Abstract
Background
There have been several published reports on the use of orally administered, specific centrally acting medicines for the treatment of idiopathic cough; however, there is no extant systematic review of randomized controlled trials (RCTs) that evaluated their efficacy and safety for the treatment of idiopathic cough in human beings.
Methods
We conducted a series of definitive systematic reviews and meta-analyses of RCTs. Claims data from the MEDLINE, EMBASE, LILACS, CBM, CNKI, VIP, Wan Fang, and Cochrane Library databases were used. We also reviewed articles and reference lists of relevant articles pertaining to human subjects published prior to March 26, 2016. No language restrictions were imposed. Two authors independently reviewed the titles and abstracts of the retrieved studies, which were matched using Review Manager 5.3 software. Disagreements were resolved by consensus. The outcome data were the number of subjects whose symptoms declined, measured by cough or Leicester Cough Questionnaire (LCQ) score. Random effect meta-analyses were used to pool the findings. Publication bias was assessed using funnel plots.
Results
Three RCTs, regarding the medicines baclofen, amitriptyline, and gabapentin, were conducted involving 92 persons in total. Our reviews confirmed that baclofen, amitriptyline, and gabapentin show promise in the treatment of cough for select cases of refractory chronic cough. After-treatment relief of cough symptoms was significant (risk ratio =2.41; 95% CI: 1.15–5.04, n=84). Each of the medicines was well tolerated with minimal side effects. Methodological biases in the design and execution of cluster randomized trials might contribute to any selection bias in this review.
Conclusions
Baclofen, amitriptyline, and gabapentin may be effective ‘non-specific’ antitussives in clinical settings, although none of them are used in medical assessments or routinely included in the anatomic diagnostic protocol.
Keywords: Cough, neurotransmitter agents, anti-depressive agents, randomized controlled trial (RCT), drug therapy, oral medicine
Cough is one of the most distressing symptoms in patients with cough hypersensitivity syndrome (CHS), which emphasizes cough reflex hypersensitivity as a key feature (1). Chronic cough can be controlled by addressing the “cause” of the cough, although this approach is not always successful. For the majority of patients, a systematic investigation reveals an underlying cause, usually asthma, upper airway disorders, gastroesophageal reflux, or various combinations of these conditions. Subsequent treatments specific to the underlying cause usually lead to an improvement or resolution of the cough. However, in a minority of patients, no underlying cause can be identified, despite appropriate investigation. Their coughs do not respond to conventional medications and are referred to as “refractory coughs”. These cases are often a medical challenge. Non-specific antitussives are needed for symptomatic relief in these patients with idiopathic cough and for non-responders to treatments of the cause(s).
Since the increased sensitivity of airway sensory nerves is important in the pathogenesis of an idiopathic or refractory cough, an ideal antitussive would effectively reduce this increased sensitivity to normal levels, without significant adverse effects. Unfortunately, the antitussives currently available fall short of this expectation in two aspects. First, antitussives such as opioids are not consistently effective, for example, on coughs induced by upper airway disorders. Second, they achieve therapeutic effect at the expense of unpleasant or intolerable side effects. Treatment with anesthetics or sedatives causes significant drowsiness and giddiness, affecting the patient’s quality of life. Safer and more effective cough suppressants against disorders of any etiology are desperately needed.
There has been some progress in the new therapeutic options of orally administered, specific neuromodulators for ‘non-specific’ antitussives (2). However, there is a paucity of well-controlled clinical studies documenting evidence for the use of many of the drug classes today. Moreover, the current study is limited by a relatively small sample size, and there is no systematic review of studies for antitussive therapies. Thus, we conducted a retrospective study to evaluate the efficacy and safety of specific, orally administered centrally acting medicines in the treatment of' idiopathic cough.
Methods
Literature search
Electronic searches
Two authors independently searched the PUBMED, EMBASE, CENTRAL, LILACS, CBM, CNKI, VIP, and Wang fang databases and the master and doctor thesis database using the search strategies detailed in Figure 1. Studies were published before March 26, 2016, and studies were confined to humans.
Figure 1.
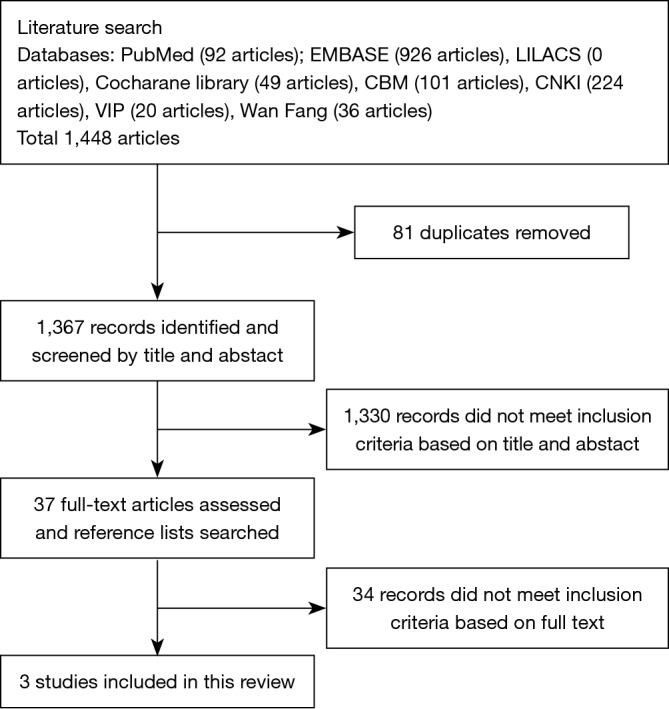
Flowchart for study selection.
