Abstract
Background
Pulmonary cryptococcosis (PC) was not a rare infectious disease in non-AIDS patients. However, data on the immune status were lacking in southern China for comparative analysis of differences between immunocompromised and immunocompetent hosts. This study was to investigate the epidemiological, clinical, radiological, and treatment profiles for patients with PC.
Methods
We performed a retrospective review of 88 patients diagnosed with tissue-confirmed PC who were not HIV-infected from 2003 to 2013.
Results
Of 88 patients, 35(39.7%) were immunocompromised host. Fever and CNS symptom were significantly common in immunocompromised patients compared to immunocompetent patients (P=0.019 and P=0.036, respectively). The most frequent radiologic abnormalities were solitary or multiple pulmonary nodules, and masses or consolidations, and most lesions were located in the peripheral lung field. Cavitations and halo sign were significantly frequent in immunocompromised patients than in immunocompetent patients (P<0.05). The most frequently applied and reliable diagnostic procedure was CT-guided percutaneous translung biopsy. Treatment included antifungal drug alone in 20 patients, surgery alone in 20 including 3 treated by VATS, surgery plus antifungal drugs in 20 patients.
Conclusions
PC was not rare in immunocompetent host in southern China. Special differences remained in clinical manifestation and radiological findings of PC between immunocompromised and immunocompetent patients. Future work on the mechanisms of possible differences is required.
Keywords: Pulmonary cryptococcosis, computed tomography, Non-AIDS patient, immune status
Introduction
Cryptococcosis is a fungal infection that can result in pulmonary involvement after the inhalation of cryptococcus neoformans and cryptococcus gattii spores (1). PC is not rare in china (2-4). PC is more common in immunocompromised patients, including those with impaired cell-mediated immunity, as in HIV, solid-organ transplant recipients, and hematologic malignancies, and patients on chronic corticosteroids or other immunosuppressive therapy (5-7). With a declining incidence of AIDS-related cryptococcosis in the highly active antiretroviral therapy era, and increasing use of immunosuppressants worldwide, non-HIV-infected individuals may become the predominant infected group (6). PC also occurs in immunocompetent host (8,9). Recent data showed that the incidence of PC is increasing (10,11). A multicentre retrospective study demonstrated that PC was the third fungal infection in china (74 cases out 474 cases, 15.6%) (10). Most PC patients in china had no apparent underlying disease, which is in stark contrast with reports from other countries (3,4). Some reports differ with regard to PC in immunocompromised and immunocompetent host (3,5). Data were lack of comparisons in such patients with regard to their immune status in China. The aim of our study was to clarify the epidemiological, clinical, radiological characteristics, as well as treatments of PC in these two groups of patients in southern China.
Methods
Study subjects
Through a computer-assisted search of medical records, we retrospectively reviewed all pathological diagnoses of PC at the First Affiliated Hospital (a 2500-bed tertiary referral hospital) of Fujian Medical University, Fuzhou, China from January 2003 to December 2013. HIV serologic testing was routinely performed. AIDS patients were excluded. Diagnosis of PC was made by cytology of histopathology of lung tissue. All samples were fixed using neutral formalin, embedded in paraffin, stained with haematoxylin—eosin and histochemically stained with Grocott’s methenamine silver periodic acid—Schiff, and mucus card Red. Then, the samples were examined by light microscopy. Data collected included demographics, predisposing factors; clinical findings; and radiological, treatment, and outcome data. Acute-onset could be defined as symptoms of PC lasting less than 3 weeks, and chronic-onset could be defined as symptoms lasting more than 3 weeks. Patients were considered to be immunocompromised hosts with at least one predisposing condition, including use of immunosuppressive drugs, uncontrolled diabetes mellitus, malignancies, autoimmune disorders, end-stage renal failure, splenectomy and idiopathic CD4 T-cell lymphopenia. The study was approved by the Institutional Review Board of the First Affiliated Hospital, Fujian Medical University (No, 2014-hx-02). Patient information was anonymized and de-identified prior to analysis.
