Abstract
AIM
To evaluate the diagnostic and prognostic value of presepsin in cirrhosis-associated bacterial infections.
METHODS
Two hundred and sixteen patients with cirrhosis were enrolled. At admission, the presence of bacterial infections and level of plasma presepsin, serum C-reactive protein (CRP) and procalcitonin (PCT) were evaluated. Patients were followed for three months to assess the possible association between presepsin level and short-term mortality.
RESULTS
Present 34.7 of patients had bacterial infection. Presepsin levels were significantly higher in patients with infection than without (median, 1002 pg/mL vs 477 pg/mL, P < 0.001), increasing with the severity of infection [organ failure (OF): Yes vs No, 2358 pg/mL vs 710 pg/mL, P < 0.001]. Diagnostic accuracy of presepsin for severe infections was similar to PCT and superior to CRP (AUC-ROC: 0.85, 0.85 and 0.66, respectively, P = NS for presepsin vs PCT and P < 0.01 for presepsin vs CRP). At the optimal cut-off value of presepsin > 1206 pg/mL sensitivity, specificity, positive predictive values and negative predictive values were as follows: 87.5%, 74.5%, 61.8% and 92.7%. The accuracy of presepsin, however, decreased in advanced stage of the disease or in the presence of renal failure, most probably because of the significantly elevated presepsin levels in non-infected patients. 28-d mortality rate was higher among patients with > 1277 pg/mL compared to those with ≤ 1277 pg/mL (46.9% vs 11.6%, P < 0.001). In a binary logistic regression analysis, however, only PCT (OR = 1.81, 95%CI: 1.09-3.01, P = 0.022) but neither presepsin nor CRP were independent risk factor for 28-d mortality after adjusting with MELD score and leukocyte count.
CONCLUSION
Presepsin is a valuable new biomarker for defining severe infections in cirrhosis, proving same efficacy as PCT. However, it is not a useful marker of short-term mortality.
Keywords: Presepsin, Cirrhosis, Bacterial infection, Organ failure, Mortality
Core tip: C-reactive protein (CRP) and procalcitonin (PCT) are broadly used in clinical practice to aid early diagnosis of bacterial infections, but they have limitations in cirrhosis. Additional biomarkers with enhanced accuracy are highly needed. Presepsin is a novel biomarker of infection and sepsis, but has not been assessed in cirrhosis so far. In the present study we evaluated the diagnostic and prognostic performance of presepsin in cirrhosis-associated infections in comparison with classic acute phase proteins. Presepsin measurement enhanced diagnostic utility of CRP and reflected the severity of infections more accurately, with a similar efficacy as PCT. Advanced disease stage and renal failure limited the diagnostic accuracy. The increase in PCT level but not in presepsin concentration was an independent predictor of short-term mortality during infectious episodes.
INTRODUCTION
Infectious episodes represent particularly important causes of progression of liver failure and the development of liver-related complications[1]. Due to altered sensitivity, the end-organ damaging effect of bacterial infection is greater in cirrhosis and often culminates in newly developed liver and/or extrahepatic organ failures, which is associated with a very high short-term mortality rate[2,3].
Early recognition of bacterial infections is essential, however, in the clinical practice their accurate identification is challenging from both the clinical[4] and the laboratory point of view[5]. In cirrhosis, usual clinical presentations lack up to 50% of the bacterial infections and are replaced by non-specific complaints or just revealed by organ dysfunctions. Due to some disease specific characteristics, there is an evident lack of sensitivity and specificity of the conventional laboratory and clinical parameters for the definition of systemic inflammatory response (SIRS)[6,7], which often makes it difficult to diagnose sepsis.
Currently C-reactive protein (CRP) and procalcitonin (PCT) are broadly used in the clinical practice to aid the early diagnosis of bacterial infection[8]. In cirrhosis, these conventional markers, however, perform somewhat differently in comparison with the non-cirrhotic patient populations for various reasons. For the first, if the main source of the molecule is the liver, as in the case of CRP, synthesis of the molecule is affected by liver failure and its severity. As a result the diagnostic accuracy of liver synthesised acute phase proteins (APPs) decreases in advanced stage of cirrhosis[9]. Moreover, peak levels can be misleading and do not indicate the severity of the infection adequately, since the more severe the underlying liver dysfunction, the lower the CRP response to bacteraemia is[10]. Secondly, elimination of certain molecules can be affected by renal failure and also renal replacement therapy. Acute kidney injury (AKI) is frequent in patients with cirrhosis, especially in bacterial infections[11]. While CRP has a high molecular weight (MW) (115-kDa) and its renal clearance is negligible[12,13], PCT is small with a MW of 13 kDa, and renal elimination is thought to be one of the major pathways for its elimination[14]. Accordingly, false or inappropriate increase of the PCT level has been reported in end-stage renal disease patients due to the prolonged elimination rate[15,16]. Similarly, artificial reduction of the PCT level was also found after renal replacement therapy (HCO-CVVHDF). Proteins with MW < 60 kDa are filtered out by the dialysis membrane[17]. Thirdly, inflammatory state sustained by bacterial translocation (BT) and without overt infection is alone sufficient to elevate inflammatory markers to a significant level[5,9]. Bacterial translocation is an increasing problem with disease severity[18].
Accordingly, data are not homogeneous about the optimal cut-off for either of CRP and PCT to differentiate patients with infection from those without[19-23]. Probably using a single threshold is not appropriate. Additional biomarkers are highly needed to optimize the rule in and rule out processes necessary for the diagnosis and also for the severity assessment of the infectious episodes in cirrhosis.
Presepsin (soluble CD14 subtype, sCD14-ST) is a 13-kDa-cleavage product of CD14 receptor that recognizes different cell surface structure of both Gram-negative and positive bacteria. Presepsin in the circulation can be perceived as a witness of activated monocyte-macrophage in response to pathogens[24]. Several recent clinical studies have shown that presepsin is a specific and sensitive novel marker for the diagnosis of sepsis[25], for evaluating the severity of sepsis and for predicting the outcome[26,27]. Beyond sepsis, presepsin is worthy of studying in those clinical settings, where systemic infections are frequently associated with severe diseases course such in cirrhosis [acute decompensation (AD), organ failure]. Contributive role of presepsin for the diagnosis and prognosis of cirrhosis associated bacterial infection has not been assessed extensively so far.
In the present study, we aimed to assess (1) performance of presepsin in the diagnosis of cirrhosis associated bacterial infections in comparison with routinely used APPs such as CRP and PCT; (2) whether presepsin is devoid of the limitations of classic APPs related to cirrhosis; and (3) whether presepsin is able to provide prognostic information during infectious episodes in cirrhosis.
MATERIALS AND METHODS
Patient population
Two hundred and sixteen well-characterized patients with cirrhosis (male/female: 118/99, age: 57.5 ± 10.3 years, disease duration: 3.9 ± 4.2 years) were included consecutively from our in- and outpatient Gastroenterology Department between May 2010 and April 2011. The diagnosis of cirrhosis was considered either histologically proven or considered obvious by clinical, biochemical and morphological criteria[28].
Data collection
The clinical and laboratory characteristics of the patients are presented in Table 1. Clinical data were recorded at enrolment. These included demographics, co-morbidities (including cardio- and cerebrovascular, respiratory, renal disorders, diabetes and extrahepatic cancers), etiology of cirrhosis, history and severity of liver disease, presence of hepatocellular carcinoma (HCC), reason for AD, clinical status of patients, and also presence, type and location of bacterial infection. Severity of the cirrhosis was graded according to liver-oriented scores [Child-Pugh score (CPs) and the model for end-stage liver disease (MELD)][29]. Episodes of AD were defined by acute development of large ascites, hepatic encephalopathy, gastrointestinal hemorrhage, bacterial infection or any combination of these warranting hospital admission[3]. The enrolled patients were followed for 90 d or until death. Presence and grade of organ system failure(s) [OF] were determined retrospectively based on the available clinical and laboratory data after accessibility of CLIF-C Organ Failure Score[30].
Table 1.