Searching other resources
The references of identified studies were also manually searched for additional studies.
Screening of literature
Inclusion criteria
(I) Types of studies: all randomized controlled clinical trials were included; (II) types of participants: patients with chronic cough who had a history consistent with a daily dry, nonproductive cough lasting longer than 8 weeks’ duration with a negative chest X-ray or an unremarkable physical examination with minimal to no positive computer tomography before presentation. All participants were either nonsmokers or ex-smokers of at least ten years without chronic obstructive pulmonary diseases, and all had no history of asthma or positive responses to treatments for asthma. Patients on angiotensin-converting enzyme inhibitors or infection (had a cough that was producing purulent sputum) were excluded from the study; (III) types of interventions: centrally acting medicines except for anesthetics or sedatives delivered orally and trials of crossover design were included if all participants had equal access to such medications; (IV) types of outcome measures: proportions of participants whose cough symptom or LCQ score decreased.
Exclusion criteria
(I) Data were abnormal or incomplete, or data extraction was impossible; (II) various studies focused on the sample patient population or the same data were used repeatedly; studies with incomplete data; (III) nonhuman studies; (IV) review, case report, comment, meeting minutes and studies with incomplete publication; (V) studies with incomplete or unclear information.
Data extraction and analysis
Selection of studies
The literatures was entered into EndnoteX5, and repeated literature was excluded. Then, two authors screened the remaining literature independently. Initial screening was conducted by reviewing the titles and abstracts, which was confirmed by reading the full text and then cross-checked, and any discrepancy was resolved by discussion with a third author. The full texts of potential studies for inclusion were obtained to decide if they should be included.
Data extraction and management
Trial data were extracted by both review authors and entered into the Cochrane Collaboration software program Review Manager. Data were entered for individual trials. The study setting, patient recruitment, numbers of patients, dose and type of therapy, and side effects were noted (Table 1).
Table 1. Characteristics of included studies.
| Study | Age, y, mean (range) | Type of study | Location | Patients | Num | Type of outcome | Control group or arm | Medicine dose/day | Therapy duration | Side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Ryan 2012 | 61–63 | Randomized double-blind placebo-controlled | Australia | refractory chronic cough | 62 (52 completed) | +clinical improvement in LCQ score =20:12 mean VAS score (cm) =36:42 | Placebo (n=26) | Gabapentin in maximum tolerable of 1,800 mg (n=26) | 12 weeks | Nausea, stomach pain, fatigue, dizziness, dry mouth |
| Jeyakumar 2006 | 55 | Randomized | USA | chronic cough resulting from postviral vagal neuropathy | 28 | # Patients’ subjective cough responses: complete response =11:0 partial response =3:3 no response =1:10 QOL data correlated strongly with the patients’ subjective responses | Codeine/guaifenesin (n=13) | Amitriptyline 10 mg qn (n=15) | 10 days | No |
| Dicpinigaitis 1998 | 37–69 | Randomized double-blind placebo-controlled, cross-over | USA | refractory cough | 2 | Cough episodes per 24 hours by diaries documenting 2:0 | Placebo (n=2) | Baclofen 10 mg tid (n=2) | 28 days | No |
#, those patients experiencing a 75% to 100% cough reduction were recorded as having a complete response, 25% to 50% a partial response, and 0% as having no response; LCQ, Leicester cough questionnaire; QOL, quality-of-life; +, a clinical improvement in LCQ score of greater than 1∙3 the smallest change in score regarded as clinically meaningful; VAS, visual analogue scale.
Assessment of risk of bias in included studies
Studies that met the inclusion criteria underwent quality assessment, which was performed independently by both review authors using two methods. The first method involved using the Cochrane approach to assess concealment of allocation. Trials were scored using the following principles.
Grade A: adequate concealment.
Grade B: unclear concealment.
Grade C: clearly inadequate concealment.
Second, each study was also assessed using a 1 to 5 scale as described by Jaded 1996 and summarized as follows.
Was the study described as randomized? (1= yes; 0= no).
Was the study described as double blind? (1= yes; 0= no).
Was there a description of withdrawals and dropouts? (1= yes; 0= no).
Was the method of randomization clearly described and appropriate? (1= yes; 0= no).
Was the method of double blinding well described and appropriate? (1= yes; 0= no).
One point was deducted if the methods for randomization or blinding were inappropriate.
Data synthesis
All included trials were entered into Review Manager 5.3 software.
