Abstract
Bifidobacterium animalis ssp. lactis GCL2505 (B. lactis GCL2505) is able to survive passage through the intestine and then proliferate, leading to an increase in the amount of gut bifidobacteria. In the present study, we evaluated the impact of B. lactis GCL2505 on abdominal visceral fat storage in overweight and mildly obese Japanese adults. This clinical study was a double-blind, randomized, placebo-controlled, parallel-group comparative trial performed for 12 weeks. Healthy Japanese subjects (N=137) with body mass indices ranging from 23 to 30 kg/m2 consumed either fermented milk containing B. lactis GCL2505 or a placebo every day, and then visceral and subcutaneous abdominal fat areas were measured by computed tomography as the primary endpoints. The number of fecal bifidobacteria was also measured. Visceral fat area, but not subcutaneous fat area, was significantly reduced from baseline at 8 and 12 weeks in the GCL2505 group, compared with the placebo group. The total number of fecal bifidobacteria was significantly increased in the GCL2505 group. These results indicate that B. lactis GCL2505 reduces abdominal visceral fat, a key factor associated with metabolic disorders. This finding suggests that this probiotic strain can potentially serve as a specific functional food to achieve visceral fat reduction in overweight or mildly obese individuals.
Keywords: probiotics, Bifidobacterium, visceral fat, randomized trial, overweight
INTRODUCTION
Abdominal visceral fat accumulation is a known underlying component of metabolic syndrome (MS), which is an independent risk factor for coronary heart disease, hypertension, type-2 diabetes, and impaired glucose tolerance [1,2,3,4]. Visceral fat accumulation is a form of obesity related to environmental factors such as diet and physical inactivity [5, 6].
Although inappropriate dietary habits and inadequate physical activity are the main causes of overweight, obesity, and excess accumulation of abdominal visceral fat, several other environmental factors, including gut microbiota, are also being recognized as important factors [7, 8]. The human intestinal tract harbors a large, active, and complex community of microorganisms comprising over 500 different taxa and 100 trillion cells [9]. The gut microbiota plays important roles in digestion, metabolism, nutrient extraction, vitamin synthesis, prevention of colonization by pathogens, and immunomodulation [9]. In addition to an increased energy harvest from the diet, several other mechanisms, including lipopolysaccharide-induced chronic inflammation, modulation of tissue fatty acid composition, and gut hormone secretion, have been proposed as links between the gut microbiota and obesity [10].
Attention has recently focused on the association between the composition of the microbiota, especially the Firmicutes/Bacteroidetes ratio, and obesity [11, 12]. Furthermore, Bifidobacterium (phylum Actinobacteria) has also been implicated in obesity and overweight [13]. For instance, the number of bifidobacteria in feces is lower in overweight and obese subjects than in lean subjects [13]. These findings suggest that an increase in gut bifidobacteria elicits beneficial effects on obesity, overweight, and metabolic disorders. Thus, probiotics that include bifidobacteria have been reported to exert beneficial effects on weight loss, lowering fat mass, and improvement of the insulin sensitivity in most animal studies and in some human studies [14,15,16,17,18]. A systematic review of probiotics for weight loss and glycemic control indicated that probiotics were effective for improvement of glycemic control, but have limited efficacy in terms of decreasing body weight and body mass index (BMI) in humans [17, 18]. Until now, however, there have been only limited human studies on abdominal visceral fat.
The probiotic species B. animalis ssp. lactis (B. lactis), which is used widely as a daily product, has been reported to have a large number of health benefits for gastrointestinal and immune function [16, 19, 20]. B. lactis GCL2505 originated from the healthy human gut and is used in fermented milk products in the Japanese market. We previously showed that B. lactis GCL2505 reaches the intestine in a viable form and subsequently proliferates to increase the total number of gut bifidobacteria [21, 22].
The purpose of this study was to examine the effects of probiotic B. lactis GCL2505 on abdominal fat storage, especially abdominal visceral fat, in overweight or mildly obese Japanese adults (BMI: 23–30 kg/m2).
MATERIALS AND METHODS
Study design
This study was a multicenter, randomized, double-blind, placebo-controlled intervention trial that was conducted according to the Consolidated Standards of Reporting Trials (CONSORT) statement [23]. The study was also conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of C’est Lavie Shimbashi Clinic (Tokyo, Japan). Subjects provided written informed consent before study initiation. This study was performed by a contract research organization, KSO Co., Ltd. (Tokyo, Japan), from December 2014 to June 2015 at the following two facilities in Tokyo, Japan: C’est Lavie Shimbashi Clinic and Oval Coat Kenshin Clinic. The principle investigator of this study was one of the authors, Haruhi Sugimura, M.D.
Subjects
Healthy Japanese participants were recruited based on their age (20–65 years) and BMI (23–30 kg/m2) and were categorized as overweight and obesity according to World Health Organization standards [24]. Subjects had not received medications that would interfere with the results and had no serious diseases, including hepatic, cardiovascular, respiratory, endocrine, or metabolic disorders. Other exclusion criteria were as follows: 1) food allergy; 2) antibiotic use within 4 weeks before entering the study; 3) use of supplements that would interfere with the results; 4) pregnancy, lactation, or planning to become pregnant; 5) participation in another clinical study; and 6) unsuitable for computed tomography (CT) (e.g., metal devices in the body). The subjects were considered to be healthy by the principle investigator based on the results of blood and urine tests and subjects’ self-reports. Subjects were asked to maintain their normal lifestyle and to avoid any other probiotic-containing products during the study.
