Abstract
Background
Nutritional assessment is important in patients with pulmonary nontuberculous mycobacterial (PNTM) disease. The therapeutic effect of a cholesterol-rich diet in tuberculosis (TB) patients has been demonstrated, but the role of cholesterol in PNTM disease is unclear. This study evaluated the sequential changes in nutritional markers, including cholesterol, total lymphocyte count and visceral fat volume, according to the PNTM disease course.
Methods
This was an age-, sex- and number of comorbid diseases-matched case-control analysis of 89 patients with PNTM disease and 356 controls, who were participants in a Korean national survey.
Results
The median body mass index (BMI) and cholesterol level in the PNTM group [BMI =19.7 kg/m2; interquartile range (IQR): 17.8–21.6; cholesterol: 159 mg/dL; IQR, 135–185] were lower than those in controls (BMI: 23.1 kg/m2; IQR, 21.3–25.3; cholesterol: 188 mg/dL; IQR, 164-217; both P<0.001). In a multivariate analysis, Age more than 70 years (OR =3.38; 95% CI: 1.13–10.15, P=0.029), BMI <19.5 kg/m2 (OR =5.09; 95% CI: 1.67–15.48; P=0.004) and cavitary lesions (OR: 3.86; 95% CI: 1.30–11.47; P=0.015) were independently associated with extensive pulmonary lesions involving more than four lobes. The total cholesterol level, total lymphocyte count showed a tendency to decrease in PNTM patients with disease progression (both, P value <0.05), but not in those with a stable disease course. A decrease in cholesterol concentration of >20 mg/dL and a decrease in lymphocyte count more than 200/µL were predictive factors for disease progression (cholesterol: OR =10.50, 95% CI: 2.51–43.98, P=0.001; lymphocyte count: OR =5.32, 95% CI: 1.46–19.35, P=0.011).
Conclusions
These findings suggest that the change in cholesterol level may be a marker of disease progression in patients with PNTM disease.
Keywords: Nontuberculous mycobacterial disease, cholesterol, body mass index, visceral fat
Introduction
The incidence of pulmonary nontuberculous mycobacterial (PNTM) disease is increasing in many regions of the world (1-3). PNTM disease frequently occurs in slender middle-aged and older females (4). Patients with Mycobacterium avium complex (MAC) lung disease have a small visceral fat area and low nutrient intake (5). BMI as a marker for nutritional assessment is correlated with disease spread (6). Nutritional assessment is thus important in PNTM disease.
Cholesterol is a component of the cell membrane involved in lymphocyte proliferation and their differentiation into cytotoxic cells (7,8). Patients with tuberculosis have been reported to have low cholesterol levels (9), and cholesterol supplementation accelerated the sterilization rate of sputum cultures in pulmonary tuberculosis patients (10). These results suggest that the efficacy of anti-tubercular treatment could be influenced by nutritional status through its effects on cellular immunity. Moreover, hypocholesterolemia is associated with poor outcomes in elderly patients and critically ill surgical patients (11-13).
There are no published reports on the role of cholesterol levels in patients with PNTM disease. Accordingly, in this study, we compared cholesterol levels in patients with PNTM disease with those of healthy controls from a nationwide survey and investigated the nutritional factors related to disease extension and progression in patients with PNTM disease.
Methods
Study design and subjects
This case-control, retrospective study evaluated the nutritional status of PNTM patients and control subjects at Chuncheon Sacred Heart Hospital, Kangdong Sacred Heart Hospital and Hallym University Sacred Heart Hospital. The Ethics Review Committee approved the study protocol. Diagnosis of pulmonary nontuberculous mycobacterial (PNTM) disease was based on the criteria of the American Thoracic Society (ATS) guidelines (14).
After retrospective analysis of the medical records of 206 patients with PNTM lung disease admitted to our hospitals between October 2007 and June 2015, 89 patients with PNTM were included in this study.
The exclusion criteria were as follows: patients who could not be followed up for at least 12 months, those who did not undergo initial or follow-up chest computed tomography (CT), those without initial or follow-up laboratory tests, those with abnormal liver function, dyslipidemia, end-stage renal disease or any neoplasms within 5 years, and those undergoing treatment with a corticosteroid, immunosuppressive agent, psychotic drug or statin.
The control group was randomly recruited from subjects who participated in the Korea National Health and Nutrition Examination Surveys (KAHANES) V study from 2011 to 2014. KAHANES is a nationwide survey of the health and nutritional status of the Korean population (https://knhanes.cdc.go.kr/knhanes). Of 32,144 subjects who participated in the survey from February 2011 to December 2014, 22,766 were selected after excluding those with missing BMI, cholesterol or hemoglobin data. Finally, a total of 12,954 subjects without a history of dyslipidemia, TB malignancy, depression, chronic renal disease or cirrhosis were included in the study.
