Abstract
Obstructive sleep apnoea (OSA) is increasingly prevalent, particularly in the context of the obesity epidemic, and is associated with a significant social, health and economic impact. The gold standard of treatment for moderate to severe OSA is continuous positive airway pressure (CPAP). However compliance rates can be low. Methodology to improve patient tolerance to CPAP alongside with alternative, non-surgical and surgical, management strategies are discussed. All patients that fail CPAP therapy would benefit from formal upper airway evaluation by the otolaryngologist to identify any obvious causes and consider site-specific surgical therapies. Patient selection is integral to ensuring successful outcomes. A multidisciplinary team is needed to manage these patients.
Keywords: Obstructive sleep apnoea (OSA), continuous positive airway pressure (CPAP), compliance, failure, surgery
Introduction
Sleep-related breathing disorders comprise of a wide range of conditions, including obstructive sleep apnoea (OSA), where recurrent partial or complete cessation of breathing occurs. This spectrum of disorders is common and negatively impacts patient health alongside conferring a significant socio-economic burden (1). In addition, with the ensuing obesity epidemic, many of these conditions are increasing in prevalence.
OSA affects 2–4% of males and 1–2% of females but is thought to be underdiagnosed, with one large population study indicating that 93% of females and 82% of males with moderate to severe OSA had not been clinically diagnosed, despite access to healthcare (2-4). More recent estimates indicate that 13% of men and 6% of women have moderate to severe OSA (5). There is robust evidence demonstrating that moderate to severe OSA correlates independently with a large increased risk of all-cause mortality, alongside with an increased risk of, amongst others, cardiac arrhythmias, myocardial infarction, insulin resistance, pulmonary and systemic hypertension, stroke, impaired cognition and road traffic accidents (6-11).
In accordance with the UK national guidelines, the gold standard treatment for moderate to severe OSA is continuous positive airway pressure (CPAP) (12). This is most frequently in the form of nasal CPAP although auto-titrating CPAP, which responds to the individual’s airflow patterns, and bilevel positive airway pressure (BiPAP) have also been used, although the latter currently has a limited evidence base as compared to CPAP. However compliance, particularly long-term, can be poor and has been estimated to be as low as 40–85% (12-15).
CPAP
CPAP was first developed in the 1980s and its underlying principle is that of continuous mild air pressure which serves to stent open the airway, and thereby overcome anatomical areas of collapse or obstruction. The key elements of the system include a CPAP machine (which creates the pressure gradient) and tubing, which attaches to, and transmits pressure to, the CPAP mask. The CPAP mask usually covers the nose only (nasal CPAP, nCPAP) but can be used via a nose and mouth mask (full face mask), or, in the form of nasal prongs.
A large body of literature, including higher level evidence in the form of meta-analyses and randomized controlled trials, describes the benefits of CPAP in terms of both symptomatic improvement and long term outcomes (15). By preventing airway collapse and vibration, CPAP eliminates snoring and improves sleep quality for the partner along with nocturnal symptoms such as choking, awakenings and nocturia. Furthermore, daytime somnolence is improved both subjectively and objectively with a resultant improvement in concentration. CPAP has also consistently demonstrated improvements in OSA-specific quality of life studies (15-17). From a long-term cardiovascular risk perspective, CPAP has been shown to have a positive impact, with, for example, randomized trials demonstrating reduction in blood pressure. Interestingly a meta-analysis of these studies suggested that a confounding factor may be non-adherence to CPAP, particularly if it was used for less than 4 hours per night, highlighting the importance of compliance (18). The long-term effects regarding cerebrovascular accidents, triglyceride levels and insulin resistance are less clear (13,15,19).
Despite the highly effective treatment CPAP offers, poor adherence limits its efficacy. Compliance has been variably classified in the literature and thus adherence rates range from 40–85% (1,15). In the US, compliance has been arbitrarily defined as usage for more than 4 hours per night for more than 70% of nights. Of course, this does not correlate to a specific threshold beyond which efficacy is absolute—in short, the greater the use of CPAP, the better the outcomes in terms of symptomatic quality of life markers and longer term blood pressure/cardiovascular readings. Hence, there has been great interest in improving tolerability of the CPAP system. Commonly cited side effects include dermatitis, rhinitis, epistaxis, nasal discomfort, congestion, mask leak, aerophagia, barotrauma and claustrophobia. There may therefore be specific otolaryngological factors contributing to failure of CPAP, particularly in relation to the nasal cavity and paranasal sinuses. Contributing nasal conditions include anatomical, physiological and pathological factors. Anatomical considerations incorporate deviated nasal septum (DNS), external framework deformities, valve collapse, enlarged turbinates and nasopharyngeal pathology occluding the posterior choanae (e.g., adenoids). These can be corrected with surgical intervention. Pathophysiological conditions are common and include allergic or vasomotor rhinitis, for which patients require appropriate education and counselling, skin prick allergy testing, allergen avoidance advice and treatment with antihistamines and intranasal steroids (20). CPAP rhinitis is due to inflammatory changes in the nasal mucosa as a result of the persistent high air pressures—this also requires similar treatment with saline douching and intranasal steroids (21). Pathological processes such as sinusitis and nasal polyposis are often problematic and can be missed during routine respiratory review as they are better evaluated with rigid and flexible endoscopes in otolaryngology outpatients. This can be treated effectively, either medically, or surgically, in the form of endoscopic sinus surgery (22,23). Correction of these factors can lead to an improvement in CPAP compliance via a reduction in pressure requirements but rarely, alone, can it lead to resolution of OSA (24).
