Highlights
-
•
Case describes a response to sunitinib in clear cell ovarian cancer.
-
•
Discussion of unique molecular characteristics of clear cell ovarian cancers
-
•
Practical points regarding dosing and toxicity when using sunitinib discussed
Keywords: Targeted therapy, Clear cell, Drug resistance
1. Introduction
Clear cell ovarian cancer (OCCA) is an uncommon subtype of epithelial ovarian cancer accounting for less than 10% of clinical cases in most reported cohorts (Fujiwara et al., 2016). Whereas the precursor cell of the more common high grade serous ovarian (HGS) cancer has been hypothesised to be secretory epithelium of the distal fallopian tube the precursor of clear cell ovarian cancer is less clear. The recent association of clear cell histology (Wentzensen et al., 2016) with previous areas of endometriosis has pointed towards an entirely different cell of origin and indeed molecular characterisation has confirmed this fundamental disparity between HGS and OCCA.
The poor response rates to cytotoxic chemotherapy in the setting of advanced disease in the order of 10–30% (Magazzino et al., 2011) and molecular characteristics of these tumours (Anon, 2013) have prompted investigators to propose treatment of OCCA with sunitinib maleate, a multi-active RTK inhibitor with selectivity for PDGFRa, VEGFR and c-MET, as a potential therapeutic option in this disease (Friedlander et al., 2016). To our knowledge only one previously published report demonstrating activity of sunitinib in clear cell ovarian cancer exists (Anglesio et al., 2011). This report illustrates a response to sunitinib in a patient with parenchymal hepatic deposits and chemotherapy refractory OCCA.
1.1. Case description
A 59 year old patient was discussed at the gynaecologic oncology multi-disciplinary meeting having presented with abdominal distension with radiological features of an ovarian malignancy. A CT scan demonstrated a complex 16 cm × 13 cm mass arising in the pelvis from the right ovary. Her baseline serum tumour marker ca125 was 34 U/ml She had no medical history of note and was previously fit and well. Her ECOG performance status was 1. There was no family history of malignancy.
A primary debulking procedure was carried out including Total abdominal hysterectomy, Bilateral salpingo-oophorectomy, omentectomy and peritoneal stripping, to no residual macroscopic disease. The histopathologic assessment of the tumour revealed a FIGO Stage IIIA2 clear cell ovarian carcinoma by virtue of omental involvement. There was no background of co-existing endometriosis noted. A course of adjuvant chemotherapy with carboplatin and paclitaxel was prescribed but following the first cycle of platinum doublet therapy the patient developed a significant myocardial infarction, and a worsening of her performance status to ECOG P2. Adjuvant treatment was then withheld and the patient was placed on outpatient surveillance.
15 months later she reported increasing lethargy and abdominal distension. A CT scan performed at this time demonstrated a recurrence of her disease with multiple parenchymal liver deposits, the largest measuring 3 cm in diameter, peritoneal deposits and lymphadenopathy at the porta hepatis. A modified retreatment regiment of platinum containing chemotherapy with nitrate cover was utilised. The patient tolerated this well with no further cardiac events. Unfortunately after 2 cycles a re-staging CT demonstrated progression in the size of the nodal disease and hepatic deposits - the largest lesion measuring 5.5 cm in diameter.
