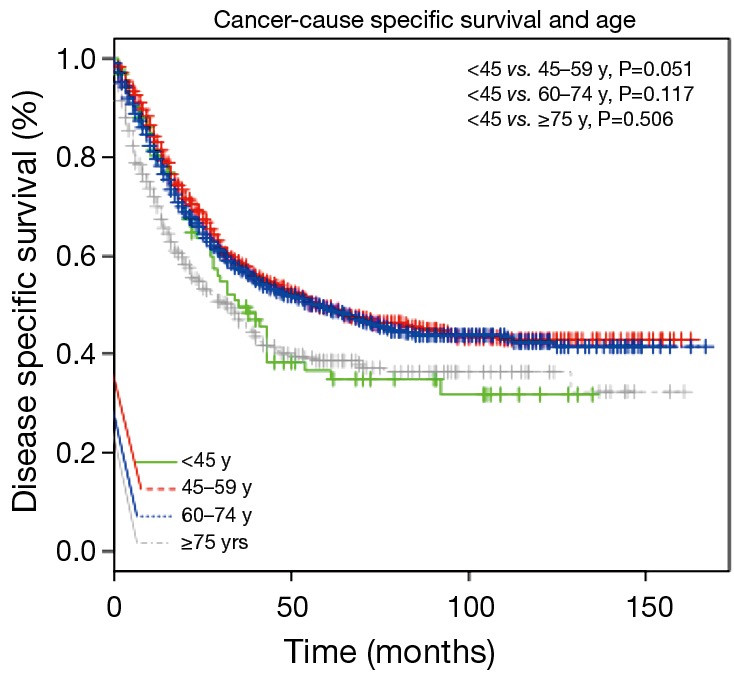
Unadjusted cancer-cause specific survival between each group.
Clinicopathological features
As shown in Table 1, all the clinicopathological features of each group were showed significant differences. White male patients comprised the majority of the whole cohort. Overall, the patients <45 y had fewer lymph nodes (LN) examined (13.8±9.0, P=0.046), but had a higher incidence of LN metastasis (LNM) (60.6%, P=0.002) and more advanced pT and pN stage (P=0.003, 0.014). The proportion of advanced pT stage (T3, T4), pN stage (N3, N4) and LNM rate was highest among patients <45 y. Figure 2 have manifested a downtrend between age and LNM.
Table 1. Clinicopathological features of patients in four different age groups.
| Characteristics | Age (years) (%) | P | |||
|---|---|---|---|---|---|
| <45 | 45–59 | 60–74 | ≥75 | ||
| Number | 94 (3.6) | 813 (31.3) | 1,272 (48.9) | 422 (16.2) | |
| Age* | 39.1±5.2 | 53.8±3.9 | 66.8±4.3 | 78.7±3.3 | <0.001 |
| LN examined* | 13.8±9.0 | 15.8±15.1 | 14.7±13.7 | 13.6±13.5 | 0.046 |
| Survival (M)* | 38.7±35.0 | 43.9±40.1 | 37.5±35.7 | 28.8±32.9 | <0.001 |
| Race | 0.003 | ||||
| White | 84 (89.4) | 778 (95.7) | 1,220 (95.9) | 408 (96.7) | |
| Black | 5 (5.3) | 20 (2.5) | 15 (1.2) | 3 (0.7) | |
| Others and unknown | 5 (5.3) | 15 (1.8) | 37 (2.9) | 11 (2.6) | |
| Gender | 0.009 | ||||
| Male | 84 (89.4) | 736 (90.5) | 1,144 (89.9) | 357 (84.6) | |
| Female | 10 (10.6) | 77 (9.5) | 128 (10.1) | 65 (15.4) | |
| Grade | 0.016 | ||||
| Well | 8 (8.5) | 63 (7.7) | 107 (8.4) | 27 (6.4) | |
| Moderately | 40 (42.6) | 296 (36.4) | 465 (36.6) | 143 (33.9) | |
| Poor | 38 (40.4) | 370 (45.5) | 570 (44.8) | 206 (48.8) | |
| Undifferentiated | 4 (4.3) | 17 (2.1) | 22 (1.7) | 21 (5.0) | |
| Unknown | 4 (4.3) | 67 (8.2) | 108 (8.5) | 25 (5.9) | |
| Radiotherapy | <0.001 | ||||
| Yes | 41 (43.6) | 213 (26.2) | 261 (20.5) | 54 (12.8) | |
| No | 53 (56.4) | 600 (73.8) | 1,011 (79.5) | 368 (87.2) | |
| LNM | 0.002 | ||||
| Yes | 57 (60.6) | 390 (48.0) | 543 (42.7) | 187 (44.3) | |
| No | 37 (39.4) | 423 (52.0) | 729 (57.3) | 235 (55.7) | |
| pN stage | 0.014 | ||||
| pN0 | 37 (39.4) | 423 (52.0) | 729 (57.3) | 235 (55.7) | |
| pN1 | 17 (18.1) | 170 (20.9) | 231 (18.2) | 81 (19.2) | |
| pN2 | 24 (25.5%) | 125 (15.4) | 179 (14.1) | 64 (15.2) | |
| pN3 | 16 (17.0) | 95 (11.7) | 133 (10.5) | 42 (10.0) | |
| pT stage | 0.003 | ||||
| pT1 | 24 (25.5) | 308 (37.9) | 515 (40.5) | 130 (30.8) | |
| pT2 | 12 (12.8) | 111 (13.7) | 179 (14.1) | 61 (14.5) | |
| pT3 | 44 (46.8) | 329 (40.5) | 490 (38.5) | 192 (45.5) | |
| pT4 | 14 (14.9) | 65 (8.0) | 88 (6.9) | 39 (9.2) | |
*, mean ± SD. LNM, LN metastasis.