Application modifications and patient interventions
Over the last 20 years there have been developments in both non-surgical and surgical options available for patients who fail to tolerate CPAP. Advances in positive airway pressure technologies have allowed lower pressures to be delivered to the patient’s airway, with the underlying concept being that higher pressures lead to more patient discomfort and side effects. However, this has not been substantiated in observational studies and prospective, randomized trials—pressure levels do not necessarily correlate with adherence (25,26). Nevertheless, BiPAP was initially developed in order to vary the pressure delivered during the respiratory cycle. Very few comparative studies with CPAP are available but in the largest, compliance rates were similar (27). More recently, expiratory pressure relief system, which is, in effect, a further refinement of BiPAP, with variable pressures for each breath depending on the flow rates, has been used. Similarly however, randomized controlled trials have demonstrated no real benefit (28). Another avenue of interest is auto-titrating devices which provide the lowest pressure required to stent the airways open. This technique has demonstrated better tolerability but this benefit may possibly be negated by one study suggesting superior long-term cardiovascular outcomes with CPAP (15). The most recent Cochrane reviews however found no statistically significant difference between the various available appliances (28,29). Research continues in predicting CPAP pressure requirements and many parameters have been suggested including BMI, mean oxygen saturation, mean respiratory disturbance index, gender, depression and mask leak—with the strongest mathematical weighting for BMI and mean oxygen saturation (30-33).
Some clinicians recommend adjuvant hypnotic use as a short course to alleviate initial insomnia and anxiety with the use of CPAP. As with all chronic diseases, patient education is vital and this may aid in adherence. As a corollary to this, peer/partner support groups alongside motivational interviewing and cognitive behavioural therapy may have a role (29). Other patient interventions and lifestyle modifications include weight loss (including referral for bariatric surgery if indicated), reducing alcohol intake and positional therapy. Recent studies have indicated that sleep position therapy can be highly efficacious (1). However, this must be countered by the fact that non-CPAP therapies, such as positional therapies and oral appliances, have no significant long-term data, including for cardiovascular outcomes. Medications to treat contributing conditions such as rhinitis or hypothyroidism can also be of use. Simpler modifications to the CPAP system such as implementation of a chin strap may also be beneficial (34). In addition, patients with nasal congestion may benefit from the use of the full face mask system. Humidification of the CPAP system may also be beneficial although research has been conflicting in this regard, with more recent reports suggesting no positive impact on compliance but an improvement in the overall side effect profile, particularly nasal symptoms (35,36).
Oral appliances can be an excellent adjunct in carefully selected patients, as outlined by Giles et al. in their Cochrane review (37). Mandibular advancement splints (MAS) or devices function by protruding the hyoid bone anteriorly along with the mandible, contracting genioglossus and thus increasing retroglossal distance. Similar to CPAP however, the main drawback is patient compliance due to discomfort and the need to use it every night. MAS is contraindicated in those with uncontrolled epilepsy, poor dentition and edentulous patients (1). Randomized trials have demonstrated the efficacy of MAS therapy alone or in combination with CPAP (1,15,38). This may be particularly useful in a retrognathic patient with a bulky tongue for example, where the MAS will bring the jaw forward, improve the retroglossal dimension and therefore reduce CPAP pressure. Further work has demonstrated not only their clinical efficacy but also their cost effectiveness in the long-term, with a suggestion that a MAS is an appropriate first choice in most patients in the short-term (39). Again, however, long-term analysis of cardiovascular risk stratification is deficient, particularly in comparison with CPAP-related studies.
Otorhinolaryngological consultation
At this juncture, should the patient still not tolerate CPAP, then a surgical consultation is indicated. A thorough clinical history and examination is warranted to elicit potential therapeutic targets. A full assessment of co-morbidities and specifically body mass index is required, as the latter has been shown to correlate with surgical outcomes. In fact, surgery is often not recommended unless the BMI is less than 35 kg/m2 (40,41). It is important to specifically query patients regarding any rhinological symptoms. Nasal problems such as allergic rhinitis, CPAP rhinitis, polyposis, DNS, alar collapse can all contribute to not only intolerance to CPAP but also, in some cases, part of the multilevel obstruction synonymous with OSA and other sleep-related breathing disorders (Figures 1,2,3,4,5,6) (1). Patients with nasal congestion are mouth breathers and as a result, during sleep, the temporomandibular joint retracts, thereby further reducing the retroglossal airway. An open mouth during sleep may also exacerbate palatal vibrations and may impact retro-palatal dimensions. These clinical factors highlight the need to address nasal patency and obstruction. A recent computational fluid dynamics analysis has highlighted this effect of nasal obstruction on CPAP treatment, with a particular correlation between inspiratory pressures and maximal airflow velocity (42).