Probiotic fermented milk
The test product was fermented milk (FM) containing B. lactis GCL2505 (approximately 8 × 1010 colony forming units [cfu]/100 g), which was not combined with any other starter cultures. The FM was delivered to each subject weekly under refrigeration and stored in a refrigerator until consumption to maintain the number of viable B. lactis GCL2505 cells. The placebo was prepared with the same ingredients and by adding food-grade acetic acid and lactic acid to adjust its pH to 4.5 without any starter cultures. The FM and placebo had the same nutritional content and flavor. They both consisted of approximately 9% skim milk powder, sweeteners, and a small amount of flavoring, and they were identical in terms of energy (44 kcal), protein (3.0 g), fat (0.1 g), carbohydrate (7.7 g), sodium (54 mg), and calcium (96 mg) content per 100 g. The number of viable B. lactis GCL2505 cells was determined using a transoligosaccharide propionate agar medium (Yakult Pharmaceutical Industry Co., Ltd., Tokyo, Japan).
Study protocol
The study period consisted of a 2-week screening, followed by a 12-week treatment period. During the screening period, each subject was assessed with regard to the eligibility criteria. Randomization was carried out by a study statistician not directly involved in the trial. All of the 160 eligible subjects were randomly allocated to two groups defined as the GCL2505 and placebo groups, matching them for age, sex, body weight, BMI, and visceral fat area, according to information obtained during the screening period. The linkage between the identification number and treatment group was kept in a sealed document by the allocation officer. The investigators, subjects, and study statistician had no knowledge of the treatment groups until data analyses were complete.
Each subject consumed 100 g FM or placebo every day for 12 weeks. They were instructed to maintain their regular lifestyle and to keep a daily record of FM or placebo consumption, diet, and number of steps walked as the amount of physical activity. Abdominal CT scans for the measurement of abdominal fat area were conducted at 0, 8, and 12 weeks. Anthropometry and fasting blood, urine, and fecal sampling were performed at 0, 4, 8, and 12 weeks. Fecal samples were delivered to the laboratory in a refrigerated, anaerobic state using an AnaeroPack Kenki (Mitsubishi GAS Chemical Co., Inc., Tokyo, Japan). These samples were diluted 10-fold with phosphate-buffered saline (pH 7.4) and homogenized. Suspensions were kept at −80°C until required for analysis.
Anthropometric measures and body composition
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the subject standing. BMI was calculated in the standard way: weight (kg) divided by the square of height (m). Waist and hip circumferences were measured to the nearest 0.1 cm in a standing position. Waist circumference was measured around the abdomen at the level of the umbilicus. Hip circumference was measured at the level of maximum extension of the buttocks posteriorly in a horizontal plane.
Abdominal fat area
The abdominal visceral fat area (VFA) and subcutaneous fat area (SFA) were measured using CT. Four-slice CT images (120 kVp, 200 mAs tube current, 8.0 mm slice thickness, and 430 mm field of view) were acquired at the level of the lumbar 4–5 vertebrae. Abdominal VFA, SFA, and total (the sum of visceral and subcutaneous) fat area (TFA) were measured using the Fat Scan ver. 4 software (East Japan Institute of Technology Co., Ltd., Hitachi, Ibaraki, Japan). To avoid unnecessary radiation exposure, CT scans were conducted only once at each measurement point (0, 8, and 12 weeks). The measurement of VFA by CT is reportedly easily affected by the respiration phase in subjects and the slice site [25]. Therefore, to investigate the time course changes of VFA accurately in the present study, the scanner and principal investigator strictly assessed a series of CT images obtained from the same subjects at each measurement point and excluded any inappropriate data.
Biochemical parameters
The concentrations of several plasma biochemical parameters were measured at 0, 4, 8, and 12 weeks. Blood was drawn from each subject in a fasting state, in which they had consumed no food or drink except water for at least 10 hours. Biochemical parameters, including total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, free fatty acid, albumin, aspartate transaminase, alanine aminotransaminase, lactate dehydrogenase, bilirubin, alkaline phosphatase, γ-GTP, creatine phosphokinase, white blood cells, red blood cells, hemoglobin, platelets, fasting blood glucose, hemoglobin A1c, total protein, uric nitrogen, creatinine, uric acid, sodium, chloride, potassium, calcium, inorganic phosphorus, magnesium, and serum iron were analyzed by LSI Medience Corporation (Tokyo, Japan). The values of urine parameters were measured at 0, 4, 8, and 12 weeks. Urine parameters, including urine protein, sugar, urobilinogen, bilirubin, ketone bodies, and occult blood, were also analyzed by LSI Medience Corporation.
