Abstract
Background
Bullous lung disease is characterized by formation of blebs, bullae and emphysema. We investigate the role of oxidative stress in the pathogenesis of bullous lung disease and compare between conventional thoracotomy versus video assisted thoracoscopic approach in surgical management of such patients.
Methods
This study was a prospective case control study and it was carried out on 21 patients (16 males and 5 females) with bullous lung disease selected as candidate for surgical interference. This was in addition to 21 apparently healthy age and sex matched subjects selected as control group. Plasma levels of α1-antitrypsin were estimated using commercially available ELISA assay kit, while plasma levels of malondialdehyde (MDA), β-carotene, vitamin A, vitamin C and vitamin E were estimated using spectrophotometric methods. Conventional thoracotomy approach was done in thirteen patients, while, videothoracoscopic approach was done in eight patients.
Results
There were significant higher plasma levels of MDA (P<0.001) and lower plasma levels of β-carotene (P<0.01), vitamin A, vitamin C and vitamin E (P<0.001 for each) among patients with bullous lung disease when compared with the control group. There was non-significant difference regarding the air leakage and the hospital stay among patients with bullous lung disease who managed via conventional thoracotomy approach when compared with those managed via videothoracoscopic approach.
Conclusions
This study proves that the oxidative stress plays an important role in the pathogenesis of bullous lung disease. Also there are no significant outcome differences between conventional thoracotomy versus video assisted thoracoscopic approach in surgical treatment of such patients.
Keywords: Oxidative stress, bollous lung, surgical approach, α1-antitrypsin
Introduction
Bullous lung disease is characterized by formation of blebs, bullae and emphysema. The term bulla is used to describe an air-filled space within the lung parenchyma resulting from deterioration of the alveolar tissue (1). Most patients with bullae have a significant cigarette smoking history, although cocaine smoking, pulmonary sarcoidosis, α1-antitrypsin deficiency “AATD”, α1-antichymotrypsin deficiency, Marfan’s syndrome, Ehlers-Danlos syndrome and inhaled fiberglass exposure have all been implicated (2,3). AATD is a strong genetic risk factor of the chronic obstructive pulmonary disease “COPD” (4).
Patients who have bullous lung disease in the presence of diffuse parenchymal involvement (emphysematous or non emphysematous) should be evaluated on an individual basis and surgery should be performed for patients in whom even a small increase in pulmonary function might be of major benefit (1). Surgical therapy is indicated when patients have incapacitating dyspnea or for patients who have complications related to bullous disease, such as infection or pneumothorax (5). Bullectomy or resection of the entire bulla, either through a video assisted thoracoscopic surgery (VATS) or a standard open thoracotomy, is the most common surgical technique used for treatment (1).
The balance between oxidants and antioxidants is complex and the interactions between these pathways have received little attention (6). The lung is continuously exposed to endogenous and exogenous oxidants (cigarette smoke, mineral dust, ozone, radiation). Reactive oxygen and nitrogen species are mainly produced by phagocytes as well as by, polymorphonuclear, alveolar, bronchial and endothelial cells (7).
Malondialdehyde “MDA” is a low-molecular weight aldehyde that can be produced from free radical attack on polyunsaturated fatty acids. It is used as a marker of oxidative stress (8). The lungs are protected from the negative effects of oxidant persistence in tissues by endogenous agents named antioxidants. Antioxidants may be non- enzyme or enzyme proteins. The former include glutathione, vitamins (α-tocopherol and ascorbic acid), beta-carotene and uric acid; the latter, superoxide dismutase, catalases, and peroxidases (9). Dietary antioxidants may have beneficial effects on respiratory health (10). α-tocopherol is a form of vitamin E which helps maintain integrity of membrane fatty acids by inhibiting lipid peroxidation. Carotenoids are plant pigments and include; α- and β-carotene, lycopene, lutein and β-cryptoxanthin. This group of fat soluble antioxidants has been shown to benefit respiratory health due to their ability to scavenge reactive oxygen species “ROS” and reduce oxidative stress (11). Vitamin C is the major water soluble antioxidant and a powerful inhibitor of lipid peroxidation and regenerates vitamin E in lipoproteins and membranes (12).