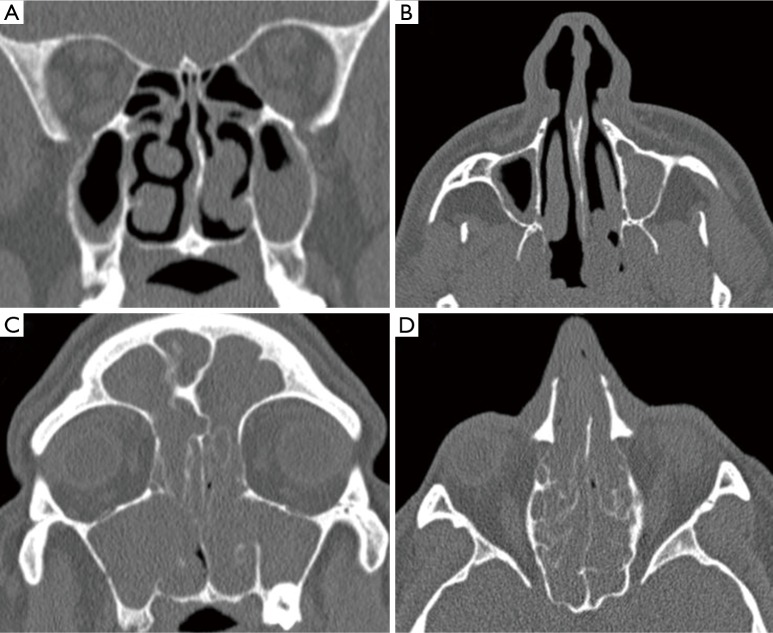
Figure 1.
Computed tomography images of two obstructive sleep apnoea (OSA) patients requiring continuous positive airway pressure (CPAP) with nasal pathology. (A,B) Coronal and axial slices of the first patient demonstrating a left sided polyp occluding part of the post nasal space, maxillary sinus disease and a slightly deviated septum to the left; (C,D) coronal and axial images of a second patient demonstrating extensive sinonasal polyposis, which ultimately failed medical management and required endoscopic sinus surgery.
Figure 2.
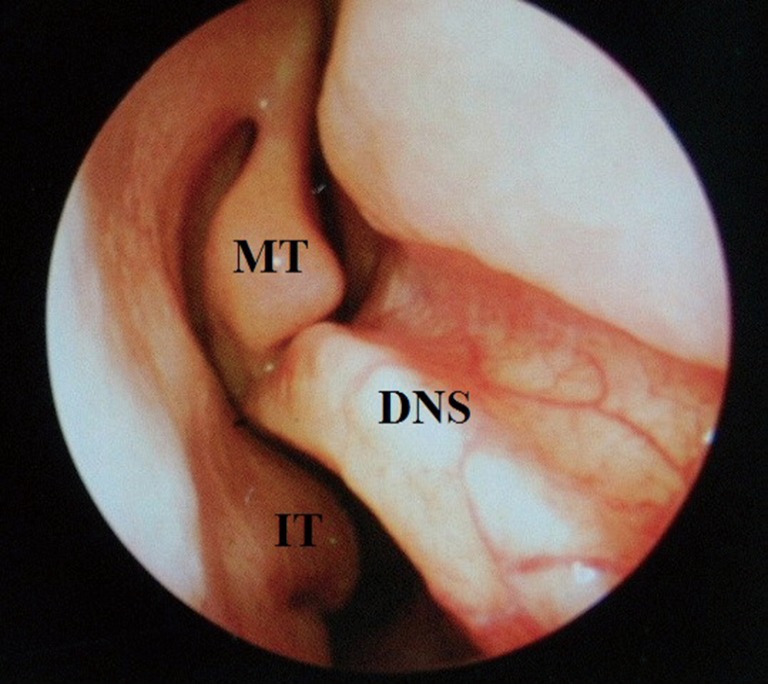
Rigid endoscope image of right sided nasal cavity with a deviated nasal septum (DNS) with a large spur opposing the right middle turbinate (MT) and part of the inferior turbinate (IT).
Figure 3.
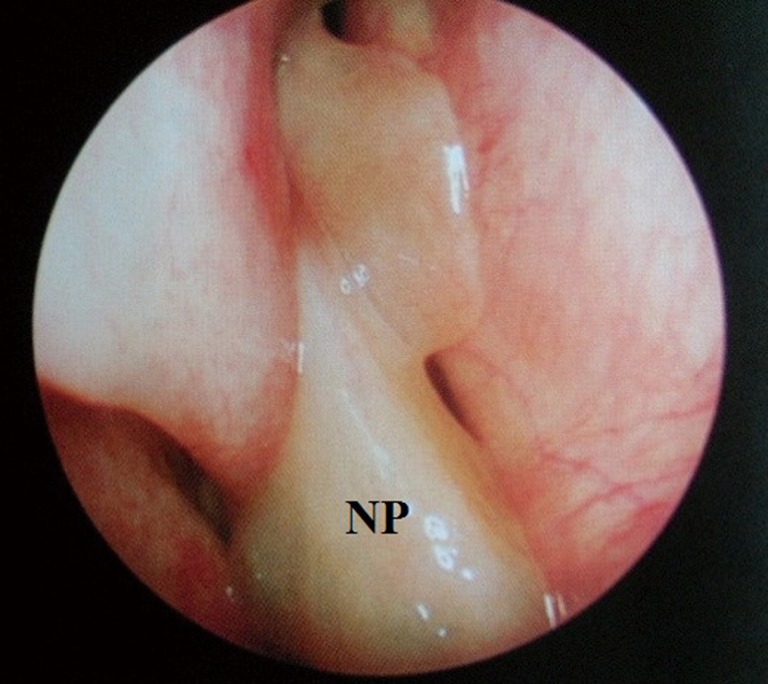
Rigid endoscope image of nasal polyposis (NP); note the differing texture, colour and position of the polyp in comparison to the normal nasal mucosa; polyps are also insensate.
Figure 4.