Demographic, clinical and laboratory characteristics of patients with or without bacterial infections
| Non-infected | Infected | P value | ||||
| n | 141 | 75 | ||||
| Gender (male/female) | 77/64 | 41/34 | NS | |||
| Age (yr) | 57.3 ± 10.7 | 58.1 ± 9.7 | NS | |||
| Child-Pugh score | 6.9 ± 1.7 | 9.3 ± 2.2 | < 0.001 | |||
| Child-Pugh stage, n (%) | ||||||
| A | 58 (41.1) | 6 (8.0) | < 0.001 | |||
| B | 65 (46.1) | 28 (37.3) | ||||
| C | 18 (12.8) | 41 (54.7) | ||||
| MELD score | 12.3 ± 4.1 | 19.1 ± 9.1 | < 0.001 | |||
| Serum bilirubin (μmol/L) | 41.4 ± 38.3 | 124.1 ± 147.4 | < 0.001 | |||
| Serum albumin (g/L) | 35.4 ± 7.1 | 28.1 ± 6.5 | < 0.001 | |||
| INR | 1.3 ± 0.2 | 1.6 ± 0.5 | < 0.001 | |||
| Serum creatinine (μmol/L) | 83.8 ± 74.3 | 131.2 ± 129.9 | < 0.001 | |||
| Ascites present, n (%) | 58 (41.1) | 59 (78.7) | < 0.001 | |||
| HCC, n (%) | 4 (2.8) | 11 (14.7) | 0.003 | |||
| Comorbidities present, n (%) | 72 (51.1) | 45 (60.0) | NS | |||
| Type of bacterial infections, n (%) | ||||||
| UTI | 25 (29.4) | |||||
| SBP | 20 (23.5) | |||||
| Pneumonia | 18 (21.2) | |||||
| SSTI | 4 (4.7) | |||||
| Miscellaneous | 18 (21.2) | |||||
| Multiple | 9 (10.6) | |||||
| Acute phase proteins, median (IQR) | ||||||
| Presepsin (pg/mL) | Overall | 477 (332-680) | 1002 (575-2149) | < 0.001 | ||
| OF absent | OF present | 710 (533-1277) | 2357 (1398-3666) | < 0.001 | ||
| CRP (mg/L) | Overall | 4.6 (1.8-8.8) | 30.1 (11.3-57.4) | < 0.001 | ||
| OF absent | OF present | 25 (9.6-40.5) | 52.2 (23.4-84) | 0.027 | ||
| PCT (μmol/L) | Overall | 0.1 (0.1-0.2) | 0.4 (0.1-1.2) | < 0.001 | ||
| OF absent | OF present | 0.2 (0.1-0.5) | 1.7 (0.6-5.3) | < 0.001 | ||
Data are presented as mean ± SD or n (%) if not otherwise indicated. CRP: C-reactive protein; HCC: Hepatocellular carcinoma; IQR: Interquartile range; INR: International normalized ratio; MELD: Model for end-stage liver disease score; NS: Non-significant; PCT: Procalcitonin; SD: Standard deviation; UTI: Urinary tract infection; SBP: Spontaneous bacterial peritonitis; SSTI: Skin and soft tissue infection.
The diagnosis of bacterial infection was established by assessment of clinical symptoms and laboratory reports, including microbiological culture results (if available), compatible findings of imaging techniques, and the effect of antibiotic treatment by two independent gastroenterologists (M.P. and Zs.V.). Following bacterial infections were considered and diagnosed on the basis of conventional criteria: (1) skin and soft tissue infections[31]; (2) lower respiratory tract infections (acute bronchitis, pneumonia)[32]; (3) urinary tract infections (UTI) (cystitis, pyelonephritis)[33]; (4) some rare causes of infections, such as biliary tract infections (cholecystitis, cholangitis, liver abscess), osteomyelitis, and endocarditis; (5) spontaneous bacterial peritonitis (SBP), diagnosis of which was based on ascitic fluid polymorphonuclear cell (PMN) count exceeding 250/mm3 and3/or positive culture if secondary causes of peritonitis were excluded (EASL guidelines[34]); and (6) bacterial infection of unknown origin was defined when clinical symptoms and signs of infection were present and confirmed by microbiological demonstration of the causative organism from blood culture in the absence of site-specific infection. Bacterial infection was considered severe when the infectious episode was complicated by OF.
Measurements of presepsin and other laboratory parameters
Venous blood samples were captured at enrolment. Routine laboratory data, such as liver biochemistry, renal function, blood count and serum CRP and PCT levels were determined directly at the Department of Laboratory Medicine. Methods for qualitative assessment of serum CRP and PCT levels were reported previously[9].
For presepsin measurements, blood samples were immediately centrifuged at 3000 g for 10 min, and plasma was stored at -70 °C until use. Presepsin levels were measured by means of a PATHFAST® presepsin analyzer (Mitsubishi Chemical Medience Corporation, Tokyo, Japan) which is based on chemiluminescent enzyme immunoassay, with a detection limit of 20 pg/mL.
Outcome
For outcome assessment, a follow-up examination was set up at the 28th day after enrolment in the study. Patients who survived until follow-up were counted as survivors, whereas patients who died within the follow-up period were counted as non-survivors.
Statistical analysis
Variables were tested for normality using Shapiro Wilk’s W test. Continuous variables were summarized as mean ± SD or as medians [interquartile range (IQR)] according to their homogeneity. Categorical variables were compared with the χ2 test or χ2 test with Yates correction as appropriate. Continuous variables were compared with the Mann-Whitney U test or Student’s t test. Relationship between continuous variables was assessed with the non-parametric Spearman correlation. Diagnostic accuracy of presepsin and other APPs for defining various study-endpoints: (1) presence of bacterial infection; (2) presence of severe infection; and (3) short-term mortality was estimated using receiver operating curve (ROC) analysis by plotting sensitivity vs 1-specificity. Area under the curve (AUC-ROC) and corresponding 95%CI were calculated. ROC curves were compared with the method of DeLong et al[35] in Medcalc. Youden index was chosen, calculated as the maximum (sensitivity + specificity - 1) value, to estimate the best discriminate thresholds. Sensitivities, specificities, positive predictive values (PPV) and negative predictive values (NPV) were calculated to determine the predictive power of individual APPs or their combinations in all the three clinical settings. Binary logistic regression was used to assess the relationship between APPs and short-term mortality adjusted for the MELD score and WBC count. For the analysis APPs were loge-transformed to ensure normal distribution. Associations are given as odds ratios (OR) or likelihood ratios (LR) with a 95%CI. A 2-sided probability value < 0.05 was considered to be significant. For statistical analysis and graphical presentation SPSS 22.0 (SPSS Inc., Chicago, IL, United States) and GraphPad Prism 7 (San Diego, CA, United States) were used.
The statistical methods of this study were reviewed by Professor Elek Dinya, PhD, DSc, Semmelweis University, Institute of Health Informatics, Budapest, Hungary.
Review of the literature
We performed a systematic review of studies reporting on CRP and PCT in prognosis of cirrhosis. Papers were eligible if they presented original research in adult patients with cirrhosis and reported association of CRP and/ or PCT with the disease outcome either in patients with or without bacterial infection. Studies had to have been published in peer-reviewed journals. We started searching PubMed using the following search terms: [“C-reactive protein” OR “procalcitonin”] AND “liver cirrhosis”. Limits were human and time ranging from 1991 until 2016 (1st June). Only articles reporting short or long-term outcome in cirrhosis were included. This search revealed 20 articles. In Table 2 we summarize the clinical significance of CRP and PCT in the prediction of disease course in cirrhosis based on findings reported in relevant literature.
Table 2.