Methods
This study was a prospective case control study and it was carried out on 21 patients (16 males and 5 females) with bullous lung disease selected according to the inclusion criteria as candidate for surgical treatment who admitted at the department of Cardiothoracic surgery-Faculty of Medicine-Assiut University, after obtaining approval of university hospital ethics committee and informed consent from the included patients. This was in addition to 21 apparently healthy age and sex matched subjects selected as control group (group C). The study was carried out during the period from July 2011 to July 2012. Conventional thoracotomy was done in thirteen patients (group A), while, VATS was done in eight patients (group B).
The inclusion criteria were (13), the presence of bulla(e) occupying at least one third or more of the hemithorax or presence of bulla(e) regardless their sizes in the presence of symptoms or complications or failure of medical treatment. Patients with liver affection were excluded from the study.
Three cc venous blood were obtained by venepuncture from all included patients preoperatively and from the control group, on EDTA tubes, for assay of alpha-1 antitrypsin, malondialdehyde, β-carotene, vitamin A and vitamin C, blood samples were then centrifuged at 3,500 rpm for 15 min at 4 °C and the plasma transferred into 1 mL cryotubes, and stored at −80 °C for later analyses.
Using commercially available assay kit according to manufacturer protocol for measurements of:
Plasma α1-antitrypsin [supplied by Assaypro LLC. Catalog No. EA5001-1]. By using an enzyme-linked immune-sorbent (ELISA) assay kits based on sandwich-ELISA principle by enzyme-linked immune-sorbent assay (ELISA) multiskan EX microplate photometer, thermo scientific, STAT FAX-2100, USA;
Plasma lipid peroxidation products (malondialdehyde) assay was by the method of Aruoma et al. (14). Plasma vitamin A and β-carotene assay was carried out by the method of Caarr-Price as modified by Kaser and Stekol (15). Vitamin C assay was by the method of Roe (16). Vitamin E assay was by the method of Baker and Frank (17). All the previous biochemical parameters of oxidative stress were measured using T60 UV visible spectrophotometer. PG INSTRUMENTS LIMITED, Alma park wibtoft, Leicester shreshire, England. LE17SBE. Serial No. 20-1650-01-0010.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS-version 17) software. The results were expressed as mean ± standard deviation. One way ANOVA (analysis of variance) test was used to compare more than two groups as regard quantitative variable [least significant difference (LSD)]. Pearson correlation analysis was used to evaluate the correlations between different parameters. P values of less than 0.05 were considered significant.
Results
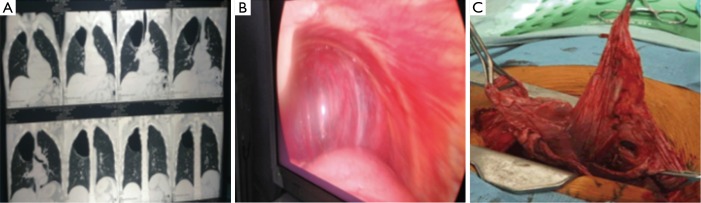
Table 1 showed the demographic and postoperative outcome data of group A and group B. There was no significant outcome differences between the two groups regarding the hospital stay and air leakage. The clinical and radiological findings of the studied patients were presented in Table 2. All patients have dyspnea grade-III and unilateral lung bulle. 90% of cases were associated with chronic obstructive pulmonary disease “COPD” GOLD-III. Table 3: revealed significant changes in the plasma levels of malondialdehyde (MDA) and antioxidant vitamins (vitamin A, β-Carotenes, vitamin C and vitamin E) among the included patients versus the control group. There was no significant difference in the plasma levels of α1-antitrypsin among the studied patients when compared with the control group except for one non-smoker male patient marked decrease in the plasma level of α-1 antitrypsin (93 mg/dL), Reference range 100–360 mg/dL) and diagnosed as having α-1 antitrypsin deficiency. Figure 1 revealed a positive correlation between vitamin C and MDA. Figure 2 showed a computed tomography of chest (coronal section) of a right unilateral lung bulla (A), A picture captured during resection of lung bulla through videothoracoscopic approach (B), a picture captured during resection of lung bulla through conventional thoracotomy approach (C).