Figure 2.
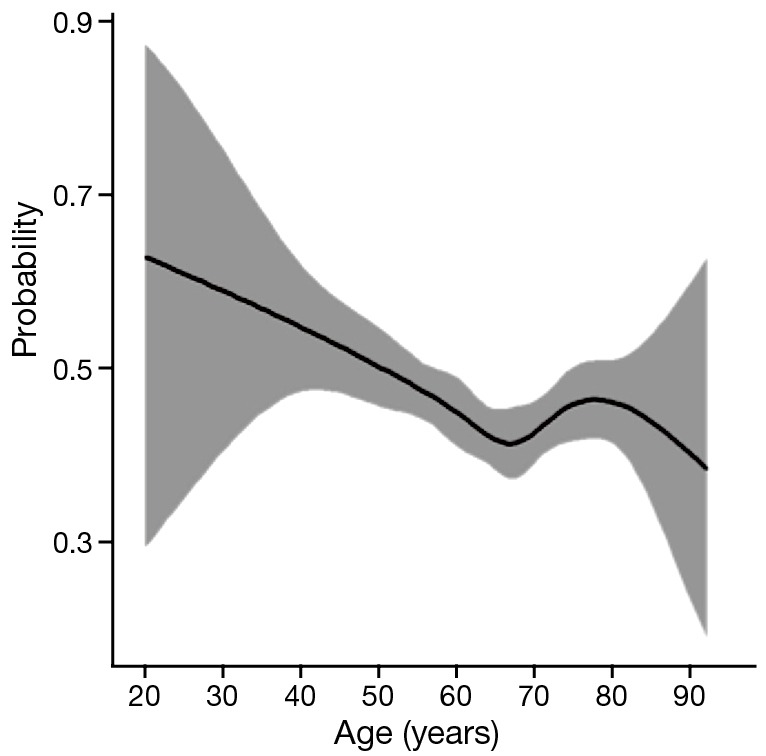
The shape of the dose-response relation between N status and age and their 95% confidence intervals (CI) (shape scope), which using generalized smoothing splines with five knots (13).
Postoperative radiotherapy and survival
Younger patients were more likely to received postoperative radiotherapy compared to patients >45 y (43.6%, P<0.001). Despite this additional treatment modality, their survival rate was relatively poor (38.7±35.0 months, P<0.001). After a median follow up duration of 25 months (interquartile range, 10–56 months), EAC-specific death occurred in 54 patients (57.4%) in the age <45 y group, 371 patients (45.6%) in the 45–59 years group, 562 patients (44.2%) in the 60–74 years group, 211 patients (50.0%) in the age ≥75 years group, respectively. Cancer-cause specific survivals of each group were shown in Figure 2, the survival of patients <45 and ≥75 y was worse compared to that of patients between 45–74 y.
For age group comparison, we used the patients group between 45–59 y as the reference group, because this cohort had the best prognosis. Compared with the reference group of 45–59 y, the HR (95% CI) of other three groups were 1.34 (1.01–1.78) for the age <45 y cohort, 1.06 (0.93–1.21) for the 60–74 y cohort and 1.48 (1.25–1.75) for the age ≥75 y cohort using the Cox proportional hazards model adjusted for sex, race (shown in Table 2, Model 1). When the data was further adjusted the number of LN examined, histological grade and postoperative radiation, the results were unchanged (shown in Table 2, Model 2). These two statistically models confirmed that the prognosis of patients younger than 45 y was significantly worse compared to patients between 45–59 y (P=0.046, 0.039).