Association of classic acute phase proteins with mortality in patients with cirrhosis
| Ref. | Year | Journal | Population | n | Outcome | Measure | Value |
| C-reactive protein | |||||||
| Reuken et al[72] | 2013 | Liver Int | Ascites | 108 | 90-d mortality | AUC | 0.69 (0.59-0.79), P = 0.001 |
| Moreno et al[69] | 2013 | Liver Int | Non-septic | 95 | 1-yr mortality | AUC | 0.71 (0.6-0.8), P < 0.001 |
| Mortensen et al[70] | 2012 | Eur J Gastroenterol Hepatol | Stable alcohol cirrhosis | 45 | Long term mortality (about 6- yr) | HR1 | |
| 1.074 (1.001-1.153), P = 0.046 | |||||||
| Wiese et al[74] | 2014 | Liver Int | Stable cirrhotic | 193 | 1- yr survival | HR | 1.18, P = 0.0483 |
| Lim et al[68] | 2014 | Plos One | SBP | 75 | 30-d mortality | AUC | 0.64 (0.49-0.79), P = 0.076 |
| HR | ND, P = 0.064 | ||||||
| Schwabl et al[73] | 2015 | Liver Int | SBP | 168 | 30-d mortality | HR1 | 1.067 (1.004-1.134), P = 0.037 |
| Ha et al[65] | 2011 | Korean J Intern Med | Bacteraemia | 202 | 30-d mortality | Difference | Survivor vs non-survivor2 |
| 37.8 vs 34.3 mg/L, P = 0.721 | |||||||
| Cervoni et al[20] | 2012 | J Hepatol | CPs > 7 | 148 | 180-d mortality | AUC | 0.63 (0.51-0.73), P = ND |
| HR14 | 2.73 (1.41-5.26), P = 0.003 | ||||||
| Di Martino et al[64] | 2015 | Liver Transplant | CPs > 7 | 109 | 90-d mortality | HR14 | 2.21 (1.03-4.76), P = 0.042 |
| Cervoni et al[63] | 2016 | Eur J Gastroenterol Hepatol | CPs > 7 | 583 | 90-d mortality | HR14 | 1.69 (1.01-2.81), P = 0.046 |
| Park et al[71] | 2015 | J Korean Med Sci | Alcoholic cirrhosis various reasons for admission | 409 | 30-d mortality | OR | "CRP > 20 not independent predictor" |
| Ximenes et al[75] | 2016 | Am J Emerg Med | Hepatic decompensation | 149 | Inhospital mortality | OR | OR: ND, P > 0.100 |
| Kwon et al[67] | 2015 | BMC Gastroenterol | Acute decompensation | 184 | 30 d survival | OR | OR: ND, P = 0.122 |
| Lazzarotto et al[19] | 2013 | Ann Hepatol | Acute decompensation | 64 | 90 d survival | Difference | Survivor vs non-survivor2 |
| 7 vs 41 mg/L, P = 0.026 | |||||||
| Kronenberger et al[66] | 2012 | BMC Med | Acute decompensation + outpatients | 120 | Overall survival (196 ± 165 d) | HR | Univariate: 0.314 (0.141-0.702) , P = 0.005 |
| Multivariate: ND, P = 0.077 | |||||||
| Procalcitonin | |||||||
| Lin et al[80] | 2015 | J Crit Care | Acute decompensation | 96 | Sepsis in-hospital mortality | AUC | 0.692 |
| Kotecha et al[79] | 2013 | Eur J Gastroenterol Hepatol | Cirrhosis + SIRS | 100 | In-hospital survival | OR1 | 0.949 (0.868-1.037), P = 0.249 |
| Connert et al[78] | 2003 | Z Gastroenterol | Compensated + decompensated | 100 | 60-d mortality | Percent died | < 0.58 vs ≥ 0.58: |
| 9.1% vs 46.7%, P < 0.001 | |||||||
| Al-Dorzi et al[76] | 2014 | Clin Lab | Septic shock | 45 | 28-d mortality | Difference | Low PCT vs high PCT: |
| 80% vs 77%, P = 0.61 | |||||||
| Berres et al[77] | 2009 | Liver Int | Critically ill | 38 | ICU mortality | Difference | NA, P = NS |
Adjusted for MELD score;
Median values;
Not significant in the log-rank test;
CRP variation over 15 d. SBP: Spontaneous bacterial peritonitis; HR: Hazard ratio; AUC: Area under the curve; OR: Odds ratio; CPs: Child-Pugh score; ICU: Intensive care unit; SIRS: Systemic inflammatory response syndrome.
Ethical permission
All patients were informed of the nature of the study and signed an informed consent form. The regional and national committee (DEOEC RKEB/IKEB 5306-9/ 2011, 3885/2012/EKU [60/PI/2012]) for research ethics approved the study protocol.
RESULTS
Study population
Two hundred and sixteen patients with cirrhosis were enrolled in this cohort. The main characteristics of patients with or without infection are summarized in Table 1. There were 118 men with a mean age of 57.6 ± 10.3 years. The median Child-Pugh and MELD score were 7 (95%CI: 6-9) and 13 (95%CI: 10-17), respectively. The main baseline characteristics were as follows: alcoholic liver disease in 159 patients (73.6%), HCC in 15 patients (6.9%) and renal impairment in 33 (15.3%) based on creatinine cut-off values ≥ 133 μmol/L. One-hundred and seventeen patients (54.2%) had extrahepatic co-morbidities. Acute decompensation of the disease warranting hospital admission occurred in a total of 101 patients (46.8%) of whom 27 (26.7%) had at least one OF.
Documented bacterial infection was present in 75 (34.7%) patients of whom 9 (12.0%) suffered from multifocal episode. Bacteria were Gram-negative in 52.6% and Gram-positive in 47.4% of culture-positive cases. No cases of invasive fungal infections were detected. The distribution of infections is shown in Table 1. The infected and the non-infected patient groups did not differ in gender, age, and presence of comorbidities. However, patients with infections had more advanced disease stage, as indicated by median values of Child-Pugh and MELD score and presence of ascites. Renal impairment (29.3% vs 7.8%, P < 0.001) and occurrence of HCC (14.7% vs 2.8%, P = 0.003) were more frequent in patients with infection as compared to those without as well. The occurrence of AD episodes and the development of OF were more common in the presence of infections (AD: INF vs non-INF, 85.3% vs 26.2%, P < 0.001 and OF: INF vs non-INF, 37.5% vs 8.1%, P = 0.001).
Association between presepsin levels and bacterial infections
Presepsin values ranged from 142 to 5950 pg/mL [median (IQR), 576 pg/mL (376-972)] and were significantly higher in patients with infection as compared to those without [1002 (575-2149) pg/mL vs 477 (332-680) pg/mL, P < 0.001] (Figure 1). This association was also confirmed in the different disease severity subgroups according to Child-Pugh stage (Figure 2A) or the presence of ascites (Figure 2B). In the subgroup of patients with renal failure, presepsin levels were also different numerically between infected and non-infected patient groups however it did not reach statistically significance (P = 0.08) (Figure 2C). Further evaluating non-infected patients, a significant increase was observed in presepsin levels in case of more advanced disease stage and also in the presence of renal failure (P < 0.001 for both).
Figure 1.
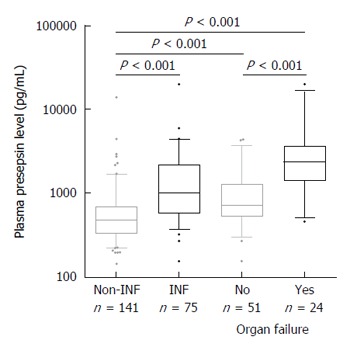
Presepsin levels in patients with cirrhosis according to presence or absence and severity of bacterial infections (n = 216). Median presepsin levels are significantly higher in patients with infection as compared to those without and associated to the severity of the infection. Lines denote median values, boxes represent 25th-75th percentiles and whiskers indicate the 5th-95th range. P values were calculated by Mann-Whitney U-test or Kruskal-Wallis H-test as appropriate. INF: Bacterial infection.
Figure 2.
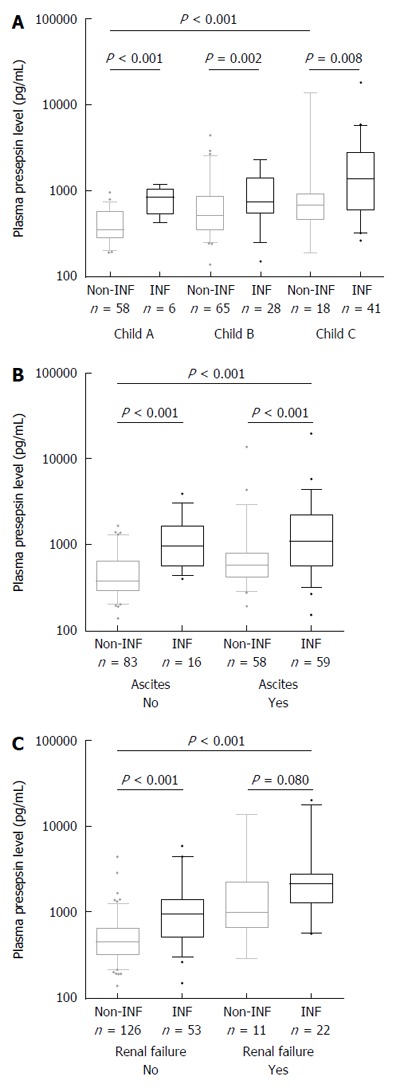
Presepsin levels in subgroups of patients with different diseases severity according to presence or absence of bacterial infections (n = 216). Significant differences in median presepsin levels between non-infected and infected patients are observed in all disease severity subgroups according to Child-Pugh stage (A) or the presence of ascites (B); However, no significant difference is observed in renal failure subgroup (C). Among non-infected patients, a significant increase in median presepsin levels is observed according to disease severity or in the presence of renal failure. Lines denote median values, boxes represent 25th-75th percentiles and whiskers indicate the 5th-95th range. P values were calculated by the Mann-Whitney U or the Kruskal-Wallis H-test as appropriate. Creatinine values of 4 patients were missing in the non-infected group.
Presepsin level was positively correlated with classic markers of bacterial infections, such as CRP, PCT, and different WBC parameters, but also with renal and liver function tests (Table 3) and accordingly with liver liver-oriented scores (CPs and MELD).
Table 3.
