Abstract
Lung cancer (LC) has become one of the leading causes of preventable death in the last few decades. Cigarette smoking (CS) stays as the main etiologic factor of LC despite that many other causes such as occupational exposures, air pollution, asbestos, or radiation have also been implicated. Patients with chronic obstructive pulmonary disease (COPD), which also represents a major cause of morbidity and mortality in developed countries, exhibit a significantly greater risk of LC. The study of the underlying biological mechanisms that may predispose patients with chronic respiratory diseases to a higher incidence of LC has also gained much attention in the last few years. The present review has been divided into three major sections in which different aspects have been addressed: (I) relevant etiologic agents of LC; (II) studies confirming the hypothesis that COPD patients are exposed to a greater risk of developing LC; and (III) evidence on the most relevant underlying biological mechanisms that support the links between COPD and LC. Several carcinogenic agents have been described in the last decades but CS remains to be the leading etiologic agent in most geographical regions in which the incidence of LC is very high. Growing evidence has put the line forward the implications of COPD and especially of emphysema in LC development. Hence, COPD represents a major risk factor of LC in patients. Different avenues of research have demonstrated the presence of relevant biological mechanisms that may predispose COPD patients to develop LC. Importantly, the so far identified biological mechanisms offer targets for the design of specific therapeutic strategies that will further the current treatment options for patients with LC. Prospective screening studies, in which patients with COPD should be followed up for several years will help identify biomarkers that may predict the risk of LC among these patients.
Keywords: Chronic obstructive pulmonary disease (COPD), lung cancer (LC), etiologic agents, epidemics, underlying biological mechanisms
Introduction
Lung cancer (LC) has become one of the leading causes of preventable death in the last few decades (1). Cigarette smoking (CS) stands as the main etiologic factor of LC despite that many other causes such as occupational exposures, air pollution, asbestos, or radiation have also been implicated (1-6). In the 1950s and 1960s, several epidemiologic studies were conducted, in which the links between CS and LC were clearly established (1,7-9). On the other hand, the combination of CS with several environmental or occupational agents may increase the risk of LC in exposed individuals (1). Presently research on the epidemiology of LC is still very active as primary prevention continues to be the most relevant target. Moreover, indoor and outdoor pollutants, which may vary with time, and the components of CS (proportions of tar and nicotine), are also a matter of research nowadays (1). Additionally, the changes in the histopathological characteristics of LC in many developed countries, with a significant rise in the frequency of adenocarcinoma, have also prompted research in this field.
Molecular epidemiology focuses on the elucidation of the biological mechanisms that favor malignancy in the lung parenchyma and airways of smokers and on the factors that enhance susceptibility to LC. In line with this, it has also been well established that patients with underlying respiratory conditions such as chronic obstructive pulmonary disease (COPD), which also represents a major cause of morbidity and mortality in developed countries, exhibit a significantly greater risk of LC (3,10-17). Importantly, in patients with moderate-to-severe COPD, the prevalence of LC can go up as high as five-fold that of smokers without the disease (18-24). Furthermore, the epidemiologic relevance of emphysema in the development of LC in patients with COPD has also been highlighted (2-5,16). The need for well-validated and practical LC screening tools to be implemented in clinical settings has also been underscored in several recent studies (2-5,17).
The study of the underlying biological mechanisms that may predispose patients with chronic respiratory diseases to a higher incidence of LC has also gained much attention in the last few years by several investigators including those listed in this review (25-31). For instance, oxidative and nitrosative stress as a result of reactive oxygen and nitrogen species (ROS and RNS, respectively) were shown to favor carcinogenesis through the activation of cellular processes that result in neoplastic transformation or the induction of DNA mutations (32). Other investigations have also demonstrated the contribution of oxidative damage, inflammatory events, and tumor microenvironment to lung carcinogenesis in patients and animal models (19,20,25-31,33-37).
The present review has been divided into three different sections in which the main topics introduced herein have been reviewed. First of all, the most relevant etiologic agents of LC are being briefly described. Secondly, the different studies that have confirmed the underlying hypothesis that COPD patients are exposed to a greater risk of developing LC are also reviewed. Finally, in the third place, evidence on the most relevant underlying biological mechanisms that support the links between COPD and LC, which account for the greater predisposition of these patients to lung tumorigenesis, is also described in the current review.
Main etiologic agents of LC
As abovementioned, in this century, CS still continues to be the most important etiologic factor of LC. Nonetheless, several other agents such as indoor and outdoor pollutants, which may act synergistically with CS, also play a relevant role in the etiology of LC. A brief description of the most relevant etiologic factors follows below (Figure 1).
Figure 1.
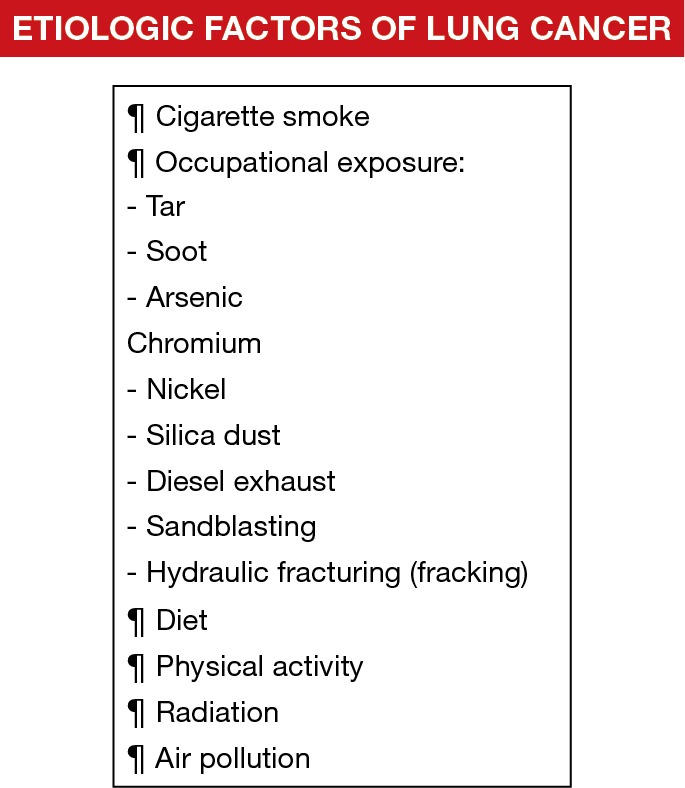
Schematic representation as highlighted by the sign ¶ of the most relevant etiologic factors of lung cancer in patients. Cigarette smoking, occupational exposure (including several procedures and materials), diet, physical activity, radiation, and air pollution are the most relevant etiologic agents of lung cancer.
Carcinogenic effects of CS
We need to go back in history as far as the 1950s in order to understand the etiology of LC. Indeed, the first epidemiologic studies that demonstrated the links between the deleterious effects of CS on the airways and lungs of smokers and LC development were published by British and American scientists in the 1950s and 1960s (1,7-9). Importantly, the duration of smoking and the number of cigarettes smoked were shown to increase the risk of LC among the smokers (38,39). Specifically, in other seminal studies, the duration of CS predicted a higher risk of LC than the amount of cigarettes smoked daily (38,39). Thus, these findings have important implications on the age at which individuals start smoking, which is presently more common in younger adolescents.