Statistical analysis
The extracted data were subjected to a meta-analysis. For dichotomous variables, individual and pooled statistics were calculated as relative risks with 95% confidence intervals. For continuous outcomes, individual and pooled statistics were calculated as weighted mean differences or standard mean differences, as indicated, with 95% confidence intervals. Heterogeneity was examined with the Homogeneity test (Q test; α=0.1) and quantified with I2. A P of ≥0.10 and I2 of ≤50% suggested that the homogeneity and fixed effects model was appropriate for meta-analysis. In contrast, if there was heterogeneity, and a random effects model was used. Publication bias was tested with Eggers’ test.
Subgroup analysis
Because there were few available articles, a subgroup analysis was not conducted according to the factors potentially affecting the heterogeneity among studies.
Assessment of clinical heterogeneity
The trial characteristics that may influence the observed treatment effect were examined. Clinical heterogeneity was investigated using a sensitivity analysis.
Sensitivity analysis
After removing the studies with the lowest quality, the combined value was re-calculated for the analysis of sensitivity with α=0.05. Two factors were investigated: (II) publication bias, examined using a funnel plot; and (II) the effects of overall trial quality on the pooled result, examined using both the Cochrane approach and that of Jaded 1996.
Results
Results of the search
Electronic searches retrieved a total of 1,448 citations. We removed 81 duplicated articles and excluded 1,330 after screening the abstracts. Thirty-seven full-text articles were assessed, and reference lists were searched (Figure 1). The last three studies met the inclusion criteria of the review. The studies were about baclofen, amitriptyline and gabapentin. A detailed description of the studies is provided (Table 1). Publication bias was tested with Eggers’ test (Figure 2).
Figure 2.
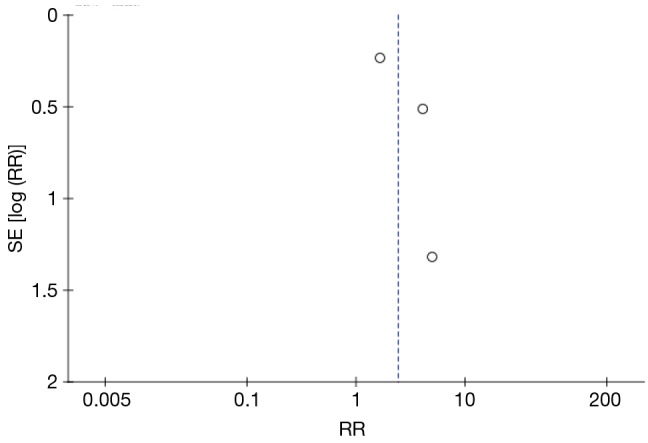
Funnel plot of oral specific neuromodulators.
Effects of interventions
Subjects
Patient characteristics are summarized in Table 2. The majority of subjects were female and middle aged, as is typical of patients presenting with refractory cough. At screening, subjects were highly sensitive to capsaicin; the cough reflex sensitivity defined by the quantity of capsaicin needed to induce five coughs LogC5 was −0.3 to 0.80. Ten participants withdrew from the study, and 82 completed it. The effective fill participants were 84 person-times [arm of specific neuromodulators, n=49; arm of control medicine Codeine/Guaifenesin (3), n=13; arm of control medicine placebo (4,5), n=32]. Of all patients screened and randomized, four withdrew because of possible side-effects, two were lost to follow-up after the first treatment period, two perceived lack of efficacy, one received treatment for comorbidity and one withdrew for personal reasons.
Table 2. Baseline characteristics of randomized subjects.
| Demographics | Content |
|---|---|
| Age (mean range) | 49.7–62.7 |
| Male, no. (%) | 36 (39.1) |
| Cough duration (months) | 12–156 |
| Smoking history, no. (%) | 92 (100.0) |
| Never smoked, no. (%) | 65 (70.7) |
| Ex-smoker, no. (%) | 27 (29.3) |
| Perform pulmonary function (%) | 63 (68.5) |
| Normal spirometry, no. (%) | 62 (67.4) |
| FEV1/FVC <70% predicted | 0 (0) |
| Efficacy variables | 92 (100.0) |
| Symptom, no. (%) | 92 (100.0) |
| LCQ score, no. (%) | 90 (97.8) |
| CRS C5 (ìM), no. (%) | 64 (70.1) |
| LogC5 (μm) | −0.3 to 0.80 |
| Endoscopy, no. (%) | 29 (31.5) |
| FeNO (ppb), no. (%) | 62 (67.4) |
| History of nasal allergy, no. (%) | 5 (5.4) |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CRS C5, cough reflex sensitivity defined by quantity of capsaicin needed to induce five coughs; symptom, cough frequency and severity (VAS score or 4-point Likert-type scale); FeNO, fraction of exhaled nitric oxide, ppb, parts per billion.
Objective cough frequency, severity and LCQ score
There was no significant difference between cough count profiles between the control and specific central antitussives based on a negative binomial model, with fixed-effects terms for subject and period baselines, treatment, period, and time after dosing with a treatment by time after dosing interaction and subject as random effects. There appeared to be significant differences in either cough frequency or cough severity in comparing baclofen and gabapentin with placebo or amitriptyline with codeine/guaifenesin (Figures 3,4,5). Similarly, LCQ scores suggested significant improvement with gabapentin and amitriptyline compared with the control (Figures 4,5).