Table 1. Demographic and postoperative evaluation data of the studied groups.
| Variables | Group A (n=13, conventional thoracotomy approach) | Group B (n=8, video assisted thoracoscopic approach) | P value |
|---|---|---|---|
| Age (mean ± SD, years) | 55.1±10.2 | 53.6±9.6 | – |
| Sex (No, %) | – | ||
| Male | 10 (76.9%) | 6 (75%) | |
| Female | 3 (23.1%) | 2 (25%) | |
| Postoperative evaluation data | |||
| Dyspnea | Totally improved | Totally improved | – |
| Radiological | Fully expanded lung with no residual | Fully expanded lung with no residual | |
| ICU stay | No need | No need | – |
| Hospital stay (mean ± SD, days) | 6.64±1.01 | 5.08±1.26 | *0.85ns |
| Air leakage (mean ± SD, days) | 4.57±1.22 | 3.23±1.30 | *0.84ns |
*ns, non significant; ICU, intensike care unit.
Table 2. Clinical, etiological and radiological findings of the studied patients.
| Findings (n=21) | No, (%) | |
|---|---|---|
| Dyspnea grade-III (No, %) | 21 [100] | |
| FEV1/FVC ≤50% (No, %) | 21 [100] | |
| Pneumothorax (No, %) | 11 [52] | 6 females [36] |
| 7 males [64] | ||
| Accidentally discovery (No, %) | 2 (9.5) | 1 female [50] |
| 1 male [50] | ||
| Smoking history | ||
| Heavy smokers (No, %) | 15 (71.5) | All are males |
| Non-smoker (No, %) | 6 (28.5) | 1 male, 5 females |
| Etiological classification | ||
| COPD (No, %) | 19 [90] | 4 female [21] |
| 15 male [79] | ||
| α1-antitrypsin deficiency (No, %) | 1 [5] | Male |
| Idiopathic (No, %) | 1 [5] | Female |
| Radiological findings | ||
| Right unilateral bulla≥ 1/3 of hemithorax (No, %) | 13 [62] | |
| Left unilateral bulla ≥1/3 of hemithorax (No, %) | 8 [38] | |
Table 3. Mean±SD of plasma levels of malondialdehyde (MDA) and antioxidant vitamins (vitamin A, B-Carotenes, vitamin C and vitamin E) among the included patients versus the control group.
| Variables | Control (n=21) | Patients (n=21) | **P value |
|---|---|---|---|
| MDA, mmol/L | 0.23±0.06 | 0.42±0.09 | 0.000*** |
| VitaminA, ìg/dL | 42.86±5.52 | 27.9±3.77 | 0.000*** |
| β-Carotene, ìg/dL | 26.24±5.99 | 18.96±4.89 | 0.001** |
| Vitamin C, mg/dL | 11.22±2.55 | 8.19±2.18 | 0.000*** |
| Vitamin E, mg/L | 3.72±0.93 | 2.01±0.65 | 0.000*** |
| *α-1 antitrypsin, mg/dL | 256.1±49.35 | 254.1±49.47 | 0.9920ns |
*, Only one non-smoker male patient showed marked decrease in the plasma level of α-1 antitrypsin (93 mg/dL), Reference range 100–360 mg/dL) and diagnosed as having α-1 antitrypsin deficiency. **, P value marked as ** indicate significant change at P<0.01; ***, indicate significant change at P<0.001, ns means non-significant.
Figure 1.
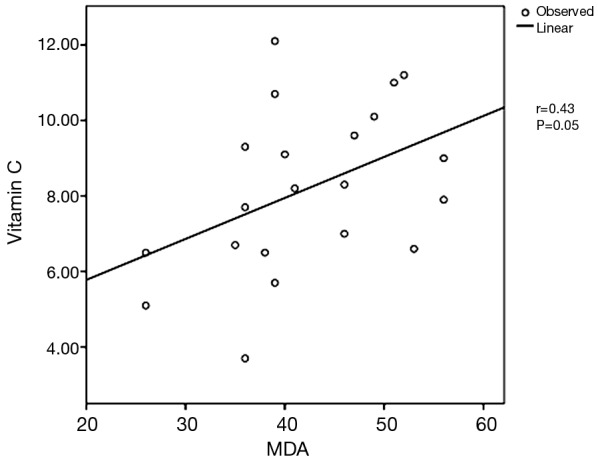
Positive correlation between the plasma levels of MDA and vitamin C. MDA, malondialdehyde.