Correlations between presepsin and different laboratory parameters or liver-orientated scores
| Variable | Spearman’s rho | P value |
| CRP | 0.63 | < 0.001 |
| PCT | 0.53 | < 0.001 |
| Leucocyte count | 0.27 | < 0.001 |
| Serum creatinine | 0.36 | < 0.001 |
| Serum bilirubin | 0.28 | < 0.001 |
| Serum albumin | -0.40 | < 0.001 |
| INR | 0.15 | 0.032 |
| CPs | 0.42 | < 0.001 |
| MELD score | 0.45 | < 0.001 |
CRP: C-reactive protein; CPs: Child-Pugh score; INR: International normalized ratio; MELD score: Model for end-stage liver disease; PCT: Procalcitonin.
Considering the type of infectious episodes, presepsin level was not different according to the location or Gram specificity of the infection (data not shown). Patients with multifocal infections (10.6%) showed numerically higher presepsin levels than those with unifocal ones without reaching statistically significance [2470 pg/mL (729-2671) vs 983 pg/mL (560-1774), P = 0.065]. Nonetheless, presepsin level was associated with the severity of the infection. Twenty-four infections (32%) were complicated with at least one OF. Presepsin level was significantly higher in patients with OF as compared to those without [2358 pg/mL (1398-3666) vs 710 pg/mL (533-1277), P < 0.001] (Figure 1).
Accuracy of presepsin level in the diagnosis of bacterial infections compared to classic acute phase proteins
The diagnostic accuracy of presepsin for identifying patients with infection was established by ROC analysis and compared to CRP and PCT. Presepsin was a similar predictor of bacterial infection in overall [AUC-ROC, 95%CI: 0.79 (0.73-0.84)] vs PCT [0.77 (0.71-0.83), P = 0.668] but somewhat lower than CRP [0.86 (0.80-0.90), P = 0.057] (Figure 3A). Combination of CRP with presepsin, however, increased the sensitivity and NPV, compared with CRP on its own, by 9 % and 4 % respectively. A similar trend was found with the combination of CRP and PCT (Table 4). On the contrary, the diagnostic accuracy of presepsin [AUC-ROC 95%CI: 0.85 (0.74-0.92)] for identifying patients with infection complicated by OF was similar to PCT [0.85 (0.74-0.92)] and clearly superior to CRP [0.66 (0.54-0.77), P = 0.994 for presepsin vs PCT, and P < 0.01 for both presepsin vs CRP and PCT vs CRP] (Figure 3B). The optimum diagnostic thresholds for each individual biomarker based on ROC analysis and their performances belonging to the cut-off points are shown in Table 4.
Figure 3.
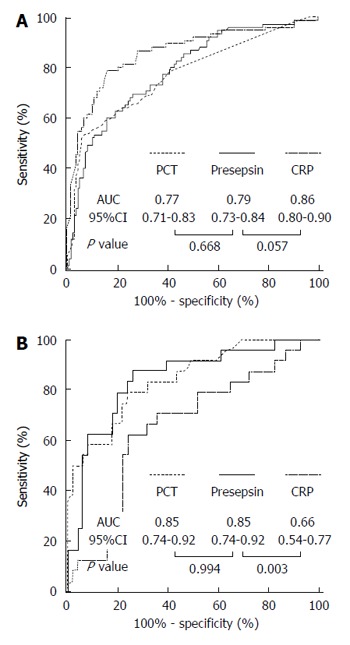
Receiver-operating characteristic curves of presepsin, procalcitonin and C-reactive protein for the identification of bacterial infection overall (A) or bacterial infection complicated by organ failure (B). ROC analysis were performed (A) in the whole cohort (n = 216) or (B) in patients with bacterial infection (n = 75). The control group comprised (A) patients without bacterial infection (n = 141), or (B) patients with bacterial infection without organ failure (n = 51). AUC: Area under curve; CI: Confidence interval; CRP: C-reactive protein; PCT: Procalcitonin.
Table 4.
Performance characteristics of presepsin and other acute phase proteins during bacterial infections in patients with cirrhosis in various clinical settings
| Variable | Cut-off values | Sensitivity | Specificity | PPV | NPV | LR+ | LR- | |
| INF overall | Presepsin (pg/mL) | 844 | 60.0% | 84.45 | 67.2% | 79.9% | 3.85 | 0.47 |
| PCT (μmol/L) | 0.39 | 53.3% | 93.65 | 81.6% | 79.0% | 8.36 | 0.50 | |
| CRP (mg/L) | 10.8 | 78.7% | 84.4% | 78.2% | 88.1% | 5.04 | 0.25 | |
| At least one marker positive (Presepsin/CRP) | 88.0% | 74.5% | 64.7% | 92.1% | 3.45 | 0.16 | ||
| At least one marker positive (PCT/CRP) | 81.3% | 84.2% | 69.3% | 91.1% | 5.15 | 0.22 | ||
| INF + OF | Presepsin (pg/mL) | 1206 | 87.5% | 74.5% | 61.8% | 92.75 | 3.43 | 0.17 |
| PCT (μmol/L) | 0.5 | 79.2% | 76.5% | 61.3% | 88.6% | 3.36 | 0.27 | |
| CRP (mg/L) | 40.5 | 62.5% | 76.5% | 55.6% | 81.2% | 2.66 | 0.49 |
PPV: Positive predictive value; NPV: Negative predictive value; LR+: Positive likelihood ratio; LR-: Negative likelihood ratio; PCT: Procalcitonin; CRP: C-reactive protein; MELD: Model for end-stage liver disease; INF: Infection; OF: Organ failure.
Diagnostic accuracy of presepsin level according to the disease severity
Diagnostic accuracy of presepsin for identifying patients with infection decreased in advanced stage of the disease and also in the presence of renal failure. Specificity and LR values of presepsin > 844 pg/mL were obviously lower in patients with Child B or C stage cirrhosis (74%; LR+: 2.28, LR-: 0.56) compared with those with Child A (96%; LR+: 15.6, LR-: 0.31), whereas the difference in sensitivity was somewhat less (70% vs 58%). A similar trend was found when the performance of presepsin > 844 pg/mL was evaluated in patients with or without renal failure (specificity: 46% vs 87%; sensitivity: 86% vs 49%; LR+: 1.58 vs 3.86, LR-: 0.30 vs 0.58, respectively).
Association of presepsin level with short-term mortality during infectious episodes
Seventy-five patients with bacterial infection were eligible for evaluation of short-term mortality. Twenty-three patients (31.5%) died within 3 mo of follow-up. Of these, 20 patients (27.4%) died within the first 28 d. Plasma presepsin levels at admission were significantly higher in non-survivors than in survivors at the 28-d follow-up [2323 (1172-3688) pg/mL vs 852 (549-1451) pg/mL, P < 0.001]. Discriminative ability (AUC-ROC) of presepsin was 0.76 with the best cut-off value of 1277 pg/mL. 28-d mortality rate was significantly higher among patients with presepsin level above this threshold (46.9% vs 11.6%, P < 0.001). The optimum cut-off values and the belonging sensitivities, specificities, PPVs and NPVs of all three APPs for identifying non-survivors are summarized in Table 5.
Table 5.
Performance characteristics of presepsin, procalcitonin and C-reactive protein for prediction of short-term (28-d) mortality in patients with bacterial infection (n = 75)
| Variable | Cut-off values | AUC-ROC (95%CI) | Sensitivity | Specificity | PPV | NPV | LR+ | LR- |
| Presepsin (pg/mL) | 1277 | 0.76 (0.64-0.85) | 75.0% | 69.1% | 46.9% | 88.4% | 2.43 | 0.36 |
| PCT (μmol/L) | 0.48 | 0.87 (0.77-0.93) | 90.0% | 74.6% | 56.2% | 95.3% | 3.54 | 0.13 |
| CRP (mg/L) | 39.6 | 0.74 (0.63-0.84) | 75.0% | 74.6% | 51.7% | 89.1% | 2.95 | 0.34 |
PPV: Positive predictive value; NPV: Negative predictive value; LR+: Positive likelihood ratio; LR-: Negative likelihood ratio; PCT: Procalcitonin; CRP: C-reactive protein; MELD: Model for end-stage liver disease.
In the univariate logistic regression analysis, increased presepsin level was found to be a risk factor of short-term mortality during bacterial infection [OR = 3.59 (95%CI: 1.65-7.84), P = 0.001] similarly to CRP and PCT. Presepsin level however lost it significance after adjusting for MELD score and leukocyte count [OR = 1.61, (95%CI: 0.65-3.97), P = 0.303], with multivariate binary logistic regression analysis. PCT was the only APP that was independently associated with the risk of short-term mortality [OR = 1.81, (95%CI: 1.09-3.01), P = 0.022] in this model (Table 6).
Table 6.