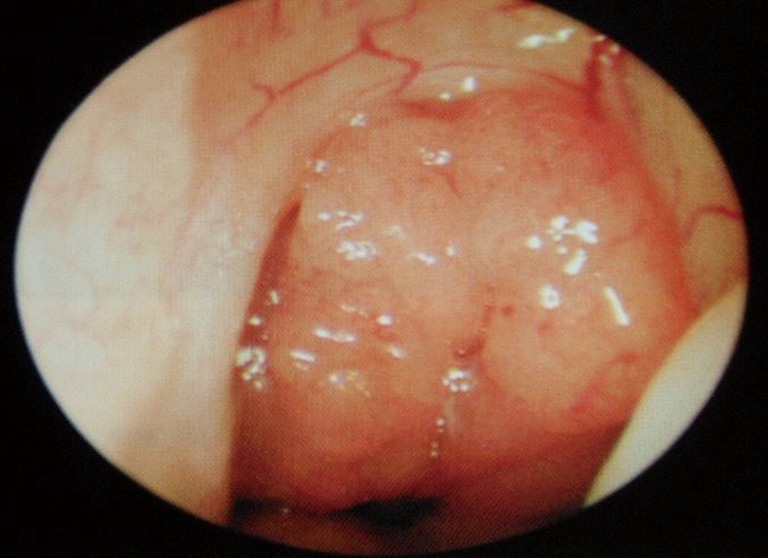
Rigid endoscope image of adenoidal hypertrophy in adult, occluding post nasal space.
Figure 5.
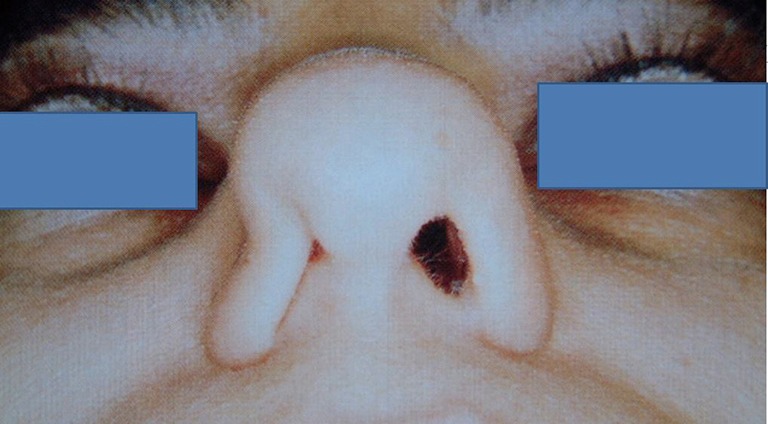
Clinical photograph demonstrating right nasal valve collapse on gentle inspiration; nasal valve can be primary or secondary to other pathology (e.g., deviated septum).
Figure 6.
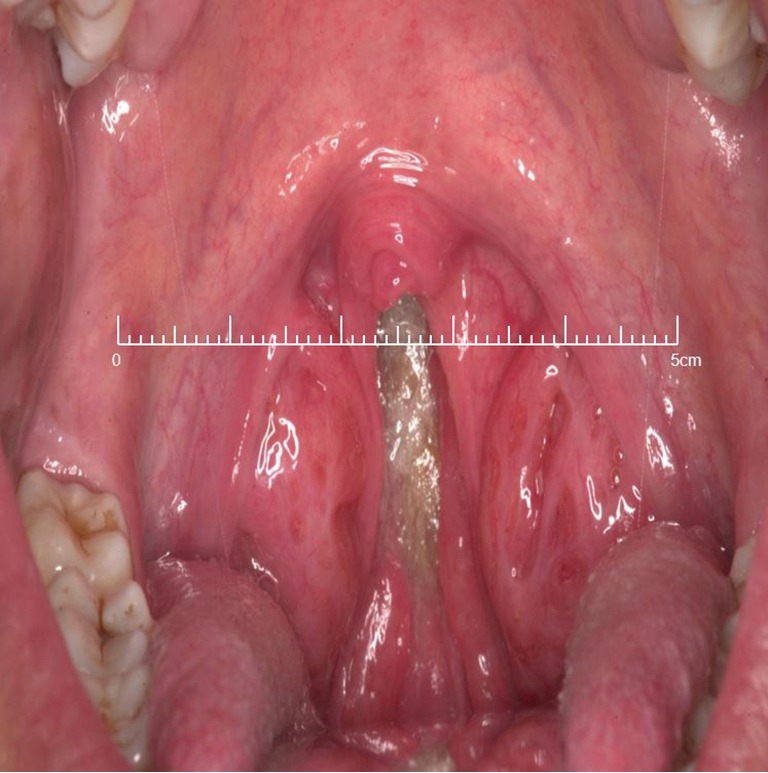
Clinical image of an overcrowded oropharynx secondary to tonsillar hypertrophy, lax palate and redundant pharyngeal mucosa.