Figure 2.
Imaging and surgical removal of a lung bulla. (A) Computed tomography of chest (coronal section) showing right unilateral bulla; (B) a picture captured during resection of lung bulla through video assisted thoracoscopic approach (C) a picture captured during resection of lung bulla through conventional thoracotomy approach.
Discussion
Because of their anatomy, location and functions, the lungs are highly susceptible to oxidative damage. Research into oxidants/antioxidants balance in lung diseases is a promising field that can provide insights into pathogenetic mechanisms and open new therapeutic perspectives (6). Regarding the underlying etiology of lung bulla in this study, there was no significant difference in the plasma levels of α1-antitrypsin among the studied patients when compared with the control group except for one non-smoker male patient showed marked decrease in the plasma level of α-1 antitrypsin and diagnosed as having α-1 antitrypsin deficiency which represent 5% of cases, while, 90% of cases were associated with chronic obstructive pulmonary disease “COPD”, whom diagnosed as having bullous emphysema i.e., bullae occurring in conjunction with underlying COPD (18), and 5% of cases were idiopathic. Also there was significant smoking history among the included patients in this study because all included males except one, were heavy smokers. The present study revealed significant higher plasma levels of MDA (P<0.001) which is one of oxidative stress markers and lower antioxidant vitamins in the form of lower plasma levels of β-carotene (P<0.01), vitamin A, vitamin C and vitamin E (P<0.001 for each) among the studied patients when compared with the control group. The present findings indicate the presence of oxidative stress in patients with bullous lung disease which support its role in the pathogenesis of such lung disorder. As smoking and oxidative stress play a pivotal role in the pathogenesis of COPD (19), and 90% of patients with lung bullae in this study have COPD, this prove that the persistence of oxidative stress has role in the progression of COPD into bullous emphysema.
To the best of our knowledge, no previous studies could be traced in literature regarding the comparison in the outcome when using conventional thoracotomy versus VATS in patients whom surgical interference is indicated. In the present study, patients were divided into two groups according to the surgical approach. Group A, where conventional thoracotomy approach was used and group B, where videothoracoscopic approach was used and to compare the outcome in both groups, improvement of dyspnea, radiological findings, post-operative intensive care unit “I.C.U” requirement, hospital stay and air leakage, all these parameters were used in comparison for judgment. There was improvement of dyspnea in both groups equally with fully expanded lung with no residual on radiological evaluation and no requirement for post-operative I.C.U in both. Although the hospital stay and the air leakage were less, in duration, in group B than group A, but the difference was statistically not significant.
In conclusion: the present study prove that oxidative stress play an important role in the development of lung bullae and a more important role in the progression of patients with COPD into bullous emphysema, so administration of antioxidant vitamins (A, C and E) may be beneficial in such patients even after surgical treatment. There are no significant outcome differences between conventional thoracotomy versus VATS approach in surgical treatment of such patients.
Recommendations
Additional more comprehensive large-scale studies to compare conventional thoracotomy versus VATS approach in surgical treatment of patients with bullous lung disease are recommended. Also future studies to compare the oxidative stress in COPD patients with bullous lung disease versus COPD patients without bullous lung disease are required.
Acknowledgements
We would like to acknowledge the team work of the Metabolic and Genetic Disorders Unit, Faculty of Medicine, Assiut University, where the laboratory work of this study has been done.