Table 2. HRs for death due to EAC by age group.
| Age (years) | N | No. with outcome (%) | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI)a | P valueb | HR (95% CI)a | P valueb | ||||
| <45 | 94 | 54 (57.4) | 1.34 (1.01–1.78) | 0.046 | 1.35 (1.02–1.80) | 0.039 | |
| 45–59 | 813 | 371 (45.6) | Reference | — | Reference | — | |
| 60–74 | 1,272 | 562 (44.2) | 1.06 (0.93–1.21) | 0.413 | 1.11 (0.97–1.26) | 0.137 | |
| ≥75 | 422 | 211 (50.0) | 1.48 (1.25–1.75) | <0.001 | 1.52 (1.28–1.81) | <0.001 | |
a, hazard ratio estimated using the Cox proportional hazards model; b, derived from the log-rank test. Model 1, adjustment for sex, race; Model 2, adjustment for sex, race, number of LN examined, tumor grade, radiotherapy. HR, hazard ratio; EAC, esophageal adenocarcinoma.
Discussion
EAC is uncommon in young people (14), but the incidence among younger patients with EAC has increased substantially over the past three decades (11,15). This observation partly attributed to the increasing risk factor among younger adults such as obesity, lack of physical activity and gastroesophageal reflux disease (GERD) (16-18). Some researchers try to investigate whether age influences the clinicopathological features and prognosis of EAC, but the results of previous studies were inconsistent.
Some researchers believe that EAC among younger patients may have more aggressive biology and metastatic risk. Thus, Oezcelik et al. reported that patients <40 y had a higher prevalence of stage IV disease and a poorer survival (9). Portale et al. reported that patients ≤50 y were more likely to present with advanced stage and a more than 70% LNM rate, significantly higher than patients >50 y. However, the survival rates were having no significant difference (11). Hashemi et al. reported that patients ≤50 y were more likely to have LNM but had similar survival rate compared with patients >50 y (19). Moreover, some other reports showed there was no significant difference in the tumor pathology between different age group (8,20,21).
These conflicting results are difficult to reconcile. We speculate that one of the primary reasons is that the sample sizes of younger patients of EAC in these reports were usually quite small; Secondly, these studies did not adjust the variables among each group, and this may have led to some statistical bias; In addition, most studies defined only one or two thresholds for all age groups. Fewer groups may cause unprecise age stratification, and may have unknown effect on the prognosis of each group. To address the limitation of small sample size, we used the SEER database with a large sample size and the ability to perform cohort analysis among subgroups. Besides, generalized smoothing splines were used to show the relationship between age and LNM (Figure 1), this statistical method manifest a nonlinear relationship between continuous variable and categorical variable, and have a high fitting degree. Although the number of LN examined had no significant difference among each age subgroup, the ratio of metastatic LN compared to number of nodes examined decreased as the patient age increased. We also excluded the patients who received preoperative radiotherapy to reduce the impact on the pathological stage as much as possible. For more accurate comparison, we subdivided all the patients into four groups to make the age distribution more accurate. Although the unadjusted survival did not show significant difference when using Kaplan–Meier methods, we created Cox proportional hazards models to adjust the variables such as sex, race, the number of LN examined, tumor grade and radiotherapy, the results demonstrated patients <45 y had a higher HR and significantly worse survival compared to the patients between 45–59 y.
Portale et al. found that young patients with EAC were asymptomatic for a longer time before diagnosis (11), but this criterion may not the only reason for a poor survival. A retrospective study by Hashemi et al. showed that patients ≤50 y were more likely than older patients to receive neoadjuvant or adjuvant therapy (19). Therefore despite lack of data on the use chemotherapy in SEER database is a shortcoming, we still considered the young patients were able to tolerate chemotherapy compared to older patients because of better physical condition and advanced pathological stage. But why young patients underwent more adjuvant or neoadjuvant therapy, but still have a worse prognosis is worth considering. Solomon et al reported have find out that patients with localize EAC (confined to the organ of origin) without LM metastasis had no significant survival benefit from additional chemotherapies using the Florida Cancer Data System (FCDS) (22). Vallböhmer et al. reported that patients ≤50 y had a significantly higher 5-year survival rate that they attributed to neoadjuvant therapy (10). However the pathological stage of young patients was similar to that of older patients receiving neoadjuvant therapy. Thus neoadjuvant therapy may lead the tumor downstage and an enhanced ability to perform complete surgery and have lower perioperative morbidity that may improve the prognosis of younger patients.
It is undeniable that our studies still have some limitations given its retrospective nature. Due to lack of some information such as lymph-vascular invasion, we do not know whether it is more prone to happened in young patients; Besides, we speculate that unknown gene influence were also could be the potential factor for poor survival.