Each patient with PNTM disease was matched with four controls by age, sex and the number of comorbid diseases. The comorbid diseases included hypertension, stroke, angina, arthritis, airway diseases such as asthma or COPD, diabetes mellitus and thyroid disorders. The tolerance limit for age matching was five. The data were analyzed using the greedy matching algorithm, GMATCHSAS macro (Mayo Clinic, Rochester, MN, USA).
Data collection
We investigated the presence of a history of TB, body mass index (BMI), comorbid diseases, chest CT scans and laboratory tests in 89 patients with PNTM disease. The PNTM disease patients were classified into stable (n=52) and progressed (n=37) groups according to the disease course after follow-up, irrespective of anti-NTM treatment. The progressed group was defined as patients with continued positive sputum and radiographic deterioration after follow-up for >1 year. The stable group was defined as patients with sputum conversion or patients without radiographic deterioration after follow-up for >1 year. Sputum culture conversion was defined as three consecutive negative conversions from the date of the first negative culture in cases who had undergone anti-NTM treatment (15). Radiologic deterioration was defined as increased radiographic extent with newly developed cavitary lesions or centrilobular nodules more than two lobes. Disease duration was defined as the period from diagnosis of PNTM disease to sputum culture conversion or last visit in the clinic. We investigated the factors influencing cholesterol level such as smoking, diabetes mellitus (DM), hypothyroidism, hyperthyroidism and menopause in patients with PNTM disease (16-19).
We identified NTM species via a polymerase chain reaction (PCR)-restriction fragment length polymorphism method based on the rpoB gene (20). The number of involved lung segments was used as an indicator of radiological severity, and extensive lung involvement was defined as a radiographic disease extent of more than four lobes. Radiological features were classified as cavity and bronchiectasis. AFB smear positivity was defined as >1 AFB per 100 high-power fields by Ziehl-Neelsen staining (21).
Multi-detector CT (MDCT) was performed using a 64-channel CT scanner (Sensation 64, Siemens Medical Solutions, Forchheim, Germany). Measurement of the visceral fat volume was obtained from chest CT taken at the level of the L1 vertebra using image-analysis software (Aquarius 3D Workstation, TeraRecon, San Mateo, CA, USA). Regions of interest (ROIs) were defined by tracing the margin of the abdominal cavity on serial axial CT images. Intraperitoneal tissues of fat density (−195 to −45 HU) within the ROI were segmented and calculated to yield the total visceral fat volume.
Total lymphocyte count was examined as a useful indicator of nutritional status and outcome (22). The cholesterol levels, total lymphocyte count and visceral fat volume from chest CT were compared between diagnosis and after follow-up in the stable and progressed groups.
Statistical analysis
Categorical variables were analyzed using the χ2 test and continuous variables using the Mann-Whitney test. The non-parametric Wilcoxon signed-rank test was used to evaluate longitudinal changes in albumin, hemoglobin and cholesterol levels.
Multivariate logistic regression analysis was performed to evaluate the risk factors for extensive lung involvement and disease progression in patients with PNTM disease. Variables with a P<0.1 in the univariate analyses were entered into the multiple logistic regression model.
All statistical analyses were conducted using PASW Statistics ver. 20 (SPSS Inc., Chicago IL, USA), GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA) and SAS University edition (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of the participants
The baseline clinical characteristics of the participants are shown in Table 1. This study included 89 patients with PNTM disease (49 females and 40 males) and 356 controls (196 females and 160 males).
Table 1. Basic characteristics of the study participants.