Clinical examination allows assessment of the nasal and oral cavities alongside the anatomical segments of the pharynx. A general inspection can elicit dental pathology, retrognathia, craniofacial abnormalities, neck circumference and body habitus. The former may be particularly relevant for the putative use of MAS. Nasal examination can identify the rhinological factors outlined above. Examination of the oral cavity and oropharynx highlights the grade of the palatine tonsils (tonsillar hypertrophy of grade 2 or above may be significant as substantial lateral oropharyngeal wall collapse increases CPAP pressure requirement), dimensions of the soft palate and uvula and evidence of redundant pharyngeal tissue. In addition, Friedman and Mallampati tongue positions can be noted. These findings can help decipher which patients may benefit from palatal surgery as compared to tongue base surgery, with, for example, patients with Friedman tongue position 3 or 4 more likely to require tongue base procedures as compared to those with Friedman tongue position 1, who are more likely to benefit from palatal surgery (1,43). Flexible nasopharyngolaryngoscopy in the outpatient setting allows clearer visualization of the pharynx, larynx and tongue base and, although subjective, can allow a dynamic assessment of the airway to assess for levels of collapse, particularly with adjunct manoeuvres such as simulated snoring or Muller’s manoeuvre, although their value has been questioned (1,41).
Further investigations are myriad but there is increasing evidence for the use of drug-induced sleep endoscopy (DISE). DISE is useful in demonstrating dynamic upper airway obstruction which can help in understanding the mechanisms as to why CPAP may fail, such as epiglottic trap door phenomenon. Certainly, in comparison to the awake state and outpatient flexible endoscopy, during sleep, muscle tone and control of upper airway patency is different and so DISE is ideal in visualizing the three-dimensional upper airway dynamics during an induced sleep state. Controversy persists due to a drug-induced non-physiological state being assessed during this procedure, alongside the inherent subjectivity and lack of standardisation in definitions. However, this is countered by accumulating data of its value (with good inter-rater reliability and correlation with surgical outcomes) along with the supposition that these drugs affect all anatomic segments equally, allowing an assessment of obstruction at each anatomical level. Furthermore, a recent European consensus group meeting has sought to standardize data capture for these procedures. In addition, the advent of neurophysiological (bispectral index) monitoring may be helpful in the development of clearer anaesthetic and sedation protocols during the procedure (1,44-50).
Surgical options
The primary aims of surgery are to either bypass upper airway obstruction or to increase the upper airway dimensions. By addressing anatomical obstructions or areas of collapse in these OSA patients, CPAP requirements may be reduced and therefore improve patient compliance, although the observational studies outlined above do not necessarily support this theory. The key however remains appropriate patient selection and DISE is invaluable in this regard. Patients with a high BMI tend to do less well and may be better served, in the first instance, by weight loss measures, either with lifestyle, medical or surgical interventions. Patient counselling should highlight that multilevel obstruction is the norm and that CPAP remains the gold standard treatment. OSA, after all, is a complex, multifactorial phenomenon of heterogeneous aetiology (51). One of the confounding factors remains the variable definitions of successful outcomes or end points for either non-surgical or surgical therapies. Ravesloot and de Vries highlight this dilemma and suggest that mean apnoea-hypopnoea indices (AHI) be used in lieu of compliance rates for CPAP, which may be masking insufficient reductions in AHI in comparison to surgical interventions (52). Moreover, the lack of a robust evidence base associated with snoring/OSA surgery is well documented but is also the case for surgery in general. There is very little randomized controlled level 1 evidence and we therefore rely principally on level 3 and 4 studies.
Nasal surgery alone will rarely remove the requirement for CPAP but may facilitate its use, particularly nCPAP. There is in fact limited evidence that nasal obstruction contributes to the pathogenesis of OSA. Surgical options include septoplasty, turbinate reduction, septorhinoplasty and nasal valve surgery alongside with endoscopic sinus surgery (Figures 1-6). In a recent meta-analysis, the value of nasal surgery has been confirmed in reducing CPAP pressure requirements and patient discomfort, although the value of turbinate surgery is less clear (53,54). The main limitations with these surgical studies remain their power, level of evidence (typically retrospective level IV) and varying definitions of successful outcomes.
Oropharyngeal surgery may be beneficial in diligently selected patients (55). There has been a trend away from radical palatal surgeries such as uvulopalatopharyngoplasty and in fact there is some suggestion that this may increase mask leaks when CPAP is recommenced, one of the identified factors in poor compliance. However, Friedman et al. demonstrated reduced mouth leak in judiciously selected patients and upper airway surgeries (56). Currently, radiofrequency applications to the soft palate have shown promising long-term efficacy and has been approved by NICE in the UK (57). Repeat procedures may be required but complication rates are low (58). Additional tonsillectomy may also be beneficial either at a primary or secondary stage, depending on clinical findings and DISE evaluation. As a corollary to this, it is important to consider repeat DISE following multiple surgeries as the dynamics of the upper airway will have been affected. Another option, in lieu of radiofrequency treatments, remains laser-assisted palatoplasty, which has been shown to reduce pressure requirements and in some cases, remove the need for CPAP entirely (23). Elshaug et al., in their comprehensive review, report a pooled success rate for soft palate procedures of 55% using traditional surgical definitions (of a 50% reduction in AHI and/or ≤20) as compared to 31.5% or 13% if success is defined as AHI ≤10 or AHI ≤5 respectively. Similarly, pooled data for maxillary and/or mandibular advancement surgery success rates decrease from 86%, 45% and 43% respectively, depending on the definitions used (59,60). This not only demonstrates the potential benefits of surgical intervention for OSA but also highlights the work to be done in redefining outcomes and setting standards for this subset of patients. After surgical intervention it is prudent to repeat the sleep study as part of this ongoing assessment.