Figure 3.
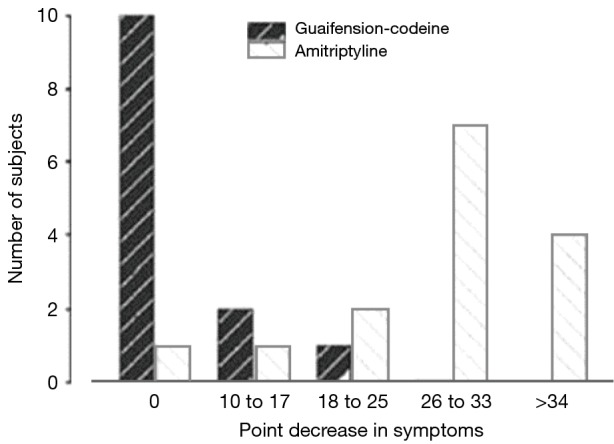
Bar chart showing the difference in response rates between amitriptyline and codeine/guaifenesin in pre- and posttreatment cough quality of life.
Figure 4.
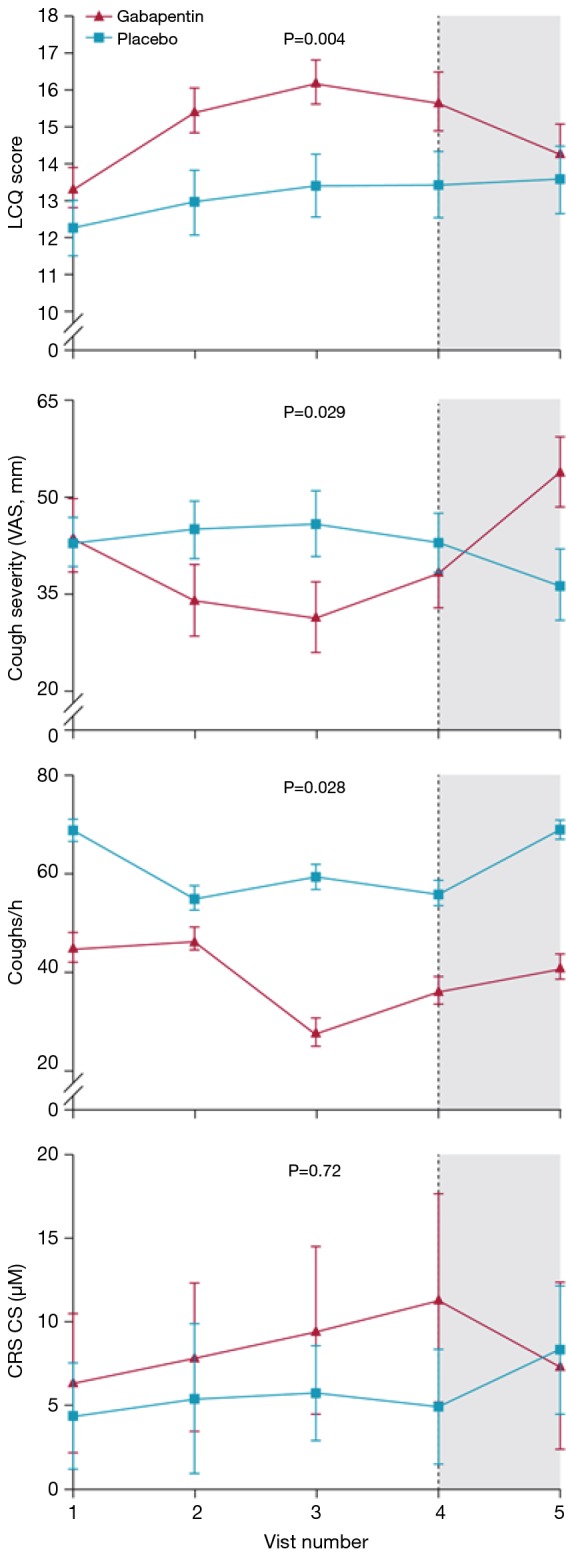
Mean efficacy variable scores for gabapentin versus placebo during and after treatment. Dose was escalated from days 1–6 and reduced from days 78–83. Treatment was stopped completely by visit 4 (dotted line). p values represent the significance of the differences between gabapentin and placebo.
Figure 5.