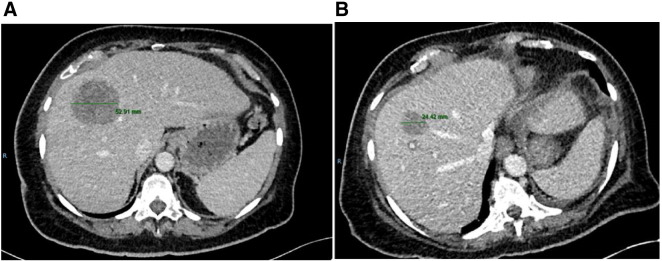
At this point sunitinib was obtained and the patient agreed to self-fund its off license use in the setting of chemotherapy refractory disease. Sunitinib was commenced at a dose of 50 mg od in the intermittent dosing schedule as per the Motzer et al. pivotal study in renal cell carcinoma (Motzer et al., 2007). After four weeks of treatment the main toxicity was grade I-II fatigue. Unfortunately the patient was then admitted with rectal bleeding and the sunitinib withheld. Colonoscopy and Oesophogastric duodenoscopy failed to identify a bleeding focus, and the bleeding settled with conservative management. However given the possible contribution of sunitinib, a decision was made that it should be discontinued. During this admission the patient underwent a re-staging CT which demonstrated a reduction in the size and volume of the multiple hepatic parenchymal metastatic deposits compared to the pre treatment imaging. The largest of these reduced in size from 55–25 mm with a concomitant improvement in symptoms of right upper quadrant discomfort. A representative image is shown in Fig. 1. The patient has now been off treatment and on follow up for 2 months. She has unfortunately since declined in her performance status and no further treatment is planned. She is currently being supported by the community palliative care team.
Fig. 1.
A) Pre treatment CT CAP demonstrating hepatic deposit measuring 55 mm. B) Following 4 weeks of sunitinib this now measures 25 mm.
2. Discussion
To our knowledge this Is only the third report in the literature demonstrating that sunitinib monotherapy can provoke a response in chemotherapy refractory clear cell ovarian cancer. Pre-clinical data has demonstrated distinct molecular characteristics of these tumours, more similar in their somatic mutation burden to clear cell renal carcinoma than the more common high grade serous subtype. Specifically in comparison to more common types of EOC, somatic mutations in p53 are usually absent, with high levels of activation of PI3Kinase pathway signalling, either through inactivation of PTEN or through missense variants causing activation in PIK3CA. Other interesting mutations enriched for in OCCA include inactivation of the chromatin remodelling gene ARID1A which is a member of the SWI/SNF complex, notably also recurrently inactivated in clear cell renal carcinoma (Friedlander et al., 2016, Jones et al., 2010, Rauh-Hain and Penson, 2008, Nature, 2013). There is a clear rationale to target angiogenesis and multiple RTKs given the overexpression of the IL6-HIF-STAT3 pathway in these cancers compared to high grade serous ovarian cancer (Anglesio et al., 2011).
Previous phase II studies of sunitinib in ovarian carcinoma have been undertaken in unselected ovarian cancer histological groups. A phase II study in relapsed epithelial ovarian/fallopian/Primary peritoneal cancer (Biagi et al., 2011) recruited 30 patients. Three patients had a measurable response - all in the platinum sensitive population and all on intermittent rather than continuous lower dose (37.5 mg) dosing regimens. The AGO study group have since evaluated the 50 mg intermittent regimen compared to continuous dosing of 37 mg in a phase 2 study in the platinum resistant setting. 73 patients recruited into the study (Baumann et al., 2012) and in the non-continuous dosing group there were 6 responses compared to 2 responses in the continuous group. Interestingly in renal cell carcinoma the intermittent sunitinib dosing regimen has a confirmed efficacy signal over the continuous regimen (Motzer et al., 2012). One other published study has specifically examined the role of sunitinib in OCCA where the above molecular findings perhaps provide more of a biological justification than in other groups of EOC. In the study reported by Bowtell and colleagues two partial responses to sunitinib were reported in chemotherapy refractory OCCA, both in patients with large volume nodal or visceral disease outside the pelvis. A further study reported the short term response of a single patient to sunitinib in the fifth line setting (Rauh-Hain and Penson, 2008). The eagerly awaited GOG254 sunitinib phase II study, although not published has been presented in abstract form. This demonstrated an overall response rate of 7% in OCCA. Given the pre-clinical data and encouraging reports of responses in chemotherapy refractory patients such as that presented here this is clearly disappointing. One could speculate that a biomarker for a sensitive subgroup exists, but this has not yet been elucidated. It may be that alternative or combination strategies are necessary to improve on the response rates in the GOG254 study and recent data has however demonstrated other avenues that may be clinically relevant to extend this efficacy spectrum of small molecule inhibitors in this disease. The epistatic relationship between the SWI/SNF complex and PIK3CA mutations in OCCA have been shown converge on and drive IL-6 signalling and a pan PI3K inhibitor has shown efficacy in pre-clinical models of OCCA (Chandler et al., 2015). Furthermore a recent paper demonstrated a novel synthetic lethal relationship between inhibition of the EZH2 methyltransferase in ARID1A mutant tumours (Bitler et al., 2015). A strategy to target IL-6 using monoclonal antibodies such as the approved therapy for rheumatoid arthritis Tocilizumab has just entered early stage trials in ovarian cancer (Dijkgraaf et al., 2015) and one could foresee its particular applicability in OCCA given the data presented above.