More recently, there has been interest and promising results in tissue repositioning procedures such as relocation and lateral pharyngoplasty (61,62). Caples et al. recent review surmises that further research is required but that newer pharyngeal techniques and multilevel procedures appear promising (55). In addition, these palatopharyngeal reconstructive surgeries for patients intolerant of CPAP have demonstrated cost-effectiveness (63). The key finding is that of minimally invasive multilevel surgery as compared to the radical palatal surgeries originally described.
Base of tongue collapse is recognized as a significant site of obstruction in patients with OSA and is often underappreciated. There may also be an associated epiglottic contribution (64). Both of these will significantly increase CPAP pressure requirement and hence cause difficulty in tolerating this form of therapy. However, this can be challenging to address, given the location. Various surgical options have been described. Minimally invasive options such as radiofrequency can be efficacious whilst more aggressive procedures such as midline glossectomy and hyoid suspension have had varying success rates (1,23). Most recently, transoral robotic surgery has had very encouraging initial results (64). Arora et al., in their prospective study with long-term follow-up, treated 14 patients with moderate to severe OSA with transoral robotic surgery to the tongue base, and additional wedge epiglottoplasty in ten of the patients; there were statistically significant improvements in mean AHI (overall 51% reduction, with normal postoperative sleep study results in 36% of patients), mean Epworth Sleepiness Score, mean oxygen saturations and in quality of life markers (64). It is worth noting that in this study the robust selection criteria included an AHI of at least 15, failure to tolerate CPAP and MAS and importantly, in the context of this article, a BMI of less than 35 kg/m2 and DISE evaluation demonstrating tongue base collapse with or without epiglottic collapse, highlighting the importance of these latter two factors in successful outcomes (64). Another recent area of interest has been hypoglossal nerve stimulation synchronized with inspiration via the surgical introduction of an electrical implant, with the underlying theory that reduced upper airway muscle activity is fundamental to OSA (65).
Apart from tracheostomy, the most successful outcomes have been with maxillomandibular advancement surgery, which increases retropalatal and retroglossal dimensions. This surgery is often neglected due to the requirement for a soft diet for a number of weeks and potentially serious side effects. However low complication rates have been reported in a recent review (1,66).
Combined modality treatment individualized for patients is necessary, within a multidisciplinary team setting including respiratory physicians, maxillofacial and otolaryngology surgeons. Typically multilevel surgery is required and all patients intolerant of CPAP should be referred for an otolaryngology opinion, to assess for surgical targets to reduce upper airway obstruction. Further long-term prospective studies are required with an emphasis on standardized data capture, definitions of success and outcomes.
Discussion
Sleep-related breathing disorders are increasingly common and confer a significant health and socioeconomic burden. Moderate to severe OSA is treated with CPAP but patient tolerance and compliance can be poor. These patients require alternative options and should be managed in the multidisciplinary team.
Non-surgical options include technical modifications, lifestyle changes and support alongside oral appliances. Surgery can be effective in either facilitating the use of CPAP or in bypassing and improving anatomical obstructions or areas of collapse, which are typically multilevel.
As with all management algorithms, patient selection is critical and an important investigative tool includes DISE. Therefore, all patients that fail a trial of CPAP should be referred for otolaryngology review to exclude upper airway obstruction and undergo consideration for site-specific surgical intervention.
Further research is however required to improve the evidence base for these interventions. Long-term prospective randomized controlled trials are required. In addition, standardized data capture alongside with agreed definitions of success and outcomes (which are likely to be a combination of patient scoring systems and objective polysomnography outcomes) are essential.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Virk JS, Kotecha B. Otorhinolaryngological aspects of sleep-related breathing disorders. J Thorac Dis 2016;8:213-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 3.Young T, Evans L, Finn L, et al. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997;20:705-6. [DOI] [PubMed] [Google Scholar]
- 4.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax 1991;46:85-90. 10.1136/thx.46.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079-85. [PMC free article] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-41. 10.1056/NEJMoa043104 [DOI] [PubMed] [Google Scholar]
- 8.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002;165:670-6. 10.1164/ajrccm.165.5.2103001 [DOI] [PubMed] [Google Scholar]
- 9.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest 1988;94:9-14. 10.1378/chest.94.1.9 [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490-4. 10.1016/0002-9149(83)90013-9 [DOI] [PubMed] [Google Scholar]
- 11.George CF, Smiley A. Sleep apnea & automobile crashes. Sleep 1999;22:790-5. [PubMed] [Google Scholar]