The composition of cigarettes has also changed considerably throughout time. Currently, the use of filtered cigarettes is predominant over the unfiltered cigarettes that had been used in the last century. The proportions of nicotine and tar, which includes several types of cancer promoter chemicals in the condensable residue of CS, have varied throughout time. Studies have demonstrated that in smokers consuming filtered cigarettes with lower tar proportions the risk of LC was reduced compared to those exposed to unfiltered cigarettes with higher tar yields (40-42). In another investigation (43), in smokers consuming high-yield tar cigarettes the risk of LC was significantly greater than in low- and medium-yield smokers, suggesting that tar yields are a risk factor of LC. The risks for current and past-smokers have also been reported. As such, compared to lifelong non-smokers, the risk of LC was estimated to be four-fold higher among former smokers, while it was 14-fold greater among current smokers (44). The composition of chemicals included in the cigarette may also greatly account for the pattern changes in histological types of LC that have been observed in the last few decades. In this regard, a shift towards a predominance of adenocarcinoma over squamous and small cell carcinomas has been reported since late 1970s in several investigations (45-47). Moreover, evidence has also shown that passive smokers who are exposed to second-hand smoke have a higher risk of LC, especially that of nonsmoking women with a smoker husband (48,49).
Diet and physical activity
Importantly, factors such as the diet and physical activity also seem to play a role in the risk of LC in smokers. For instance, fruits, vegetables, and antioxidant micronutrients may exert protective effects against LC (50). Results obtained from case-control and cohort studies have demonstrated that smokers with an elevated daily intake of vegetables and fruits, especially the latter, had a lower risk of LC than those subjects who did not follow this type of diet (51-53). Moreover, diets rich in other types of nutrients that are abundantly present in tomatoes and cruciferous vegetables (cross-like shape as a result of the four equal-sized petals in their flowers) such as vitamins A carotenoids, C, and folic acid, and fiber induced protective effects against LC in smokers compared to those who did not include this sort of elements in their diet (52,54-56). Despite that the studies aimed to evaluate the potential beneficial effects of certain nutrients in the reduction of LC risk in smokers are hard to conduct and interpret (confounding lifestyle factors associated with smoking), it is currently possible to conclude that vegetable consumption protects against LC (1). Finally, high levels of physical activity have also been shown to correlate with a lower risk of LC among active smokers than those with a more sedentary lifestyle, even after adjusting for CS (1). In this regard, pulmonary rehabilitation and exercise training programs for several weeks also induced beneficial effects in patients with LC who underwent thoracic surgery for the treatment of their lung neoplasm (57).
Occupational exposure
The contribution of occupational exposure to LC has been estimated to range from 9% to 15%, which is relatively low in industrialized regions when compared to the implications of CS (1). LC has been associated especially with exposure to the following agents: tar, soot, arsenic, chromium, nickel, and silica dust (1). Nonetheless, exposure to these agents has been well controlled in developed countries in the last decades (1). Additionally, in epidemiologic studies, exposure to diesel exhaust was also demonstrated to induce LC in truck drivers (58,59), railroad workers (60), and operators of heavy construction equipment (61). More recently, exposure to new technologies included in the environment and workplace such as sandblasting jean workers and hydraulic fracturing (fracking) have introduced new hazards that may lead to the development of occupational diseases including LC (62).
Asbestos, which consists of fibers of silicate minerals, may also cause LC in exposed individuals such as coal miners (63). First evidence comes from studies conducted in the United Kingdom and United States of America, where textile (63) and insulation (64,65) workers exhibited a significantly higher risk of LC than non-exposed individuals. Importantly, asbestos-induced LC depends on the duration of the exposure and is significantly increased by the influence of CS, which may favor the retention of asbestos fibers in the lungs (66,67).
Radiation
Exposure to ionizing radiation has shown a strong association with LC (68). Despite that exposure to radiation produced by X-rays, gamma rays, neutrons, and radon was shown to cause LC, the levels required to induce lung carcinogenesis were significantly greater than those usually experienced by the general population (1). As such, among atomic bomb non-smoker survivors, the radiation-related risks for LC were similar to those estimated for other solid neoplasms: 0.9 with a female:male sex ratio of 1.6 (69). Radon is a chemical inert gas that occurs naturally as a decay product of radium. The decay products of radon emit alpha particles of high energy and mass that may damage nuclear DNA of cells in the lungs and airways. In fact, a very high risk of LC (40%) was observed in underground miners of uranium who had been exposed to radon chronically (70). In the study, the rates of deaths from LC were 70% and 39% for never-smokers and current smokers, respectively (70). Furthermore, in buildings, radon is ubiquitously distributed as it enters directly from the soil, and its concentrations may vary from room to room, depending on the level of ventilation. The effects of indoor exposure to radon are significantly lower than those seen as a result of occupational exposure as in uranium miners (1,70). Recently, it has been demonstrated that residential radon may increase LC risk up to 30% among never-smokers (71,72). However, in the studies, the potential influence of environmental CS on the risk of LC could not be ruled out (1,71,72). In conclusion, despite that indoor exposure to radon has been suggested to cause LC, these assumptions still need to be definitely confirmed in future epidemiologic studies (1,71,72).
Air pollution
The line has been recently put forward that air pollutants also contain carcinogens, which may favor LC development (1,73). Potential carcinogens include polycyclic aromatic hydrocarbons, arsenic, nickel, and chromium, which are all produced by the combustion of fossil fuels (1). Descriptive studies have highlighted a potential role of air pollution in LC development, especially in urbanized areas (1). In line with this, the relationships between long-term exposure to particulate matter <10 micrometer in diameter (PM10), sulfur dioxide, nitrogen dioxide and mortality of LC have been analyzed in a cohort of Northern China for several years [1998–2009] (74). The results were not conclusive as age, the assignment method for air pollution exposure, and smoking history influenced the analyses of the study results (74). In another study conducted in Tianjin (Northern China), exposure to high concentrations of polycyclic aromatic hydrocarbons induced a greater risk of LC among the elderly in a similar fashion for both men and women (75). On the other hand, ambient fine particulate matter (PM2.5) has recently been shown to account for 32% of total reported deaths in the 74 leading cities of China (76). Specifically, 20% of the reported deaths were attributed to cardiovascular, respiratory and LC conditions (76). The investigators concluded that in certain regions of China, PM2.5 imposes significant health risks that are even greater than those so far attributed to CS, thus action plans for Air Pollution Prevention and Control should be enforced, at least in specific geographical areas (76).
Recently, strong associations between small cell LC and adenocarcinoma (hazard ratios: 1.53 and 1.44, respectively) and exposure to high levels of PM2.5 have also been reported in a cohort of women of the Canadian Cancer Registry (77). In other studies, however, the effects of air pollution as a risk factor of LC were significantly attenuated after adjusting for several factors such as CS and occupational exposure, even if the influence of urbanization persisted (1,78). Other approaches have included the analyses of the effects of factories and smelters in populations residing nearby. However, the results were not entirely conclusive (1). As a summary from different reports (1), it is possible to conclude that 1–2% of LC can be attributed to air pollution, although these proportions may vary widely across geographical regions.
Susceptibility to LC: influence of COPD and other chronic respiratory conditions
Evidence and epidemics
Underlying chronic respiratory conditions such as COPD and lung fibrosis, especially pneumoconiosis (1), increase the susceptibility of the patients to LC. Importantly, COPD and chronic airway obstruction have long been associated with LC development (2-5,10-13,16,79). Interestingly, the prevalence of LC has been consistently greater in men than in females. However, in the last decades, evidence has shown a rising incidence of LC in women probably as a result of the increased numbers of female smokers, who were diagnosed with LC (1,80). Furthermore, among never-smokers the prevalence of LC in females was also significantly higher than in men (81). Most of women were diagnosed at advanced stages of tumor progression, even among never-smokers, who exhibited similar survival rates to those reported in smokers with LC (81).
Therefore, the deployment of screening programs in patients with underlying respiratory diseases has recently emerged as an actual medical need in Western and Eastern societies (2,3,82-84). Progress has been made in the identification of the best screening programs for the early diagnosis of LC. In this regard, low-dose computerized tomography (LDCT) has been proposed as a useful screening tool for LC (85). In keeping with, several authors (3,4,86,87) have demonstrated the benefits of LDCT in the early diagnosis of LC among smokers. Nonetheless, other authors have claimed that in LC screening programs, LDCT should not be still widely applied in the general population up until more convincing results are published (2).