Image acquisition and evaluation
All radiologic data were reviewed retrospectively by two radiologists (RX You and Z Xing). A final reading was reached by consensus. Nodules were defined as round or oval opacities that were less than 3 cm in greatest diameter. If these opacities were 3 cm or larger in diameter, they were referred to as masses. The nodules were characterized as well or ill defined, and the margins, as smooth or irregular. Halo sign was a micronodule (1 cm in diameter) surrounded by a perimeter of ground-glass opacity. Consolidation was defined as parenchymal non nonnodular opacities that obscured underlying pulmonary architecture and that often included air bronchograms. Lymphadenopathy was determined when the short-axis diameter exceeded 1cm. The distribution of each pattern was classified as being predominantly central, peripheral, or random. The brain CT and magnetic resonance imaging were performed to evaluate who may have central nervous system (CNS) involvement. The corrected PET images were evaluated qualitatively by visual inspection and semi-quantitatively by analysis of standard uptake value (SUV).
Follow-up studies
All patients were followed up to 1 June 2014, except for the deceased and those lost to follow up. Final outcome was classified as follows. Complete resolution was defined as the disappearance of radiographic evidence of pulmonary abnormalities, while deterioration was defined as clinical symptoms having deteriorated or progressive radiographic worsening of pulmonary abnormalities. Partial resolution was defined as clinical symptoms having improved and imaging findings indicating that the lesions had partially resolved. A stable condition was defined as clinical symptoms and imaging findings having not changed.
Statistical analysis
We performed statistical analysis using SPSS software for Windows, version 17.0 (SPSS), with the Pearson’s Chi-square test and Fisher exact test used for qualitative variables and Student’s test used for quantitative variables. A significant difference was considered to be present for P values <0.05.
Results
Patient characteristics
Our retrospective analysis identified 88 patients with tissue-proven PC. The diagnosis was confirmed histologically by the presence of Cryptococcus in lung tissues. Table 1 summarizes patient characteristics. A total of 55 males (62.5%) and 33 females (37.5%) were included. Mean age was 47.7 years (standard deviation, 13.57; range, 17–81). Fifty (56.9%) patients resided in rural or semirural regions. 22.7% of patients had a clear history of environmental exposure. Two patients had a close contact with pigeons that frequently flew to the veranda of his residence. Ten patients worked in agricultural fields of rice or fruits, and two were construction laborers. The environmental exposure was not significantly different (P=0.202) for immunocompromised and the immunocompetent patients. Eight patients have traveled abroad. The destinations mainly were USA, Australia and Canada.
Table 1. Demographics and clinical information of patients with PC.
| Variables | Immunocompetent patients (n=53) | Immunocompromised patients (n=35) | P value |
|---|---|---|---|
| Age | 43±12.12 | 55±12.53 | 0.000 |
| Male | 33 | 22 | 0.955 |
| Onset | |||
| Acute | 10 | 8 | 0.650 |
| Chronic | 30 | 15 | 0.207 |
| Accidental | 13 | 12 | 0.321 |
| Symptom | |||
| Asymptom | 14 | 13 | 0.286 |
| Spit phlegm | 19 | 7 | 0.111 |
| Dry cough | 13 | 6 | 0.410 |
| Fever | 5 | 10 | 0.019 |
| Dyspnea | 6 | 6 | 0.644 |
| Chest pain | 9 | 4 | 0.472 |
| Hemoptysis | 3 | 1 | 0.968 |
| CNS symptoms | 2 | 7 | 0.036 |
| Enviroment exposure history | 15 | 5 | 0.202 |
A total of 39.8% (35/88) of the patients had underlying diseases. Predisposing factors included: malignancies (n=9), cirrhosis (n=8), autoimmune disorders (n=6), diabetes mellitus (n=8), chronic use of corticosteroid or other immunosuppressive therapies (n=7), end-stage renal failure (n=2), and idiopathic CD4 T-cell lymphopenia (n=2). Seven patients had more than one underlying diseases.