- 12.National Institute for Health and Clinical Excellence. Continuous positive airway pressure for obstructive sleep apnoea/hypopnoea syndrome. Available online: https://www.nice.org.uk/guidance/ta139/resources/continuous-positive-airway-pressure-for-obstructive-sleep-apnoeahypopnoea-syndrome-374791501
- 13.Turnbull CD, Bratton DJ, Craig SE, et al. In patients with minimally symptomatic OSA can baseline characteristics and early patterns of CPAP usage predict those who are likely to be longer-term users of CPAP. J Thorac Dis 2016;8:276-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakkar RK, Berry RB. Positive airway pressure treatment for obstructive sleep apnea. Chest 2007;132:1057-72. 10.1378/chest.06-2432 [DOI] [PubMed] [Google Scholar]
- 15.Donovan LM, Boeder S, Malhotra A, et al. New developments in the use of positive airway pressure for obstructive sleep apnea. J Thorac Dis 2015;7:1323-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monasterio C, Vidal S, Duran J, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med 2001;164:939-43. 10.1164/ajrccm.164.6.2008010 [DOI] [PubMed] [Google Scholar]
- 17.Chakravorty I, Cayton RM, Szczepura A. Health utilities in evaluating intervention in the sleep apnoea/hypopnoea syndrome. Eur Respir J 2002;20:1233-8. 10.1183/09031936.00.00014401 [DOI] [PubMed] [Google Scholar]
- 18.Bratton DJ, Stradling JR, Barbé F, et al. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax 2014;69:1128-35. 10.1136/thoraxjnl-2013-204993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg E, Berne C, Elmasry A, et al. CPAP treatment of a population-based sample--what are the benefits and the treatment compliance? Sleep Med 2006;7:553-60. 10.1016/j.sleep.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 20.Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection, and diagnosis. J Allergy Clin Immunol 2001;108:S2-8. 10.1067/mai.2001.115569 [DOI] [PubMed] [Google Scholar]
- 21.Almendros I, Acerbi I, Vilaseca I, et al. Continuous positive airway pressure (CPAP) induces early nasal inflammation. Sleep 2008;31:127-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotecha B. The nose, snoring and obstructive sleep apnoea. Rhinology 2011;49:259-63. [DOI] [PubMed] [Google Scholar]
- 23.Kotecha BT, Hall AC. Role of surgery in adult obstructive sleep apnoea. Sleep Med Rev 2014;18:405-13. 10.1016/j.smrv.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 24.Friedman M, Tanyeri H, Lim JW, et al. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg 2000;122:71-4. 10.1016/S0194-5998(00)70147-1 [DOI] [PubMed] [Google Scholar]
- 25.Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One 2013;8:e64382. 10.1371/journal.pone.0064382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis 1993;147:887-95. 10.1164/ajrccm/147.4.887 [DOI] [PubMed] [Google Scholar]
- 27.Atwood CW, Jr. Progress toward a clearer understanding of the role of bilevel positive airway pressure therapy for obstructive sleep apnea. J Clin Sleep Med 2013;9:337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith I, Lasserson TJ. Pressure modification for improving usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev 2009;(4):CD003531. [DOI] [PubMed] [Google Scholar]
- 29.Haniffa M, Lasserson TJ, Smith I. Interventions to improve compliance with continuous positive airway pressure for obstructive sleep apnoea. Cochrane Database Syst Rev 2004;(4):CD003531. [DOI] [PubMed] [Google Scholar]
- 30.Loredo JS, Berry C, Nelesen RA, et al. Prediction of continuous positive airway pressure in obstructive sleep apnea. Sleep Breath 2007;11:45-51. 10.1007/s11325-006-0082-x [DOI] [PubMed] [Google Scholar]
- 31.Hussain SF, Irfan M, Waheed Z, et al. Compliance with continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea among privately paying patients- a cross sectional study. BMC Pulm Med 2014;14:188. 10.1186/1471-2466-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sopkova Z, Dorkova Z, Tkacova R. Predictors of compliance with continuous positive airway pressure treatment in patients with obstructive sleep apnea and metabolic syndrome. Wien Klin Wochenschr 2009;121:398-404. 10.1007/s00508-009-1181-z [DOI] [PubMed] [Google Scholar]
- 33.Camacho M, Riaz M, Tahoori A, et al. Mathematical Equations to Predict Positive Airway Pressures for Obstructive Sleep Apnea: A Systematic Review. Sleep Disord 2015;2015:293868. [DOI] [PMC free article] [PubMed]
- 34.Knowles SR, O'Brien DT, Zhang S, et al. Effect of addition of chin strap on PAP compliance, nightly duration of use, and other factors. J Clin Sleep Med 2014;10:377-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan S, Doherty LS, Nolan GM, et al. Effects of heated humidification and topical steroids on compliance, nasal symptoms, and quality of life in patients with obstructive sleep apnea syndrome using nasal continuous positive airway pressure. J Clin Sleep Med 2009;5:422-7. [PMC free article] [PubMed] [Google Scholar]
- 36.Ruhle KH, Franke KJ, Domanski U, et al. Quality of life, compliance, sleep and nasopharyngeal side effects during CPAP therapy with and without controlled heated humidification. Sleep Breath 2011;15:479-85. 10.1007/s11325-010-0363-2 [DOI] [PubMed] [Google Scholar]
- 37.Giles TL, Lasserson TJ, Smith BH, et al. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev 2006;(3):CD001106. [DOI] [PubMed] [Google Scholar]
- 38.Health Quality Ontario . Oral appliances for obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser 2009;9:1-51. [PMC free article] [PubMed] [Google Scholar]