Interestingly, in a LC screening program using LDCT that was conducted in a region of Spain, the presence of COPD and especially of emphysema were strong predictors of LC among the study patients (3). The authors concluded that the results obtained in the LC screening program were comparable to observations that had been previously reported in other European programs, and that LDCT was a valid and feasible diagnostic tool in this context (3). In another study, the same investigators (4) showed that LC screening programs that are exclusively based on the National Lung Screening Trial (NLST) criteria may fail to identify all the cases of LC. On this basis, the investigators suggested that the application of the NLST criteria in patients with emphysema enhanced the detection rates of LC, while lowered the number of missed cases (4). Interestingly, the use of another diagnostic/therapeutic tool such as the endobronchial insertion of one-way valves for the treatment of severe dyspnea in patients with emphysema allowed for the early diagnosis of LC, especially during follow-up (82).
Risk factors of LC
With the aim to identify potential risk factors that may help predict LC morbidity and mortality other approaches have also been used in clinical settings. For instance, pleural and vascular invasion were demonstrated to influence survival and increase the risk of death in patients with non-small cell lung cancer (NSCLC) of small sizes, thus they could be used in a predictive risk model (5,84). Other observations have underscored that in patients with early stages, pneumonectomy rather than lobectomy was associated with poorer survival (12,13), and that airway obstruction was an important predictive factor to define 30-day mortality after lung surgery even in LC patients with advanced age (12,88).
Whether long-lasting effects of CS may be found in patients already diagnosed with LC has also been the matter of recent research. In this regard, in long-term survivor patients, recurrence and the appearance of second tumors were observed in the lungs and other organs of patients with LC as early as three years after the diagnosis (12,89). As the new tumors were also related to CS, the authors concluded that the multiple carcinogenic effects of CS persist several years after the LC diagnosis in patients with long survival (12,89). Thus, novel diagnostic tools are required in order to identify patients who may be at a higher risk of LC, with a special focus on patients with a long smoking history and/or the presence of underlying respiratory conditions such as COPD (90-93).
Interestingly, the risk factors of LC hospitalization have also been recently analyzed from the National Hospital Discharge Database in Spain (79). The conclusions from the study were that age and sex influenced the incidence of hospitalization in patients with LC and that while it decreased in men, a rise was observed in women, which was partly related to the presence of comorbidities (79). In keeping with, recent results obtained from the Spanish National Statistics Institute have shown that the age-adjusted mortality rates increased among Spanish women, while they decreased in men (94). These findings were related to a rise in the prevalence of CS reported among women in Spain (94). Similar findings are probably expected in other geographical regions and deserve special attention (95,96). As a matter of fact, the incidence of CS and exposure to other carcinogenic agents represent major targets for LC prevention campaigns in Western and Eastern societies.
Potential biological mechanisms mediating LC development in patients with COPD
Chronic inflammation
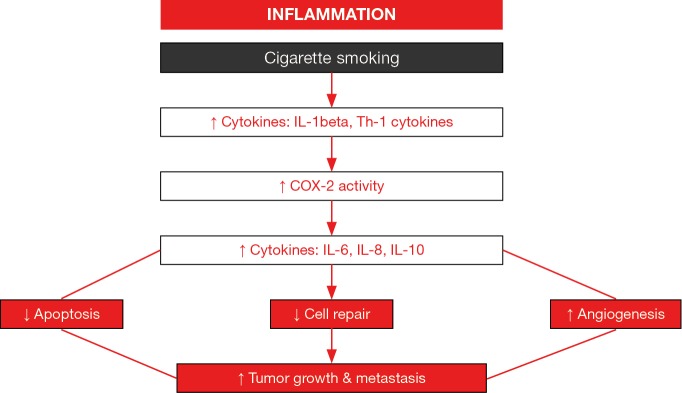
Chronic inflammation through the induction of several interleukins (IL) and cyclooxygenase-2 activity may be an important player in the lung tumor formation among patients with COPD (Figure 2) (97-100). Free radicals and proteases released by activated leukocytes together with the formation of tertiary lymphoid aggregates may conform the first step in tumor development of patients bearing underlying lung inflammatory conditions such as COPD (100). Moreover, these inflammatory molecules may interfere with key regulatory mechanisms such as cell death (apoptosis), autophagy, cell repair, and angiogenesis, which contribute to the neoproliferative process (97,98). Recently, migration (a key process in tumor progression) of NSCLC cells A549 was significantly increased in a chemotactic gradient produced by the serum of COPD patients compared to that of the healthy controls (29). In the serum of the patients, the concentrations of CCL21 and CXCL12, but not those of CXCL5, were significantly greater than levels found in the control subjects (29). Interestingly, the blockade of CCL21 and CXCL12 activities showed that the greater migration of the A549 cells observed in COPD was mediated by the former cytokine (CCL21) (29). The investigators concluded that CCL21 may favor cancer cell migration in the lungs of patients with COPD (29). As these results may offer an interesting therapeutic strategy to combat LC, future studies should be specifically designed in order to demonstrate this mechanism in actual tumors from COPD patients.
Figure 2.
Schematic representation of the potential role of cytokines in tumor development in patients with underlying COPD. Cigarette smoking induces chronic inflammatory events characterized by the induction of several interleukins (IL), cyclooxygenase-2 activity, and cytokines. These inflammatory molecules interfere with key regulatory mechanisms such as cell death (apoptosis), cell repair, and angiogenesis, which contribute to the neoproliferative including tumor growth and metastasis.
Other cytokines and growth factors such as tumor necrosis factor (TNF)-alpha, vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-beta have also been shown to participate in the development of LC in patients with underlying respiratory conditions (30,101). Identification of additional inflammatory molecules that may be involved in the development of LC among patients with COPD and/or tumor progression is of relevance as they offer potential for the design of novel therapeutic strategies in the treatment of LC (100,102).
Cytokines released by T cells may play different roles in tumorigenesis. In line with this, Th1 lymphocytes, which release TNF-alpha, IL-2, and interferon-gamma, have been shown to exert antitumor effects, while Th2 cells, which mainly produce IL-4, predominantly favor tumor growth by inhibiting the host immune system (103). Importantly, LC relapse may also rely on alterations in the balance between Th1 and Th2 cytokines in patients (104-106). For instance, a rise in the systemic levels of Th2 cytokines was observed in patients with LC, while those of Th1 cytokines were decreased (106). Interestingly, after surgical resection of the lung tumor, levels of Th1 and Th2 cytokines were modified in the same patients (106). Specifically, blood levels of both IL-10 and IL-4 were significantly reduced after tumor resection compared to baseline levels before the surgery, despite that they remained significantly greater than those detected in the healthy control group of subjects (only baseline measurements) (106). A relationship between accumulation of myeloid-derived suppressor cells in patients with COPD and LC development has also been recently suggested (107). Recently, associations have also been reported between the levels of certain immune regulators in the bronchoalveolar lavage and disease progression such as metastasis and body weight loss in patients (108).
In a recent investigation from our group (unpublished observations), levels of Th1 cytokines were significantly greater in the tumors of patients with LC and underlying COPD than in those without the chronic respiratory condition. We concluded from these findings that patients with COPD may be somehow protected against tumor development and progression by the release of Th1 cytokines. Studies underway will shed light into the potential mechanisms linking the release of cytokines, chronic inflammation and the lung tumorigenesis in patients with underlying chronic respiratory conditions.