Conclusions
In conclusion, our study showed the patients <45 y presented with advanced stage and received more aggressive treatment after esophagectomy. Worse prognosis of these patients deserved considering, screening such as endoscopy should be performed in young people at high risk. In the future, additional prospective studies such as therapeutic strategies and fundamental research should be undertaken.
Acknowledgements
Funding: This work was supported by the grant from the Shanghai Rising-Star Program (11QH1400600), National Natural Science Foundation of China (81272608) and National Natural Science Foundation of China (81102044).
Ethical Statement: The study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Cowie A, Noble F, Underwood T. Strategies to improve outcomes in esophageal adenocarcinoma. Expert Rev Anticancer Ther 2014;14:677-87. 10.1586/14737140.2014.895668 [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. 10.1136/gutjnl-2014-308124 [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology 2015;149:302-17.e1. 10.1053/j.gastro.2015.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosetti C, Levi F, Ferlay J, et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer 2008;122:1118-29. 10.1002/ijc.23232 [DOI] [PubMed] [Google Scholar]
- 6.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer 2009;101:855-9. 10.1038/sj.bjc.6605246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepage C, Rachet B, Jooste V, et al. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol 2008;103:2694-9. 10.1111/j.1572-0241.2008.02191.x [DOI] [PubMed] [Google Scholar]
- 8.Scott Bolton J, Wu TT, Yeo CJ, et al. Esophagectomy for adenocarcinoma in patients 45 years of age and younger. J Gastrointest Surg 2001;5:620-5. 10.1016/S1091-255X(01)80104-9 [DOI] [PubMed] [Google Scholar]
- 9.Oezcelik A, Ayazi S, DeMeester SR, et al. Adenocarcinoma of the esophagus in the young. J Gastrointest Surg 2013;17:1032-5. 10.1007/s11605-013-2177-6 [DOI] [PubMed] [Google Scholar]
- 10.Vallböhmer D, Hölscher AH, Brabender J, et al. Clinicopathologic and prognostic factors of young and elderly patients with esophageal adenocarcinoma: is there really a difference? Dis Esophagus 2008;21:596-600. 10.1111/j.1442-2050.2008.00817.x [DOI] [PubMed] [Google Scholar]
- 11.Portale G, Peters JH, Hsieh CC, et al. Esophageal adenocarcinoma in patients < or = 50 years old: delayed diagnosis and advanced disease at presentation. Am Surg 2004;70:954-8. [PubMed] [Google Scholar]
- 12.SEER: SEER*Stat Database: Incidence—SEER 17 Regs Public Use, Nov 2005 Sub (1973-2003 vary-ing). National Cancer Institute, Surveillance, Epidemiology, and End Results Program. Available online: http://seer.cancer.gov
- 13.Anderson-Cook CM. Generalized Additive Models: An Introduction With R. Journal of the American Statistical Association 2007;102:760-1. 10.1198/jasa.2007.s188 [DOI] [Google Scholar]
- 14.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA 1991;265:1287-9. 10.1001/jama.1991.03460100089030 [DOI] [PubMed] [Google Scholar]
- 15.Devesa SS, Blot WJ, Fraumeni JF, Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53. [DOI] [PubMed] [Google Scholar]
- 16.Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst 1995;87:104-9. 10.1093/jnci/87.2.104 [DOI] [PubMed] [Google Scholar]
- 17.Vaughan TL, Davis S, Kristal A, et al. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 1995;4:85-92. [PubMed] [Google Scholar]
- 18.La Vecchia C, Negri E, Lagiou P, et al. Oesophageal adenocarcinoma: a paradigm of mechanical carcinogenesis? Int J Cancer 2002;102:269-70. 10.1002/ijc.10697 [DOI] [PubMed] [Google Scholar]
- 19.Hashemi N, Loren D, DiMarino AJ, et al. Presentation and prognosis of esophageal adenocarcinoma in patients below age 50. Dig Dis Sci 2009;54:1708-12. 10.1007/s10620-008-0565-7 [DOI] [PubMed] [Google Scholar]
- 20.Mori M, Ohno S, Tsutsui S, et al. Esophageal carcinoma in young patients. Ann Thorac Surg 1990;49:284-6. 10.1016/0003-4975(90)90152-V [DOI] [PubMed] [Google Scholar]
- 21.Adam DJ, Craig SR, Sang CT, et al. Oesophagogastrectomy for carcinoma in patients under 50 years of age. J R Coll Surg Edinb 1996;41:371-3. [PubMed] [Google Scholar]
- 22.Solomon NL, Cheung MC, Byrne MM, et al. Does chemoradiotherapy improve outcomes for surgically resected adenocarcinoma of the stomach or esophagus? Ann Surg Oncol 2010;17:98-108. 10.1245/s10434-009-0679-y [DOI] [PubMed] [Google Scholar]