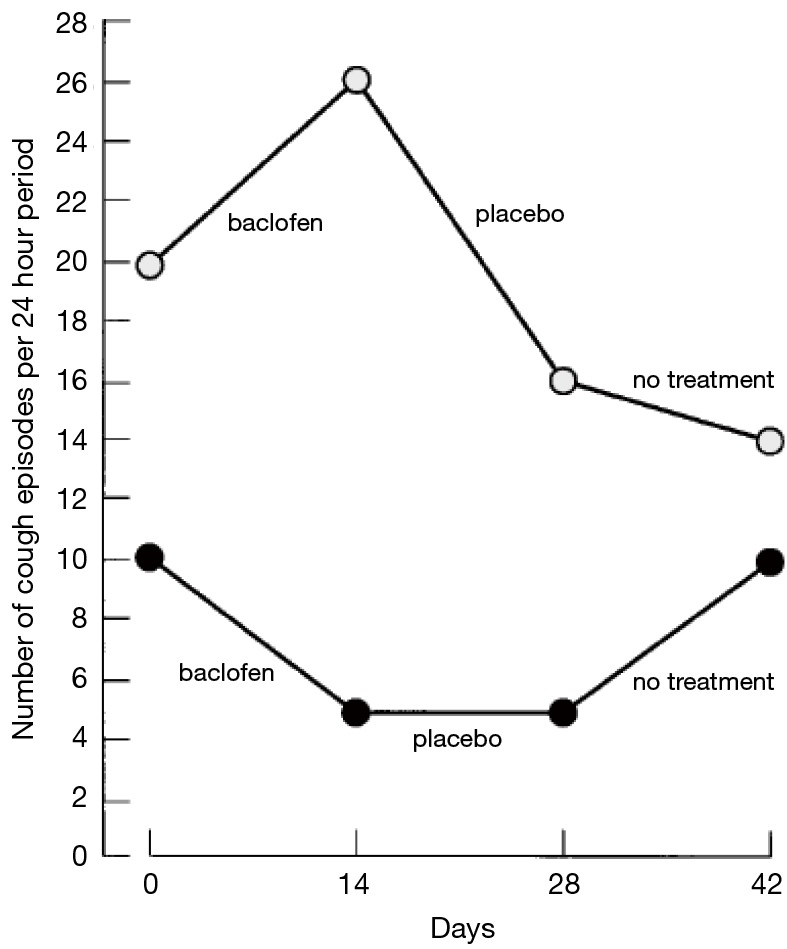
Daily number of coughing episodes during the 4-week treatment period (day 0–28) and the subsequent 2 weeks (day 28–42). Subject 1 received baclofen on days 0–14 and placebo on days 14–28. Subject 2 received placebo on days 0–14 and baclofen on days 14–28.
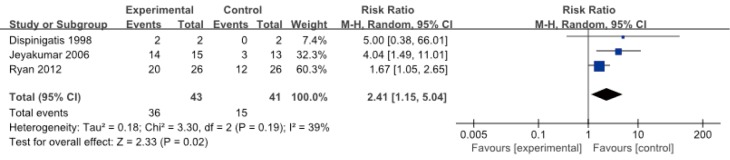
After treatment, the results favor specific neuromodulators overall (risk ratio =2.41, 95% CI: 1.15–5.04, n= 84) (Figure 6). However, the positive effects were not maintained 2 to 4 weeks after drug cessation according to the same study measurements. Cough frequency eventually returned to the previous baseline levels (4,5), and even cough severity (VAS score) significantly increased to greater than baseline values (4) (Figure 4), suggesting a possible treatment-by-period interaction.
Figure 6.
Plot of oral specific neuromodulators vs. control medicines
Capsaicin cough responses
After the placebo treatment period, a reduction of cough sensitivity to inhaled capsaicin was not sustained after treatment in both studies (4,5). After 14 days of baclofen therapy, the capsaicin cough threshold increased by three to five doubling concentrations (8- to 32-fold) in the subjects (5) (Figure 7). The reduction in efficacy of baclofen and gabapentin after withdrawal further supports its antitussive effect.
Figure 7.
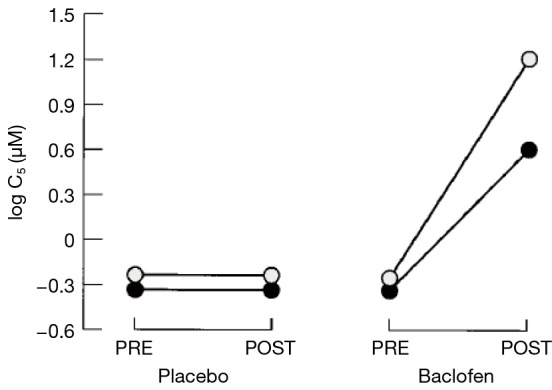
Cough sensitivity to inhaled capsaicin before and after a 14-day course of baclofen and placebo. C5 = concentration of capsaicin inducing 5 or more coughs.
Adverse events
No adverse effects of baclofen or amitriptyline were reported. Only one study mentioned side effects, in which ten (31%) of 32 patients assigned gabapentin had one or more adverse effects compared with three (10%) of 30 assigned placebos (P=0.059). To manage the adverse effects, the dose was temporarily reduced [in six (19%) in the gabapentin group vs. three (10%) in the placebo group] or patients were withdrawn from the study [one (3%) vs. one (3%)] (Table 3).
Table 3. Adverse events of gabapentin.
| Adverse effects | Gabapentin (n=17) | Placebo (n=6) |
|---|---|---|
| Blurred vision | 1 (6%) | 0 |
| Depression | 0 | 1 (17%) |
| Disorientation, confusion | 2 (12%) | 0 |
| Dizziness | 3 (18%) | 1 (17%) |
| Dry or very dry mouth | 2 (12%) | 1 (17%) |
| Fatigue | 3 (18%) | 1 (17%) |
| Headache | 1 (6%) | 0 |
| Memory loss | 1 (6%) | 0 |
| Nausea, stomach pain | 4 (24%) | 2 (33%) |
Data are number of events (%); n, total number of events associated with study drug.