Association of presepsin, procalcitonin and C-reactive protein levels with short-term (28-d) mortality in patients with cirrhosis and bacterial infection
|
Binary logistic regression analysis |
||||||||
|
Univariate |
Multivariate |
|||||||
| Unadjusted | P value | Adjusted for MELD | P value | Adjusted for leucocyte count | P value | Adjusted for MELD and leucocyte count | P value | |
| ln(Presepsin) | 3.59 (1.65-7.84) | 0.001 | 1.9 (0.81-4.43) | 0.138 | 2.91 (1.28-6.64) | 0.011 | 1.61 (0.65-3.97) | 0.303 |
| ln(PCT) | 2.54 (1.55-4.16) | < 0.001 | 1.89 (1.14-3.14) | 0.014 | 2.33 (1.42-3.83) | 0.001 | 1.81 (1.09-3.01) | 0.022 |
| ln(CRP) | 2.17 (1.23-3.81) | 0.007 | 1.73 (0.93-3.21) | 0.081 | 1.84 (1.03-3.31) | 0.040 | 1.56 (0.81-2.99) | 0.180 |
Associations are expressed as odds ratios and 95%CI per 1 loge-unit increase. MELD: Model for end-stage liver disease; PCT: Procalcitonin; CRP: C-reactive protein.
DISCUSSION
Infected patients with cirrhosis can be asymptomatic at initial stages, but highly susceptible to dissemination of infections due to their immunocompromised state that often leads to development of severe disease specific complications with significant mortality rate[36,37]. Accurate laboratory markers are of importance to maximize the efficacy of diagnostic procedure of bacterial infections and thus making early intervention possible. C-reactive protein is the most widely used APP in the everyday clinical practice; however, it has some limitations in patients with cirrhosis[5]. Thus identification of novel biomarkers is required to reach this unmet need in this patient group.
Primary aim of our study was to assess the performance of presepsin - a recently reported novel sepsis marker - in the diagnosis of cirrhosis-associated bacterial infections in comparison with routinely used APPs (CRP, PCT) in such a patient cohort that represents the everyday clinical practice. To the best of our knowledge, this is the first study in cirrhosis, reporting the feasibility and the usefulness of presepsin in these clinical settings. We evaluated a large cohort of patients, in which not only severe but also mild forms of infections were represented. One-third of patients had mild infections and mainly localized to the urinary tract, while another subgroup of patients (32%) suffered from severe infectious episodes. In our study severe infectious episodes were defined by the presence of hepatic and/or extrahepatic OF(s), since currently accepted clinical definition of SIRS and hence sepsis[38] is not entirely applicable to cirrhotic patients for various reasons[5]. New definition of OFs has directly been elaborated for cirrhotic patient population recently[3,30] that uses simple measures and is easy to apply in everyday clinical practice. Moreover presence of OF is predictive of worse outcome[2].
For the diagnosis of bacterial infections, the best cut-off level of presepsin was 844 pg/mL in our cirrhotic patient cohort. Diagnostic cut-off levels were different in previous studies in non-cirrhotic populations, but most reports suggest an approximate level of 400-600 pg/mL[39,40].
Presepsin alone was not suitable as a screening tool to search for infection, however adding it to CRP, we found that presepsin was clinically useful. For the first, this combination amended efficacy of identification of the infectious episode. Sensitivity and NPV were increased by 9% and 4% compared to CRP alone. Secondly, presepsin was able to distinguish severe infectious episodes from non-severe ones more properly compared to CRP; AUC-ROC values were 0.85 and 0.66, respectively. Performance of presepsin corresponds to those reported in non-cirrhotic septic patient populations. In a recent meta-analysis of Zheng et al[41], comprising a total of 8 studies and 1757 patients, the AUC of the summary ROC (SROC) was 0.82. In contrast, weak predictive power of CRP with an AUC-ROC of 0.64 was reported for the infections in critically ill patients with cirrhosis in intensive care unit (ICU)[22], which is also in agreement with our results. Regarding CRP, patients with cirrhosis may present reduced CRP in response to infection[5,10].
It is acknowledged that level of certain APPs are different according to the pathogens causing infections, while others are not. In a landmark study of Angeletti et al[42] level of PCT and mid-regional pro-adrenomedullin (MR-proADM) were found to be significantly higher in patients with sepsis caused by Gram-negative than Gram-positive strains. These data are also confirmed by other studies[43-45]. Some reports also highlighted differences in circulating cytokine levels in bloodstream infections according to Gram specificity, i.e., Gram-negative infections leaded to higher increase in the level of interleukin (IL)-6, TNF-alpha or IL-10[46]. On the contrary, levels of other APPs, such as C-reactive protein, soluble (s)CD14, sCD163 or soluble urokinase plasminogen activator receptor (SuPAR) are not in relation with the Gram specificity of the infection[47-50]. In the present study, presepsin level was not different according to Gram specificity of the infection, which is in agreement with previous literature findings[51-53].
Overall, presepsin was indisputably a valuable complementary tool in our cirrhotic patient cohort from a clinical point-of-view, but cost issues might compromise their joint use in the laboratory screening procedure of infections. Adding presepsin to CRP significantly increased the cost, from 0.9 to 12.5 $. Medico-economic evaluation, however is lacking at this time and should be performed before proposing introduction of their combined use into routine clinical practice. Presepsin had very similar discriminative ability as PCT in both above-mentioned clinical settings. Furthermore, prices of presepsin and PCT are also comparable (12.5 and 10.7 $) suggesting their interchangeability in this patient population.
Secondary aim of our study was to evaluate whether presepsin is devoid of the limitations of classic APPs in cirrhosis. Previously in a small case-control study of Park et al[10] showed that the more severe the underlying liver dysfunction, the lower the CRP response to bacteremia was. Equally in a former study[9], we reported that the diagnostic accuracy of both CRP and PCT for identifying patients with infection obviously decreased in advanced stage of the disease or in presence of ascites. Correspondingly, presepsin behaved similarly in the present study. Presepsin is not primarily synthesized in the liver, thus not the decreasing synthetic capacity is the major limitation of their diagnostic performance in advanced cirrhosis. Ongoing chronic inflammatory state is a characteristic feature of cirrhosis that is potentially able to induce the synthesis of APPs in the absence of infection[54,55] and inevitably limits their clinical utility in the diagnostic procedure of bacterial infections. Out of various explanations, BT has major importance. Bacterial translocation is frequently reported in patients with cirrhosis-associated severe liver dysfunction or ascites[56,57]. It is likely that this process resulted in higher presepsin level in our non-infected cirrhotic patients compared to healthy controls in previous studies[39,58]. Furthermore, presepsin levels were associated with diseases severity (Child A: 361, Child B: 530 and Child C: 703 pg/mL, P < 0.001) or presence of ascites (Yes vs No: 382 pg/mL vs 575 pg/mL, P < 0.001) in the non-infected patient group as well.
Another important, but rarely considered issue is the effect of renal function on the levels of APPs. Acute kidney injury (AKI) is a frequent complication of cirrhosis, occurring in up to 50% of hospitalized patients with cirrhosis[59]. Exact clearance mechanism of presepsin is unknown but considering its low MW it is presumably filtered by the glomeruli, reabsorbed, and catabolized within the proximal tubular cells[60]. From a clinical point of view, little information is available on the accurate association between presepsin level and kidney function. Nagata et al[61] reported that presepsin levels tend to increase with decreasing glomerular filtration rate - assessed by inulin renal clearance measurements - and are markedly high in patients with chronic renal failure or receiving hemodialysis. Nakamura et al[62] retrospectively analyzed presepsin levels in patients with or without sepsis presenting in the ICU, and found that presepsin levels were markedly high in patients with renal failure and end-stage kidney disease. Accordingly, we evaluated the impact of kidney function on presepsin levels. Significant correlation was found between presepsin and serum creatinine level (Spearman’s rho: +0.36, P < 0.001). Furthermore, in a small subgroup of patients with renal failure, presepsin values were markedly high even in the absence of infection, at comparable levels to those of bacterial infection but without renal failure (1011 vs 774 pg/mL). These results suggest that the evaluation of presepsin levels in cirrhosis warrants special consideration during AD episodes complicated by AKI, and probably a different cut-off is needed for diagnosing infection in such patients.
