Abstract
Using quantitative T2* at 7 Tesla (T) magnetic resonance imaging, we investigated whether impairment in selective cognitive functions in multiple sclerosis (MS) can be explained by pathology in specific areas and/or layers of the cortex.
Thirty-one MS patients underwent neuropsychological evaluation, acquisition of 7 T multi-echo T2* gradient-echo sequences, and 3 T anatomical images for cortical surfaces reconstruction. Seventeen age-matched healthy subjects served as controls.
Cortical T2* maps were sampled at various depths throughout the cortex and juxtacortex. Relation between T2*, neuropsychological scores and a cognitive index (CI), calculated from a principal component analysis on the whole battery, was tested by a general linear model.
Cognitive impairment correlated with T2* increase, independently from white matter lesions and cortical thickness, in cortical areas highly relevant for cognition belonging to the default-mode network (p < 0.05 corrected). Dysfunction in different cognitive functions correlated with longer T2* in selective cortical regions, most of which showed longer T2* relative to controls. For most tests, this association was strongest in deeper cortical layers. Executive dysfunction, however, was mainly related with pathology in juxtameningeal cortex. T2* explained up to 20% of the variance of the CI, independently of conventional imaging metrics (adjusted-R2: 52–67%, p < 5.10− 4).
Location of pathology across the cortical width and mantle showed selective correlation with impairment in differing cognitive domains. These findings may guide studies at lower field strength designed to develop surrogate markers of cognitive impairment in MS.
Abbreviations: BVMT, brief visual memory test; BVMT - DR, brief visuo-spatial memory test delayed recall; CI, cognitive index; CVLT, California verbal learning test; DB, digit span backward; DF, digit span forward; DR, delayed recall; EDSS, expanded disability status score; JLOT, judgment of line orientation test; LDCR, long delayed cued recall; LDFR, long delayed free recall; q-T2*, quantitative T2*; MS, multiple sclerosis; MRI, magnetic resonance imaging; NP, neuropsychological; PCA, principal component analysis; SDMT, symbol digit modalities test; TMT, trail making test; TOT, total recall; WCST, Wisconsin card sorting test; WM, white matter; WMLV, white matter lesion volume
Keywords: Multiple sclerosis, Cognitive impairment, Laminar cortical pathology, 7 Tesla MRI, T2*
Highlights
-
•
Cognitive deficit in multiple sclerosis is associated with cortical T2* increase.
-
•
Location of clusters of correlation varies upon affected cognitive domains.
-
•
Global cognitive deficit was associated with T2* increase in deepest cortical layers.
-
•
Executive dysfunction was associated with T2* increase in outer cortical layers.
-
•
Regional T2* explained up to 20% of the variance of cognitive performance in MS.
1. Introduction
Cortical atrophy and cortical lesions are major predictors of cognitive dysfunction in multiple sclerosis (MS), a common clinical manifestation of this disease, affecting deleteriously social and vocational activities (Amato et al., 2004, Benedict et al., 2006, Calabrese et al., 2009, Calabrese et al., 2012, Harrison et al., 2015, Nielsen et al., 2013, Portaccio et al., 2006). Previous studies highlighted that atrophy in selective cortical areas, including frontal regions, precuneus and cingulate cortex is critical for cognition in MS (Louapre et al., 2014, Morgen et al., 2006, Nocentini et al., 2014, Riccitelli et al., 2011, Sbardella et al., 2013). The specificity of cortical atrophy as primary mechanism leading to MS cognitive impairment, however, can hardly be established, as cortical tissue loss may not necessarily result from local pathological processes, but also be the consequence of retrograde axonal degeneration from distant white matter (WM) lesions (Calabrese et al., 2015b).
MRI studies suggested that the location of pathology within the cortical width has a differential impact on cognition in MS, based on the observation that juxta- and leukocortical lesions appeared to better predict neuropsychological (NP) performance than purely intracortical lesions (Harrison et al., 2015, Nelson et al., 2011, Nielsen et al., 2013). The role of subpial demyelination in the development of NP deficits in MS, however, is still uncertain due to the lack of sensitive imaging tools for detecting in vivo subpial cortical lesions.