| Parameter | Control (n=356) | NTM patients (n=89) | Stable (n=52) | Progressed (n=37) |
|---|---|---|---|---|
| Age, years¶ | 71 (62,79) | 71 (61,79) | 69.5 (59.8, 79.8) | 74 (61.5, 78.5) |
| Females, N (%) | 196 (55.1) | 49 (55.1) | 28 (53.8) | 21 (56.8) |
| BMI (kg/m2)¶ | 23.1 (21.3, 25.3) | 19.7 (17.8, 21.6) | 19.8 (18.0, 22.4) | 19.0 (17.7, 20.7) |
| Number of comorbid diseases | ||||
| 0 | 148 (41.6) | 37 (41.6) | 16 (30.8) | 21 (56.8) |
| 1 | 132 (37.1) | 33 (37.1) | 20 (38.5) | 13 (35.1) |
| 2 | 64 (18.0) | 16 (18.0) | 15 (28.8) | 1 (2.7) |
| 3 | 12 (3.4) | 3 (3.4) | 1 (1.9) | 2 (5.4) |
| Past TB history, N (%)∫ | – | 43 (48.3) | 21 (40.4) | 22 (59.5) |
| AFB smear positivity, N (%) | – | 41 (46.1) | 23 (44.2) | 18 (48.6) |
| Extensive lung involvement, N (%) | – | 30 (33.7) | 15 (28.8) | 15 (40.5) |
| Bronchiectasis, N (%) | – | 85 (95.5) | 48 (92.3) | 37 (100) |
| Cavity, N (%) | – | 36 (40.4) | 15 (28.8) | 21 (56.8) |
| Species, N (%) | ||||
| M. avium/M. intracellulare (MAC) | – | 62(69.7) | 38 (73.1) | 24 (64.9) |
| MAC + M. abscessus | – | 7 (7.9) | 2 (3.8) | 5 (13.5) |
| M. abscessus | – | 11 (12.4) | 5 (9.6) | 6 (16.2) |
| M. kansasii | – | 2 (2.2) | 2 (3.8) | 0 (0) |
| M. fortuitum | – | 2 (2.2) | 2 (3.8) | 0 (0) |
| Unknown | – | 5 (5.6) | 3 (5.8) | 2 (5.4) |
| Disease duration (month)¶ | – | 36 (24, 54) | 30 (24, 48) | 36 (24, 60) |
| Follow-up period ¶ | – | 26 (16, 39.5) | 24 (16, 37.8) | 27 (18.5, 49.0) |
| Anti-NTM treatment, N (%) | – | 44 (49.4) | 29 (55.8) | 15 (40.5) |
| Smoking, N (%) | – | 19 (21.3) | 12 (23.1) | 7 (18.9) |
| DM, N (%)∫ | − | 12 (13.5) | 9 (17.3) | 3 (8.1) |
| Hyperthyroidism, N (%)∫ | − | 3 (3.4) | 1 (1.9) | 2 (5.4) |
| Hypothyroidism, N (%)∫ | − | 4 (4.5) | 4 (7.7) | 0 (0) |
| Menopause, N (%)∫ | − | 38 (42.7) | 21 (40.4) | 17 (45.9) |
¶, median (IQR); ∫, only in patients with PNTM disease. NTM, nontuberculous mycobacterium; BMI, body mass index; TB, tuberculosis; AFB, acid fast bacilli; DM, diabetes mellitus; PNTM, pulmonary nontuberculous mycobacterial.
By design, no difference in age, sex or the number of comorbid diseases was observed. In patients with PNTM lung disease, the follow-up periods ranged from 12 to 92 months, with a median of 26 months. Patients with PNTM disease were compared according to their disease course.
Cavitary lesions were more frequent in progressed patients than stable patients (56.8% vs. 28.8%; P=0.008). Smoking, DM, hyperthyroidism and menopause were not significantly different between two groups. All four patients with hypothyroidism had stable disease course.
The median BMI was lower in patients with PNTM disease than in controls [PNTM: 19.7 kg/m2, interquartile range (IQR) 17.8–21.6 vs. control: 23.1 kg/m2, IQR, 21.3–25.3, P<0.001; Figure 1]. The median cholesterol level was also lower in patients with PNTM disease than in controls (PNTM 159 mg/dL, IQR 135–185 vs. control: 188 mg/dL, IQR 164–217, P<0.001; Figure 1). The median BMI value and cholesterol level were not significantly different between stable and progressed patients.
Figure 1.
BMI and cholesterol levels in controls and patients with PNTM disease. BMI, body mass index; PNTM, pulmonary nontuberculous mycobacterial.
Risk factors associated with extensive lung involvement
An analysis of the risk factors associated with extensive lung involvement (radiologic extension to more than four lobes) was performed (Table 2). On univariate analysis, female, age >70 years old, cavitary lesions and BMI <19.5 kg/m2 were associated with extensive lung involvement. In the multivariate analysis, age >70 years old (P=0.029), cavitary lesions (P=0.015) and BMI <19.5 kg/m2 (P=0.004) were risk factors for extensive lung involvement.