Third aim of our study was to assess whether presepsin is able to provide prognostic information in cirrhosis associated bacterial infections. Studies in this clinical setting only exist regarding CRP[19,20,63-75] and PCT[76-80] and their findings are not without controversies. In Table 2 we summarized available data on clinical significance of CRP and PCT in short or long-term mortality of patients with cirrhosis. Most of the studies included both stable outpatients and patients with ongoing AD episodes with or without bacterial infections. Furthermore, evaluations often were done as a whole of these non-homogenous patient groups rendering direct comparison and a single conclusion rather difficult. Recently, an important concept has been derived from the CANONIC study[3]. Acute-on-chronic liver failure (ACLF) is associated with systemic inflammation and robust inflammatory response as judged by presence of elevated CRP or elevated leukocyte count results in worse outcome. Higher leukocyte count was found to be an independent predictor of 28-d transplant-free mortality. Based on these results it was reasonable to assume that excessive increase in the APP levels, as a representative of the exaggerated inflammatory process could be associated with higher risk of short-term mortality in cirrhosis during bacterial infections. In patients with increased level of PCT, CRP and presepsin, short-term mortality was significantly higher. Indeed, higher level of PCT, CRP and presepsin were associated with short-term mortality in our study. However, after adjusting for diseases severity and leukocyte count, this association was only preserved for PCT and not for CRP or presepsin. From biological point of view this finding might be explained by the fact that presepsin has a different profile. It belongs to a distinctive class of molecules, so-called “hormonkines”[81]. Procalcitonin has a cytokine-like behaviour during inflammation and infection. It is produced primarily in neuroendocrine cells of various organs and represents involvement of several instead of one organ into the pro-inflammatory response[82]. Lastly, it has been demonstrated that PCT has various toxic effects and pose harm to the host. Administration of PCT to septic animals greatly increases mortality. Antibodies directed against PCT are able to ameliorate harmful effects of PCT with a marked decrease symptomatology and mortality of sepsis[83]. Presepsin represents activation of the monocyte-macrophage system during inflammatory process. Macrophages have a dual effect: production of excessive amount of inflammatory cytokines can cause tissue damage but involvement in the resolution of the inflammation promote tissue repair. This latter process is driven by M2-type macrophages in the presence of local microenvironmental anti-inflammatory signals such as IL-10[84].
Plasma presepsin was only assessed at enrolment, and thus dynamic changes of the concentration were unknown, which is inevitably one of the limitations of the present study. For this reason, additional clinical study will be needed to further investigate serial changes in presepsin levels and their possible association with worse outcome during infection.
To conclude, the present study suggests that presepsin is a promising biomarker during diagnostic procedure of bacterial infections in cirrhosis by enhancing the diagnostic capacity of CRP and reflecting more accurately the severity of infections. Performance of presepsin is equal to PCT in these clinical settings. Diagnostic accuracy of presepsin, however, decreases in advanced stage of the disease or in the presence of renal failure. Level of presepsin is not associated with the pathogens causing infections. Procalcitonin, but not presepsin, is a biomarker for predicting infection-related short-term mortality in patients with cirrhosis.
COMMENTS
Background
Bacterial infections are frequent complications in cirrhosis and often culminate in newly developed liver and/or extrahepatic organ failures, which is associated with significant mortality. Early laboratory diagnosis of these episodes is essential but challenging. There is an evident lack of sensitivity and specificity of the conventional laboratory markers due to disease specific characteristics. Advanced stage of cirrhosis affects diagnostic accuracy of liver synthesised acute phase proteins (e.g., C-reactive protein) whereas acute kidney injury affects renal clearance of small molecules (e.g., procalcitonin). Enhanced bacterial translocation induces significant elevation of inflammatory markers as well. Additional biomarkers are highly needed to optimize the diagnostic procedure and severity assessment of the infectious episodes in cirrhosis. Presepsin is a novel biomarker of activated monocyte-macrophage in response to pathogens and specific and sensitive marker of the sepsis.
Research frontiers
Presepsin is worthy of studying in cirrhosis, where systemic infections are frequently associated with severe disease course, such as acute development of liver and/or extrahepatic organ failures. Contributive role of presepsin for the diagnosis and prognosis of cirrhosis associated bacterial infection, however, has not been assessed extensively so far.
Innovations and breakthroughs
To the knowledge of the authors, this is the first study in cirrhosis to investigate the performance of presepsin in the diagnosis and prognosis of cirrhosis associated bacterial infections. Presepsin is a promising biomarker of infection in terms of diagnostic, but not the prognostic procedure. Presepsin enhances diagnostic capacity of C-reactive protein and reflects more accurately severity of infections. In these clinical settings its performance is equal to procalcitonin. Diagnostic accuracy of presepsin, however, decreases in advanced stage of the disease or in the presence of renal failure. Level of presepsin is not associated with the pathogens causing infections. A clear strength of our study is the large study population that represents the everyday clinical practice and assessment of presepsin in comparison with routinely used acute phase proteins. The authors also provide a profound overview about the significance of routinely used acute phase proteins in the prognosis of cirrhosis.
Applications
In every day clinical practice, presepsin is a useful complementary adjunct to C-reactive protein and promising alternate of procalcitonin during the diagnostic procedure of cirrhosis associated bacterial infection. However, it is not devoid of the limitations of these classic acute phase proteins in the presence of advanced stage or certain acute complications of the disease. Larger prospective studies including serial changes in presepsin levels are needed to further investigate any possible association of presepsin level with worse outcome during infection or any suggestion for more aggressive or pre-emptive antibiotic therapy according to presepsin level.
Terminology
Presepsin or soluble CD14 subtype (sCD14-ST) is a 13-kDa-cleavage product of CD14 receptor of monocyte-macrophage that recognizes different cell surface structure of both Gram-negative and positive bacteria. Bacterial translocation is defined as an enhanced passage of bacteria and/or bacterial products from the intestinal tract to systemic circulation.
Peer-review
This study suggests for the first time that presepsin is a promising biomarker during diagnostic procedure of bacterial infections in cirrhosis for enhancing diagnostic capacity of C-reactive protein and reflecting more accurately the severity of infections. Performance of presepsin is equal to procalcitonin in these clinical settings. In contrast to procalcitonin and certain cytokines, presepsin level is not associated with the pathogens causing infections. Moreover, procalcitonin but not presepsin is a biomarker for predicting infection-related short-term mortality in patients with cirrhosis. Acute phase proteins are not simply surrogate markers of on-going inflammatory processes of the host organism but might also be active participants, hence exerting harmful or beneficial effects.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Hungarian National Review Board and the Institutional Review Board of the University of Debrecen.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Data sharing statement: No additional data are available.
Peer-review started: June 29, 2016
First decision: August 8, 2016
Article in press: September 28, 2016
P- Reviewer: Angeletti S, Kim IH S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Jalan R, Fernandez J, Wiest R, Schnabl B, Moreau R, Angeli P, Stadlbauer V, Gustot T, Bernardi M, Canton R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol. 2014;60:1310–1324. doi: 10.1016/j.jhep.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj JS, O’Leary JG, Reddy KR, Wong F, Biggins SW, Patton H, Fallon MB, Garcia-Tsao G, Maliakkal B, Malik R, et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–137, 1426-137. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 4.Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut. 2005;54:718–725. doi: 10.1136/gut.2004.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pieri G, Agarwal B, Burroughs AK. C-reactive protein and bacterial infection in cirrhosis. Ann Gastroenterol. 2014;27:113–120. [PMC free article] [PubMed] [Google Scholar]