Type 1 (M1) and type 2 (M2) polarized macrophage subtypes play a significant role in tumorigenesis through the regulation of several functions such as cell adhesion, apoptosis, and senescence (104,105,109). Furthermore, macrophages may exert proinflammatory or anti-inflammatory functions depending on the secreted cytokines. In tumors, macrophages are the predominant cells within the inflammatory infiltrates. Importantly, M1 macrophages favor inflammation, whereas M2 macrophages promote anti-inflammatory actions and tissue repair. While M1 cells fight against tumor development, M2 macrophages exert the opposite effects, by promoting cancer growth, survival, progression, and dissemination (110). Recently, the profile of macrophages has been analyzed in the airways of COPD (111). As such, patients with COPD and no LC, airway macrophages did not follow the classic M1/M2 pattern, as a skewed transcriptomic profile that favors M2 macrophages was actually found (111). The authors concluded that this profile might favor tumorigenesis in COPD.
Recent unpublished observations from our group have also shown that in patients with LC, the number of M1 macrophages was reduced while a rise in M2 macrophages was observed in the same specimens. Additionally, a significantly greater M1/M2 ratio was detected in the tumors of LC patients with underlying COPD than in those of patients without this disease. These findings suggest that the prognosis of LC patients with underlying COPD may be better than in those with no COPD. Nonetheless, further research is needed in order to confirm this hypothesis.
The implications of tumor microenvironment are also relevant in the study of the underlying biology that accounts for the greater predisposition of COPD patients to develop lung tumors. In this regard, tumor microenvironment induces immune suppression, reduces the efficacy of chemotherapy, and favors epithelial-to-mesenchymal transition (EMT) in the airways (type-II EMT, obliteration of small airways), which has recently emerged as a novel target for LC treatment (112). In line with this, a recent investigation showed that treatment of lung epithelial cells with CS extracts induced alveolar EMT through a cascade of biological mechanisms characterized by a rise in TGF-beta and Rac1/Smad2 signaling pathway (113). Interestingly, the blockade of TGF-beta activity attenuated EMT expression markers (113). The authors also concluded that these results may open a new avenue for research in the treatment of patients with LC and underlying COPD (113) as type II-EMT and angiogenesis (type-III EMT) favor lung tumor development (31).
Redox balance
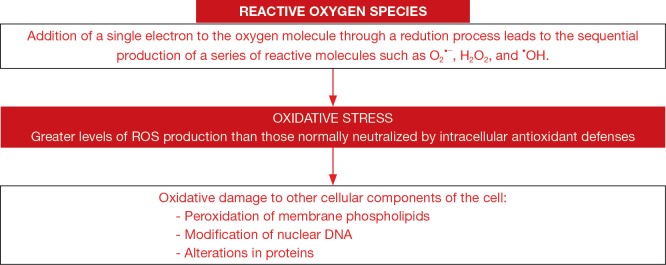
Oxidative stress, defined as the imbalance between oxidants and antioxidants in favor of the former, represents another relevant contributing factor to LC progression (18-22,25-28,33,36) (Figure 3). Oxidative and nitrosative stress were shown to favor carcinogenesis through the activation of cellular processes that result in neoplastic transformation, the induction of DNA mutations (32,114), or even through induction of macrophage dysfunction (alterations in phagocytosis) (27). A rise in oxidant production was observed in several tissues of LC patients and in smokers (18,21,22,33). Proteins and DNA are major cellular target components for the action of oxidants that escape the cellular antioxidant systems. In this regard, several plasma proteins were strongly nitrated and oxidized in LC patients (20). Proteins involved in glycolysis, oxidant scavenging, and cellular structure were more severely nitrated in the lung tumor tissue compared to the non-tumor parenchyma in LC patients in another study (19). More recently, patients with advanced LC exhibited increased systemic oxidative stress levels compared to healthy controls (18,33).
Figure 3.
Schematic representation whereby redox imbalance may induce damage in cells. Reactive oxygen species (ROS) are formed by the addition of electrons to the oxygen molecule leading to the formation of different ROS. Oxidative stress takes place in cells and tissues as a result of an imbalance between oxidants and antioxidants in favor of the former. Oxidative damage in tissues is induced through several mechanisms such as peroxidation of membrane lipids, alterations in nuclear DNA, or oxidation of cell proteins.
In a previous study from our group (25), protein carbonylation levels, as measured by malondialdehyde (MDA)-protein adducts, were also increased in the normal epithelium of patients with LC, especially in patients with underlying COPD, whose levels were significantly greater than in those without this disease. These findings were consistent with those reported in previous investigations, in which a rise in different redox markers was demonstrated in lung tissues or blood of patients with LC (19,20,25,37,101).
Several structural and functional proteins were significantly more oxidized in the lung tumors and non-tumor parenchyma in patients with LC (26). In fact, proteins such as cofilin (34), vimentin (115), and alpha-1-antitrypsin (116,117) were also shown to be more oxidized in the normal epithelium of the airways distant to the neoplasm in patients with LC (25), and their function was altered as a result of the oxidative posttranslational modifications, which may contribute to lung destruction and emphysema (116,117).
On the other hand, several proteins including vimentin, actin, and carbonic anhydrase-1 were also identified to be tyrosine nitrated in the lung tumors of patients (19). Whether these patients might also have had underlying COPD was not analyzed in that investigation (19). However, no conclusive results have been recently encountered in a study conducted in our group as protein tyrosine nitration levels did not significantly differ in the lung tumors of patients with and without COPD (19).
In a recent study (26), a significant rise in mitochondrial superoxide dismutase (SOD)2 protein content was observed in the tumors compared to non-tumor parenchyma in both groups of patients. Additionally, when patients with LC and COPD were analyzed separately on the basis of their smoking history, the heaviest smokers were those exhibiting the actual increase in SOD2 levels in the lung tumors (26). These findings suggest that chronic CS may further enhance the rise in SOD2 content observed in the tumors of those patients. The conclusions from these findings were that SOD2 may be a key survival mechanism for the cancer cells to proliferate in the tumors. In fact, another investigation (34) demonstrated that inhibition of SOD activity reduced tumor burden in mice and promoted cell death in several NSCLC lines of cells. Furthermore, SOD2 was also shown to favor cell migration and invasiveness of tumors in other investigations (117,118). More recently, mRNA and protein levels of SOD2 were also significantly increased in lung tumors and other cancer types in patients (37). The authors concluded that SOD2 may even be considered as a biomarker for cancer progression, from tumor growth to metastasis. Hence, SOD2 overexpression seems to be involved in tumorigenesis in patients with LC, particularly in those with COPD who have a history of CS. Thus, drugs targeted to block SOD2 activity could be of interest in clinical settings.
Protein levels of SOD1 were in general much greater in the patients with underlying COPD in both tumor and non-tumor lung specimens than in LC patients with no COPD (26). Interestingly, CS did not influence SOD1 levels in any of the LC patients with COPD. The conclusions were that SOD1 seems to participate in antioxidant defense of the lungs in COPD patients regardless of the presence of LC rather than in carcinogenesis (26).
The antioxidant enzyme catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen, thus protecting the cells from oxidative damage. Importantly (26), catalase protein levels were significantly reduced in the tumors compared to non-tumor parenchyma in LC patients with and without COPD. Furthermore, in LC patients with COPD, the heaviest smokers were those showing the decrease in catalase levels in the tumor lesions compared to the non-tumor lungs (26). These findings are consistent with other studies in which catalase deficiency was shown to contribute to mammary tumorigenesis in rodents (119) and cancer in patients (37). Collectively, it would be possible to conclude that catalase depletion seems to be involved in cancer development, especially in LC patients with underlying COPD, especially in those who were heavy smokers.
Systemic levels of the oxygen radical superoxide anion and oxidative stress markers were significantly greater, while blood levels of the antioxidant glutathione were reduced in the LC patients with COPD compared to those without this disease (26). Importantly, no significant differences between moderate and heavy smokers were seen in any of the markers analyzed in the blood samples when LC patients with COPD were further subdivided according to their smoking history. In this investigation (26), it was suggested that underlying COPD itself rather than chronic CS may account for the differential pattern of redox balance expression observed in the lung tumors and especially in the blood compartment of LC patients with and without COPD (26).