Gut bifidobacteria
Bacterial DNA was extracted from 10-fold dilutions of fecal samples, and the number of gut bifidobacteria was subsequently quantified by real-time PCR using Bifidobacterium species- and subspecies-specific primers according to the procedure previously described [21]. Total counts of bifidobacteria in the fecal samples were represented as the sum of nine species (B. bifidum, B. breve, B. longum, B. adolescentis, B. angulatum, B. catenulatum, B. dentium, B. infantis, and B. lactis). The detection limit of each bifidobacteria species or subspecies was 2.0 × 105 cells per gram of feces.
Statistical analysis
Results were expressed in either actual values or changes from 0 week. Unpaired t-tests (two-sided) were used to compare baseline characteristics of the subjects in the two groups, and calculated p-values <0.05 were considered statistically significant. An intergroup comparison by two-factor repeated-measures analysis of variance (ANOVA) was performed using the actual values. Interactions of group by time were considered significant for p-values <0.05. Bonferroni’s correction was used for post hoc comparisons when ANOVA revealed statistically significant differences.
All statistical analyses were performed using the IBM SPSS Statistics for Windows software version 21 (IBM Corp., Armonk, NY, USA).
RESULTS
Characteristics of subjects
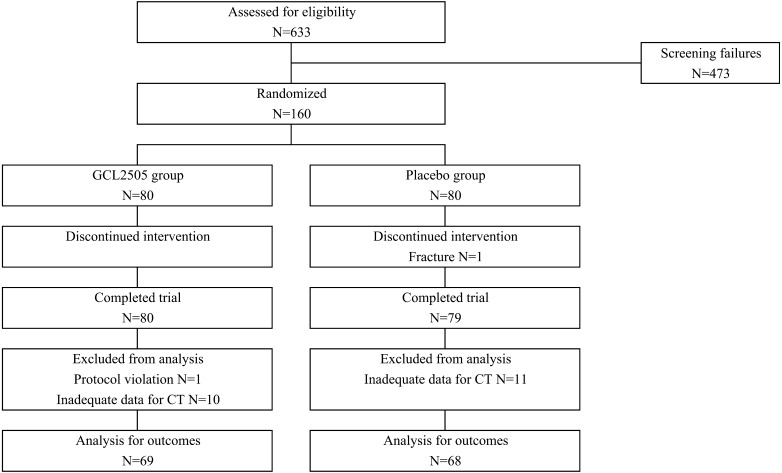
Participant flow is shown in Fig. 1. Subjects (N=160) were randomly assigned to the GCL2505 and placebo groups. One subject in the placebo group did not complete the study due to bone fracture. At 0 week, CT was not obtained for another subject due to interference of barium for the contrast X-ray; therefore, this subject was excluded from the analysis (due to protocol violation). From the viewpoint of the accuracy of the CT data as described in the Materials and Methods (abdominal fat area), we assessed a series of CT images obtained from the same subjects at each measurement point to exclude any inappropriate data. Fourteen subjects (7 in the GCL2505 group and 7 in the placebo group) were excluded due to overestimation of VFA mainly caused by compression of the abdominal cavity during inspiration, and 7 subjects (3 in the GCL2505 group and 4 in the placebo group) were excluded due to underestimation of VFA mainly caused by the inclusion of an internal organ or gas in the CT scan images. Consequently, 10 subjects in the GCL2505 group and 11 subjects in the placebo group were excluded from the analysis. Thus, the numbers of subjects for analysis were 137 (69 in the GCL2505 group and 68 in the placebo group). There were no significant differences in baseline data between the GCL2505 and placebo groups (Table 1) and in nutrient intake (dietary energy, protein, carbohydrate, and fat) and steps walked during the treatment period (Table 2). The intake rates were 99.5% and 99.8% for FM (GCL2505 group) and the placebo (placebo group), respectively. No adverse events were observed throughout this study in any subjects with regard to safety parameters including blood and urine tests.
Fig. 1.
Flow diagram of the study showing numbers of participants.
Table 1. Baseline characteristics of the subjects.
| GCL2505 | Placebo | |
|---|---|---|
| Subjects (N) | 69 | 68 |
| Male | 46 | 41 |
| Female | 23 | 27 |
| Age (years) | 46.9 ± 8.8 | 46.9 ± 8.7 |
| Height (cm) | 167.0 ± 8.5 | 166.6 ± 8.0 |
| Weight (kg) | 75.0 ± 8.4 | 74.9 ± 8.6 |
| BMI (kg/m2) | 26.8 ± 1.5 | 26.9 ± 1.5 |
| Waist circumference (cm) | 93.0 ± 4.8 | 92.9 ± 3.8 |
| Hip circumference (cm) | 98.4 ± 3.4 | 98.3 ± 3.2 |
| WHR | 0.95 ± 0.04 | 0.94 ± 0.03 |
| Visceral fat area (cm2) | 133.4 ± 29.6 | 124.3 ± 26.4 |
| Subcutaneous fat area (cm2) | 215.9 ± 55.8 | 219.9 ± 43.7 |
| Total fat area (cm2) | 349.3 ± 60.5 | 344.2 ± 45.9 |
Values are means ± SD. BMI: Body Mass Index; WHR: waist to hip ratio.
p-values were analyzed by unpaired t-test.