Surface-based assessment of T2* relaxation rates (q-T2*) at 7 Tesla (T) MRI has been shown to provide a quantitative tool for mapping tissue integrity throughout the entire cortical mantle and cortical depth without being biased by visual detection of cortical lesions (Cohen-Adad et al., 2012). Pathological-MR correlations demonstrated that cortical demyelination and/or iron loss in MS lesions were characterized by longer q-T2* (Yao et al., 2014). In a previous investigation that used T2* cortical mapping at 7 T, we demonstrated in vivo a gradient of cortical pathology across disease stages in association with worsening disability as assessed by MS Severity Score and Expanded Diseases Severity Scale (EDSS) (Mainero et al., 2015). This suggested that a pathological process driven from the pial surface contributes to disease progression.
In the present study, in a heterogeneous MS cohort, we mapped cortical tissue integrity by means of 7 T q-T2* as a function of cortical depth from the juxtameningeal cortical layers towards WM (laminar analysis) to assess: 1) whether impaired NP performance from various cognitive domains including memory, attention, information processing speed and executive function was associated with a preferential distribution pattern of pathology either across the cortical mantle or cortical width, and 2) the predictive value of laminar cortical pathology to explain cognitive impairment independently of cortical atrophy and WM lesions. We hypothesized that the pattern of cortical pathology, both across the whole cortical mantle and cortical width, was dependent upon the affected cognitive domain, as previous studies based on detection of focal cortical lesions showed that leukocortical lesions were more strongly associated with processing speed and memory in MS than subpial lesions (Nielsen et al., 2013).
2. Materials and methods
2.1. Subjects
The Institutional Review Board of our Institution approved all study procedures, and subjects gave written informed consent to participate in the study. Thirty-one patients (21 females; mean age = 42.4 ± 8.7 SD years) were prospectively recruited in the study from May 2010 to May 2013. This cohort is partly overlapping (31 out of 41 patients) with a cohort from a previously published study in which we found cortical areas characterized by longer q-T2* relative to a healthy matched population (Louapre et al., 2016, Louapre et al., 2015, Mainero et al., 2015). The remaining 10 MS subjects were not included due to the lack of NP evaluation at the time of the scanning procedure. Inclusion criteria were: age between 18 and 60 years, diagnosis of clinically isolated syndrome (CIS) or MS, years of education > 8, absence of relapse within 3 months, and corticosteroid therapy within 1 month from study enrollment. Exclusion criteria included significant depression or other psychiatric and/or neurological disease (other than MS), major medical comorbidity, pregnancy, and contraindications for MRI. Seventeen age-matched healthy subjects (9 females, mean age = 39.3 ± 8.8 SD years) were included as controls.
Twenty-six patients were on stable treatment (at least 6 months) with disease modifying therapies. Neurological disability was assessed by certified neurologists (R.P.K., J.A.S., A.S.N.) using the expanded disability status scale (EDSS) (Kurtzke, 1983) within a week from MRI scans.
2.2. Neuropsychological evaluation
All patients were administered a NP battery (performed by N.M.) within a week of MRI scans, evaluating the following domains: 1) attention and information processing speed: symbol digit modalities test (SDMT) and trail making test (TMT) A; 2) verbal and visual memory: California verbal learning test–II (CVLT) and the brief visuospatial memory test – revised (BVMT); 3) executive function: Wisconsin card sorting test-64 card version (WCST) and TMT B; 4) short-term memory: digit span forward (DF) and working memory: digit span backward (DB) 5) visuospatial processing: judgment of line orientation test (JLOT). To get a global measure of cognitive efficiency, we performed a principal component analysis (PCA) on the NP scores matrix and defined as ‘cognitive index’ (CI) the first principal component of the test performance matrix (Hawellek et al., 2011). As CVLT scores were missing for one subject of our cohort, all further results referring to CVLT or CI are based on 30 subjects. CI scores increase with greater cognitive impairment. Factor loading of each test to the first principal component is presented in Supplementary material and Supplementary Table 1.
