Abstract
Background
Extensive drug-resistant Acinetobacter baumannii (XDR A. baumannii) has emerged as an important pathogen in patients with ventilator-associated pneumonia (VAP) worldwide. This study determined whether or not combination tigecycline (TGC) treatment improved the short-term outcome of patients with XDR A. baumannii-induced VAP.
Methods: Fifty-eight patients admitted to our intensive care unit (ICU) with confirmed XDR A. baumannii VAP between January 2011 and June 2013 were retrospectively studied. Fourteen patients were excluded. The included subjects were classified into two groups depending on treatment regimens with or without TGC (TGC group, n=20; non-TGC group, n=24). Thirty-day mortality rates, and clinical and microbiologic responses were reviewed and compared in detail.
Results
Microbiological eradication was observed in 3 patients (15.0%) in the TGC group and 7 patients (29.2%) in the non-TGC group (P=0.264). The mean time-to-eradication of XDR A. baumannii was 5.3±2.1 versus 7.6±4.0 days (P=0.395). Ten of 20 (50%) patients developed resistance to TGC after initiation of TGC therapy in the TGC group. Clinical cure were achieved in 50.0% of the patients (10/20) in the TGC group and 45.8% of the patients (7/24) in the non-TGC group (P=1.000). No differences existed in the 30-day mortality, length of ICU stay, length of hospital stay (LOS), and length of invasive mechanical ventilation (MV) between the two groups. The occurrence of septic shock was significantly lower in the TGC group (20.0% vs. 54.2%; P=0.030).
Conclusions
TGC combination therapy did not improve the clinical cure and microbiologic eradication in patients with XDR A. baumannii VAP. TGC combination therapy did not decrease all-cause mortality in patients with XDR A. baumannii VAP. TGC combination therapy reduced the incidence of septic shock in patients with XDR A. baumannii VAP, and might decrease the incidence of poly-microbial VAP. TGC combination therapy can only be recommended as an option when other optimized therapeutics, such as colistin, are unavailable.
Keywords: Tigecycline (TGC), ventilator-associated pneumonia (VAP), Acinetobacter baumannii (A. baumannii), combination therapy, outcome
Introduction
Acinetobacter baumannii (A. baumannii) has emerged as an important hospital-acquired pathogen for critically ill patients admitted to the intensive care unit (ICU) (1). Ventilator-associated pneumonia (VAP) is a major hospital-acquired infection in ICU patients with invasive mechanical ventilation (MV). VAP is associated with prolonged MV, and increased ICU length of stay and mortality. Extensive drug-resistant (XDR) A. baumannii, defined as resistance to all standard antimicrobial agents except colistin and tigecycline (TGC) (2), has become one of the most common pathogens in patients with VAP, and is associated with increased ICU mortality (3).
Historically, carbapenems and sulbactam are recommended and widely utilized for the treatment of A. baumannii (4). Previous studies have suggested favorable clinical outcomes with the treatment of TGC or TGC combinations (5-8). There are few alternative antibiotics that are effective against XDR A. baumannii. Colistin is not available in mainland China. Therefore, TGC maybe the final choice for patients with VAP caused by XDR A. baumannii in mainland China, even though whether or not TGC can be administered for VAP is still controversial (9). A warning from the FDA based on a recent meta-analysis has shown an increased risk for mortality associated with TGC compared to other drugs to treat various serious infections, especially VAP (10-12).
The aim of this retrospective cohort case control study was to determine whether or not TGC combination therapy (concomitant treatment with carbapenems or cefoperazone/sulbactam) compared with conventional empirical therapy (only carbapenems or cefoperazone/sulbactam) is useful and rational for VAP due to infection with XDR A. baumannii in ICU.