Table 2. Daily nutrition intake and steps walked in the GCL2505-supplemented (N=69) and placebo (N=68) groups during the treatment period.
| Treatment period |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 week |
4 weeks |
8 weeks |
12 weeks |
||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Energy (kcal/day) | GCL2505 | 1,817 | 1,715, 1,919 | 1,853 | 1,737, 1,968 | 1,818 | 1,706, 1,931 | 1,845 | 1,724, 1,966 |
| Placebo | 1,876 | 1,768, 1,984 | 1,909 | 1,789, 2,029 | 1,914 | 1,812, 2,016 | 1,881 | 1,787, 1,974 | |
| Protein (g/day) | GCL2505 | 68.6 | 64.3, 72.9 | 69.3 | 65.0, 73.5 | 68.3 | 63.8, 72.9 | 69.0 | 64.5, 73.6 |
| Placebo | 70.8 | 66.5, 75.0 | 71.0 | 67.0, 75.0 | 72.1 | 67.6, 76.6 | 71.8 | 68.2, 75.5 | |
| Carbohydrate (g/day) | GCL2505 | 241.1 | 228.0, 254.2 | 242.3 | 226.3, 258.4 | 241.0 | 226.1, 255.9 | 241.7 | 224.5, 258.8 |
| Placebo | 252.9 | 237.5, 268.3 | 249.8 | 232.0, 267.7 | 251.9 | 236.4, 267.4 | 247.6 | 231.9, 263.3 | |
| Fat (g/day) | GCL2505 | 57.2 | 52.7, 61.8 | 60.9 | 56.2, 65.6 | 58.4 | 53.6, 63.1 | 60.2 | 55.0, 65.3 |
| Placebo | 58.6 | 54.2, 62.9 | 63.7 | 57.6, 69.8 | 62.4 | 58.4, 66.4 | 60.9 | 57.1, 64.7 | |
| Steps walked (counts/day) | GCL2505 | 7,206 | 6,552, 7,861 | 7,681 | 6,933, 8,430 | 7,696 | 6,870, 8,522 | 7,831 | 7,049, 8,613 |
| Placebo | 7,754 | 6,835, 8,674 | 8,175 | 7,080, 9,271 | 8,264 | 7,200, 9,328 | 8,048 | 7,037, 9,058 | |
Values are means and 95% confidence intervals (CIs).
Abdominal fat area
Changes in VFA, SFA, and TFA are summarized in Table 3. There was a significant group by time interaction in VFA from baseline. The mean decreases in VFA at 8 and 12 weeks from baseline were significantly greater in the GCL2505 group (−6.8 cm2 [95% confidence interval (CI), −10.6 to −2.9], −5.1 cm2 [−8.6 to −1.5]) than in the placebo group (0.9 cm2 [−2.6 to 4.4], 1.5 cm2 [−1.3 to 4.3]). In addition, the mean TFA tended to decrease from baseline in the GCL2505 group compared with the placebo group at 12 weeks (p=0.050). There were no statistically significant differences in SFA between the two groups and no changes within either group.
Table 3. Changes in abdominal fat areas by computed tomography scan in the GCL2505-supplemented (N=69) and placebo (N=68) groups during the treatment period.
| Treatment period |
Time × group a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 week |
8 weeks |
12 weeks |
|||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| VFA (cm2) | Actual values | GCL2505 | 133.4 | 126.4, 140.4 | 127.3 | 120.0, 134.6 | 128.3 | 121.2, 135.5 | 0.004 |
| Placebo | 124.3 | 118.0, 130.6 | 125.2 | 118.5, 132.0 | 125.8 | 119.3, 132.3 | |||
| Change from 0 week | GCL2505 | - | −6.8## | −10.6, −2.9 | −5.1# | −8.6, −1.5 | |||
| Placebo | - | 0.9 | −2.6, 4.4 | 1.5 | −1.3, 4.3 | ||||
| SFA (cm2) | Actual values | GCL2505 | 215.9 | 202.7, 229.0 | 213.7 | 200.9, 226.6 | 214.8 | 201.7, 227.8 | 0.676 |
| Placebo | 219.9 | 209.5, 230.2 | 216.0 | 204.9, 227.1 | 219.8 | 208.6, 231.0 | |||
| Change from 0 week | GCL2505 | - | −2.4 | −5.9, 1.2 | −1.1 | −5.2, 3.0 | |||
| Placebo | - | −3.9 | −7.6, −0.1 | −0.1 | −3.8, 3.6 | ||||
| TFA (cm2) | Actual values | GCL2505 | 349.3 | 335.0, 363.5 | 341.0 | 326.6, 355.5 | 343.1 | 328.8, 357.4 | 0.111 |
| Placebo | 344.2 | 333.3, 355.1 | 341.2 | 329.2, 353.3 | 345.6 | 333.4, 357.7 | |||
| Change from 0 week | GCL2505 | - | −9.1 | −14.2, −4.0 | −6.2 | −11.9, −0.5 | |||
| Placebo | - | −3.0 | −8.4, 2.5 | 1.4 | −3.6, 6.4 | ||||
Values are means and 95% confidence intervals (CIs). VFA: visceral fat area; SFA: subcutaneous fat area; TFA: total fat area.