2.3. MRI data acquisition
Study participants underwent two imaging sessions, within a week, at 7 T and 3 T (Siemens scanners) using 32-channel coils. The 7 T protocol included acquisition of 1) multi-echo 2D FLASH T2*-weighted spoiled gradient-echo (GRE) images with TR = 2210 ms, TE = 6.44 + 3.32n [n = 0, …,11] ms, flip angle = 55°, 2 slabs of 40 slices each to cover the supratentorial brain, resolution = 0.33 × 0.33 × 1 mm3 (25% gap), acquisition time (TA) for each slab = ~ 10 min; 2) a T1-weighted 3D magnetization-prepared rapid acquisition gradient echo sequence (MPRAGE, TR/TI/TE = 2600/1100/3.26 ms, flip angle = 9°, resolution = 0.60 × 0.60 × 1.5 mm3, TA = 5.5 min) for co-registration of 7 T GRE data with cortical surfaces.
Imaging session at 3 T session included a structural scan with 3D magnetization-prepared rapid acquisition with multiple gradient echoes (MEMPR) (TR/TI = 2530/1200 ms, TE = [1.7, 3.6, 5.4, 7.3] ms, resolution = 0.9 × 0.9 × 0.9 mm3, TA = ~ 6.5 min) for cortical surface reconstruction and co-registration with 7 T data.
2.4. MRI data processing
2.4.1. WM lesion volume
WM lesions were segmented on magnitude images from 7 T single-echo FLASH T2* scans with a semi-automated tool in 3D Slicer version 4.2.0 (http://www.slicer.org) (CL, CG, CM, 7, 3 and 15 years of experience in imaging analysis respectively). WM lesion volume (WMLV) was computed using FSL (FMRIB Software Library, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL).
2.4.2. Cortical surface reconstruction
Pial and WM surfaces reconstruction was performed using FreeSurfer, version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/), according to a multi-step procedure that calculates the grey matter (GM)/WM border and the cerebrospinal fluid (CSF)/GM (pial) border in the 3D MEMPR volume (Dale et al., 1999), currently the recommended anatomical sequence for FreeSurfer pipeline (Fischl and Dale, 2000). We applied the FreeSurfer pipeline on 3 T MEMPR sequences since the pipeline for surface reconstruction from 7 T is not optimal due to large B1 inhomogeneities (Fujimoto et al., 2014).
Topological defects in cortical surfaces due to WM and leukocortical lesions were corrected using a semi-automated procedure with lesions inpainting. Mean cortical thickness (CT) (mm) was measured in each subject as previously detailed (Fischl and Dale, 2000).
2.4.3. Quantitative T2* maps
Quantitative-T2* maps were generated from 7 T multi-echo T2* scans in each subject (Cohen-Adad et al., 2012, Govindarajan et al., 2015, Mainero et al., 2015), and registered onto the corresponding 3 T cortical surfaces using a boundary based registration (Greve and Fischl, 2009), two-step procedure as previously detailed (Cohen-Adad et al., 2012, Govindarajan et al., 2015). The registered data were then concatenated into a whole brain volume using FreeSurfer tools and resampled at 0.33 × 0.33 × 0.33 mm3 isotropic voxels. Quantitative-T2* was subsequently sampled at 25%, 50%, and 75% depth from the pial surface (0% depth) towards GM/WM boundary (100% depth) over the entire cortical surface, and at 0.5, 1, 1.5 and 2 mm below WM surface. We previously demonstrated that surface-based mapping of 7 T T2* relaxation rates as a function of cortical depth using the methods reported above is highly reproducible and could prove useful for studying the laminar architecture and pathology of the cerebral cortex in vivo (Govindarajan et al., 2015).
2.5. Statistics
Vertex wise general linear models (GLM) were used in FreeSurfer to test: 1) q-T2* differences between patients and controls; 2) the relationship between q-T2* at each depth and individual NP scores and CI. Prior to surface-based statistics, individual q-T2* surfaces were smoothed using a 5 mm full width at half maximum Gaussian kernel and normalized on the surface template ‘fsaverage’ in FreeSurfer. Age, gender, education, CT (at the vertex level) and WMLV were included as covariates of no interest in GLM analyses. Cortical thickness at the vertex level was considered as covariate of no interest as it was previously demonstrated to correlate with cortical q-T2* in MS patients (Mainero et al., 2015). We applied a cluster-wise correction for multiple comparisons using Monte-Carlo simulation with 10,000 iterations, and corrected p-values < 0.05 were considered statistically significant. Localization of significant clusters across the cortex was performed using the Desikan atlas.