Glutathione play a relevant role in the detoxification of carcinogens and polycyclic aromatic hydrocarbons (120). Several mutations or deletions of glutathione transferases have been shown to increase susceptibility to develop cancers in patients (121,122). A meta-analysis showed that a specific genotype of glutathione transferases increased the risk to develop LC in Asian populations (120). Levels of the antioxidant reduced glutathione (GSH) were significantly lower in the plasma of LC patients with COPD than in patients without this disease. Interestingly, smoking history did not influence the results encountered in LC patients with underlying COPD, since no differences were detected between moderate and heavy smokers (26). Collectively, all these findings suggest that oxidative damage and antioxidant depletion may contribute to a greater risk to lung carcinogenesis, especially in patients with underlying COPD. Systemic levels of superoxide anion, protein carbonyls, GSH, and nitrotyrosine above a specific threshold levels were predictive of the presence of underlying COPD among the study patients with LC (26). The conclusions were that underlying COPD may predispose patients to a higher risk to develop LC through the induction of increased levels of oxidative stress (26).
Collectively, oxidative stress appears to be a potential therapeutic target for the treatment of LC as antioxidants have recently demonstrated to exert antitumor effects by downregulating proliferating signaling pathways in experimental models (123,124). Furthermore, in the THP-1 cell line (macrophages), the antioxidant thymoquinone also attenuated the phagocytic alterations induced by CS exposure and lipopolysaccharide (27). Nonetheless, in another experimental mouse model of LC, the antioxidant N-acetyl cysteine did not show any beneficial effects on tumor growth (36). Taken together, these results suggest that more research is needed before antioxidants can be included in LC guidelines as potential therapies in actual patients.
Conclusions
Several carcinogenic agents have been described in the last decades but CS remains to be the leading etiologic agent in most of the geographical regions in which the incidence of LC is very high. Growing evidence has put the line forward the implications of COPD and especially of emphysema in LC development. Hence, COPD represents a major risk factor of LC in patients. Different avenues of research have demonstrated the presence of relevant biological mechanisms that may predispose COPD patients to develop LC. Importantly, the so far identified biological mechanisms offer targets for the design of specific therapeutic strategies that will further the current treatment options for patients with LC. Prospective screening studies, in which patients with COPD should be followed up for several years will help identify biomarkers that may predict the risk of LC among these patients.
Acknowledgements
SEPAR 2008, FUCAP 2009, FUCAP 2011, FUCAP 2012, FIS 11/02029 (FEDER), FIS 14/00713 (FEDER), SAF2011-26908, and CIBERES (Instituto de Salud Carlos III) (Spain) have contributed to fund part of the research described in the review.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Alberg AJ, Ford JG, Samet JM, et al. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:29S-55S. [DOI] [PubMed] [Google Scholar]
- 2.Ruano-Ravina A, Perez RM, Fernandez-Villar A. Lung cancer screening with low-dose computed tomography after the National Lung Screening Trial. The debate is still open. Arch Bronconeumol 2013;49:158-65. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Salcedo P, Berto J, de-Torres JP, et al. Lung Cancer Screening: Fourteen Year Experience of the Pamplona Early Detection Program (P-IELCAP). Arch Bronconeumol 2015;51:169-76. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Salcedo P, Wilson DO, de-Torres JP, et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med 2015;191:924-31. 10.1164/rccm.201410-1848OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sánchez de Cos-Escuín J. Prognostic Factors in Stage I Lung Cancer. Arch Bronconeumol 2015;51:427-8. 10.1016/j.arbr.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 6.Villar Álvarez F, Muguruza Trueba I, Vicente Antunes SI. Notes on Recurrence and Second Tumors in Lung Cancer. Arch Bronconeumol 2016;52:545-6. 10.1016/j.arbr.2016.03.026 [DOI] [PubMed] [Google Scholar]
- 7.Doll R, Hill AB. Smoking and carcinoma of the lung; preliminary report. Br Med J 1950;2:739-48. 10.1136/bmj.2.4682.739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levin ML, Goldstein H, Gerhardt PR. Cancer and tobacco smoking; a preliminary report. J Am Med Assoc 1950;143:336-8. 10.1001/jama.1950.02910390008002 [DOI] [PubMed] [Google Scholar]
- 9.Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc 1950;143:329-36. 10.1001/jama.1950.02910390001001 [DOI] [PubMed] [Google Scholar]
- 10.Álvarez Martínez CJ, Bastarrika Alemañ G, Disdier Vicente C, et al. Guideline on management of solitary pulmonary nodule. Arch Bronconeumol 2014;50:285-93. 10.1016/j.arbr.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 11.Leiro-Fernández V, Mouronte-Roibas C, Ramos-Hernandez C, et al. Changes in clinical presentation and staging of lung cancer over two decades. Arch Bronconeumol 2014;50:417-21. 10.1016/j.arbr.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez M, Gómez Hernández MT, Novoa NM, et al. Poorer Survival in Stage IB Lung Cancer Patients After Pneumonectomy. Arch Bronconeumol 2015;51:223-6. 10.1016/j.arbr.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez M, Gómez Hernández MT, Novoa NM, et al. Morbidity and mortality in octogenarians with lung cancer undergoing pneumonectomy. Arch Bronconeumol 2015;51:219-22. 10.1016/j.arbr.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Sánchez de Cos Escuín J, Serra Mitjans M, Hernández Hernández J, et al. The Spanish Society of Pulmonology and Thoracic Surgery Lung Cancer Cooperative Group-II registry. A descriptive study. Arch Bronconeumol 2013;49:462-7. 10.1016/j.arbr.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 15.Sánchez de Cos J, Hernández JH, López MF, et al. SEPAR guidelines for lung cancer staging. Arch Bronconeumol 2011;47:454-65. 10.1016/j.arbr.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez J, Marin M, Sanchez-Salcedo P, et al. Lung cancer screening in patients with chronic obstructive pulmonary disease. Ann Transl Med 2016;4:160. 10.21037/atm.2016.03.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leiro-Fernández V, Priegue Carrera A, Fernández-Villar A. Efficacy of Double Bronchodilation (LABA+LAMA) in Patients with Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer. Arch Bronconeumol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Srivastava S, Prasad R, et al. Oxidative stress in non-small cell lung cancer patients after chemotherapy: association with treatment response. Respirology 2010;15:349-56. 10.1111/j.1440-1843.2009.01703.x [DOI] [PubMed] [Google Scholar]
- 19.Masri FA, Comhair SA, Koeck T, et al. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med 2005;172:597-605. 10.1164/rccm.200411-1523OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pignatelli B, Li CQ, Boffetta P, et al. Nitrated and oxidized plasma proteins in smokers and lung cancer patients. Cancer Res 2001;61:778-84. [PubMed] [Google Scholar]