There was a significant difference between the groups, as determined using an unpaired t-test with Bonferroni correction. #p<0.05; ##p<0.01.
ap-value represented as a group-by-time interaction effect by two-factor repeated-measures ANOVA using actual values.
Table 4 shows changes in abdominal fat area in subjects classified as non-MS (59 in the GCL2505 group and 54 in the placebo group), defined as not having a high visceral fat area (≥100 cm2) and two or more of the following criteria according to the diagnostic criteria for MS in Japan: 1) triglycerides ≥150 mg/dl and/or HDL cholesterol <40 mg/dl; 2) fasting blood glucose ≥110 mg/dl; and 3) systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg. The mean decreases in VFA at 8 and 12 weeks from baseline were also significantly greater in the GCL2505 group than in the placebo group in non-MS subjects. Furthermore, the mean TFA also tended to decrease from baseline in the GCL2505 group compared with the placebo group at 12 weeks (p=0.053).
Table 4. Changes in abdominal fat areas in subjects classified as non-MS by computed tomography in the GCL2505-supplemented (N=59) and placebo (N=54) groups during the treatment period.
| Treatment period |
Time × group a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 week |
8 weeks |
12 weeks |
|||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||||
| VFA (cm2) | Actual values | GCL2505 | 131.6 | 123.7, 139.5 | 125.4 | 117.2, 133.5 | 125.2 | 117.3, 133.0 | 0.001 |
| Placebo | 120.2 | 113.9, 126.6 | 121.5 | 114.3, 128.7 | 122.4 | 115.7, 129.2 | |||
| Change from 0 week | GCL2505 | - | −7.0## | −10.9, −3.1 | −6.4## | −10.2, −2.7 | |||
| Placebo | - | 1.2 | −2.6, 5.1 | 2.2 | −1.0, 5.4 | ||||
| SFA (cm2) | Actual values | GCL2505 | 217.5 | 203.1, 232.0 | 216.6 | 202.5, 230.8 | 217.0 | 202.6, 231.3 | 0.497 |
| Placebo | 227.6 | 216.1, 239.0 | 223.6 | 211.4, 235.9 | 227.0 | 214.6, 239.3 | |||
| Change from 0 week | GCL2505 | - | −1.2 | −5.1, 2.7 | −0.6 | −5.1, 3.9 | |||
| Placebo | - | −3.9 | −8.4, 0.5 | −0.6 | −4.9, 3.7 | ||||
| TFA (cm2) | Actual values | GCL2505 | 349.2 | 333.7, 364.6 | 342.0 | 326.6, 357.4 | 342.1 | 326.7, 357.6 | 0.108 |
| Placebo | 347.8 | 335.4, 360.2 | 345.1 | 331.3, 358.9 | 349.4 | 335.5, 363.3 | |||
| Change from 0 week | GCL2505 | - | −8.2 | −13.8, −2.6 | −7.0 | −13.4, −0.7 | |||
| Placebo | - | −2.7 | −9.1, 3.7 | 1.6 | −4.3, 7.5 | ||||
Non-MS was defined as subjects without metabolic syndrome having high visceral fat area (≥100 cm2) and two or more of the following criteria: 1) triglyceride ≥150 mg and/or HDL-cholesterol <40 mg/dl, 2) fasting blood glucose ≥110 mg/dl and 3) systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg.
Values are means and 95% confidence intervals (CIs). VFA: visceral fat area; SFA: subcutaneous fat area; TFA: total fat area.
There was a significant difference between the groups, as determined using an unpaired t-test with Bonferroni correction. ##p<0.01.
ap-value represented as a group-by-time interaction effect by two-factor repeated-measures ANOVA using actual values.
Anthropometric parameters
Table 5 shows the anthropometric data. There were no statistically significant differences in body weight, BMI, or waist to hip ratio between the two groups.
Table 5. Changes in anthropometric parameters in the GCL2505-supplemented (N=69) and placebo (N=68) groups during the treatment period.
| Treatment period |
Time × group a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 week |
4 weeks |
8 weeks |
12 weeks |
|||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Weight (kg) | GCL2505 | 75.0 | 73.1, 77.0 | 75.5 | 73.5, 77.6 | 75.2 | 73.2, 77.2 | 75.0 | 72.9, 77.0 | 0.772 |
| Placebo | 74.9 | 72.8, 76.9 | 75.5 | 73.5, 77.5 | 75.0 | 73.0, 77.0 | 74.8 | 72.8, 76.8 | ||
| BMI (kg/m2) | GCL2505 | 26.8 | 26.5, 27.2 | 27.0 | 26.6, 27.4 | 26.9 | 26.5, 27.3 | 26.8 | 26.4, 27.2 | 0.731 |
| Placebo | 26.9 | 26.5, 27.3 | 27.2 | 26.8, 27.5 | 27.0 | 26.6, 27.3 | 26.9 | 26.5, 27.3 | ||
| WHR | GCL2505 | 0.95 | 0.94, 0.95 | 0.95 | 0.94, 0.96 | 0.95 | 0.94, 0.96 | 0.95 | 0.94, 0.96 | 0.807 |
| Placebo | 0.94 | 0.94, 0.95 | 0.95 | 0.94, 0.96 | 0.95 | 0.94, 0.96 | 0.95 | 0.94, 0.96 | ||
Values are means and 95% confidence intervals (CIs). WHR: waist to hip ratio.
ap-value represented as a group-by-time interaction effect using two-factor repeated-measures ANOVA.