- 6.Cazzaniga M, Dionigi E, Gobbo G, Fioretti A, Monti V, Salerno F. The systemic inflammatory response syndrome in cirrhotic patients: relationship with their in-hospital outcome. J Hepatol. 2009;51:475–482. doi: 10.1016/j.jhep.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 9.Papp M, Vitalis Z, Altorjay I, Tornai I, Udvardy M, Harsfalvi J, Vida A, Kappelmayer J, Lakatos PL, Antal-Szalmas P. Acute phase proteins in the diagnosis and prediction of cirrhosis associated bacterial infections. Liver Int. 2012;32:603–611. doi: 10.1111/j.1478-3231.2011.02689.x. [DOI] [PubMed] [Google Scholar]
- 10.Park WB, Lee KD, Lee CS, Jang HC, Kim HB, Lee HS, Oh MD, Choe KW. Production of C-reactive protein in Escherichia coli-infected patients with liver dysfunction due to liver cirrhosis. Diagn Microbiol Infect Dis. 2005;51:227–230. doi: 10.1016/j.diagmicrobio.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Angeli P, Tonon M, Pilutti C, Morando F, Piano S. Sepsis-induced acute kidney injury in patients with cirrhosis. Hepatol Int. 2016;10:115–123. doi: 10.1007/s12072-015-9641-1. [DOI] [PubMed] [Google Scholar]
- 12.Westhuyzen J, Healy H. Review: Biology and relevance of C-reactive protein in cardiovascular and renal disease. Ann Clin Lab Sci. 2000;30:133–143. [PubMed] [Google Scholar]
- 13.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–1357. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu XL, Xiao ZH, Yang MY, Zhu YM. Diagnostic value of serum procalcitonin in patients with chronic renal insufficiency: a systematic review and meta-analysis. Nephrol Dial Transplant. 2013;28:122–129. doi: 10.1093/ndt/gfs339. [DOI] [PubMed] [Google Scholar]
- 15.Lee WS, Kang DW, Back JH, Kim HL, Chung JH, Shin BC. Cutoff value of serum procalcitonin as a diagnostic biomarker of infection in end-stage renal disease patients. Korean J Intern Med. 2015;30:198–204. doi: 10.3904/kjim.2015.30.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Sayed D, Grotts J, Golgert WA, Sugar AM. Sensitivity and specificity of procalcitonin in predicting bacterial infections in patients with renal impairment. Open Forum Infect Dis. 2014;1:ofu068. doi: 10.1093/ofid/ofu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldini A, Chelazzi C, Terreni A, Biagioli T, Giannoni C, Villa G, Messeri G, De Gaudio AR. Is procalcitonin a reliable marker of sepsis in critically ill septic patients undergoing continuous veno-venous hemodiafiltration with “high cut-off” membranes (HCO-CVVHDF)? Clin Chem Lab Med. 2013;51:e261–e263. doi: 10.1515/cclm-2013-0257. [DOI] [PubMed] [Google Scholar]
- 18.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Lazzarotto C, Ronsoni MF, Fayad L, Nogueira CL, Bazzo ML, Narciso-Schiavon JL, de Lucca Schiavon L, Dantas-Corrêa EB. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12:599–607. [PubMed] [Google Scholar]
- 20.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299–1304. doi: 10.1016/j.jhep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Li CH, Yang RB, Pang JH, Chang SS, Lin CC, Chen CH, Chen HY, Chiu TF. Procalcitonin as a biomarker for bacterial infections in patients with liver cirrhosis in the emergency department. Acad Emerg Med. 2011;18:121–126. doi: 10.1111/j.1553-2712.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 22.Bota DP, Van Nuffelen M, Zakariah AN, Vincent JL. Serum levels of C-reactive protein and procalcitonin in critically ill patients with cirrhosis of the liver. J Lab Clin Med. 2005;146:347–351. doi: 10.1016/j.lab.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, Guyomarch S, Tardy B, Bertrand JC. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000;26:1082–1088. doi: 10.1007/s001340051321. [DOI] [PubMed] [Google Scholar]
- 24.Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97–103. doi: 10.1016/j.cca.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Dupuy AM, Philippart F, Péan Y, Lasocki S, Charles PE, Chalumeau M, Claessens YE, Quenot JP, Guen CG, Ruiz S, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review: I - currently available biomarkers for clinical use in acute infections. Ann Intensive Care. 2013;3:22. doi: 10.1186/2110-5820-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong X, Cao Y, Yu M, Han C. Presepsin as a diagnostic marker for sepsis: evidence from a bivariate meta-analysis. Ther Clin Risk Manag. 2015;11:1027–1033. doi: 10.2147/TCRM.S84811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Hu L, Zhang G, Wu F, He T. Accuracy of Presepsin in Sepsis Diagnosis: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0133057. doi: 10.1371/journal.pone.0133057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey E, Carey WD. Noninvasive tests for liver disease, fibrosis, and cirrhosis: Is liver biopsy obsolete? Cleve Clin J Med. 2010;77:519–527. doi: 10.3949/ccjm.77a.09138. [DOI] [PubMed] [Google Scholar]
- 29.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl:S100–S107. doi: 10.1016/j.jhep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Jalan R, Saliba F, Pavesi M, Amoros A, Moreau R, Ginès P, Levesque E, Durand F, Angeli P, Caraceni P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Mohan P, Ramu B, Bhaskar E, Venkataraman J. Prevalence and risk factors for bacterial skin infection and mortality in cirrhosis. Ann Hepatol. 2011;10:15–20. [PubMed] [Google Scholar]
- 32.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 33.Cadranel JF, Denis J, Pauwels A, Barbare JC, Eugène C, di Martino V, Poquet E, Medini A, Coutarel P, Latrive JP, et al. Prevalence and risk factors of bacteriuria in cirrhotic patients: a prospective case-control multicenter study in 244 patients. J Hepatol. 1999;31:464–468. doi: 10.1016/s0168-8278(99)80038-5. [DOI] [PubMed] [Google Scholar]
- 34.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 35.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 36.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 37.Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556–563. doi: 10.1136/gut.2004.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 39.Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J Infect Chemother. 2011;17:764–769. doi: 10.1007/s10156-011-0254-x. [DOI] [PubMed] [Google Scholar]
- 40.Ulla M, Pizzolato E, Lucchiari M, Loiacono M, Soardo F, Forno D, Morello F, Lupia E, Moiraghi C, Mengozzi G, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care. 2013;17:R168. doi: 10.1186/cc12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Z, Jiang L, Ye L, Gao Y, Tang L, Zhang M. The accuracy of presepsin for the diagnosis of sepsis from SIRS: a systematic review and meta-analysis. Ann Intensive Care. 2015;5:48. doi: 10.1186/s13613-015-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angeletti S, Spoto S, Fogolari M, Cortigiani M, Fioravanti M, De Florio L, Curcio B, Cavalieri D, Costantino S, Dicuonzo G. Diagnostic and prognostic role of procalcitonin (PCT) and MR-pro-Adrenomedullin (MR-proADM) in bacterial infections. APMIS. 2015;123:740–748. doi: 10.1111/apm.12406. [DOI] [PubMed] [Google Scholar]
- 43.Leli C, Ferranti M, Moretti A, Al Dhahab ZS, Cenci E, Mencacci A. Procalcitonin levels in gram-positive, gram-negative, and fungal bloodstream infections. Dis Markers. 2015;2015:701480. doi: 10.1155/2015/701480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brodská H, Malíčková K, Adámková V, Benáková H, Šťastná MM, Zima T. Significantly higher procalcitonin levels could differentiate Gram-negative sepsis from Gram-positive and fungal sepsis. Clin Exp Med. 2013;13:165–170. doi: 10.1007/s10238-012-0191-8. [DOI] [PubMed] [Google Scholar]
- 45.Charles PE, Ladoire S, Aho S, Quenot JP, Doise JM, Prin S, Olsson NO, Blettery B. Serum procalcitonin elevation in critically ill patients at the onset of bacteremia caused by either Gram negative or Gram positive bacteria. BMC Infect Dis. 2008;8:38. doi: 10.1186/1471-2334-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu XJ, Tang YM, Liao C, Song H, Yang SL, Xu WQ, Shi SW, Zhao N. Inflammatory cytokine measurement quickly discriminates gram-negative from gram-positive bacteremia in pediatric hematology/oncology patients with septic shock. Intensive Care Med. 2013;39:319–326. doi: 10.1007/s00134-012-2752-4. [DOI] [PubMed] [Google Scholar]
- 47.Huttunen R, Syrjänen J, Vuento R, Hurme M, Huhtala H, Laine J, Pessi T, Aittoniemi J. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med. 2011;270:32–40. doi: 10.1111/j.1365-2796.2011.02363.x. [DOI] [PubMed] [Google Scholar]
- 48.Gaïni S, Pedersen SS, Koldkaer OG, Pedersen C, Moestrup SK, Møller HJ. New immunological serum markers in bacteraemia: anti-inflammatory soluble CD163, but not proinflammatory high mobility group-box 1 protein, is related to prognosis. Clin Exp Immunol. 2008;151:423–431. doi: 10.1111/j.1365-2249.2007.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgmann H, Winkler S, Locker GJ, Presterl E, Laczika K, Staudinger T, Knapp S, Thalhammer F, Wenisch C, Zedwitz-Liebenstein K, et al. Increased serum concentration of soluble CD14 is a prognostic marker in gram-positive sepsis. Clin Immunol Immunopathol. 1996;80:307–310. doi: 10.1006/clin.1996.0128. [DOI] [PubMed] [Google Scholar]