In terms of practical use of this agent the report presented here also highlights the specific toxicities that treating oncologists should be aware of, specifically the well reported complication of gastro-intestinal bleeding events. In our patient this necessitated stopping the drug despite an impressive response in the burden of hepatic parenchymal metastases. It must be noted that this is an unusual toxicity with the most common adverse events seen being fatigue, diarrhea, nausea and diastolic hypertension (Motzer et al., 2012). It is useful to note that in the pivotal studies in renal cancer the discontinuation rate due to AE's is around 15% with approximately 40% of patients requiring dose adjustment to manage these toxicities.
In summary sunitinib used off license in clear cell histology has demonstrated meaningful and encouraging responses in patients with otherwise chemotherapy refractory disease. This has not however been replicated widely in a recently reported phase II study. In the absence of validated biomarkers to identify responders to sunitinib novel strategies are urgently needed. Early data exists for alternative small molecule inhibitors which may give hope in this population of chemo-refractory patients.
Consent and conflict of interest statements
Informed consent for publication of this case was obtained in writing from the patient prior to publication.
None of the authors has any conflicting interests regarding this manuscript.
References
- Anglesio M.S. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin. Cancer Res. 2011;17(8):2538–2548. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- Baumann K.H. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann. Oncol. 2012;23(9):2265–2271. doi: 10.1093/annonc/mds003. [DOI] [PubMed] [Google Scholar]
- Biagi J.J. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC Clinical Trials Group study. Ann. Oncol. 2011;22(2):335–340. doi: 10.1093/annonc/mdq357. [DOI] [PubMed] [Google Scholar]
- Bitler B.G. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat. Med. 2015;21(3):231–238. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R.L. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nat. Commun. 2015;6:6118. doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf E.M. A phase I trial combining carboplatin/doxorubicin with tocilizumab, an anti-IL-6R monoclonal antibody, and interferon-alpha2b in patients with recurrent epithelial ovarian cancer. Ann. Oncol. 2015;26(10):2141–2149. doi: 10.1093/annonc/mdv309. [DOI] [PubMed] [Google Scholar]
- Friedlander M.L. Molecular profiling of clear cell ovarian cancers: identifying potential treatment targets for clinical trials. Int. J. Gynecol. Cancer. 2016;26(4):648–654. doi: 10.1097/IGC.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Shintani D., Nishikawa T. Clear-cell carcinoma of the ovary. Ann. Oncol. 2016;27(Suppl. 1):i50–i52. doi: 10.1093/annonc/mdw086. [DOI] [PubMed] [Google Scholar]
- Jones S. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazzino F. Surgical and medical treatment of clear cell ovarian cancer: results from the multicenter Italian Trials in Ovarian Cancer (MITO) 9 retrospective study. Int. J. Gynecol. Cancer. 2011;21(6):1063–1070. doi: 10.1097/IGC.0b013e318218f270. [DOI] [PubMed] [Google Scholar]
- Motzer R.J. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- Motzer R.J. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J. Clin. Oncol. 2012;30(12):1371–1377. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;49(7456):43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh-Hain J.A., Penson R.T. Potential benefit of sunitinib in recurrent and refractory ovarian clear cell adenocarcinoma. Int. J. Gynecol. Cancer. 2008;18(5):934–936. doi: 10.1111/j.1525-1438.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- Wentzensen N. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J. Clin. Oncol. 2016 doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]