- 21.Rahman I, Morrison D, Donaldson K, et al. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996;154:1055-60. 10.1164/ajrccm.154.4.8887607 [DOI] [PubMed] [Google Scholar]
- 22.Rahman I, van Schadewijk AA, Crowther AJ, et al. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:490-5. 10.1164/rccm.2110101 [DOI] [PubMed] [Google Scholar]
- 23.Álvarez FV, Trueba IM, Sanchis JB, et al. Recommendations of the Spanish Society of Pneumology and Thoracic Surgery on the diagnosis and treatment of non-small-cell lung cancer. Arch Bronconeumol 2016;52 Suppl 1:2-62. [DOI] [PubMed] [Google Scholar]
- 24.Villar Álvarez F, Muguruza Trueba I, Belda Sanchis J, et al. Executive summary of the SEPAR recommendations for the diagnosis and treatment of non-small cell lung cancer. Arch Bronconeumol 2016;52:378-88. 10.1016/j.arbr.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 25.Barreiro E, Fermoselle C, Mateu-Jimenez M, et al. Oxidative stress and inflammation in the normal airways and blood of patients with lung cancer and COPD. Free Radic Biol Med 2013;65:859-71. 10.1016/j.freeradbiomed.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 26.Mateu-Jiménez M, Sánchez-Font A, Rodríguez-Fuster A, et al. Redox imbalance in lung cancer of patients with underlying chronic respiratory conditions. Mol Med 2016. [Epub ahead of print]. 10.2119/molmed.2015.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnawi J, Tran HB, Roscioli E, et al. Pro-phagocytic Effects of Thymoquinone on Cigarette Smoke-exposed Macrophages Occur by Modulation of the Sphingosine-1-phosphate Signalling System. COPD 2016;13:653-61. 10.3109/15412555.2016.1153614 [DOI] [PubMed] [Google Scholar]
- 28.Bernardo I, Bozinovski S, Vlahos R. Targeting oxidant-dependent mechanisms for the treatment of COPD and its comorbidities. Pharmacol Ther 2015;155:60-79. 10.1016/j.pharmthera.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 29.Kuźnar-Kamińska B, Mikuła-Pietrasik J, Sosińska P, et al. COPD promotes migration of A549 lung cancer cells: the role of chemokine CCL21. Int J Chron Obstruct Pulmon Dis 2016;11:1061-6. 10.2147/COPD.S96490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marwick JA, Kirkham P, Gilmour PS, et al. Cigarette smoke-induced oxidative stress and TGF-beta1 increase p21waf1/cip1 expression in alveolar epithelial cells. Ann N Y Acad Sci 2002;973:278-83. 10.1111/j.1749-6632.2002.tb04649.x [DOI] [PubMed] [Google Scholar]
- 31.Sohal SS, Mahmood MQ, Walters EH. Clinical significance of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease (COPD): potential target for prevention of airway fibrosis and lung cancer. Clin Transl Med 2014;3:33. 10.1186/s40169-014-0033-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. 10.1056/NEJMra0802714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esme H, Cemek M, Sezer M, et al. High levels of oxidative stress in patients with advanced lung cancer. Respirology 2008;13:112-6. 10.1111/j.1440-1843.2007.01212.x [DOI] [PubMed] [Google Scholar]
- 34.Glasauer A, Sena LA, Diebold LP, et al. Targeting SOD1 reduces experimental non-small-cell lung cancer. J Clin Invest 2014;124:117-28. 10.1172/JCI71714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grimm EA, Sikora AG, Ekmekcioglu S. Molecular pathways: inflammation-associated nitric-oxide production as a cancer-supporting redox mechanism and a potential therapeutic target. Clin Cancer Res 2013;19:5557-63. 10.1158/1078-0432.CCR-12-1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mateu-Jiménez M, Cucarull-Martinez B, Yelamos J, et al. Reduced tumor burden through increased oxidative stress in lung adenocarcinoma cells of PARP-1 and PARP-2 knockout mice. Biochimie 2016;121:278-86. 10.1016/j.biochi.2015.11.030 [DOI] [PubMed] [Google Scholar]
- 37.Miar A, Hevia D, Munoz-Cimadevilla H, et al. Manganese superoxide dismutase (SOD2/MnSOD)/catalase and SOD2/GPx1 ratios as biomarkers for tumor progression and metastasis in prostate, colon, and lung cancer. Free Radic Biol Med 2015;85:45-55. 10.1016/j.freeradbiomed.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 38.Doll R. Mortality from lung cancer among non-smokers. Br J Cancer 1953;7:303-12. 10.1038/bjc.1953.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doll R, Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J Epidemiol Community Health 1978;32:303-13. 10.1136/jech.32.4.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garfinkel L, Stellman SD. Smoking and lung cancer in women: findings in a prospective study. Cancer Res 1988;48:6951-5. [PubMed] [Google Scholar]
- 41.Lubin JH, Blot WJ, Berrino F, et al. Patterns of lung cancer risk according to type of cigarette smoked. Int J Cancer 1984;33:569-76. 10.1002/ijc.2910330504 [DOI] [PubMed] [Google Scholar]
- 42.Sidney S, Tekawa IS, Friedman GD. A prospective study of cigarette tar yield and lung cancer. Cancer Causes Control 1993;4:3-10. 10.1007/BF00051707 [DOI] [PubMed] [Google Scholar]
- 43.Stellman SD, Garfinkel L. Lung cancer risk is proportional to cigarette tar yield: evidence from a prospective study. Prev Med 1989;18:518-25. 10.1016/0091-7435(89)90010-8 [DOI] [PubMed] [Google Scholar]
- 44.Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004;328:1519. 10.1136/bmj.38142.554479.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charloux A, Quoix E, Wolkove N, et al. The increasing incidence of lung adenocarcinoma: reality or artefact? A review of the epidemiology of lung adenocarcinoma. Int J Epidemiol 1997;26:14-23. 10.1093/ije/26.1.14 [DOI] [PubMed] [Google Scholar]
- 46.Vincent RG, Pickren JW, Lane WW, et al. The changing histopathology of lung cancer: a review of 1682 cases. Cancer 1977;39:1647-55. [DOI] [PubMed] [Google Scholar]
- 47.Furrukh M. Tobacco Smoking and Lung Cancer: Perception-changing facts. Sultan Qaboos Univ Med J 2013;13:345-58. 10.12816/0003255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirayama T. Non-smoking wives of heavy smokers have a higher risk of lung cancer: a study from Japan. Br Med J (Clin Res Ed) 1981;282:183-5. 10.1136/bmj.282.6259.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trichopoulos D, Kalandidi A, Sparros L, et al. Lung cancer and passive smoking. Int J Cancer 1981;27:1-4. 10.1002/ijc.2910270102 [DOI] [PubMed] [Google Scholar]
- 50.Peto R, Doll R, Buckley JD, et al. Can dietary beta-carotene materially reduce human cancer rates? Nature 1981;290:201-8. 10.1038/290201a0 [DOI] [PubMed] [Google Scholar]
- 51.Holick CN, Michaud DS, Stolzenberg-Solomon R, et al. Dietary carotenoids, serum beta-carotene, and retinol and risk of lung cancer in the alpha-tocopherol, beta-carotene cohort study. Am J Epidemiol 2002;156:536-47. 10.1093/aje/kwf072 [DOI] [PubMed] [Google Scholar]
- 52.Neuhouser ML, Patterson RE, Thornquist MD, et al. Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomarkers Prev 2003;12:350-8. [PubMed] [Google Scholar]
- 53.Wright ME, Mayne ST, Swanson CA, et al. Dietary carotenoids, vegetables, and lung cancer risk in women: the Missouri women's health study (United States). Cancer Causes Control 2003;14:85-96. 10.1023/A:1022565601937 [DOI] [PubMed] [Google Scholar]