Within clusters of correlation between q-T2* and CI, we estimated using multilinear regression models the additional explained variance provided by q-T2* to predict CI independently of the other covariates of no interest (age, gender, education, WMLV and mean CT of the cluster). Additional explained variance was obtained by subtracting adjusted R-squared of multilinear regression models including q-T2* in independent variables from adjusted R-squared of multilinear regression models without q-T2* in independent variables. We computed the partial correlation coefficient between q-T2* residual and CI residual, corrected for above covariates of no interest. Statistical analyses were performed with R software (version 2.13.1).
3. Results
3.1. Population characteristics
Participants' demographics, clinical, NP, and conventional MRI characteristics are reported in Table 1. About 48% of patients (8 relapsing remitting multiple sclerosis, RRMS, and 7 secondary progressive multiple sclerosis, SPMS) showed impairment in at least one test of the NP battery (Z-score ≤ − 2). Thirty-five percent of the population (4 RRMS, 7 SPMS) had impairment in at least two tests. Most frequently affected domains were information processing speed and executive function. Regional analysis of q-T2* differences between MS and controls disclosed several clusters of longer q-T2* in both RRMS and SPMS (Supplementary Table 2).
Table 1.
Demographics, clinical, imaging, and neuropsychological characteristics of the cohort.
| Demographics and clinical characteristics | |
|---|---|
| Number of patients | 31 |
| Gender (male/female) | 10/21 |
| Age (years) | 42.4 (8.7) |
| Disease duration (years) | 11.9 (8.0) |
| Clinical course (CIS/RRMS/SPMS) | 1/20/10 |
| EDSS (median, range) | 2.5 (1–8) |
| Conventional imaging characteristics | |
|---|---|
| Cortical thickness (mm) | 2.41 (0.12) |
| WM LV (mm3) | 5060 (6414) |
| Neuropsychological performance | ||
|---|---|---|
| Raw score | Z score | |
| Information processing speed | ||
| SDMT | 53 (15) | − 0.6 (1.5) |
| TMT A | 35 (17) | − 0.6 (1.7) |
| Learning-recall ability | ||
| CVLT - TOT | 53 (12.4) | 0.3 (1.4) |
| CVLT - LDFR | 12.1 (3.6) | 0.2 (1.2) |
| CVLT - LDCR | 13 (3.1) | 0.2 (1.1) |
| BVMT - TOT | 25 (7) | − 0.1 (1.3) |
| BVMT - DR | 9.1 (2.8) | − 0.1 (1.5) |
| Executive function | ||
| TMT B | 84 (50) | − 1.7 (3.3) |
| WCST - total errors | 18 (9) | − 0.6 (1) |
| WCST - total perseverations | 10 (5) | − 0.9 (0.8) |
| Working memory | ||
| DF | 8.3 (2.5) | − 0.1 (0.1)a |
| DB | 7 (3.1) | |
| Visuo-spatial function | ||
| JLOT | 12 (2) | − 0.1 (1.1) |
| Number of patients with Z < − 1.5 for at least one test | 19 (1 CIS, 9 RR, 9 SP MS) | |
| Number of patients with Z < − 1.5 for at least two tests | 14 (6 RR, 8 SP MS) | |
| Number of patients with Z < − 2 for at least one test | 15 (8 RR, 7 SP MS) | |
| Number of patients with Z < − 2 for at least two tests | 11 (4 RR, 7 SP MS) | |
Results are expressed as mean (standard deviation), except for EDSS.
EDSS: expanded disability status scale; WM LV: white matter lesion volume; SDMT: symbol digit modalities test; BVMT - TOT: brief visuospatial memory test total recall; BVMT - DR: brief visuospatial memory test delayed recall; TMT: trail making test; DF: digit span forward; DB: digit span backward; JLOT: judgment of line orientation test; WCST: Wisconsin card sorting test; CVLT - TOT: California verbal learning test total recall; LDFR: long delayed free recall; LDCR: long delayed cued recall.