Table 2. Factors associated with extensive lung involvement in patients with pulmonary nontuberculous mycobacterial (PNTM) disease.
| Parameter | Extensive lung involvement | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Female (vs. male) | 2.58 (1.02, 6.57) | 0.046 | 2.30 (0.76,6.98) | 0.14 | |
| Age >70 (vs. ≤70) years | 2.72 (1.09, 6.81) | 0.033 | 3.38 (1.13,10.15) | 0.029 | |
| Past TB history | 1.35 (0.56, 3.27) | 0.5 | – | – | |
| Cavity | 5.38 (2.08, 13.92) | 0.001 | 3.86 (1.30,11.47) | 0.015 | |
| BMI <19.5 kg/m2 | 7.48 (2.72, 20.58) | <0.001 | 5.09 (1.67,15.48) | 0.004 | |
| Cholesterol <160 mg/dL | 0.49 (0.20, 1.20) | 0.118 | – | – | |
| Albumin <4.0 g/dL | 0.84 (0.35, 2.03) | 0.705 | – | – | |
| Hb <12 g/dL | 1.71 (0.70, 4.19) | 0.243 | – | – | |
| Lymphocyte <1,500/uL | 0.74 (0.30, 1.78) | 0.495 | – | – | |
| Disease duration >3 year | 1.21 (0.49, 2.98) | 0.684 | – | – | |
| Anti-NTM treatment | 0.79 (0.33, 1.91) | 0.6 | – | – | |
| Visceral fat volume <45 cm3 | 1.67 (0.68, 4.05) | 0.265 | – | – | |
| M. abscessus | 1.78 (0.62, 5.13) | 0.284 | – | – | |
| AFB positivity | 1.55 (0.64, 3.76) | 0.328 | – | – | |
| Smoking | 0.64 (0.21, 1.99) | 0.444 | – | – | |
| DM | 0.62 (0.15, 2.47) | 0.496 | – | – | |
| Menopause | 1.42 (0.36, 5.66) | 0.622 | – | – | |
TB, tuberculosis; BMI, body mass index; AFB, acid fast bacilli; DM, diabetes mellitus.
Changes in nutritional markers according to disease course
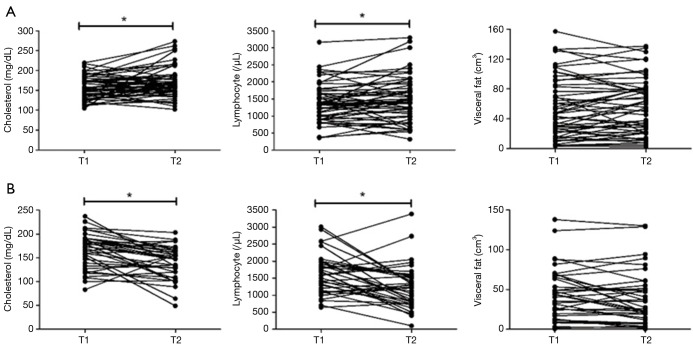
Serial measurements of total cholesterol, total lymphocyte count and visceral fat using chest CT were performed in 89 patients with PNTM disease (Figure 2). The trend differed according to the disease course. In patients with progressive disease, the serum cholesterol level decreased significantly from a median of 173 mg/dL (IQR, 136–190.5) to 147 mg/dL (IQR, 114.5–164.5) at the end of the follow-up (P=0.001). In comparison, in patients with stable disease the serum cholesterol level increased significantly from a median of 154.5 mg/dL (IQR, 133.3-178.8) to 163.5 mg/dL (IQR, 146.5–187.5) at the end of follow-up (P=0.005).
Figure 2.
Trends in cholesterol level, total lymphocyte count and visceral fat area between diagnosis and the end of follow-up in patients with PNTM disease. (A) Stable patients: patients with sputum conversion or without radiographic deterioration after follow-up for >1 year; (B) progressed patients: patients with continued positive sputum and radiographic deterioration after follow-up for >1 year. T1, diagnosis; T2, follow-up time. PNTM, pulmonary nontuberculous mycobacterial.
In patients with progressive disease, total lymphocyte count decreased significantly from a median of 1,680/µL (IQR, 1,172–1,981) to 1,244/µL (IQR, 776–1,559) at the end of follow-up (P=0.002). In comparison, in patients with stable disease total lymphocyte count increased significantly from a median of 1,351/µL (IQR, 990–1,716) to 1,435/µL (IQR, 1,017–1,878) at the end of follow-up (P=0.031).
The changes in visceral fat volume were not significant (progressive disease, initial: 43.6 cm3, follow-up: 27.3 cm3, P=0.142; stable disease, initial: 47.2 cm3, follow-up: 54.9 cm3, P=0.339) in both groups.
Risk factors associated with disease progression in patients with PNTM disease
We conducted a statistical analysis to identify the risk factors associated with PNTM disease progression (Table 3). On univariate analysis, a history of TB (P=0.078), cavitary lesions (P=0.009), BMI <19.5 kg/m2 (P=0.09), a decrease in cholesterol level >20 mg/dL (P<0.001), a decrease in total lymphocyte count > 200/µL (P<0.001) and M. abscessus (P=0.065) were associated with PNTM disease progression. In the multivariate logistic regression analysis, a decrease in cholesterol level >20 mg/dL (P=0.001) and a decrease in lymphocyte count >200/µL (P=0.011) were predictors of PNTM disease progression.