Methods
Study population and data collection
This study was conducted in a 16-bed respiratory ICU at Beijing Chao-Yang Hospital, a teaching hospital affiliated with Capital Medical University. Approximately 150 patients are admitted to the respiratory ICU at Beijing Chao-Yang Hospital per year. The medical records of all patients consecutively admitted to the ICU from January 2011 to June 2013 were reviewed. All patients with XDR A. baumannii infections according to bronchoalveolar lavage fluid (BALF) or endotracheal aspiration (ETA) cultures were selected and patients who were diagnosed with VAP were included in our study. Patients with a positive A. baumannii culture prior to intubation or with a therapeutic duration <72 h were excluded. All enrolled patients received conventional empirical antibiotic treatment (carbepenems or cefoperazone/sulbactam) with or without TGC. Because TGC exhibited good activity according to drug sensitivity testing, TGC was first recommended when the pathogen was shown to be XDR A. baumannii. Effective antibiotic treatments were initiated as soon as the pathogen was isolated. Patients were divided into two groups depending on treatment regimens. The first dose of intravenous TGC was 100 mg, followed by 50 mg every 12 h. The dosages for carbepenems was 1g every 8 h intravenously (imipenem/cilastatin or meropenem), and 3 g every 8 h for cefoperazone/sulbactam, and were adjusted according to estimated creatinine clearance rate for patients with renal injuries. The duration of treatment mainly depended on the clinical response, but was administered for at least 72 h.
The following data were retrieved from electronic medical records and stored in a data file: patient characteristics, including age, gender, medical history, and indication for ICU admission; presence of typical symptoms and signs; standard ICU laboratory findings; clinical and microbiologic findings; antibiotic therapy; and outcome. The length of hospital stay (LOS), MV days, and history of previous antibiotic use within 90 days before VAP onset were also recorded.
The study was approved by the Ethics Committee of Beijing Chao-Yang Hospital (Capital Medical University, No. 2014-Ke-1). Due to the retrospective nature of the study, written informed consent for participation in the study was waived.
Definitions
VAP was defined according to ATS/IDSA 2005 criteria as “a new or progressive pulmonary infiltration occurring more than 48 hours after receiving invasive MV or within 48 hours after extubation, plus at least two of the following: (I) temperature >38.0 or <36.0 °C; (II) leukocytosis or leukopenia; and (III) purulent tracheal secretions or sputum”.
XDR A. baumannii was defined according to the new definition of drug resistant A. baumannii by the European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) in 2011. XDR A. baumannii was defined as resistance to all standard antimicrobial agents except colistin or TGC.
In agreement with the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee (13), septic shock was defined as low blood pressure with evidence of sepsis, and the need for sufficient fluid resuscitation and vasopressors.
Bacteremia was defined as the presence of viable bacteria in the blood as demonstrated by at least two repeat positive cultures.
Microbiological testing
Quantitative cultures of lower respiratory tract secretions were obtained by BALF or ETA. The culture data were followed up to 14 days after initiating treatment for A. baumannii VAP or until discharge from the hospital. The etiologic agent for VAP was considered to be determined if the BALF yielded >105 cfu/mL or if the ETA culture yielded >106 cfu/mL. Microbiological evaluation and identification were performed using conventional methods and a Phoenix 100 automated system (ABD; BD Diagnostic Systems). The method of susceptibility testing was studied using the Kirby-Bauer Disk Diffusion Agar method for TGC, amikacin, and cefoperazone/sulbactam, and a MIC for carbapenems, quinolones, and other β-lactams. As far as we know, there are no susceptibility breakpoints for TGC against A. baumannii from EUCAST or CLSI, thus an adjustment of TGC disk diffusion breakpoints (susceptible/resistant) to ≥16/≤12 mm was used to reduce inter-method errors to an acceptable level (only 9.7%, all minor) (14). BALF and ETA cultures were routinely collected twice a week to monitor pathogen eradication.
Efficacy of antibiotic therapy
The microbiologic response was considered positive if the organism was not isolated in at least three repeated samples during or after the course of targeted antibiotic treatment, and negative if cultures were persistently positive.
Clinical cure was defined as resolution of clinical signs and symptoms of pneumonia without progression in radiographic findings after 72 h of targeted antibiotic therapies. Persistent symptoms and signs that acquired discontinuation of initial therapy or addition of other antibiotics, or death, were regarded as failures.