Biochemical parameters
There were no statistically significant differences in any of the plasma or urine biochemical parameters throughout the study between the two groups (data not shown).
Gut bifidobacteria
Changes in fecal bifidobacteria are presented in Table 6. There were significant group by time interactions in the total number of bifidobacteria and B. lactis cells. At 4 weeks, the total number of bifidobacteria significantly increased in the GCL2505 group compared with the placebo group, and this difference was maintained during the study period. Similarly, the number of B. lactis cells significantly increased in feces after 4 weeks of B. lactis GCL2505 ingestion, and this difference was maintained until 12 weeks. However, the levels of other endogenous bifidobacteria (B. bifidum, B. breve, B. longum, B. adolescentis, B. angulatum, B. catenulatum, B. dentium, and B. infantis) were not significantly different between the two groups.
Table 6. Changes in fecal bifidobacteria in the GCL2505-supplemented (N=69) and placebo (N=68) groups during the treatment period.
| Treatment period |
Time × group a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 week |
4 weeks |
8 weeks |
12 weeks |
|||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| B. lactis | GCL2505 | 5.43 | 5.29, 5.56 | 8.96## | 8.77, 9.16 | 9.17## | 9.02, 9.32 | 9.33## | 9.16, 9.49 | <0.001 |
| Placebo | 5.48 | 5.31, 5.64 | 5.42 | 5.28, 5.56 | 5.37 | 5.29, 5.45 | 5.31 | 5.30, 5.31 | ||
| Total | GCL2505 | 9.06 | 8.79, 9.33 | 9.60## | 9.50, 9.71 | 9.60## | 9.46, 9.74 | 9.76## | 9.63, 9.88 | <0.001 |
| bifidobacteria b | Placebo | 9.07 | 8.82, 9.31 | 9.14 | 8.89, 9.40 | 9.13 | 8.86, 9.41 | 9.20 | 8.93, 9.47 | |
| Endogenous | GCL2505 | 9.05 | 8.77, 9.32 | 9.08 | 8.83, 9.33 | 8.94 | 8.65, 9.22 | 9.04 | 8.76, 9.32 | 0.271 |
| bifidobacteria c | Placebo | 9.04 | 8.78, 9.29 | 9.13 | 8.88, 9.39 | 9.11 | 8.83, 9.40 | 9.19 | 8.92, 9.47 | |
Values are means and 95% confidence intervals (CIs) of common logarithms of the number of bacteria per 1 g feces.
There was a significant difference between the groups, as determined using an unpaired t-test with Bonferroni correction. #p<0.05; ##p<0.01.
a p-value represented as a group-by-time interaction effect using two-factor repeated-measures ANOVA.
b Total bifidobacteria are expressed as the sum of the counts of nine species (B. bifidum, B. breve, B. longum, B. adolescentis, B. angulatum, B. catenulatum, B. dentium, B. infantis, and B. lactis).
c Endogenous bifidobacteria are expressed as the counts of total bifidobacteria excluding B. lactis.
DISCUSSION
FM containing 1010 cfu of B. lactis GCL2505 was given to healthy overweight or mildly obese Japanese adults, and its effects on the amount of abdominal fat, especially visceral fat, were investigated. The results of this study showed that VFA was significantly decreased in the GCL2505 group compared with the placebo group at 8 and 12 weeks. Abdominal visceral fat accumulation is affected by exercise and food intake [3], but there were no significant differences in the daily number of steps walked or nutrient intake between the GCL2505 and placebo groups during the study period. Therefore, the present results clearly demonstrate that B. lactis GCL2505 consumption decreases VFA. Other anthropometric parameters such as SFA and body weight also did not change significantly, in agreement with a meta-analysis reported by Park et al. [18].
Several studies have shown that excess accumulation of visceral fat rather than increased body weight, BMI, or subcutaneous fat correlates with metabolic disorders [25, 26]. The visceral adipose tissue compartment is described as a unique pathogenic fat depot [6] and as an endocrine organ that secretes various bioactive substances such as adipocytokines that can influence the risk of developing metabolic disorders [27]. Additionally, although Asians including Japanese are less obese, they tend to be more susceptible to metabolic disorders than Europeans and Americans [28]. Japanese men are also reported to have a larger visceral adipose depot at the level of the waist circumference despite substantially less obesity overall [28]. Accordingly, in terms of preventing the development of metabolic disorders in Japanese adults, it is relatively more important to reduce visceral fat rather than body weight or total body fat, and B. lactis GCL2505 may be useful for overweight and mildly obese adults.
Furthermore, the subjects in the present study with a BMI of 23–30 kg/m2 comprised both MS and non-MS subjects, as defined by the guidelines of the Japanese committee organized to establish the definition and diagnostic criteria for MS in Japanese [29]. In analysis stratified by the presence of MS, we also found that B. lactis GCL2505 consumption decreased VFA in non-MS subjects. Therefore, our present results also suggest that B. lactis GCL2505 consumption may help to prevent progression to MS in non-MS individuals, which could reduce the risk of MS-associated disorders such as type-2 diabetes and cardiovascular disease.