Combined Z-score from the Wechsler Adult Intelligence Scale comprising digit span. Z scores are calculated from normative data of the literature, corrected for age, sex and education as appropriate.
3.2. Regional association between cortical q-T2* and cognitive impairment
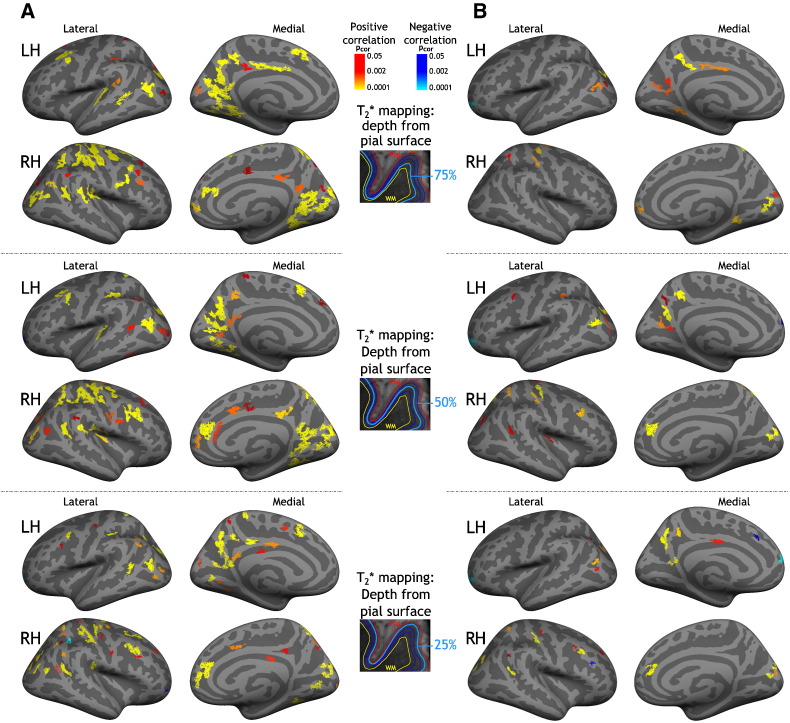
Fig. 1 illustrates results of the GLM laminar analysis exploring the relationship between CI and q-T2* at 25%, 50%, and 75% depth from the pial surface. Including age, gender, education and CT as covariates of no interest, we found, in both hemispheres and for all three depths, clusters of positive correlation between q-T2* (consistent with myelin and iron loss) and CI (reflecting greater cognitive impairment), mainly located in bilateral frontal and prefrontal areas, in bilateral parietal cortex (precuneus, inferior and superior parietal gyri), and in bilateral cingulate cortex (Fig. 1-A). When we included WMLV as covariate of no interest in the GLM, we found focal clusters of positive correlation between q-T2* and CI, mainly in bilateral frontal and prefrontal cortex, left precuneus, right inferior parietal, and left posterior cingulate cortex (Fig. 1-B). An overlap map of clusters of correlation between CI and q-T2* at 25%, 50%, and 75% depth from the pial surface is presented in Supplementary Fig. 1.
Fig. 1.
Areas of correlation between cortical quantitative T2* and cognitive index in multiple sclerosis. Overlay of the general linear model (GLM) significance maps (p < 0.05 corrected for multiple comparisons) showing clusters with a positive (red yellow) or negative (blue) correlation between q-T2* at 25%, 50%, and 75% depth from the pial surface and cognitive impairment as measured by the cognitive index (CI) in MS subjects.
A) Covariates of no interest included in the model were age, gender, education and cortical thickness at the vertex level.
B) Covariates of no interest included in the model were age, gender, education, cortical thickness at the vertex level and white matter lesion volume. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Clusters of correlation between CI and juxta-cortical q-T2* (for all 4 subcortical sampling) were minimal relative to clusters of correlation between CI and intracortical q-T2* (data not shown).