Table 3. Factors associated with disease progression in patients with pulmonary nontuberculous mycobacterial (PNTM) disease.
| Parameter | Disease progression | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Female (vs. male) | 1.13 (0.48, 2.63) | 0.786 | |||
| Age >70 (vs. ≤70) years | 1.85(0.79, 4.35) | 0.159 | |||
| Disease extension of more than two-thirds | 1.68 (0.69, 4.09) | 0.252 | |||
| Past TB history | 2.17 (0.92, 5.11) | 0.078 | 1.72 (0.49, 6.03) | 0.396 | |
| Cavity | 3.24 (1.34, 7.84) | 0.009 | 2.83 (0.83, 9.69) | 0.097 | |
| BMI <19.5 mg/kg2 | 2.10 (0.89, 4.95) | 0.09 | 0.93 (0.28, 3.06) | 0.907 | |
| Decrease in cholesterol level >20 mg/dL | 11.06 (3.59, 34.10) | <0.001 | 10.50 (2.51, 43.98) | 0.001 | |
| Decrease in cholesterol level >30 mg/dL | 7.84 (2.03, 30.36) | 0.003 | |||
| Decrease in lymphocyte count >200/μL | 9.43 (3.36, 24.46) | <0.001 | 5.32 (1.46, 19.35) | 0.011 | |
| Decrease in lymphocyte count >300/μL | 9.92 (3.22, 30.56) | <0.001 | |||
| Decrease in visceral fat area >20 cm3 | 2.47 (0.79, 7.67) | 0.119 | |||
| Decrease in visceral fat area >30 cm3 | 1.06 (0.22, 5.04) | 0.943 | |||
| Anti-NTM treatment | 1.85 (0.79, 4.35) | 0.159 | |||
| Disease duration >3 year | 1.91 (0.79, 4.59) | 0.146 | |||
| M. abscessus | 2.72 (0.94, 7.88) | 0.065 | 1.36 (0.29, 6.33) | 0.692 | |
| AFB positivity | 1.20 (0.51, 2.78) | 0.68 | |||
TB, tuberculosis; BMI, body mass index; AFB, acid fast bacilli.
Risk factors associated with decrease in cholesterol in patients with PNTM disease
Smoking, DM, hyperthyroidism, hypothyroidism and menopause were not associated with decrease in cholesterol level more than 20 mg/dL (Table 4). In the multivariate analysis, disease duration more than 3 years and disease progression was associated with decrease cholesterol level more than 20 mg/dL.
Table 4. Factors associated with decrease in cholesterol level >20 mg/dL in patients with pulmonary nontuberculous mycobacterial (PNTM) disease.
| Parameter | Decrease in cholesterol level >20 mg/dL | ||||
|---|---|---|---|---|---|
| Univariate | Multivariate | ||||
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Female (vs. male) | 2.00 (0.78, 5.13) | 0.149 | – | – | |
| Age >70 (vs. ≤70) years | 1.08 (0.44, 2.66) | 0.872 | – | – | |
| Disease extension of more than two-thirds | 1.96 (0.77, 4.99) | 0.16 | – | – | |
| Past TB history | 0.98 (0.40, 2.45) | 0.99 | – | – | |
| Cavity | 1.27 (0.51, 3.16) | 0.613 | – | – | |
| BMI <19.5 mg/kg2 | 1.73 (0.70, 4.31) | 0.238 | – | – | |
| Disease progression | 13.79 (4.45, 42.75) | <0.001 | 7.95 (2.12, 29.77) | 0.002 | |
| Smoking | 0.78 (0.25, 2.43) | 0.668 | – | – | |
| DM | 1.79 (0.51, 6.23) | 0.363 | – | – | |
| Hypothyroidism | 0.76 (0.08, 7.62) | 0.813 | – | – | |
| Hyperthyroidism | 1.15 (0.11, 14.07) | 0.909 | – | – | |
| Menopause | 0.62 (0.16, 2.43) | 0.498 | – | – | |
| Decrease in visceral fat area >20 cm3 | 4.67 (1.46. 14.91) | 0.009 | 3.58 (0.81, 15.78) | 0.092 | |
| Decrease in lymphocyte count >200/μL | 5.48 (2.05, 14.63) | 0.001 | 3.32 (0.83, 13.31) | 0.091 | |
| Disease duration >3 year | 3.06 (1.20, 7.79) | 0.019 | 4.01 (1.11, 14.45) | 0.034 | |
| M. abscessus | 3.97 (1.35, 11.67) | 0.012 | 3.22 (0.75, 13.75) | 0.114 | |
| AFB positivity | 1.13 (0.46, 2.79) | 0.795 | – | – | |
TB, tuberculosis; BMI, body mass index; AFB, acid fast bacilli; DM, diabetes mellitus.