- 50.Tornai T, Vitalis Z, Sipeki N, Dinya T, Tornai D, Antal-Szalmas P, Karanyi Z, Tornai I, Papp M. Macrophage activation marker, soluble CD163 is an independent predictor of short-term mortality in patients with cirrhosis and bacterial infection. Liver Int. 2016 doi: 10.1111/liv.13133. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Endo S, Suzuki Y, Takahashi G, Shozushima T, Ishikura H, Murai A, Nishida T, Irie Y, Miura M, Iguchi H, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. 2012;18:891–897. doi: 10.1007/s10156-012-0435-2. [DOI] [PubMed] [Google Scholar]
- 52.Enguix-Armada A, Escobar-Conesa R, García-De La Torre A, De La Torre-Prados MV. Usefulness of several biomarkers in the management of septic patients: C-reactive protein, procalcitonin, presepsin and mid-regional pro-adrenomedullin. Clin Chem Lab Med. 2016;54:163–168. doi: 10.1515/cclm-2015-0243. [DOI] [PubMed] [Google Scholar]
- 53.Plesko M, Suvada J, Makohusova M, Waczulikova I, Behulova D, Vasilenkova A, Vargova M, Stecova A, Kaiserova E, Kolenova A. The role of CRP, PCT, IL-6 and presepsin in early diagnosis of bacterial infectious complications in paediatric haemato-oncological patients. Neoplasma. 2016;63:752–760. doi: 10.4149/neo_2016_512. [DOI] [PubMed] [Google Scholar]
- 54.Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–217. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 55.Márquez M, Fernández-Gutiérrez C, Montes-de-Oca M, Blanco MJ, Brun F, Rodríguez-Ramos C, Girón-González JA. Chronic antigenic stimuli as a possible explanation for the immunodepression caused by liver cirrhosis. Clin Exp Immunol. 2009;158:219–229. doi: 10.1111/j.1365-2249.2009.04005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, Fuster J, García-Valdecasas JC, Lacy A, Suárez MJ, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Tsao G, Lee FY, Barden GE, Cartun R, West AB. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 1995;108:1835–1841. doi: 10.1016/0016-5085(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 58.Okamura Y, Yokoi H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST) Clin Chim Acta. 2011;412:2157–2161. doi: 10.1016/j.cca.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Regner KR, Singbartl K. Kidney Injury in Liver Disease. Crit Care Clin. 2016;32:343–355. doi: 10.1016/j.ccc.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Chenevier-Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens YE. Presepsin (sCD14-ST) in emergency department: the need for adapted threshold values? Clin Chim Acta. 2014;427:34–36. doi: 10.1016/j.cca.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 61.Nagata T, Yasuda Y, Ando M, Abe T, Katsuno T, Kato S, Tsuboi N, Matsuo S, Maruyama S. Clinical impact of kidney function on presepsin levels. PLoS One. 2015;10:e0129159. doi: 10.1371/journal.pone.0129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura Y, Ishikura H, Nishida T, Kawano Y, Yuge R, Ichiki R, Murai A. Usefulness of presepsin in the diagnosis of sepsis in patients with or without acute kidney injury. BMC Anesthesiol. 2014;14:88. doi: 10.1186/1471-2253-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cervoni JP, Amorós À, Bañares R, Luis Montero J, Soriano G, Weil D, Moreau R, Pavesi M, Thévenot T, Di Martino V. Prognostic value of C-reactive protein in cirrhosis: external validation from the CANONIC cohort. Eur J Gastroenterol Hepatol. 2016;28:1028–1034. doi: 10.1097/MEG.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 64.Di Martino V, Coutris C, Cervoni JP, Dritsas S, Weil D, Richou C, Vanlemmens C, Thevenot T. Prognostic value of C-reactive protein levels in patients with cirrhosis. Liver Transpl. 2015;21:753–760. doi: 10.1002/lt.24088. [DOI] [PubMed] [Google Scholar]
- 65.Ha YE, Kang CI, Joo EJ, Joung MK, Chung DR, Peck KR, Lee NY, Song JH. Usefulness of C-reactive protein for evaluating clinical outcomes in cirrhotic patients with bacteremia. Korean J Intern Med. 2011;26:195–200. doi: 10.3904/kjim.2011.26.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kronenberger B, Rudloff I, Bachmann M, Brunner F, Kapper L, Filmann N, Waidmann O, Herrmann E, Pfeilschifter J, Zeuzem S, et al. Interleukin-22 predicts severity and death in advanced liver cirrhosis: a prospective cohort study. BMC Med. 2012;10:102. doi: 10.1186/1741-7015-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwon JH, Jang JW, Kim YW, Lee SW, Nam SW, Jaegal D, Lee S, Bae SH. The usefulness of C-reactive protein and neutrophil-to-lymphocyte ratio for predicting the outcome in hospitalized patients with liver cirrhosis. BMC Gastroenterol. 2015;15:146. doi: 10.1186/s12876-015-0378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim TS, Kim BK, Lee JW, Lee YK, Chang S, Kim SU, Kim DY, Ahn SH, Han KH, Chon CY, et al. Use of the delta neutrophil index as a prognostic factor of mortality in patients with spontaneous bacterial peritonitis: implications of a simple and useful marker. PLoS One. 2014;9:e86884. doi: 10.1371/journal.pone.0086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno JP, Grandclement E, Monnet E, Clerc B, Agin A, Cervoni JP, Richou C, Vanlemmens C, Dritsas S, Dumoulin G, et al. Plasma copeptin, a possible prognostic marker in cirrhosis. Liver Int. 2013;33:843–851. doi: 10.1111/liv.12175. [DOI] [PubMed] [Google Scholar]
- 70.Mortensen C, Andersen O, Krag A, Bendtsen F, Møller S. High-sensitivity C-reactive protein levels predict survival and are related to haemodynamics in alcoholic cirrhosis. Eur J Gastroenterol Hepatol. 2012;24:619–626. doi: 10.1097/MEG.0b013e328351db6e. [DOI] [PubMed] [Google Scholar]
- 71.Park JK, Lee CH, Kim IH, Kim SM, Jang JW, Kim SH, Kim SW, Lee SO, Lee ST, Kim DG. Clinical characteristics and prognostic impact of bacterial infection in hospitalized patients with alcoholic liver disease. J Korean Med Sci. 2015;30:598–605. doi: 10.3346/jkms.2015.30.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reuken PA, Stallmach A, Bruns T. Mortality after urinary tract infections in patients with advanced cirrhosis - Relevance of acute kidney injury and comorbidities. Liver Int. 2013;33:220–230. doi: 10.1111/liv.12029. [DOI] [PubMed] [Google Scholar]
- 73.Schwabl P, Bucsics T, Soucek K, Mandorfer M, Bota S, Blacky A, Hirschl AM, Ferlitsch A, Trauner M, Peck-Radosavljevic M, et al. Risk factors for development of spontaneous bacterial peritonitis and subsequent mortality in cirrhotic patients with ascites. Liver Int. 2015;35:2121–2128. doi: 10.1111/liv.12795. [DOI] [PubMed] [Google Scholar]
- 74.Wiese S, Mortensen C, Gøtze JP, Christensen E, Andersen O, Bendtsen F, Møller S. Cardiac and proinflammatory markers predict prognosis in cirrhosis. Liver Int. 2014;34:e19–e30. doi: 10.1111/liv.12428. [DOI] [PubMed] [Google Scholar]
- 75.Ximenes RO, Farias AQ, Scalabrini Neto A, Diniz MA, Kubota GT, Ivo MM, Colacique CG, D’Albuquerque LA, Daglius Dias R. Patients with cirrhosis in the ED: early predictors of infection and mortality. Am J Emerg Med. 2016;34:25–29. doi: 10.1016/j.ajem.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Al-Dorzi HM, Rishu AH, Tamim HM, Aljumah A, Al-Tamimi W, Baharoon S, Al Dabbagh T, Arabi YM. Serum procalcitonin in cirrhotic patients with septic shock: relationship with adrenal insufficiency and clinical outcomes. Clin Lab. 2014;60:1105–1114. doi: 10.7754/clin.lab.2013.130636. [DOI] [PubMed] [Google Scholar]
- 77.Berres ML, Schnyder B, Yagmur E, Inglis B, Stanzel S, Tischendorf JJ, Koch A, Winograd R, Trautwein C, Wasmuth HE. Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. 2009;29:536–543. doi: 10.1111/j.1478-3231.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- 78.Connert S, Stremmel W, Elsing C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z Gastroenterol. 2003;41:165–170. doi: 10.1055/s-2003-37314. [DOI] [PubMed] [Google Scholar]
- 79.Kotecha HL, Arora A, Chawlani R, Toshniwal J, Bansal N, Tyagi P, Sharma P, Kumar M, Kumar A. Low eosinophil count predicts in-hospital mortality in cirrhosis with systemic inflammatory response syndrome. Eur J Gastroenterol Hepatol. 2013;25:676–682. doi: 10.1097/MEG.0b013e32835eb8f7. [DOI] [PubMed] [Google Scholar]
- 80.Lin S, Huang Z, Wang M, Weng Z, Zeng D, Zhang Y, Zhu Y, Jiang J. Interleukin-6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J Crit Care. 2015;30:732–738. doi: 10.1016/j.jcrc.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 81.Müller B, White JC, Nylén ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001;86:396–404. doi: 10.1210/jcem.86.1.7089. [DOI] [PubMed] [Google Scholar]
- 82.Matwiyoff GN, Prahl JD, Miller RJ, Carmichael JJ, Amundson DE, Seda G, Daheshia M. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res. 2012;61:401–409. doi: 10.1007/s00011-012-0439-5. [DOI] [PubMed] [Google Scholar]
- 83.Nylen ES, Whang KT, Snider RH, Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit Care Med. 1998;26:1001–1006. doi: 10.1097/00003246-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 84.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]