Episodes of superinfection were defined as the isolation of two or more different organisms (bacterial and/or fungal) from the same culture which were clinically significant during or <14 days after the start of the treatment.
All patients were followed for 30 days by telephone or clinical visits. No patients were lost to follow-up. The primary endpoints were defined as all-cause death 30 days after the onset of VAP. The secondary endpoints were non-fatal complications, including critical organic or systematic dysfunction and microbiologic eradication.
Statistics
Quantitative variables are expressed as the mean ± standard deviation or median, and qualitative variables are expressed as numbers and percentages. Qualitative variables were compared using the chi-square test or Fisher’s exact test, whereas all quantitative variables were compared by a t-test or Mann-Whitney test, as appropriate. The normal distribution of quantitative variables was checked using the Kolmogorov-Smirnov test. All tests were two-sided. Event-free survival curves were constructed using the Kaplan-Meier methods and compared using a log-rank test. A P value <0.05 was considered statistically significant. Variables were subjected to multivariate analysis with a logistic regression procedure and forward stepwise selection of a P<0.10. Odds ratios (OR) were calculated with 95% confidence intervals (95% CIs). All data analyses were carried out with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
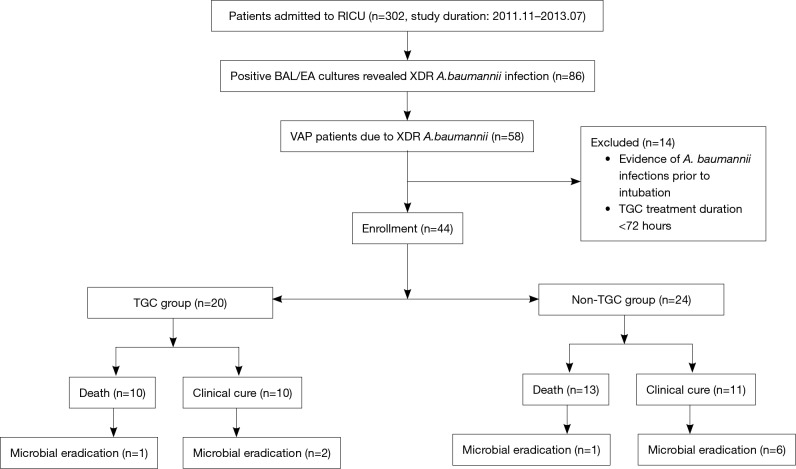
From January 2011 to June 2013, the medical records of all 302 patients admitted to our ICU were reviewed. Eighty-six patients with positive BALF or ETA XDR A. baumannii cultures were selected, and 58 patients were confirmed to have VAP. Eight patients were excluded due to positive cultures before intubation and six patients were excluded due to TGC use <72 h. Finally, 44 patients were included in our analysis. The median age of the 44 patients was 61 years (age range, 16–88 years) and 29 patients were males. Twenty patients (45.5%) administered TGC combination therapy were included in the TGC group, and 24 patients (54.5%) who received conventional antibiotics without TGC comprised the non-TGC group (Figure 1). Carbapenems were used in ten patients in the TGC group and eight patients in the non-TGC group.
Figure 1.
Flow chart of study inclusion process. RICU, respiratory intensive care unit; BAL/EA, bronchoalveolar lavage/endotracheal aspiration; XDR A. baumannii, extensive drug-resistant Acinetobacter baumannii; VAP, ventilator-associated pneumonia; TGC, tigecycline.
The baseline characteristics of the two groups are summarized in Table 1. There were no significant statistical differences between the two groups with respect to demographic and clinical characteristics, such as underlying diseases, prior therapeutics, and prior intubation time before the onset of VAP.