Clinical presentation
Table 1 showed the prominent symptoms and signs in all patients with PC at diagnosis. The onset of symptoms in PC patients was generally chronic. Immunocompetent patients had PC at a younger age than immunocompromised patients (43 vs. 55 years, P=0.000). Twenty-five patients did not have any symptoms and their diseases were detected by incident radiological examination (chest radiography or CT scan). PC can cause vague symptoms such as fever, malaise, weight loss, dyspnea, or cough, or can be asymptomatic. Fever was much more common in immunocompromised patients than in immunocompetent patients (P=0.019). Only 13 patients received lumbar puncture. Nine cases out of all cases of lung infection had concomitant CNS infection. 88.9% (8/9) of these patients had elevated CSF pressure beyond 200 mm H2O. Cultures of CSF after the first lumbar puncture was positive for Cryptococcus in 4/9 (44.4%) patients (Figures 1,2,3,4 showed chest CT scans of four representative patients), and Indian ink smear was positive in 7/9 (77.8%) patients. Immunocompromised patients were more likely to have CNS involvement (3.8% vs. 20.0%, P=0.036). Initially 40 patients (83.3%) were misdiagnosed with other pulmonary diseases, including pulmonary tuberculosis, bacterial pneumonia, and lung cancer. PC were diagnosed by percutaneous lung biopsy guided by CT in 42 (66.7%) cases, TBLB in 21 (25.9%), thoracoscopy biopsies in 3 and open lung surgery in 8 (7.4%) cases. The definite diagnoses of all patients were confirmed by histopathological examination. None of these procedures were associated with major complications (Table 2).
Figure 1.
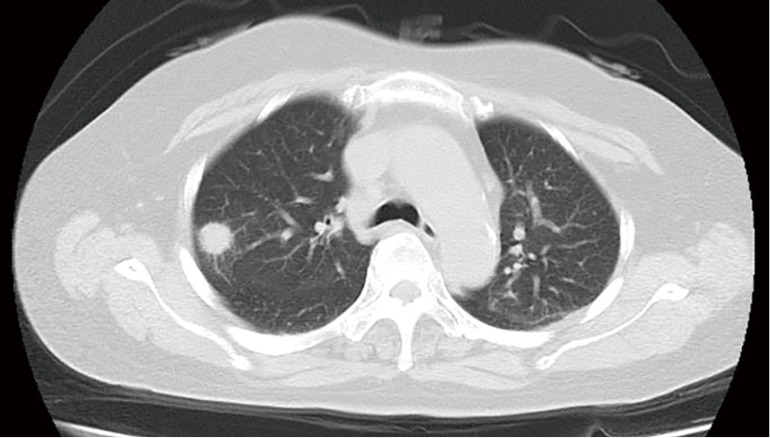
A 36-year-old immunocompetent man with PC. CT image obtained with 7-mm collimation shows smoothly marginated nodule in right lower lobe.
Figure 2.
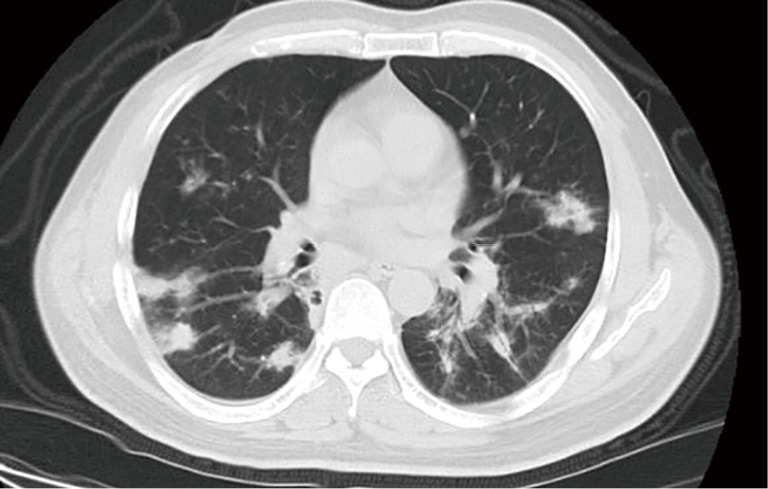
A 59-year-old woman with PC who has been diagnosed with type 2 diabetes for 16 years. CT image obtained with 7-mm collimation shows multiple ill-defined nodules predominantly in periphery of both lower lobes. Also noted are areas of halo sign.
Figure 3.