Discussion
Several previous studies have assessed nutritional status in patients with PNTM disease (5,6). The BMI of patients with PNTM disease was lower than that of age-, sex- and race-matched controls or uninfected healthy controls in previous studies (4,23,24). Ikegame et al. reported that BMI at baseline is associated with disease spread in terms of the number of affected lung segments in PNTM disease (6). In the study of Yamazaki et al., the baseline mean BMI was lower in the deteriorated group than in the non-deteriorated group (25).
Adipocyte-derived adipokines and related inflammatory cytokines have been suggested to contribute to increased susceptibility to PNTM disease (26). Tasaka et al. reported elevated adiponectin levels and reduced leptin levels in patients with PNTM disease compared with healthy controls (27). Leptin deficiency results in impaired innate and adaptive immune functions. Also, increased adiponectin decreases tumor necrosis factors and increases production of interleukin (IL)-10 and IL-1 receptor antagonist (IL-1Ra). Katalija et al. showed that immune-modulating adipokines were abnormally regulated in patients with PNTM disease (23). Several studies reported decreased cytokine production and interferon-γ secretion in patients with PNTM disease (28-30).
In our study, BMI was lower in patients with PNTM disease, in agreement with previous reports. In addition, a BMI less than 19.5 mg/kg2 and cavitary lesions were predictive of extensive lung involvement, but a visceral fat volume of less than 45 cm3 was not.
A suggested by one study (6), use of visceral fat as a universal marker in place of BMI is problematic due to sex-related differences, although it is strongly related to both BMI and adiponectin secretion (31).
Cholesterol is important for adequate functioning of the cellular immune system(7,8,32). Cell membrane lipid rafts are involved in receptor-mediated signaling and cell-to-cell interactions (33). Gordon et al. identified a relationship between the degree of inflammation and extent of cholesterolemia (13).
Hypocholesterolemia is commonly observed in critical and chronic illnesses (34). The cholesterol level of TB patients was lower than that of healthy controls (35), and a cholesterol-rich diet accelerated the bacteriologic sterilization of sputum cultures in patients with pulmonary tuberculosis (10). In patients with severe trauma, persistence of hypocholesterolemia indicates a progressive organ/metabolic dysfunction (36). Lee et al. showed that the changes in lipid profiles in septic patients differed between survivors and nonsurvivors (37).
This study aimed to examine the effect of cholesterol as a nutritional factor in patients with PNTM disease according to disease course. To our knowledge, this is the first study to evaluate the relationships of nutritional factors, including cholesterol level, total lymphocyte count and visceral fat, with disease course in patients with PNTM disease.
In this study, the cholesterol level of patients with PNTM disease was lower than that of age-, sex- and number of comorbid diseases-matched controls. Interestingly, the initial cholesterol level, total lymphocyte count and visceral fat volume were not significantly different between progressed and stable patients. While the cholesterol level and total lymphocyte count decreased concomitantly with clinical and radiological deterioration, they tended to increase with stable disease course including culture conversion or no radiographic deterioration. Thus, changes in cholesterol level and total lymphocyte count may mirror the clinical course of PNTM disease. On contrary, visceral fat volume did not significantly change according to disease course.
Multivariate analysis revealed that a decrease in cholesterol level of >20 mg/dL and a decrease in total lymphocyte count of >200/µL were positively associated with progression. A history of TB, cavitary lesions, BMI less than 19.5 mg/kg2 and M. abscessus were predictive factors for progression in univariate analyses, but not in the multivariate analysis.
Known factors that could have an impact in cholesterol, such as smoking, DM, hypothyroidism, hyperthyroidism and menopause were not related with decrease of cholesterol levels in our study. The long disease duration more than 3 years may induce the decrease of cholesterol level leading to disease progression.
Our results suggest that a cholesterol-related mechanism other than that involving adipokines and cytokines may exist in PNTM disease. Gatfield et al. demonstrated that murine macrophages depleted of cholesterol had a reduced capacity to phagocytose mycobacteria (32). Because inhaled NTM are taken up by alveolar macrophages and survive within vacuoles in the cytoplasm (38), similar to M. tuberculosis, macrophage membrane cholesterol may also play an important role in phagocytosis of NTM. It is possible that nutrient intake decreased as disease progressed. However, Wakamatsu et al. demonstrated that underlying factors other than low nutrient intake may be related to low visceral fat volume in patients with PNTM disease (5). Another possibility is that the disease progression may result in reduced cholesterol despite adequate nutrient intake. Cholesterol metabolism is found to be related to reaction of the organism to inflammation and immune function (26).