Table 1. Baseline characteristics of the study cohort.
| Baseline characteristics | TGC group (n=20) | Non-TGC group (n=24) | P value |
|---|---|---|---|
| Average age (years), median (range) | 40.5 (34.0–80.0) | 7.5 (50.8–79) | 0.099 |
| Male gender | 12(60%) | 17(70.8%) | 0.532 |
| Major comorbidities, n (%) | |||
| Obstructive pulmonary disease | 2 (10.0) | 6 (25.0) | 0.259 |
| Cardiovascular disease | 7 (35.0) | 8 (33.3) | 1.000 |
| Diabetes mellitus | 2 (10.0) | 5 (20.8) | 0.428 |
| Neurological disease | 1 (5.0) | 4 (16.7) | 0.356 |
| Chronic renal failure | 0 | 1 (4.2) | 1.000 |
| Connective tissue disease | 2 (10.0) | 3 (12.5) | 1.000 |
| Hematologic disease | 2 (10.0) | 1 (4.2) | 0.583 |
| Recently thoracolaparotomy (<30 d) | 2 (10.0) | 0 | 0.201 |
| History of tumor | 1 (5.0) | 2 (8.3) | 1.000 |
| Support of invasive device, n (%) | |||
| ECMO | 4 (20.0) | 5 (20.8) | 1.000 |
| CVVH | 4 (20.0) | 6 (25.0) | 0.734 |
| APACHE II scores, median (range) | 19.0 (15.3–23.5) | 15.0 (13.3–21.8) | 0.057 |
| Serum ALB level (g/L), median (range) | 23.2 (18.9–26.8) | 21.7 (19.1–27.5) | 0.841 |
| Serum creatinine (μmol/L), median (range) | 68.3 (52.0–122.4) | 82.2 (73.9–124.4) | 0.191 |
| PCT (ng/mL), median (range) | 0.62 (0.28–5.89) | 0.69 (0.185–5.58) | 0.629 |
| Prior intubation time (days), median (range) | 9.0 (6.25–11.0) | 11 (5.5–18.5) | 0.223 |
| Initial infection, n (%) | |||
| Bacteria | 4 (20.0) | 9 (37.5) | 0.321 |
| Fungal | 7 (35.0) | 9 (37.5) | 1.000 |
| Virus | 7 (35.0) | 6 (25.0) | 0.522 |
| Prior therapy, n (%) | |||
| Carbapenems | 10 (50.0) | 9 (37.5) | 0.543 |
| Sulbactam | 9 (45.0) | 13 (54.2) | 0.763 |
| Vancomycin/teicoplanin/linezolid | 9 (45.0) | 9 (37.5) | 0.760 |
| Antifungal | 17 (85.0) | 17 (70.8) | 0.306 |
| Anti-virus | 7 (35.0) | 5 (20.8) | 0.329 |
| Glucocorticoids | 11 (55.0) | 10 (41.7) | 0.545 |
| Immunosuppressor | 2 (10.0) | 3 (12.5) | 1.000 |
ECMO, extracorporeal membrane oxygenation; CVVH, continuous veno-venous hemofiltration; PCT, procalcitonin.
Microbiological response and clinical cure
Microbiologic eradication was successful in 3 patients (15.0%) in the TGC group and 7 patients (29.2%) in the non-TGC group (P=0.264). The mean eradication time for XDR A. baumannii was 6.0 versus 9.0 days (P=0.395), respectively. Ten of 20 patients (50%) in the TGC group developed resistance to TGC after initiation of TGC therapy.
Clinical cure was achieved in 50.0% of the patients (10/20) in the TGC group and 45.8% of the patients (7/24) in the non-TGC group (P=1.000) (Table 2).
Table 2. Microbiological characteristic of the study cohort.