Meta-analysis of obesity-associated alterations in the gut microbiota has shown a consistent difference between obese and lean subjects [11]. Gut bifidobacteria could be a potential nutritional and pharmacological target for the prevention of overweight and excess fat storage. This hypothesis has stimulated the performance of several studies investigating the effects of probiotic bifidobacteria on visceral fat in rodents [30,31,32]. For example, administration of B. adolescentis increased gut bifidobacteria, which led to amelioration of visceral fat accumulation and insulin sensitivity in rats [31]. Supplementation with B. breve strain B-3 in mice was also shown to suppress the accumulation of epididymal fat mass through an increase in the number of gut bifidobacteria [32]. Both of these reports demonstrated the efficacy in rodents of supplementation with a specific strain of Bifidobacterium and suggested an association between increased gut bifidobacteria and suppressed fat accumulation. In contrast, there are few clinical studies evaluating the efficacy of probiotic bifidobacteria on visceral fat in humans. Minami et al. reported that consumption of B. breve B-3 reduced fat mass measured by a bioelectrical impedance method in adults but did not reveal a change in visceral adipose tissue [33]. In the present study, we clearly demonstrated the utility of probiotic bifidobacteria to reduce visceral fat in humans, as in previous animal studies [30,31,32].
B. lactis GCL2505 can reach the intestine in a viable form and subsequently proliferate, leading to an increase in the number of gut bifidobacteria [21, 22]. The present study also showed that the total numbers of fecal bifidobacteria (0 week, 9.06 log cells/g feces; 12 weeks, 9.76 log cells/g feces) and fecal B. lactis cells (0 week, 5.43 log cells/g feces; 12 weeks, 9.33 log cells/g feces) were significantly increased in the GCL2505 group. Such significant differences in the total numbers of bifidobacteria and B. lactis cells between the two groups were maintained from 4 weeks to 12 weeks. Previously, we found in a clinical study that B. lactis GCL2505 increased the numbers of fecal bifidobacteria more effectively than non-proliferating bifidobacteria and lactic acid bacterial strains [22]. Because several previous studies have shown that a higher abundance of gut bifidobacteria was associated with suppressed excess accumulation of fat mass [30,31,32], we propose that increasing gut bifidobacteria via ingestion of B. lactis GCL2505 can play an effective role in reducing VFA.
In addition, B. lactis GCL2505 reportedly caused a significant increase of IgA production and mucin secretion in the murine gut when compared with a type strain of a bifidobacterial species (B. longum JCM1217T), and the proliferation level of the administered bifidobacteria in the intestine affected these physiological responses of the host [34]. IgA production and mucin secretion in the gut are associated with effective intestinal barrier function. Cani et al. suggested that oligofructose improved gut barrier function by increasing gut bifidobacteria and lowered plasma lipopolysaccharide levels, which may contribute to decreased adipose mass and the expression of pro-inflammatory markers in adipose tissue [35]. Similarly, the effect of B. lactis 420 on fat mass reduction might be related to an improvement in gut barrier function [30]. Improved gut barrier function might also be a mechanism underlying the reduction in abdominal fat upon supplementation with B. lactis GCL2505. However, no studies have been able to explain fully the underlying association between fat metabolism and gut bifidobacteria, especially in humans. The mechanism of action of B. lactis GCL2505 observed in the present study is also not clear. Further studies are warranted to elucidate how this strain affects fat metabolism and storage.
In conclusion, the present results indicate that consumption of FM containing B. lactis GCL2505 reduces abdominal visceral fat, a key factor associated with metabolic disorders. Therefore, the present findings suggest that a specific strain of B. lactis GCL2505 is safe and could be useful for the reduction of abdominal visceral fat, and they support daily consumption of this proliferative bifidobacteria as a potential way to prevent metabolic disorders in overweight or mildly obese individuals.
Acknowledgments
We are grateful to the subjects for their cooperation and participation in this study. S. Takahashi, D. Anzawa, K. Takami, A. Ishizuka, T. Mawatari, K. Kamikado, and T. Nishijima are employees of Ezaki Glico Co., Ltd., which produces dairy products using B. lactis GCL2505.