This study had several limitations. First, because this was a case-control study, whether hypocholesterolemia actively contributes to disease progression or is an unrelated phenomenon is unclear. Second, we did not measure the levels of cytokines, adiponectin, acute-phase reactants or other lipoproteins, such as HDL cholesterol. Third, we did not compare the cholesterol levels and nutritional statuses of patients with PNTM disease with those with other inflammatory lung diseases; such a comparison may have clarified the clinical significance of hypocholesterolemia. The strength of this study was that the control group was representative of the Korean general population after adjusting for age, sex and number of comorbidities.
In conclusion, our study revealed that BMI and cholesterol levels are lower in patients with PNTM disease than in healthy controls. BMI and cavitary lesions are significantly related to extensive lung involvement in PNTM disease. Although cholesterol was not a marker of extensive lung involvement, the decrease in cholesterol level may be reflective of the disease progression. The present findings warrant further studies of the role of cholesterol and the therapeutic effect of cholesterol supplementation in PNTM disease.
Acknowledgements
Funding: This work was supported by Hallym University Research Fund [HURF-2016-20].
Ethical Statement: The study was approved by institutional/regional/national ethics committee/ethics board of Chuncheon Sacred Heart Hospital (No. 2016-95).
Footnotes
Conflicts of Interest: The authors have no conflict of interest to declare.
References
- 1.Billinger ME, Olivier KN, Viboud C, et al. Nontuberculous mycobacteria-associated lung disease in hospitalized persons, United States, 1998-2005. Emerg Infect Dis 2009;15:1562-9. 10.3201/eid1510.090196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013;34:87-94. 10.1055/s-0033-1333567 [DOI] [PubMed] [Google Scholar]
- 3.Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012;185:881-6. 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-74. 10.1164/rccm.200805-686OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wakamatsu K, Nagata N, Maki S, et al. Patients with MAC Lung Disease Have a Low Visceral Fat Area and Low Nutrient Intake. Pulm Med 2015;2015:218253. [DOI] [PMC free article] [PubMed]
- 6.Ikegame S, Maki S, Wakamatsu K, et al. Nutritional assessment in patients with pulmonary nontuberculous mycobacteriosis. Intern Med 2011;50:2541-6. 10.2169/internalmedicine.50.5853 [DOI] [PubMed] [Google Scholar]
- 7.Cooper RA. Abnormalities of cell-membrane fluidity in the pathogenesis of disease. N Engl J Med 1977;297:371-7. 10.1056/NEJM197708182970707 [DOI] [PubMed] [Google Scholar]
- 8.Dabrowski MP, Peel WE, Thomson AE. Plasma membrane cholesterol regulates human lymphocyte cytotoxic function. Eur J Immunol 1980;10:821-7. 10.1002/eji.1830101105 [DOI] [PubMed] [Google Scholar]
- 9.Deniz O, Gumus S, Yaman H, et al. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clin Biochem 2007;40:162-6. 10.1016/j.clinbiochem.2006.10.015 [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Guzmán C, Vargas MH, Quiñonez F, et al. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005;127:643-51. 10.1378/chest.127.2.643 [DOI] [PubMed] [Google Scholar]
- 11.Verdery RB, Goldberg AP. Hypocholesterolemia as a predictor of death: a prospective study of 224 nursing home residents. J Gerontol 1991;46:M84-90. 10.1093/geronj/46.3.M84 [DOI] [PubMed] [Google Scholar]
- 12.Noel MA, Smith TK, Ettinger WH. Characteristics and outcomes of hospitalized older patients who develop hypocholesterolemia. J Am Geriatr Soc 1991;39:455-61. 10.1111/j.1532-5415.1991.tb02489.x [DOI] [PubMed] [Google Scholar]
- 13.Gordon BR, Parker TS, Levine DM, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med 2001;29:1563-8. 10.1097/00003246-200108000-00011 [DOI] [PubMed] [Google Scholar]
- 14.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 15.Sim YS, Park HY, Jeon K, et al. Standardized combination antibiotic treatment of Mycobacterium avium complex lung disease. Yonsei Med J 2010;51:888-94. 10.3349/ymj.2010.51.6.888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien T, Dinneen SF, O'Brien PC, et al. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc 1993;68:860-6. 10.1016/S0025-6196(12)60694-6 [DOI] [PubMed] [Google Scholar]
- 17.Moffatt RJ. Effects of cessation of smoking on serum lipids and high density lipoprotein-cholesterol. Atherosclerosis 1988;74:85-9. 10.1016/0021-9150(88)90194-3 [DOI] [PubMed] [Google Scholar]