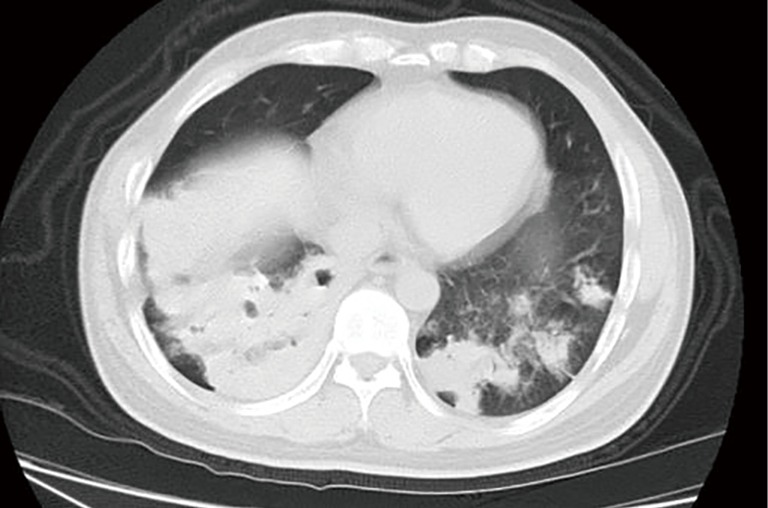
A 76-year-old woman with PC who was diagnosed with type 2 diabetes. CT image obtained with 7-mm collimation shows multifocal dense consolidation in bilateral lower lobes with cavity, multiple and ill-defined nodules in left lower lobe.
Figure 4.
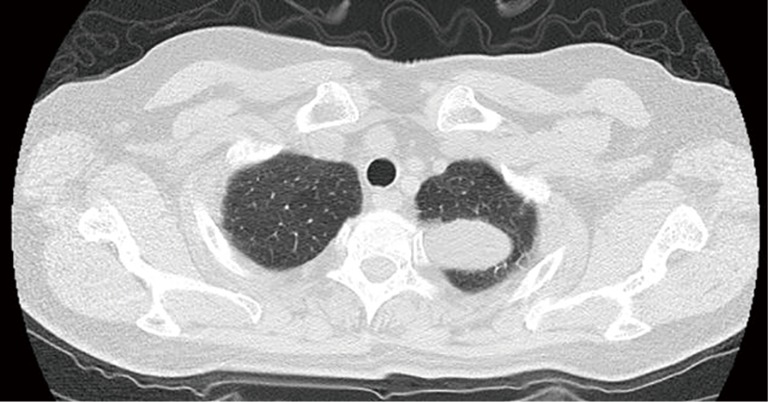
A 28-year-old immunocompetent man with PC. CT image obtained with 2-mm collimation shows well-defined shape of the mass in left upper lobes.
Table 2. Diagnostic yield of procedures in PC patients.
Radiographic findings
Abnormal chest radiographs were summarized in Tables 3,4. Lung lesions of 51.1% (45 out 88) patients were located mostly in the peripheral lung field. Lesions were usually unilaterally distributed (73.9%). The most common CT finding was pulmonary nodules which present alone or with other radiographic abnormalities. Multiple nodules were more common than solitary nodules (50.0% vs. 23.9%). Other less common associated radiographic finding included pleural effusion (3.40%), mediastinal lymphadenopathy (5.70%). Solitary nodules were more common in immunocompetent patients versus immunocompromised patients (P=0.026). Well-defined nodules were again more common in immunocompetent cases (P=0.015). The second most common findings were mass and consolidation. The presence of cavitations and halo sign were significantly frequent in immunocompromised patients than in immunocompetent patients (P<0.05). Fifty patients underwent contrast CT evaluation, with thirty-five (66.7%) showing moderate to marked enhancement, and fifteen (33.3%) showing mild and no enhancement. Brain imaging was performed in 25 patients. Meningeal enhancement occurred in 12 patients. Sixteen patients underwent 18FDG-PET.The SUV ranged from 0.9–8.0. 18FDG-PET appearances in 11patients manifested high uptakes lesions with SUV max ranged from 2.6–8.0.
Table 3. Radiological characteristics of patients with PC.