Discussion
Main findings
Based on randomized controlled trials (RCTs), we conducted an interdisciplinary meta-analysis of particular neuromodulators that have shown promising results in relieving sensory neuropathic cough. What do baclofen, amitriptyline and gabapentin have in common? They are all used to treat conditions in addition to those they were originally developed to treat. Drug repositioning, or repurposing, describes the process of deploying therapeutics to new indications. The impetus to find additional applications for currently prescribed drugs, as well as novel uses for shelved compounds, is becoming increasingly important as the drug development pipeline dwindles.
A major obstacle to the development of such therapy has been an imprecise understanding of the pathophysiological mechanisms responsible for cough. There have been remarkable advances recently in our understanding of the neuroregulation of cough in three areas: the properties of the sensory nerves, in particular their receptors and membrane channels; the plasticity of the pathways; and the central nervous mechanisms of cough. All these studies are relevant to our understanding of the pharmacology and therapy of cough. The cough reflex is mediated by the stimulation of the vagal primary afferent nerve distributed along the tracheobronchial tree (6) as well as in the extra pulmonary areas (larynx, trachea, main stem bronchi), although it is evoked by various stimuli (7). Studies indicate that rapidly adapting receptors of the vagal subserve a primary role in the cough reflex (8). Chronic refractory cough has factors in common with laryngeal hypersensitivity syndromes and chronic pain syndromes, and these similarities help to shed light on the pathophysiology of the condition. Its pathophysiology is complex and includes cough reflex sensitivity, central sensitization, peripheral sensitization, and paradoxical vocal fold movement. During the past decade, several treatments have been developed. These include centrally acting neuromodulators such as gabapentin (9).
The cornerstone of cough-relieving treatment is connected to the pharmacological treatment of pain. A multidisciplinary approach has been found in pain management, in which psychologic and physical approaches have been increasingly employed and validated as valuable modalities in the relief of pain. The focus has shifted from simply applying pain-relieving modalities to helping patients develop cough-relieving treatment. General anesthetics produce loss of consciousness and pain reduction, for example, and the cannabinoids can be useful in the management of neuropathic pain, spasticity due to multiple sclerosis, and possibly other indications (10). However, opioid analgesics and nonnarcotic analgesics—all of which are currently used in human antitussive therapy—were not included in our study. Only novel targets that may result in effective antitussives have been identified.
GABAB receptor antagonist
The muscle relaxant baclofen is a GABA agonist that inhibits spinal cord reflexes resulting in the relief of painful muscle spasms. Functional GABAB receptor heterodimer is composed of two subunits, GB1 and GB2 subunits. Each subunit contains an extracellular domain, seven transmembrane domains and an intracellular domain. GABAB agonists and competitive antagonists bind to the agonist-binding site of GABAB type 1 subunit (Venus flytrap, VFT) (11). Baclofen focuses on the presynaptic GABAB receptors to increase their activity with a secondary inhibitory effect on the presynaptic release of glutamate (12). GABA and glycine-gated channels are inhibitory. Activation of these channels causes membrane hyperpolarization inhibiting electrical activity. The GABA-gated channels are permeable to the anion Cl. The major excitatory ion channels are glutamate and acetylcholine. Activation of these channels causes depolarization and increased electrical activity. The GABAB receptor antagonist baclofen has been proved to suppress cough in cats with no effect on breathing pattern (13). Baclofen has shown good evidence that GABA8 receptors inhibit the activity of peripheral sensory afferent nerve fibers such as pulmonary C-fibers (14).
GABAB receptor agonists have also been shown to possess antitussive effects in patients and in animals independent of their effects on transient lower esophageal sphincter relaxation (TLESR; the major cause of reflux) (15,16). Use of GABAB receptor agonists such as baclofen acting both in the central nervous system and peripheral tissues may represent novel therapeutic approaches (2,17). Baclofen also has been used in both experimental models (18) and in humans (19,20) with promising results.
GABA agonists inhibit various responses in the airways, including cholinergic and tachykinin-mediated smooth muscle contraction, microvascular leakage, anaphylactic bronchospasm and cough in guinea pigs (18). Use of baclofen to suppress cough induced by angiotensin-converting enzyme inhibitors and a diminished cough reflex has been associated with increased risk of developing aspiration pneumonia in stroke patients (14) and in the elderly (19). Gamma-aminobutyric acid (GABA), an inhibitory transmitter of the central nervous system, is also found in peripheral tissues, including the lungs (17). With oral administration, initiation of therapeutic effects may require 3 to 5 days, and maximal clinical effects may not be observed until 5 to 10 days. Oral baclofen may produce sedation, hypotonia, and gastric upset. It was demonstrated that a 14-day course of baclofen given 20 mg daily significantly inhibits the cough reflex in healthy volunteers, but not 10 mg and not even a 28-day course (5).