| Microbiological characteristics | TGC group (n=20) | Non-TGC group (n=24) | P value |
|---|---|---|---|
| Microbial eradication, n (%) | 3 (15.0) | 7 (29.2) | 0.264 |
| Eradication time (days), median (range) | 6.0 (3.0–7.0) | 9.0 (2.0–20.0) | 0.395 |
| Super-infection, n (%) | 5 (25.0) | 10 (41.7) | 0.342 |
| Pseudomonas aeruginosa | 2 (10.0) | 6 (25.0) | 0.259 |
| K. oxytoca | 0 | 2 (8.3) | 0.492 |
| K. pneumoniae | 0 | 2 (8.3) | 0.492 |
| E. cloacae | 1 (5.0) | 1 (4.17) | 1.000 |
| E. aerogenes | 0 | 1 (4.17) | 1.000 |
| Stenotrophomonas maltophilia | 1 (5.0) | 1 (4.17) | 1.000 |
| Staphylococcus aureus | 0 | 1 (4.17) | 1.000 |
| Burkholderia cepacia | 1 (5.0) | 0 | 0.455 |
| Candida albicans | 0 | 1 (4.17) | 1.000 |
| Onset time of superinfection (days), median (range) | 14 (10.5–20) | 8.5 (5.75–11.25) | 0.539 |
| Combined therapy | |||
| Carbapenems | 10 (50.0) | 8 (33.3) | 0.319 |
| Cefoperazone/sulbactam | 18 (90.0) | 16 (66.7) | 0.104 |
Episodes of superinfections
Superinfections occurred in 15 patients (25.0% in the TGC-group vs. 41.7% in the non-TGC group; P=0.342); the superinfection rate was lower in the TGC group, although without statistical significance. The frequencies of microorganisms for superinfections are shown in Table 2. Pseudomonas aeruginosa was the most common pathogen (8/15) and was identified within days. Candida albicans (C. albicans) was not usually considered to be a superinfection pathogen when cultured in lower respiratory tract secretions, but C. albicans was detected in a pleural effusion and thus considered to be pathogenic. Furthermore, 5 of the 15 patients with superinfections developed two other pathogens simultaneously, all of whom were in the non-TGC group (0% vs. 20.8%; P=0.053). All patients were appropriately treated for concurrent organisms with additional antimicrobial agents that demonstrated in vitro susceptibilities.
Clinical outcome
No differences existed in the rate of 30-day all-cause deaths after the onset of VAP between the two groups [50% (TGC group) vs. 54.2% (non-TGC group); P=1.000]. Survival analysis of the two groups is displayed in Figure 2.
Figure 2.
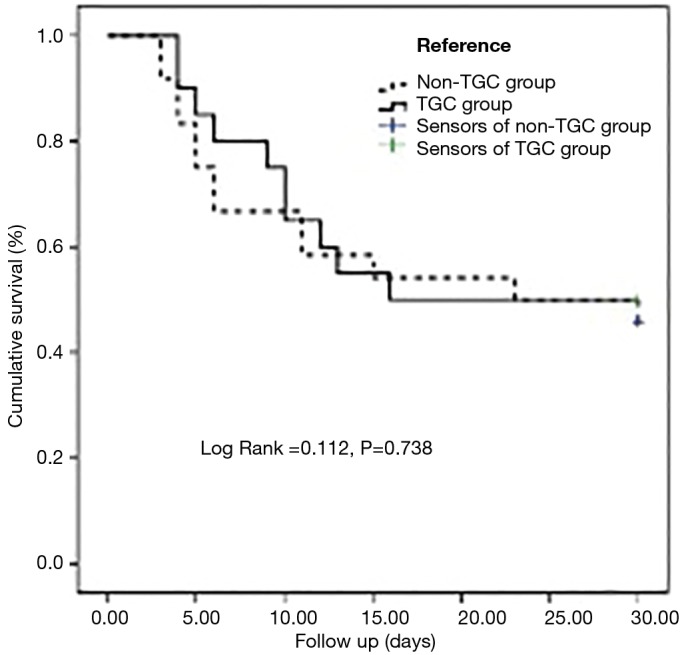
Kaplan-Meier curves for 30-day cumulative survival analysis.
No differences existed in the length of the ICU stay, LOS in hospital, and length of invasive MV between the two groups. The incidences of most serious complications between the two groups were not statistically significant, as shown in Table 3. The occurrence of septic shock was significantly lower in the TGC group (20.0% vs. 54.2%; P=0.030) (Table 3). The onset time of shock was 3 days (range, 1 to 4 days) after the onset of evaluated regimen.