| Lesion patterns | Total (%) | Immunocompetent patients (n=53) | Immunocompromised patients (n=35) | P value |
|---|---|---|---|---|
| Nodules | 65 (73.9) | 40 | 25 | 0.673 |
| Single | 21 (23.9) | 17 | 4 | 0.026 |
| Multi | 44 (50.0) | 23 | 21 | 0.127 |
| Well-defined | 31 (35.2) | 24 | 7 | 0.015 |
| Poorly-defined | 35 (39.8) | 19 | 16 | 0.069 |
| Spiculation | 17 (19.3) | 10 | 7 | 0.895 |
| Lobulation | 9 (10.2) | 6 | 3 | 0.954 |
| Mass | 20 (22.7) | 14 | 6 | 0.310 |
| Consolidation | 20 (22.7) | 12 | 8 | 0.981 |
| Unfocal | 8 (9.1) | 6 | 2 | 0.605 |
| Mutilfocal | 12 (13.6) | 6 | 6 | 0.644 |
| Cavity | 16 (18.2) | 5 | 11 | 0.009 |
| Halo-sign | 19 (21.6) | 7 | 12 | 0.019 |
| Air bronchgram | 17 (20.5) | 13 | 4 | 0.244 |
| Pleural effusion | 3 (3.40) | 1 | 2 | 0.713 |
| Mediastinal Lymphadenopathy | 5 (5.70) | 1 | 4 | 0.155 |
Table 4. Lesions distributions in patients with PC.
| Lesions distribution | Immunocompetent patients (n=53) | Immunocompromised patients (n=35) | P value |
|---|---|---|---|
| Unilateral | 43 | 22 | 0.056 |
| Bilateral | 10 | 13 | 0.056 |
| Single lobe | 35 | 13 | 0.008 |
| Multiple lobes | 18 | 22 | 0.008 |
| Peripheral | 31 | 14 | 0.089 |
| Central location | 8 | 8 | 0.355 |
| Random distribution | 14 | 13 | 0.286 |
Treatment and outcome
Six patients were lost to follow-up. Three patients died of progression of an underlying disease. Treatments were categorized as antifungal drugs, surgery, both or clinical observation (shown in Table 5). Treatment duration ranged from 2 weeks to 1.5 years. Of 50 patients (30 immunocompetent and 20 immunocompromised patients) treated with antifungal drugs. Fluconazole was initially prescribed in 29 cases, itraconazole in 3 cases, voriconazole in 1 case, combination therapy in 18 cases. Surgical intervention were considered when the lung lesions enlarged following 3 months of standard antifungal treatment; the symptoms and signs were uncontrollable; large mass lesions limited to one lung lobe or when the lesions were difficult to differentiate from other pulmonary disease especially tumors. Open thoracotomy was performed for 9 patients, thoracoscopic surgery or video-assisted minithoractomy surgery was performed for 17 patients. Lobectomy was performed for 11 patients, pulmonary wedge resection was done for 15 patients. In recent years, the thoracoscopic procedures and atypical resection were more often performed (data not shown). There was no post-operative death. Surgery in addition to administration of antifungal drug, was performed in 12 patients (5 immunocompetent and 7 immunocompromised patients). In the 79 patients, 68 cases showed improvement (overall efficiency 86%). Deterioration occurred in 15cases who received mostly antifungal agent alone. Therapeutic management changed radically during the study period in 10 patients. Of these patients, AmBd preparation in six (with voriconazole in 2, fluconazole in 2, 5-fluorocytosine in 2), voriconazole alone in four, itraconazole in 2 cases.
Table 5. Treatment and outcome of patients with PC.
| Initially treatment methods | Immunocompetent patients (n=53) | Immunocompromised patients (n=35) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CR | PR | NC | PD | CR | PR | NC | PD | ||
| Clinical observation | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Antifugal drugs | |||||||||
| Fluconazole | 10 | 7 | 2 | 1 | 5 | 2 | 1 | 1 | |
| Itraconzole | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Voriconzole | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Drugs combination | 4 | 3 | 1 | 1 | 5 | 2 | 1 | 1 | |
| Fluconazole + 5-fluorocytosine | 3 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | |
| AmBd + 5-fluorocytosine | 1 | 1 | 0 | 0 | 4 | 2 | 0 | 1 | |
| Surgery | 9 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | |
| Pulmonary wedged excisions | 6 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
| Lobectomy | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Surgery + antifungal drugs | 5 | 0 | 0 | 0 | 6 | 0 | 1 | 0 | |
| Pulmonary wedged excisions+ antifungal drugs | 2 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | |
| Lobectomy + antifungal drugs | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
Discussion
To our knowledge, the present study represents one of the largest series to describe differences in clinical characteristics, as well as treatments and outcomes of PC compared immunocompromised and immunocompetent patients. Till now no data were available in China concerning the incidence of PC based on national epidemiological survey. Fujian province was located in the temperate and subtropical region, a climate amenable for the growth and spread of cryptococcosis. An identifiable environmental exposure is not typically apparent in our study. Compared with other countries, PC in China were of own characteristics. Notably immuocompetent patients accounted for more than half of the patients in our study, similar to the series of Zhang. The study by Zhang collected 113 reports of 728 case