Amitriptyline
Amitriptyline, a tricyclic antidepressant, inhibits 5-hydroxytryptamine (5-HT) uptake and was an effective antidepressant drug. The content and functionality of 5-HT in the central nervous system may be associated with the onset of psychosis. It is generally believed that increased 5-HT is the key to the treatment of depression. It is a neurotransmitter, mainly distributed in the pineal gland and the hypothalamus, and may be involved in pain. Chronic pain and depression are associated with decreased serotonin in the CNS. The interaction of vagal afferent nerve subtypes and airway function, made possible by their convergence at key sites of integration in the brainstem, may lead to central sensitization analogous to that described in somatic pathways regulating pain sensation. Antidepressants increase the synaptic concentration of serotonin or norepinephrine by inhibiting their reuptake at the presynaptic neuronal membrane.
The use of antidepressants to potentiate analgesic activity and to reduce depression is an effective therapeutic adjunct, particularly in patients for whom pain and depression coexist. Analgesic effects of tricyclics are independent of and may occur sooner than their antidepressant effects. Therapeutic effects may not occur for more than 2 to 3 weeks; therefore, dosage must be optimized for 3 to 4 weeks before discontinuing the medication trial (21).
Gabapentin
Gabapentin is a lipophilic structural analog of the neurotransmitter gamma aminobutyric acid, which is proven to have a central action. Although structurally related to γ-aminobutyric acid (GABA), gabapentin is functionally inactive at GABAA, GABAB, and benzodiazepine receptors and is not converted metabolically into GABA or a GABA receptor agonist (9). It is known to act on the alpha 2 and delta receptor of calcium channels, inhibiting the release of neurotransmitters such as substance P, a tussigenic agent, and possibly inhibiting N-methyl-D-aspartate receptors (22).
Gabapentin is known to be effective in treating neuropathic pain with central sensitization. Patients with post herpetic neuralgia treated with once-daily gastroretentive gabapentin provide in-depth insight into how disease characteristics may influence the effectiveness of treatment and help describe complex interactions among reductions in pain intensity, interference of pain with various aspects of patient function, and overall improvement (23). Refractory cough is known to have a central sensitization mechanism similar to neuropathic pain. It shows features such as abnormal throat sensation (laryngeal paresthesia), increased cough sensitivity to tussigens (hyperdipsia), and cough triggered by nontussive stimuli such as cold or talking. Reports have shown gabapentin to be effective in sensory laryngeal neuropathy and symptom conditions that have a proven neural origin (24). There was a significant improvement in cough related quality of life with the use of gabapentin (4).
Gabapentin should be used with caution because it has pharmaceutical properties. It has rapid dose-dependent absorption with peak levels 2 to 3 hours after administration, and the effective dose is much higher than that of specific calcium channel blockers because of its low specificity for calcium ion channels. In a study conducted during the early postoperative period after coronary artery bypass graft surgery, therapy duration efficiency of reduced pain intensity did not significantly differ between the groups at 1 and 3 months (25). The common side effects of gabapentin include dizziness, fatigue, headache, and confusion, which were not reported in all studies (4).
In summary, there are few effective current treatments for refractory cough with an acceptable therapeutic ratio, and more selective drugs with a more favorable side effect profile are needed.
Limitations
Although the current literature shows promise for these neuromodulators in the treatment of cough, further investigation is warranted. Our review highlighted RCTs, but the current study was limited by a relatively small sample size. Publication bias and selective reporting bias lead to data that are not missing in RCT studies. There was considerable variation in the quality of articles (Figure 2). The use of different outcome measures may have introduced some heterogeneity.
Conclusions
We confirm the recent observation that baclofen, amitriptyline and gabapentin may be effective 'non-specific' antitussives in the clinical setting. Different methodologies might have contributed to a selection bias in this result.
Significance
In addition to providing insights into the treatment of specific neuromodulators on refractory chronic cough in humans, this study also has important implications for the design of future studies testing novel neuromodulator agents. First, human subjects with refractory cough take advantage of oral baclofen, amitriptyline and gabapentin for decreased cough reflex sensitivity, which highlights novel therapeutic options for a CHS. Physicians should prescribe drugs that inhibit neural propagation. In addition to the medicine mentioned above, effective cough treatments may include other potential medications used in the neurological and psychiatric fields, such as botulinum toxin and the selective serotonin reuptake inhibitor paroxetine. Second, sensory neuropathic cough is emerging as a distinct clinical entity. Therefore, there is a need to explore the use of neuromodulatory agents in symptom conditions which are known to have a neural origin such as pruritus and hiccoughs.
Acknowledgements
We would like to thank Prof Chunbo Li from the Shanghai Key Laboratory of Psychotic Disorders, Shanghai Mental Health Center, School of Medicine, Shanghai Jiao Tong University, for professional advice.