Table 3. Clinical outcomes of the study cohort.
| Outcomes | TGC group (n=20) | Non-TGC group (n=24) | P value |
|---|---|---|---|
| Clinical cure, n (%) | 10 (50.0) | 11 (45.8) | 1.000 |
| Critical complications, n (%) | |||
| Renal dysfunction | 4 (20.0) | 3 (12.5) | 0.684 |
| Liver dysfunction | 6 (30.0) | 7 (29.2) | 1.000 |
| Cardiac dysfunction | 5 (25.0) | 3 (12.5) | 0.436 |
| Disseminated intravascular coagulation | 1 (5.0) | 0 | 0.455 |
| Gastrointestinal hemorrhage | 1 (5.0) | 2 (8.3) | 1.000 |
| Septic shock | 4 (20.0) | 13 (54.2) | 0.030* |
| Bacteremia | 6 (30.0) | 5 (20.8) | 0.509 |
| Total ICU length of stay (days), median (range) | 22.5 (17.0–38.75) | 21 (11.3–40) | 0.637 |
| Total hospital length stay (days), median (range) | 23.5 (18.25–41.0) | 30.5 (18.3–49) | 0.620 |
| Total mechanical ventilation duration (days), median (range) | 22.5 (14.5–26.75) | 16.5 (10.0–27.5) | 0.229 |
| All-cause mortality, n (%) | 10 (50.0) | 13 (54.2) | 1.000 |
| Survival time after the onset of VAP (days) | 10.8±6.37 | 7.46±6.10 | 0.220 |
ICU, intensive care unit; VAP, ventilator associated pneumonia. *, P<0.05.
Based on multivariate analysis, septic shock (OR =0.110; 95% CI, 0.026–0.456; P=0.002) was a predictor of poor outcome, as shown in Table 4.
Table 4. Multivariate analysis of factors predicting 30-day mortality.
| Factors | P value | OR | 95% CI | |
|---|---|---|---|---|
| Minimum | Maximum | |||
| Sex | 0.689 | 0.782 | 0.234 | 2.608 |
| Age | 0.293 | 1.013 | 0.989 | 1.037 |
| Prior intubation time | 0.777 | 1.009 | 0.950 | 1.071 |
| Septic shock | 0.002* | 0.110 | 0.026 | 0.456 |
| Bacteremia | 0.343 | 0.607 | 0.216 | 1.705 |
| APACHE II scores | 0.950 | 0.997 | 0.915 | 1.087 |
| Prior glucocorticoids use | 0.105 | 0.341 | 0.093 | 1.253 |
| Superinfection | 0.228 | 2.045 | 0.640 | 6.533 |
*, P<0.05.
No severe adverse events associated with TGC were detected in the TGC group.
Discussion
The main strength of our study was that XDR A. baumannii-infected patients who were not treated with TGC were included as the control group. This enabled us to discriminate between TGC efficacy in the VAP population and efficacy by conventional therapy itself. This is the first study to determine whether or not TGC regimens might be beneficial for patients with XDR A. baumannii-associated VAP in a Chinese population. The main findings of this study were as follows: (I) TGC combination therapy did not improve the clinical cure or the microbiologic eradication of XDR A. baumannii in patients with VAP; (II) TGC combination therapy did not decrease all-cause mortality in patients with XDR A. baumannii-associated VAP; and (III) TGC combination therapy reduced the incidence of septic shock, and might decrease the incidence of poly-microbial VAP. A major limitation of the current study was that the patients in the non-TGC group did not receive any effective medication, as all of the strains were sensitive to colistin or TGC only. This finding may explain the trend toward a lower incidence of septic shock in the TGC group of patients.
In our case series, TGC was the only drug to which XDR A. baumannii was sensitive. We reported clinical cures in only 50% of patients treated with TGC combination therapy compared with the 45.8% of patients treated with non-TGC conventional antibiotics. Our clinical cure rate was lower than reported (84–90%) in previous studies for carbapenem-resistant A. baumannii (5,15). Possible explanations for the relatively low clinical cure rate may be attributed to varying resistance of A. baumannii, and the relatively low-dose of TGC therapy.