- 54.Bond GG, Thompson FE, Cook RR. Dietary vitamin A and lung cancer: results of a case-control study among chemical workers. Nutr Cancer 1987;9:109-21. 10.1080/01635588709513918 [DOI] [PubMed] [Google Scholar]
- 55.Brennan P, Fortes C, Butler J, et al. A multicenter case-control study of diet and lung cancer among non-smokers. Cancer Causes Control 2000;11:49-58. 10.1023/A:1008909519435 [DOI] [PubMed] [Google Scholar]
- 56.Hu J, Mao Y, Dryer D, et al. Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev 2002;26:129-38. 10.1016/S0361-090X(02)00038-7 [DOI] [PubMed] [Google Scholar]
- 57.Sebio R, Yanez-Brage MI, Gimenez-Moolhuyzen E, et al. Impact of a pre-operative pulmonary rehabilitation program on functional performance in patients undergoing video-assisted thoracic surgery for lung cancer. Arch Bronconeumol 2016;52:231-2. [DOI] [PubMed] [Google Scholar]
- 58.Guo J, Kauppinen T, Kyyronen P, et al. Occupational exposure to diesel and gasoline engine exhausts and risk of lung cancer among Finnish workers. Am J Ind Med 2004;45:483-90. 10.1002/ajim.20013 [DOI] [PubMed] [Google Scholar]
- 59.Parent ME, Rousseau MC, Boffetta P, et al. Exposure to diesel and gasoline engine emissions and the risk of lung cancer. Am J Epidemiol 2007;165:53-62. 10.1093/aje/kwj343 [DOI] [PubMed] [Google Scholar]
- 60.Garshick E, Laden F, Hart JE, et al. Lung cancer in railroad workers exposed to diesel exhaust. Environ Health Perspect 2004;112:1539-43. 10.1289/ehp.7195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Järvholm B, Silverman D. Lung cancer in heavy equipment operators and truck drivers with diesel exhaust exposure in the construction industry. Occup Environ Med 2003;60:516-20. 10.1136/oem.60.7.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moitra S, Puri R, Paul D, et al. Global perspectives of emerging occupational and environmental lung diseases. Curr Opin Pulm Med 2015;21:114-20. 10.1097/MCP.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 63.Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med 1955;12:81-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selikoff IJ, Churg J, Hammond EC. Asbestos exposure and neoplasia. JAMA 1964;188:22-6. 10.1001/jama.1964.03060270028006 [DOI] [PubMed] [Google Scholar]
- 65.Selikoff IJ, Hammond EC, Seidman H. Mortality experience of insulation workers in the United States and Canada, 1943--1976. Ann N Y Acad Sci 1979;330:91-116. 10.1111/j.1749-6632.1979.tb18711.x [DOI] [PubMed] [Google Scholar]
- 66.Churg A, Stevens B. Enhanced retention of asbestos fibers in the airways of human smokers. Am J Respir Crit Care Med 1995;151:1409-13. 10.1164/ajrccm.151.5.7735593 [DOI] [PubMed] [Google Scholar]
- 67.Hammond EC, Selikoff IJ, Seidman H. Asbestos exposure, cigarette smoking and death rates. Ann N Y Acad Sci 1979;330:473-90. 10.1111/j.1749-6632.1979.tb18749.x [DOI] [PubMed] [Google Scholar]
- 68.Boice JD, Jr. Studies of atomic bomb survivors. Understanding radiation effects. JAMA 1990;264:622-3. 10.1001/jama.1990.03450050080033 [DOI] [PubMed] [Google Scholar]
- 69.Pierce DA, Sharp GB, Mabuchi K. Joint effects of radiation and smoking on lung cancer risk among atomic bomb survivors. Radiat Res 2003;159:511-20. 10.1667/0033-7587(2003)159[0511:JEORAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 70.Lubin JH, Boice JD, Jr, Edling C, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst 1995;87:817-27. 10.1093/jnci/87.11.817 [DOI] [PubMed] [Google Scholar]
- 71.Ruano-Ravina A, Prini-Guadalupe L, Barros-Dios JM, et al. Exposure to residential radon and lung cancer in never-smokers: the preliminary results of the LCRINS study. Arch Bronconeumol 2012;48:405-9. [DOI] [PubMed] [Google Scholar]
- 72.Torres-Durán M, Ruano-Ravina A, Parente-Lamelas I, et al. Lung cancer in never-smokers: a case-control study in a radon-prone area (Galicia, Spain). Eur Respir J 2014;44:994-1001. 10.1183/09031936.00017114 [DOI] [PubMed] [Google Scholar]
- 73.Carazo Fernández L, Fernández Alvarez R, González-Barcala FJ, et al. Indoor air contaminants and their impact on respiratory pathologies. Arch Bronconeumol 2013;49:22-7. 10.1016/j.arbr.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Zhang LW, Huang JJ, et al. Long-term exposure to urban air pollution and lung cancer mortality: A 12-year cohort study in Northern China. Sci Total Environ 2016;571:855-61. 10.1016/j.scitotenv.2016.07.064 [DOI] [PubMed] [Google Scholar]
- 75.Han B, Liu Y, You Y, et al. Assessing the inhalation cancer risk of particulate matter bound polycyclic aromatic hydrocarbons (PAHs) for the elderly in a retirement community of a mega city in North China. Environ Sci Pollut Res Int 2016. [Epub ahead of print]. 10.1007/s11356-016-7209-9 [DOI] [PubMed] [Google Scholar]
- 76.Fang D, Wang Q, Li H, et al. Mortality effects assessment of ambient PM2.5 pollution in the 74 leading cities of China. Sci Total Environ 2016;569-570:1545-52. 10.1016/j.scitotenv.2016.06.248 [DOI] [PubMed] [Google Scholar]
- 77.Tomczak A, Miller AB, Weichenthal SA, et al. Long-term exposure to fine particulate matter air pollution and the risk of lung cancer among participants of the Canadian National Breast Screening Study. Int J Cancer 2016;139:1958-66. 10.1002/ijc.30255 [DOI] [PubMed] [Google Scholar]
- 78.Jaakkola MS, Samet JM. Occupational exposure to environmental tobacco smoke and health risk assessment. Environ Health Perspect 1999;107 Suppl 6:829-35. 10.1289/ehp.99107s6829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palacio Nebreda MM, de Miguel-Diez J, Villegas Fernández FR, et al. Trends in the Incidence of Lung Cancer Hospitalizations in Spain, 2001-2011. Arch Bronconeumol 2016;52:411-9. [DOI] [PubMed] [Google Scholar]
- 80.Molina AJ, Garcia-Martinez L, Zapata-Alvarado J, et al. Trends in Lung Cancer Incidence in a Healthcare Area. Arch Bronconeumol 2015;51:e53-e55. [DOI] [PubMed] [Google Scholar]
- 81.Parente Lamelas I, Abal Arca J, Blanco Cid N, et al. Clinical characteristics and survival in never smokers with lung cancer. Arch Bronconeumol 2014;50:62-6. [DOI] [PubMed] [Google Scholar]
- 82.Fiorelli A, Costanzo S, di Costanzo E, et al. The early detection of lung cancer during follow-up of patients undergoing endobronchial one-way valve treatment for emphysema. Arch Bronconeumol 2015;51:e13-5. [DOI] [PubMed] [Google Scholar]
- 83.Monsó E, Montuenga LM, Sánchez de Cos J, et al. Biological Marker Analysis as Part of the CIBERES-RTIC Cancer-SEPAR Strategic Project on Lung Cancer. Arch Bronconeumol 2015;51:462-7. 10.1016/j.arbr.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 84.Peñalver Cuesta JC, Jordá Aragón C, Mancheño Franch N, et al. Prognostic Factors in Non-Small Cell Lung Cancer Less Than 3 Centimeters: Actuarial Analysis, Accumulative Incidence and Risk Groups. Arch Bronconeumol 2015;51:431-9. 10.1016/j.arbr.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 85.Gould MK. Clinical practice. Lung-cancer screening with low-dose computed tomography. N Engl J Med 2014;371:1813-20. 10.1056/NEJMcp1404071 [DOI] [PubMed] [Google Scholar]
- 86.de-Torres JP, Wilson DO, Sanchez-Salcedo P, et al. Lung cancer in patients with chronic obstructive pulmonary disease. Development and validation of the COPD Lung Cancer Screening Score. Am J Respir Crit Care Med 2015;191:285-91. 10.1164/rccm.201407-1210OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932-8. 10.1378/chest.07-1490 [DOI] [PubMed] [Google Scholar]