Ethical Statement: The Research Committee at Faculty of Medicine, Assiut University approved this study (IRB00008718 20/01/016) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Greenberg JA, Singhal S, Kaiser LR. Giant bullous lung disease: evaluation, selection, techniques, and outcomes. Chest Surg Clin N Am 2003;13:631-49. 10.1016/S1052-3359(03)00095-4 [DOI] [PubMed] [Google Scholar]
- 2.Hii SW, Tam JD, Thompson BR, et al. Bullous lung disease due to marijuana. Respirology 2008;13:122-7. 10.1111/j.1440-1843.2007.01186.x [DOI] [PubMed] [Google Scholar]
- 3.Hutchison DC, Cooper D, British Thoracic Society . Alpha-1-antitrypsin deficiency: smoking, decline in lung function and implications for therapeutic trials. Respir Med 2002;96:872-80. 10.1053/rmed.2002.1381 [DOI] [PubMed] [Google Scholar]
- 4.Buda N, Kosiak W, Drozdowski J. Transthoracic Lung Ultrasonography as a Tool of Alpha-1-Antitripsine Deficiency Assessment. J Clin Case Rep 2015;5:591-2. 10.4172/2165-7920.1000591 [DOI] [Google Scholar]
- 5.Vigneswaran WT, Townsend ER, Fountain SW. Surgery for bullous disease of the lung. Eur J Cardiothorac Surg 1992;6:427-30. 10.1016/1010-7940(92)90067-8 [DOI] [PubMed] [Google Scholar]
- 6.Kinnula VL. Focus on antioxidant enzymes and antioxidant strategies in smoking related airway diseases. Thorax 2005;60:693-700. 10.1136/thx.2004.037473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bargagli E, Olivieri C, Bennett D, et al. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 2009;103:1245-56. 10.1016/j.rmed.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 8.do Val Carneiro JL, Nixdorf SL, Mantovani MS, et al. Plasma malondialdehyde levels and CXCR4 expression in peripheral blood cells of breast cancer patients. J Cancer Res Clin Oncol 2009;135:997-1004. 10.1007/s00432-008-0535-7 [DOI] [PubMed] [Google Scholar]
- 9.Hakim F, Kerem E, Rivlin J, et al. Vitamins A and E and pulmonary exacerbations in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2007;45:347-53. 10.1097/MPG.0b013e31804069e5 [DOI] [PubMed] [Google Scholar]
- 10.Berthon BS, Wood LG. Nutrition and respiratory health--feature review. Nutrients 2015;7:1618-43. 10.3390/nu7031618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieger JA, Wood LG, Clifton VL. Improving asthma during pregnancy with dietary antioxidants: the current evidence. Nutrients 2013;5:3212-34. 10.3390/nu5083212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebretsadik G, Seifu D, Yimer G, et al. The Non-Enzymatic Antioxidant and Level of Oxidative Stress of Tuberculosis Patients in Selected Treatment Center in Addis Ababa Ethiopia. Journal of Tuberculosis Research 2015;3;63-71. 10.4236/jtr.2015.33010 [DOI] [Google Scholar]
- 13.Lone YA, Dar AM, Sharma ML, et al. Outcome of the surgical treatment of bullous lung disease: a prospective study. Tanaffos 2012;11:27-33. [PMC free article] [PubMed] [Google Scholar]
- 14.Aruoma OI, Halliwell B, Laughton MJ, et al. The mechanism of initiation of lipid peroxidation. Evidence against a requirement for an iron(II)-iron(III) complex. Biochem J 1989;258:617-20. 10.1042/bj2580617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaser E, Stekol I. In Practical Clinical Biochemistry edited by H. Varley and A.H. Gowenlock, Elsevier, Amsterdam. 1943. [Google Scholar]
- 16.Roe JH. Standard Methods in Clinical Chemistry. In: Seigson D, Ed. New York:Academic Process. 1961;3. [Google Scholar]
- 17.Baker H, Frank O. Determination of Vitamin E. Clinical Vitaminology. New York: Wiley. 1968:172. [Google Scholar]
- 18.Alfred PF, Jack AE, Jay AF, et al. Fishman's Pulmonary Diseases and Disorders (4th Edition). McGraw-Hill Professional. 2008. [Google Scholar]
- 19.Repine JE, Bast A, Lankhorst I. Oxidative stress in chronic obstructive pulmonary disease. Oxidative Stress Study Group. Am J Respir Crit Care Med 1997;156:341-57. 10.1164/ajrccm.156.2.9611013 [DOI] [PubMed] [Google Scholar]