It is important to note that emergence of resistance while on treatment is of concern. In previous studies, among 42 critically ill patients, TGC (in combination with other antibiotics in 28 patients) was effective in 32 patients (76%), but resistance to TGC developed in 3 patients during treatment (16). In another study, 4 of 6 clinical isolates developed intermediate resistance to TGC after initiation of therapy (15). In our cohort, 10 of 20 patients (50%) developed resistance to TGC after initiation of therapy. This resistance to TGC may increase the therapeutic failure rate in VAP patients, and a higher dosage of TGC may decrease the incidence of this problem.
Furthermore, TGC exhibits time-dependent killing and has a prolonged post-antibiotic effect. The best pharmacokinetics and pharmacodynamics (PK/PD) index that correlates with the in vivo activity of TGC is the ratio of the 24-h area under the curve to the MIC (AUC/MIC) (17). According to a previous study (18), the AUC under this dosage was lower (15%) and the 24-h AUC/MIC was less (70%) in the patients with VAP than the patients without VAP. This finding suggested that the standard TGC dose we used in the study might be a sub-optimal dosage. In addition, standard TGC doses provided serum concentrations that were below the MIC for XDR A. baumannii (5,19), and the exposure to relatively low serum levels might promote the development of drug resistance (16). In a recent study, a higher dose of TGC (100 mg every 12 h) was prescribed to VAP patients and was associated with better outcomes than conventional administration (50 mg every 12 h) (20).
In the current study, the TGC combination showed a trend to decrease the incidence of poly-microbial VAP. This observation may reflect the benefit from the spectrum of TGC with activity to multidrug-resistant gram-positive, gram-negative, and anaerobic bacteria, which may be resistant to conventional therapy, such as carbapenems and cefoperazone/sulbactam (5).
In our series, TGC combination therapy significantly reduced the rate of septic shock in VAP patients compared to conventional therapy. Indeed, the effect of TGC combination therapy for bacteremia or bloodstream infection caused by MDR A. baumannii has been reported in previous studies (5,15) and case reports of septic shock patients complicated with trauma and acute pancreatitis (21,22). The addition of TGC to conventional therapy in patients with XDR A. baumannii-associated VAP decreased the incidence of septic shock, as first reported in the current study.
Our study was limited by the retrospective design, relatively small number of cases, and the lack of any pharmacokinetic and pharmacodynamic data as we were unable to measure TGC levels. This is the largest single center series to evaluate the microbiologic and clinical efficacy of TGC in XDR A. baumannii VAP and compare TGC combination therapy with conventional therapy. The heterogeneity of the TGC group regarding the combination regimen may help to explain the lower incidence of septic shock in the combination therapy group. Because colistin is not available in mainland China and could not be evaluated in our study, all patients in our study may have benefitted from colistin if available.
Conclusions
TGC combination therapy did not improve the clinical cure or the microbiologic eradication in patients with XDR A. baumannii-associated VAP. The TGC combination did not decrease all-cause mortality in VAP patients caused by XDR A. baumannii. TGC combination therapy reduced the incidence of septic shock in VAP patients caused by XDR A. baumannii, and might decrease the incidence of poly-microbial VAP. Although TGC combination therapy did not provide prognostic benefit for the patients with XDR A. baunmannii-associated VAP, TGC combination therapy might decrease the bacteria load and reduce the incidence of septic shock. Therefore, TGC combination therapy can be regarded as a reasonable option before the occurrence of more optimal therapeutics. Nevertheless, as TGC is a bacteriostatic and did not benefit the final outcome according to the current study, TGC may not be recommended as a first-line therapy. Further studies based on the PK/PD parameters and combination therapies in the VAP population should be performed to explore the optimal dosage and route of TGC and the best combinations.
Acknowledgements
Funding: Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150301).