- 88.Freixinet Gilart J, Rodríguez Suárez PM. Morbidity, mortality and survival after surgery for lung cancer. Arch Bronconeumol 2015;51:211-2. [DOI] [PubMed] [Google Scholar]
- 89.Sánchez de Cos Escuín J, Rodríguez López DP, Utrabo Delgado I, et al. Disease Recurrence and Second Tumors in Long-term Survivors of Lung Cancer. Arch Bronconeumol 2016;52:183-8. 10.1016/j.arbr.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 90.Andreo García F, Centeno Clemente CÁ, Sanz Santos J, et al. Initial experience with real-time elastography using an ultrasound bronchoscope for the evaluation of mediastinal lymph nodes. Arch Bronconeumol 2015;51:e8-11. 10.1016/j.arbr.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 91.Casutt A, Prella M, Beigelman-Aubry C, et al. Fluoroscopic-Guided Radial Endobronchial Ultrasound Without Guide Sheath For Peripheral Pulmonary Lesions: A Safe And Efficient Combination. Arch Bronconeumol 2015;51:338-43. [DOI] [PubMed] [Google Scholar]
- 92.Fernández-Villar A, Mouronte-Roibás C, Botana-Rial M, et al. Ten Years of Linear Endobronchial Ultrasound: Evidence of Efficacy, Safety and Cost-effectiveness. Arch Bronconeumol 2016;52:96-102. 10.1016/j.arbr.2015.08.008 [DOI] [PubMed] [Google Scholar]
- 93.García-Ortega A, Briones-Gómez A, Fabregat S, et al. Benefit of Chest Ultrasonography in the Diagnosis of Peripheral Thoracic Lesions in an Interventional Pulmonology Unit. Arch Bronconeumol 2016;52:244-9. 10.1016/j.arbr.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 94.Martín-Sánchez JC, Clèries R, Lidón-Moyano C, et al. Differences between Men and Women in Time Trends in Lung Cancer Mortality in Spain (1980-2013). Arch Bronconeumol 2016;52:316-20. 10.1016/j.arbr.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 95.Bao PP, Zheng Y, Wu CX, et al. Cancer incidence in urban Shanghai, 1973-2010: an updated trend and age-period-cohort effects. BMC Cancer 2016;16:284. 10.1186/s12885-016-2313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho WC, Kwan CK, Yau S, et al. The role of inflammation in the pathogenesis of lung cancer. Expert Opin Ther Targets 2011;15:1127-37. 10.1517/14728222.2011.599801 [DOI] [PubMed] [Google Scholar]
- 98.O'Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer 2001;85:473-83. 10.1054/bjoc.2001.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao B, Liu H, Gu Z, et al. Expression of microRNA-133 inhibits epithelial-mesenchymal transition in lung cancer cells by directly targeting FOXQ1. Arch Bronconeumol 2016. [Epub ahead of print]. 10.1016/j.arbr.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 100.Bozinovski S, Vlahos R, Anthony D, et al. COPD and squamous cell lung cancer: aberrant inflammation and immunity is the common link. Br J Pharmacol 2016;173:635-48. 10.1111/bph.13198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carpagnano GE, Spanevello A, Curci C, et al. IL-2, TNF-alpha, and leptin: local versus systemic concentrations in NSCLC patients. Oncol Res 2007;16:375-81. [DOI] [PubMed] [Google Scholar]
- 102.Bozinovski S, Anthony D, Vlahos R. Targeting pro-resolution pathways to combat chronic inflammation in COPD. J Thorac Dis 2014;6:1548-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy--a review. Virus Genes 2006;33:235-52. [DOI] [PubMed] [Google Scholar]
- 104.Conway EM, Pikor LA, Kung SH, et al. Macrophages, Inflammation, and Lung Cancer. Am J Respir Crit Care Med 2016;193:116-30. 10.1164/rccm.201508-1545CI [DOI] [PubMed] [Google Scholar]
- 105.Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [DOI] [PMC free article] [PubMed]
- 106.Li J, Wang Z, Mao K, et al. Clinical significance of serum T helper 1/T helper 2 cytokine shift in patients with non-small cell lung cancer. Oncol Lett 2014;8:1682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scrimini S, Pons J, Sauleda J. Myeloid-Derived Suppressor Cells: Possible Link Between Chronic Obstrucive Pulmonary Disease and Lung Cancer. Arch Bronconeumol 2016;52:29-35. [DOI] [PubMed] [Google Scholar]
- 108.Montilla D, Pérez M, Borges L, et al. Soluble Human Leukocyte Antigen-G in the Bronchoalveolar Lavage of Lung Cancer Patients. Arch Bronconeumol 2016;52:420-4. [DOI] [PubMed] [Google Scholar]
- 109.Yuan A, Hsiao YJ, Chen HY, et al. Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci Rep 2015;5:14273. 10.1038/srep14273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 2008;180:2011-7. 10.4049/jimmunol.180.4.2011 [DOI] [PubMed] [Google Scholar]
- 111.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol 2014;5:435. 10.3389/fimmu.2014.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mittal V, El Rayes T, Narula N, et al. The Microenvironment of Lung Cancer and Therapeutic Implications. Adv Exp Med Biol 2016;890:75-110. 10.1007/978-3-319-24932-2_5 [DOI] [PubMed] [Google Scholar]
- 113.Shen HJ, Sun YH, Zhang SJ, et al. Cigarette smoke-induced alveolar epithelial-mesenchymal transition is mediated by Rac1 activation. Biochim Biophys Acta 2014;1840:1838-49. [DOI] [PubMed]
- 114.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 115.Young JF, Larsen LB, Malmendal A, et al. Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J Int Soc Sports Nutr 2010;7:9. 10.1186/1550-2783-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alam S, Li Z, Janciauskiene S, et al. Oxidation of Z alpha1-antitrypsin by cigarette smoke induces polymerization: a novel mechanism of early-onset emphysema. Am J Respir Cell Mol Biol 2011;45:261-9. 10.1165/rcmb.2010-0328OC [DOI] [PubMed] [Google Scholar]
- 117.Li Z, Alam S, Wang J, et al. Oxidized {alpha}1-antitrypsin stimulates the release of monocyte chemotactic protein-1 from lung epithelial cells: potential role in emphysema. Am J Physiol Lung Cell Mol Physiol 2009;297:L388-L400. 10.1152/ajplung.90373.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Connor KM, Hempel N, Nelson KK, et al. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res 2007;67:10260-7. 10.1158/0008-5472.CAN-07-1204 [DOI] [PubMed] [Google Scholar]
- 119.Hempel N, Carrico PM, Melendez JA. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anticancer Agents Med Chem 2011;11:191-201. 10.2174/187152011795255911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao Y, Wang B, Hu K, et al. Glutathione S-transferase theta1 polymorphism contributes to lung cancer susceptibility: A meta-analysis of 26 case-control studies. Oncol Lett 2015;9:1947-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gallegos-Arreola MP, Gómez-Meda BC, Morgan-Villela G, et al. GSTT1 gene deletion is associated with lung cancer in Mexican patients. Dis Markers 2003-2004;19:259-61. 10.1155/2004/826408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Litwack G, Ketterer B, Arias IM. Ligandin: a hepatic protein which binds steroids, bilirubin, carcinogens and a number of exogenous organic anions. Nature 1971;234:466-7. 10.1038/234466a0 [DOI] [PubMed] [Google Scholar]
- 123.Kasala ER, Bodduluru LN, Barua CC, et al. Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed Pharmacother 2016;82:568-77. 10.1016/j.biopha.2016.05.042 [DOI] [PubMed] [Google Scholar]
- 124.Menon S, Lu C, Menon R, et al. Effects of Antioxidants in Human Cancers: Differential Effects on Non-Coding Intronic RNA Expression. Antioxidants (Basel) 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]