of PC in China mainland (2). About 69.7% patients had no underlying diseases. Our study was also consistent with study from Hong Kong (8). Data from Australia, New Zealand, and France have indicated that a relatively low proportion of non-HIV infected patients were non-immunocompromised (12,13). Genetic factors may contribute to the unusually high non-AIDS-associated PC in China (14). Why immunocompetent individuals are more susceptible to cryptococcal infections remain to be further elucidated. The most common underlying disease in current study were malignancies, the second most one was cirrhosis, possibly owing to the high prevalence of hepatitis B in southern China (15). This was very close to a study in Taiwan (16). Patients with hepatic cirrhosis have impaired cellular and humoral immunity, they may fail to eliminate the cryptococcosis when inhaled and may tend to be infected (17). Clinical manifestations of PC were highly variable and affected by host immune status (18). The typical constellation of symptoms of PC in non-AIDS patients included cough, fever, chest pain, and dyspnea (2,3). Some cases were delayed diagnosis and misdiagnosed due to the lack of specificity in clinical manifestations (3). In immunocompromised hosts, symptoms related to the systemic dissemination of the organism, predominately to the CNS.
Our data suggested that lesions of PC were mostly located in the peripheral lung field, as described in the previous reports (3). In agreement with previous CT studies, the common CT manifestations of PC were solitary or multiple pulmonary nodules (19,20). Pulmonary nodules were varied in size and margin may range from smooth to spiculated (20). Well-circumscribed pulmonary nodules represented walled-off or quiescent granulomas which implicated an effective host response against Cryptococcus species (21). Disrupted granulomatous lesions with spread to surrounding lung parenchyma represented more-advanced stages of infection and reflected an inability of the host to contain the mycose. These pathophysiologic considerations yielded important insights into several radiographic patterns in our study. Focal or multifocal airspace consolidation was the second most common radiographic pattern among non-AIDS individuals, which was also consistent with data from Taiwan and America (5,22). Cavitation and halo sign were also recognized features, but were more common seen in immunocompromised hosts (3). Pleural effusion was found in less than 5% of cases, and was small in size.
Since most hospitals in China had not yet initiated the serum cryptococcal antigen test because China Food and Drug Administration did not approve it, TBLB, CT-guided percutaneous lung biopsy, thoracoscopic biopsy or open-lung biopsy were often necessary to definitely make the histopathological diagnosis (23-25). No studies had previously compared the different diagnostic procedures head-to-head. Risks versus benefits must be considered prior to the procedure. Percutaneous lung biopsy was more commonly used in our center than other medical centers, because lesions of PC were mostly located in the peripheral of lung field (3,4). However, its utility was limited when a definitive diagnosis could not be determined. These patients often need surgical procedures which provided a large sample for histologic evaluation to determine the diagnosis (20).
The treatment of PC in non-AIDS patients in our centre largely followed the guidelines released by the Infectious Diseases Society of America, and Chinese expert consensus (1,26). We could not exactly evaluate treatment responses of each drug since regimens, dosages, duration were distinctive in the patients and confounded by the underlying conditions and follow-up time point. For mild to moderate pulmonary disease in non-AIDS patients, fluconazole had gained favor due to its ease of use and effectiveness. There were reports of favorable outcomes with observation alone in asymptomatic PC patients. However, two immunocompetent patients in our study developed cryptococcal meningitis and disseminated infection. Thus, the opinion that observation is a reasonable option for non-immunocompromised subjects with pulmonary cryptococcosis in the absence of systemic symptoms or evidence of dissemination should be debated (27). For patients with severe disease, treatment should be similar to the regimens for meningitis, amphotericin B plus flucytosine. Voriconazole have contributed to success in treatment in some refractory patients in our study (28). VATS could spare some patients with benign lesions from the risk of open thoracotomy and could be useful for wedging out lesions in patients who have limited pulmonary reserve who could not otherwise tolerate lobectomy (29). Postoperative antifungal treatment was necessary to prevent systemic dissemination when immunocompromised factors persist; lesions rupture during the surgery; the symptoms and signs relapse (30). The duration of postoperative antifungal therapy in such patients remain unknown.