References
- 1.Nikolopoulou A, Kadoglou NP. 2012. Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther 10: 933–939. [DOI] [PubMed] [Google Scholar]
- 2.Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, Hagishita T, Mori M, Moriuchi T, Kobayashi M, Katsuda S, Kawashiri MA, Nohara A, Takeda Y, Mabuchi H, Yamagishi M. 2008. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract 79: 474–481. [DOI] [PubMed] [Google Scholar]
- 3.Wajchenberg BL. 2000. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21: 697–738. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KG, Zimmet P, Shaw J. 2006. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469–480. [DOI] [PubMed] [Google Scholar]
- 5.Montague CT, O’Rahilly S. 2000. The perils of portliness: causes and consequences of visceral adiposity. Diabetes 49: 883–888. [DOI] [PubMed] [Google Scholar]
- 6.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB, Sr, O’Donnell CJ. 2007. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48. [DOI] [PubMed] [Google Scholar]
- 7.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuño MI, Queipo-Ortuño M. 2014. Impact of the gut microbiota on the development of obesity and type 2 diabetes mellitus. Front Microbiol 5: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musso G, Gambino R, Cassader M. 2010. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. Curr Opin Lipidol 21: 76–83. [DOI] [PubMed] [Google Scholar]
- 9.Sekirov I, Russell SL, Antunes LC, Finlay BB. 2010. Gut microbiota in health and disease. Physiol Rev 90: 859–904. [DOI] [PubMed] [Google Scholar]
- 10.Musso G, Gambino R, Cassader M. 2010. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care 33: 2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelakis E, Armougom F, Million M, Raoult D. 2012. The relationship between gut microbiota and weight gain in humans. Future Microbiol 7: 91–109. [DOI] [PubMed] [Google Scholar]
- 12.John K, Daniel N, Ruchi M. 2012. Impact of the gut microbiota on the development of obesity: current concepts. Am J Gastroenterol Suppl 1: 22–27. [Google Scholar]
- 13.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18: 190–195. [DOI] [PubMed] [Google Scholar]
- 14.Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. 2011. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7: 639–646. [DOI] [PubMed] [Google Scholar]
- 15.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. 2014. Gut microbiota and metabolic syndrome. World J Gastroenterol 20: 16079–16094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu YH, Lin SL, Tsai JJ, Lin MY. 2014. Probiotic actions on diseases: implications for therapeutic treatments. Food Funct 5: 625–634. [DOI] [PubMed] [Google Scholar]
- 17.Ruan Y, Sun J, He J, Chen F, Chen R, Chen H. 2015. Effect of probiotics on glycemic control: a systematic review and meta-analysis of randomized, controlled trials. PLoS One 10: e0132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S, Bae JH. 2015. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res 35: 566–575. [DOI] [PubMed] [Google Scholar]
- 19.Gill HS, Rutherfurd KJ, Prasad J, Gopal PK. 2000. Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019). Br J Nutr 83: 167–176. [DOI] [PubMed] [Google Scholar]
- 20.Taipale T, Pienihäkkinen K, Isolauri E, Larsen C, Brockmann E, Alanen P, Jokela J, Söderling E. 2011. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr 105: 409–416. [DOI] [PubMed] [Google Scholar]
- 21.Ishizuka A, Tomizuka K, Aoki R, Nishijima T, Saito Y, Inoue R, Ushida K, Mawatari T, Ikeda T. 2012. Effects of administration of Bifidobacterium animalis subsp. lactis GCL2505 on defecation frequency and bifidobacterial microbiota composition in humans. J Biosci Bioeng 113: 587–591. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Takami K, Nishijima T, Aoki R, Mawatari T, Ikeda T. 2015. Short- and long-term dynamics in the intestinal microbiota following ingestion of Bifidobacterium animalis subsp. lactis GCL2505. Biosci Microbiota Food Health 34: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D, CONSORT Group. 2011. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 9: 672–677. [DOI] [PubMed] [Google Scholar]
- 24.Barba C, Cavalli-Sforza T, Cutter J, Darnton-Hill I, Deurenberg P, Deurenberg-Yap M, Gill T, WHO Expert Consultation. 2004. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 25.Ryo M, Kishida K, Nakamura T, Yoshizumi T, Funahashi T, Shimomura I. 2014. Clinical significance of visceral adiposity assessed by computed tomography: a Japanese perspective. World J Radiol 6: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaston TB, Dixon JB. 2008. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes 32: 619–628. [DOI] [PubMed] [Google Scholar]
- 27.Farb MG, Gokce N, Reynolds JV. 2015. Visceral adiposopathy: a vascular perspective. Horm Mol Biol Clin Investig 21: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kadowaki T, Sekikawa A, Murata K, Maegawa H, Takamiya T, Okamura T, El-Saed A, Miyamatsu N, Edmundowicz D, Kita Y, Sutton-Tyrrell K, Kuller LH, Ueshima H. 2006. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes 30: 1163–1165. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzawa Y. 2005. Metabolic syndrome—definition and diagnostic criteria in Japan. J Atheroscler Thromb 12: 301. [DOI] [PubMed] [Google Scholar]
- 30.Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. 2014. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes 5: 437–445. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Wang R, Li XF, Wang RL. 2012. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr 107: 1429–1434. [DOI] [PubMed] [Google Scholar]
- 32.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, Yaeshima T, Iwatsuki K, Kamei A, Abe K. 2010. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem 74: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 33.Minami J, Kondo S, Yanagisawa N, Odamaki T, Xiao JZ, Abe F, Nakajima S, Hamamoto Y, Saitoh S, Shimoda T. 2015. Oral administration of Bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci 4: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoki R, Tsuchida S, Arai Y, Ohno K, Nishijima T, Mawatari T, Mikami Y, Ushida K. 2016. Effect of Bifidobacterium animalis subsp. lactis GCL2505 on the physiological function of intestine in a rat model. Food Sci Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. 2007. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50: 2374–2383. [DOI] [PubMed] [Google Scholar]