- 18.Garvey WT, Kwon S, Zheng D, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003;52:453-62. 10.2337/diabetes.52.2.453 [DOI] [PubMed] [Google Scholar]
- 19.Akahoshi M, Soda M, Nakashima E, et al. Effects of menopause on trends of serum cholesterol, blood pressure, and body mass index. Circulation 1996;94:61-6. 10.1161/01.CIR.94.1.61 [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Park HJ, Cho SN, et al. Species identification of mycobacteria by PCR-restriction fragment length polymorphism of the rpoB gene. J Clin Microbiol 2000;38:2966-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diagnostic Standards and Classification of Tuberculosis in Adults and Children . This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161:1376-95. 10.1164/ajrccm.161.4.16141 [DOI] [PubMed] [Google Scholar]
- 22.Seiler WO. Clinical pictures of malnutrition in ill elderly subjects. Nutrition 2001;17:496-8. 10.1016/S0899-9007(01)00558-5 [DOI] [PubMed] [Google Scholar]
- 23.Kartalija M, Ovrutsky AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med 2013;187:197-205. 10.1164/rccm.201206-1035OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okumura M, Iwai K, Ogata H, et al. Clinical factors on cavitary and nodular bronchiectatic types in pulmonary Mycobacterium avium complex disease. Intern Med 2008;47:1465-72. 10.2169/internalmedicine.47.1114 [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki Y, Kubo K, Takamizawa A, et al. Markers indicating deterioration of pulmonary Mycobacterium avium-intracellulare infection. Am J Respir Crit Care Med 1999;160:1851-5. 10.1164/ajrccm.160.6.9902019 [DOI] [PubMed] [Google Scholar]
- 26.Chan ED, Iseman MD. Slender, older women appear to be more susceptible to nontuberculous mycobacterial lung disease. Gend Med 2010;7:5-18. 10.1016/j.genm.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 27.Tasaka S, Hasegawa N, Nishimura T, et al. Elevated serum adiponectin level in patients with Mycobacterium avium-intracellulare complex pulmonary disease. Respiration 2010;79:383-7. 10.1159/000231975 [DOI] [PubMed] [Google Scholar]
- 28.Kwon YS, Kim EJ, Lee SH, et al. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung 2007;185:337-41. 10.1007/s00408-007-9040-z [DOI] [PubMed] [Google Scholar]
- 29.Safdar A, Armstrong D, Murray HW. A novel defect in interferon-gamma secretion in patients with refractory nontuberculous pulmonary mycobacteriosis. Ann Intern Med 2003;138:521. 10.7326/0003-4819-138-6-200303180-00030 [DOI] [PubMed] [Google Scholar]
- 30.Vankayalapati R, Wizel B, Samten B, et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis 2001;183:478-84. 10.1086/318087 [DOI] [PubMed] [Google Scholar]
- 31.Staiger H, Tschritter O, Machann J, et al. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes Res 2003;11:368-72. 10.1038/oby.2003.48 [DOI] [PubMed] [Google Scholar]
- 32.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science 2000;288:1647-50. 10.1126/science.288.5471.1647 [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Guzmán C, Vargas MH. Hypocholesterolemia: a major risk factor for developing pulmonary tuberculosis? Med Hypotheses 2006;66:1227-30. 10.1016/j.mehy.2005.12.041 [DOI] [PubMed] [Google Scholar]
- 34.Vyroubal P, Chiarla C, Giovannini I, et al. Hypocholesterolemia in clinically serious conditions--review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008;152:181-9. 10.5507/bp.2008.029 [DOI] [PubMed] [Google Scholar]
- 35.Deniz O, Tozkoparan E, Yaman H, et al. Serum HDL-C levels, log (TG/HDL-C) values and serum total cholesterol/HDL-C ratios significantly correlate with radiological extent of disease in patients with community-acquired pneumonia. Clin Biochem 2006;39:287-92. 10.1016/j.clinbiochem.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 36.Dunham CM, Fealk MH, Sever WE, 3rd. Following severe injury, hypocholesterolemia improves with convalescence but persists with organ failure or onset of infection. Crit Care 2003;7:R145-53. 10.1186/cc2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, Park MS, Park BH, et al. Prognostic Implications of Serum Lipid Metabolism over Time during Sepsis. Biomed Res Int 2015;2015:789298. [DOI] [PMC free article] [PubMed]
- 38.Honda JR, Knight V, Chan ED. Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med 2015;36:1-11. 10.1016/j.ccm.2014.10.001 [DOI] [PubMed] [Google Scholar]