Several limitations of this study deserved to be acknowledged. First, its retrospective design may introduce the possibility of unrecognized biases, incomplete data collection. Therefore, we were unable to perform a comparative analysis on the clinical presentation and risk profiles associated with individual manifestations of PC. Our patients were not evaluated or treated in a standardized manner. Some patients with cryptococcal meningitis who referred to the department of neurology in our hospital often failed to receive the pulmonary tissue biopsy, thus it may precede to bias. Second, it was not easy to clearly define an immunocompetent or immunocompromised host, and we identified immunocompromised host by clinical history according to published literature (3). Third, cryptococcus isolates were unavailable in most patients. Histological stains did not differentiate between the species of cryptococcosis. Only culture leads to cryptococcus species and variety identification. We did not know the distributions of the neoformans and the gattii in immunocompetent and immunocompromised hosts. Also, we cannot speculate that the variation in the amount of fungal exposure, and the differences in cryptococcal varieties and their virulence factors in different geographic regions might influence the difference in clinical features and course of the infection among patients.
Conclusions
PC in immunocompetent host was becoming more commonly identified in southern China. Physicians should heighten suspicious for PC although clinical and radiographic features of PC are nonspecific Selection of the optimal procedure for definitely diagnosis of PC should be based on several factors. We speculated that the differences in cryptococcal varieties and their virulence factors in different geographic regions, and the variation of host immunity might influence the difference in clinical features and course of the infection among patients. Future work on genotypic variability and its influence on phenotype as well as further characterization of the features of the host immune response should be launched.
Acknowledgements
This research was sponsored by the Natural Science Foundation of China (81370182), the National Basic Research Program (973 Program) in China (2013CB531402), Medical Scientific Research Foundation of Fujian Province (No.2012-CX-23), the Shanghai Subject Chief Scientist Program (07XD14012), and the Shanghai Leading Talent Projects (No. 036, 2010).
Ethical Statement: The study was approved by the Institutional Review Board of the First Affiliated Hospital, Fujian Medical University (No, 2014-hx-02). Patient information was anonymized and de-identified prior to analysis.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis 2010;50:291-322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang BQ, Zhang HZ, Fan BJ, et al. Meta-analysis of clinical manifestations of pulmonary cryptococcosis in China mainland. Chinese Journal of Clinical Medicine 2013;20:351-4. [Google Scholar]
- 3.Zhang Y, Li N, Zhang Y, et al. Clinical analysis of 76 patients pathologically diagnosed with pulmonary cryptococcosis. Eur Respir J 2012;40:1191-200. 10.1183/09031936.00168011 [DOI] [PubMed] [Google Scholar]
- 4.Ye F, Xie JX, Zeng QS, et al. Retrospective analysis of 76 immunocompetent patients with primary pulmonary cryptococcosis. Lung 2012;190:339-46. 10.1007/s00408-011-9362-8 [DOI] [PubMed] [Google Scholar]
- 5.Chang WC, Tzao C, Hsu HH, et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest 2006;129:333-40. 10.1378/chest.129.2.333 [DOI] [PubMed] [Google Scholar]
- 6.Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis 2001;33:690-9. 10.1086/322597 [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Alexander BD, Lortholary O, et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis 2008;46:e12-8. 10.1086/524738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lui G, Lee N, Ip M, et al. Cryptococcosis in apparently immunocompetent patients. QJM 2006;99:143-5. 10.1093/qjmed/hcl014 [DOI] [PubMed] [Google Scholar]
- 9.Choe YH, Moon H, Park SJ, et al. Pulmonary cryptococcosis in asymptomatic immunocompetent hosts. Scand J Infect Dis 2009;41:602-7. 10.1080/00365540903036212 [DOI] [PubMed] [Google Scholar]