- 39.Sharples L, Glover M, Clutterbuck-James A, et al. Clinical effectiveness and cost-effectiveness results from the randomised controlled Trial of Oral Mandibular Advancement Devices for Obstructive sleep apnoea-hypopnoea (TOMADO) and long-term economic analysis of oral devices and continuous positive airway pressure. Health Technol Assess 2014;18:1-296. 10.3310/hta18670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Virk JS, Kumar G, Al-Okati D, et al. Radiofrequency ablation in snoring surgery: local tissue effects and safety measures. Eur Arch Otorhinolaryngol 2014;271:3313-8. 10.1007/s00405-014-3152-x [DOI] [PubMed] [Google Scholar]
- 41.Virk JS, Nouraei R, Kotecha B. Multilevel radiofrequency ablation to the soft palate and tongue base: tips and pitfalls. Eur Arch Otorhinolaryngol 2014;271:1809-13. 10.1007/s00405-013-2858-5 [DOI] [PubMed] [Google Scholar]
- 42.Wakayama T, Suzuki M, Tanuma T. Effect of Nasal Obstruction on Continuous Positive Airway Pressure Treatment: Computational Fluid Dynamics Analyses. PLoS One 2016;11:e0150951. 10.1371/journal.pone.0150951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope 2004;114:454-9. 10.1097/00005537-200403000-00013 [DOI] [PubMed] [Google Scholar]
- 44.Georgalas C, Kotecha B. Predictive value of sleep nasendoscopy in the management of habitual snorers. Ann Otol Rhinol Laryngol 2004;113:420. 10.1177/000348940411300516 [DOI] [PubMed] [Google Scholar]
- 45.Hewitt RJ, Dasgupta A, Singh A, et al. Is sleep nasendoscopy a valuable adjunct to clinical examination in the evaluation of upper airway obstruction? Eur Arch Otorhinolaryngol 2009;266:691-7. 10.1007/s00405-008-0831-5 [DOI] [PubMed] [Google Scholar]
- 46.Abdullah VJ, Lee DL, Ha SC, et al. Sleep endoscopy with midazolam: sedation level evaluation with bispectral analysis. Otolaryngol Head Neck Surg 2013;148:331-7. 10.1177/0194599812464865 [DOI] [PubMed] [Google Scholar]
- 47.Kezirian EJ, White DP, Malhotra A, et al. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg 2010;136:393-7. 10.1001/archoto.2010.26 [DOI] [PubMed] [Google Scholar]
- 48.Ravesloot MJ, de Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope 2011;121:2710-6. 10.1002/lary.22369 [DOI] [PubMed] [Google Scholar]
- 49.Chisholm E, Kotecha B. Oropharyngeal surgery for obstructive sleep apnoea in CPAP failures. Eur Arch Otorhinolaryngol 2007;264:51-5. 10.1007/s00405-006-0139-2 [DOI] [PubMed] [Google Scholar]
- 50.Kotecha BT, Hannan SA, Khalil HM, et al. Sleep nasendoscopy: a 10-year retrospective audit study. Eur Arch Otorhinolaryngol 2007;264:1361-7. 10.1007/s00405-007-0366-1 [DOI] [PubMed] [Google Scholar]
- 51.Carvalho B, Hsia J, Capasso R. Surgical therapy of obstructive sleep apnea: a review. Neurotherapeutics 2012;9:710-6. 10.1007/s13311-012-0141-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravesloot MJ, de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep 2011;34:105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camacho M, Riaz M, Capasso R, et al. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta-analysis. Sleep 2015;38:279-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camacho M, Zaghi S, Tran D, et al. Inferior Turbinate Size and CPAP Titration Based Treatment Pressures: No Association Found among Patients Who Have Not Had Nasal Surgery. Int J Otolaryngol 2016;2016:5951273. [DOI] [PMC free article] [PubMed]
- 55.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010;33:1396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friedman M, Soans R, Joseph N, et al. The effect of multilevel upper airway surgery on continuous positive airway pressure therapy in obstructive sleep apnea/hypopnea syndrome. Laryngoscope 2009;119:193-6. 10.1002/lary.20021 [DOI] [PubMed] [Google Scholar]
- 57.National Institute for Health and Care Excellence. Interventional procedure overview of radiofrequency ablation of the soft palate for snoring. Available online: https://www.nice.org.uk/guidance/ipg476/documents/radiofrequency-ablation-of-the-soft-palate-for-snoring-overview2
- 58.Veer V, Yang WY, Green R, et al. Long-term safety and efficacy of radiofrequency ablation in the treatment of sleep disordered breathing: a meta-analysis. Eur Arch Otorhinolaryngol 2014;271:2863-70. 10.1007/s00405-014-2909-6 [DOI] [PubMed] [Google Scholar]
- 59.Elshaug AG, Moss JR, Hiller JE, et al. Upper airway surgery should not be first line treatment for obstructive sleep apnoea in adults. BMJ 2008;336:44-5. 10.1136/bmj.39381.509213.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elshaug AG, Moss JR, Southcott AM, et al. Redefining success in airway surgery for obstructive sleep apnea: a meta analysis and synthesis of the evidence. Sleep 2007;30:461-7. [DOI] [PubMed] [Google Scholar]
- 61.Li HY, Lee LA. Relocation pharyngoplasty for obstructive sleep apnea. Laryngoscope 2009;119:2472-7. 10.1002/lary.20634 [DOI] [PubMed] [Google Scholar]
- 62.Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007;137:110-4. 10.1016/j.otohns.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 63.Tan KB, Toh ST, Guilleminault C, et al. A Cost-Effectiveness Analysis of Surgery for Middle-Aged Men with Severe Obstructive Sleep Apnea Intolerant of CPAP. J Clin Sleep Med 2015;11:525-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arora A, Chaidas K, Garas G, et al. Outcome of TORS to tongue base and epiglottis in patients with OSA intolerant of conventional treatment. Sleep Breath 2016;20:739-47. 10.1007/s11325-015-1293-9 [DOI] [PubMed] [Google Scholar]
- 65.Kezirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev 2010;14:299-305. 10.1016/j.smrv.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 66.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev 2010;14:287-97. 10.1016/j.smrv.2009.11.003 [DOI] [PubMed] [Google Scholar]