Ethical Statement: The study was approved by the Ethics Committee of Beijing Chao-Yang Hospital (Capital Medical University, No. 2014-Ke-1). Due to the retrospective nature of the study, written informed consent for participation in the study was waived.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Perez F, Hujer AM, Hujer KM, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2007;51:3471-84. 10.1128/AAC.01464-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 3.Kollef KE, Schramm GE, Wills AR, et al. Predictors of 30-day mortality and hospital costs in patients with ventilator-associated pneumonia attributed to potentially antibiotic-resistant gram-negative bacteria. Chest 2008;134:281-7. 10.1378/chest.08-1116 [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society, Infectious Diseases Society of America . Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. 10.1164/rccm.200405-644ST [DOI] [PubMed] [Google Scholar]
- 5.Schafer JJ, Goff DA, Stevenson KB, et al. Early experience with tigecycline for ventilator-associated pneumonia and bacteremia caused by multidrug-resistant Acinetobacter baumannii. Pharmacotherapy 2007;27:980-7. 10.1592/phco.27.7.980 [DOI] [PubMed] [Google Scholar]
- 6.Curcio D, Castagnino J, Vazquez W, et al. Tigecycline in the treatment of ventilator-associated pneumonia: experience from the Latin American Tigecycline Use Registry. Infez Med 2010;18:27-34. [PubMed] [Google Scholar]
- 7.Tasbakan MS, Pullukcu H, Sipahi OR, et al. Is tigecyclin a good choice in the treatment of multidrug-resistant Acinetobacter baumannii pneumonia? J Chemother 2011;23:345-9. 10.1179/joc.2011.23.6.345 [DOI] [PubMed] [Google Scholar]
- 8.Nakwan N, Wannaro J, Thongmak T, et al. Safety in treatment of ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii with aerosolized colistin in neonates: a preliminary report. Pediatr Pulmonol 2011;46:60-6. 10.1002/ppul.21324 [DOI] [PubMed] [Google Scholar]
- 9.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 10.Yahav D, Lador A, Paul M, et al. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011;66:1963-71. 10.1093/jac/dkr242 [DOI] [PubMed] [Google Scholar]
- 11.Cai Y, Wang R, Liang B, et al. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011;55:1162-72. 10.1128/AAC.01402-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasad P, Sun J, Danner RL, et al. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012;54:1699-709. 10.1093/cid/cis270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RN, Ferraro MJ, Reller LB, et al. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J Clin Microbiol 2007;45:227-30. 10.1128/JCM.01588-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JD, Graves JA, Dellit TH. Antimicrobial treatment and clinical outcomes of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care Med 2010;25:343-8. 10.1177/0885066610377975 [DOI] [PubMed] [Google Scholar]
- 16.Karageorgopoulos DE, Kelesidis T, Kelesidis I, et al. Tigecycline for the treatment of multidrug-resistant (including carbapenem-resistant) Acinetobacter infections: a review of the scientific evidence. J Antimicrob Chemother 2008;62:45-55. 10.1093/jac/dkn165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006;58:256-65. 10.1093/jac/dkl224 [DOI] [PubMed] [Google Scholar]
- 18.Freire AT, Melnyk V, Kim MJ, et al. Comparison of tigecycline with imipenem/cilastatin for the treatment of hospital-acquired pneumonia. Diagn Microbiol Infect Dis 2010;68:140-51. 10.1016/j.diagmicrobio.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 19.Gordon NC, Wareham DW. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J Antimicrob Chemother 2009;63:775-80. 10.1093/jac/dkn555 [DOI] [PubMed] [Google Scholar]
- 20.De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014;18:R90. 10.1186/cc13858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerc T, Perez JP, Debien B, et al. Treatment of a septic shock due to multiresistant Acinetobacter baumannii with tigecycline in combination. Ann Fr Anesth Reanim 2007;26:1056-8. 10.1016/j.annfar.2007.08.015 [DOI] [PubMed] [Google Scholar]
- 22.Taccone FS, Rodriguez-Villalobos H, De Backer D, et al. Successful treatment of septic shock due to pan-resistant Acinetobacter baumannii using combined antimicrobial therapy including tigecycline. Eur J Clin Microbiol Infect Dis 2006;25:257-60. 10.1007/s10096-006-0123-1 [DOI] [PubMed] [Google Scholar]