- 10.Liu YN, She DY, Sun TY, et al. A multicentre retrospective study of pulmonary mycosis clinically proven from 1998 to 2007. Zhonghua Jie He He Hu Xi Za Zhi 2011;34:86-90. [PubMed] [Google Scholar]
- 11.Yuchong C, Fubin C, Jianghan C, et al. Cryptococcosis in China (1985-2010): review of cases from Chinese database. Mycopathologia 2012;173:329-35. 10.1007/s11046-011-9471-1 [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Sorrell T, Nimmo G, et al. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 2000;31:499-508. 10.1086/313992 [DOI] [PubMed] [Google Scholar]
- 13.Dromer F, Mathoulin S, Dupont B, et al. Epidemiology of cryptococcosis in France: a 9-year survey (1985-1993). French Cryptococcosis Study Group. Clin Infect Dis 1996;23:82-90. 10.1093/clinids/23.1.82 [DOI] [PubMed] [Google Scholar]
- 14.Hu XP, Wang RY, Wang X, et al. Dectin-2 polymorphism associated with pulmonary cryptococcosis in HIV-uninfected Chinese patients. Med Mycol 2015;53:810-6. 10.1093/mmy/myv043 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Jia J. Control of hepatitis B in China: prevention and treatment. Expert Rev Anti Infect Ther 2011;9:21-5. 10.1586/eri.10.143 [DOI] [PubMed] [Google Scholar]
- 16.Tseng HK, Liu CP, Ho MW, et al. Microbiological, epidemiological, and clinical characteristics and outcomes of patients with cryptococcosis in Taiwan, 1997-2010. PLoS One 2013;8:e61921. 10.1371/journal.pone.0061921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Husain S, De Vera M, et al. Cryptococcus neoformans Infection in Patients With Cirrhosis, Including Liver Transplant Candidates. Medicine (Baltimore) 2004;83:188-92. 10.1097/01.md.0000126760.45299.69 [DOI] [PubMed] [Google Scholar]
- 18.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One 2013;8:e60431. 10.1371/journal.pone.0060431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadrous HF, Antonios VS, Terrell CL, et al. Pulmonary cryptococcosis in nonimmunocompromised patients. Chest 2003;124:2143-7. 10.1016/S0012-3692(15)31671-8 [DOI] [PubMed] [Google Scholar]
- 20.Lindell RM, Hartman TE, Nadrous HF, et al. Pulmonary cryptococcosis: CT findings in immunocompetent patients. Radiology 2005;236:326-31. 10.1148/radiol.2361040460 [DOI] [PubMed] [Google Scholar]
- 21.Shibuya K, Hirata A, Omuta J, et al. Granuloma and cryptococcosis. J Infect Chemother 2005;11:115-22. 10.1007/s10156-005-0387-X [DOI] [PubMed] [Google Scholar]
- 22.Baddley JW, Perfect JR, Oster RA, et al. Pulmonary cryptococcosis in patients without HIV infection: factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis 2008;27:937-43. 10.1007/s10096-008-0529-z [DOI] [PubMed] [Google Scholar]
- 23.Lee LN, Yang PC, Kuo SH, et al. Diagnosis of pulmonary cryptococcosis by ultrasound guided percutaneous aspiration. Thorax 1993;48:75-8. 10.1136/thx.48.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. 10.1164/rccm.200612-1866OC [DOI] [PubMed] [Google Scholar]
- 25.Wagnetz U, Menezes RJ, Boerner S, et al. CT screening for lung cancer: implication of lung biopsy recommendations. AJR Am J Roentgenol 2012;198:351-8. 10.2214/AJR.11.6726 [DOI] [PubMed] [Google Scholar]
- 26.Editorial Board of Chinese Journal of Mycology. Chinese expert consensus statement on management of cryptococcal infection. Chin J Myco 2010:5:65-8. Available online: http://www.cnki.com.cn/Article/CJFDTotal-ZJXZ201002000.htm
- 27.Aberg JA. Pulmonary cryptococcosis in normal hosts: treat or observe? Chest 2003;124:2049-51. 10.1378/chest.124.6.2049 [DOI] [PubMed] [Google Scholar]
- 28.Scott LJ, Simpson D. Voriconazole: a review of its use in the management of invasive fungal infections. Drugs 2007;67:269-98. 10.2165/00003495-200767020-00009 [DOI] [PubMed] [Google Scholar]
- 29.Xie D, Chen XF, Jiang GN, et al. Clinical analysis of 81 cases of pulmonary cryptococcosis. Zhonghua Wai Ke Za Zhi 2012;50:430-3. [PubMed] [Google Scholar]
- 30.Zhou J. Discussion of the treatments after surgery in nonimmunosuppressed pulmonary cryptococcosis patients. PhD thesis, Zhejiang University, 2013. Available online: http://d.wanfangdata.com.cn/Thesis/y2421124