Funding: The research was supported by the Shanghai Municinal Health and Family Planning Commission on the Project (201440536).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Birring SS, Kavanagh J, Lai K, et al. Adult and paediatric cough guidelines: Ready for an overhaul? Pulm Pharmacol Ther 2015;35:137-44. 10.1016/j.pupt.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 2.Chung KF. NMDA and GABA receptors as potential targets in cough hypersensitivity syndrome. Curr Opin Pharmacol 2015;22:29-36. 10.1016/j.coph.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Jeyakumar A, Brickman TM, Haben M. Effectiveness of amitriptyline versus cough suppressants in the treatment of chronic cough resulting from postviral vagal neuropathy. Laryngoscope 2006;116:2108-12. 10.1097/01.mlg.0000244377.60334.e3 [DOI] [PubMed] [Google Scholar]
- 4.Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. 10.1016/S0140-6736(12)60776-4 [DOI] [PubMed] [Google Scholar]
- 5.Dicpinigaitis PV, Rauf K. Treatment of chronic, refractory cough with baclofen. Respiration 1998;65:86-8. 10.1159/000029232 [DOI] [PubMed] [Google Scholar]
- 6.Ryan NM, Gibson PG, Birring SS. Arnold's nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy. J Thorac Dis 2014;6:S748-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough: CHEST Guideline and Expert Panel report. Chest 2014;146:1633-48. 10.1378/chest.14-1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung KF, Widdicombe JG, Boushey HA. Cough: Causes, Mechanisms and Therapy. Blackwell Publishing Ltd 2003:3-10. [Google Scholar]
- 9.Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ 2015;351:h5590. 10.1136/bmj.h5590 [DOI] [PubMed] [Google Scholar]
- 10.Grant I, Atkinson JH, Gouaux B, et al. Medical marijuana: clearing away the smoke. Open Neurol J 2012;6:18-25. 10.2174/1874205X01206010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones KA, Borowsky B, Tamm JA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 1998;396:674-9. 10.1038/25348 [DOI] [PubMed] [Google Scholar]
- 12.Lozano R, Hare EB, Hagerman RJ. Modulation of the GABAergic pathway for the treatment of fragile X syndrome. Neuropsychiatr Dis Treat 2014;10:1769-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Widdicombe J. Neuroregulation of cough: implications for drug therapy. Curr Opin Pharmacol 2002;2:256-63. 10.1016/S1471-4892(02)00152-2 [DOI] [PubMed] [Google Scholar]
- 14.Prager EM, Bergstrom HC, Wynn GH, et al. The basolateral amygdala γ-aminobutyric acidergic system in health and disease. J Neurosci Res 2016;94:548-67. 10.1002/jnr.23690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv HJ, Qiu ZM. Refractory chronic cough due to gastroesophageal reflux: Definition, mechanism and management. World J Methodol 2015;5:149-56. 10.5662/wjm.v5.i3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung KF. Effective antitussives for the cough patient: an unmet need. Pulm Pharmacol Ther 2007;20:438-45. 10.1016/j.pupt.2006.10.015 [DOI] [PubMed] [Google Scholar]
- 17.Ong J, Kerr DI. GABA-receptors in peripheral tissues. Life Sci 1990;46:1489-501. 10.1016/0024-3205(90)90421-M [DOI] [PubMed] [Google Scholar]
- 18.Hoang BX, Levine SA, Graeme Shaw D, et al. Bronchial epilepsy or broncho-pulmonary hyper-excitability as a model of asthma pathogenesis. Med Hypotheses 2006;67:1042-51. 10.1016/j.mehy.2006.05.014 [DOI] [PubMed] [Google Scholar]
- 19.Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke: an interhospital comparison. Stroke 1999;30:1203-7. 10.1161/01.STR.30.6.1203 [DOI] [PubMed] [Google Scholar]
- 20.Sekizawa K, Ujiie Y, Itabashi S, et al. Lack of cough reflex in aspiration pneumonia. Lancet 1990;335:1228-9. 10.1016/0140-6736(90)92758-A [DOI] [PubMed] [Google Scholar]
- 21.Zeltzer LK, Bush JP, Chen E, et al. A psychobiologic approach to pediatric pain: Part II. Prevention and treatment. Curr Probl Pediatr 1997;27:264-84. [DOI] [PubMed] [Google Scholar]
- 22.Kimos P, Biggs C, Mah J, et al. Analgesic action of gabapentin on chronic pain in the masticatory muscles: a randomized controlled trial. Pain 2007;127:151-60. 10.1016/j.pain.2006.08.028 [DOI] [PubMed] [Google Scholar]
- 23.Kantor D, Panchal S, Patel V, et al. Treatment of Postherpetic Neuralgia With Gastroretentive Gabapentin: Interaction of Patient Demographics, Disease Characteristics, and Efficacy Outcomes. J Pain 2015;16:1300-11. 10.1016/j.jpain.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 24.Atreya S, Kumar G, Datta SS. Gabapentin for Chronic Refractory Cancer Cough. Indian J Palliat Care 2016;22:94-6. 10.4103/0973-1075.173940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ucak A, Onan B, Sen H, et al. The effects of gabapentin on acute and chronic postoperative pain after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011;25:824-9. 10.1053/j.jvca.2010.11.017 [DOI] [PubMed] [Google Scholar]