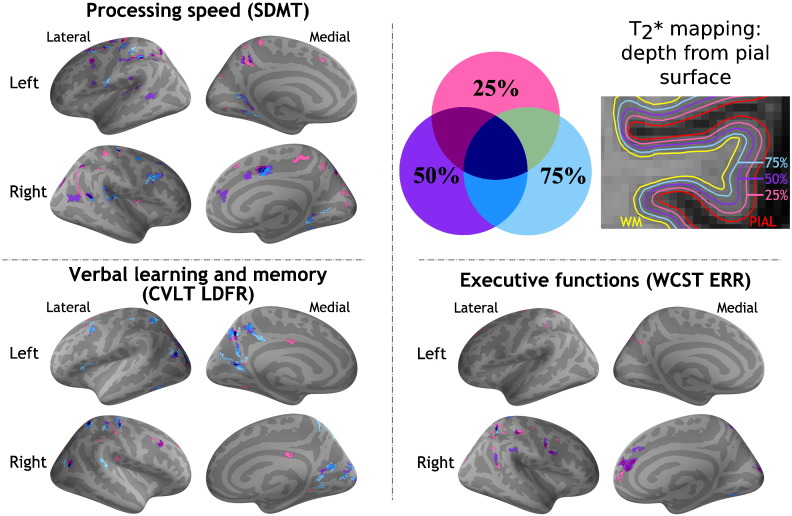
Fig. 2 displays results of the GLM laminar analysis testing the association between q-T2* at 25%, 50%, and 75% depth from the pial surface and individual NP tests' scores in selective cognitive domains: i) processing speed (SDMT); ii) verbal learning and memory (CVLT long delayed free recall); iii) executive function (WCST). Results of GLM analysis exploring the correlation between cortical q-T2* and other individual NP tests' scores are presented in Supplementary Fig. 2. We found scattered clusters of correlation between impaired information processing speed (SDMT, TMT A) and longer q-T2* across the whole cortex at all three depths, including bilateral frontal, parietal and temporal cortex (pcor < 0.0001). Verbal memory impairment (CVLT long delayed free recall, LDFR) was associated with q-T2* increase mainly in bilateral precuneus, right cuneus and left posterior cingulate cortex at 75% depth (pcor < 0.0001). We observed few clusters of correlation between visuospatial memory impairment (BVMT delayed recall, DR) and q-T2*, mainly at 75% depth from the pial surface in bilateral precentral and postcentral gyri, in the right medial prefrontal cortex and in the left precuneus. Location of clusters of correlation between q-T2* and BVMT immediate recall was similar (data not shown). For visuospatial processing, clusters of correlation between q-T2* and JLOT scores were located in the bilateral paracentral and superior frontal gyri, mainly at 75% depth. Executive function impairment (WCST) was associated with increased q-T2* predominantly in the outer cortical layers of bilateral frontal, parietal (pcor < 0.0001) and right medial-prefrontal cortex (pcor < 0.0001). Location of clusters of correlation between NP scores and cortical q-T2* is summarized in Appendix (Supplementary Table 3).
Fig. 2.
Cortical areas showing a significant correlation between quantitative T2* at 7 Tesla and neuropsychological performance at different cognitive domains in subjects with multiple sclerosis. Overlay of the general linear model (GLM) significance maps (p < 0.05 corrected for multiple comparisons) showing clusters with a correlation between q-T2* at 25%, 50%, and 75% depth from the pial surface and neuropsychological tests' scores. A negative correlation was found between cortical q-T2*, SDMT, CVLT LDFR scores (lower performance reflected in lower raw scores). A positive correlation was found between cortical q-T2* and WCST ERR (lower performance reflected in higher raw scores). Age, gender, education, WM lesion volume and cortical thickness at the vertex level were included as covariates of no interest in the model.
SDMT: symbol digit modalities test; CVLT LDFR: California verbal learning test long delayed free recall; WCST ERR: Wisconsin card sorting test total errors; WM: white matter; q-T2*: quantitative T2*.
We did not find a vertexwise correlation between cortical q-T2* and Digit Span test scores, and CVLT total recall.
3.3. Quantitative T2* and cognitive impairment: dependence on cortical depth
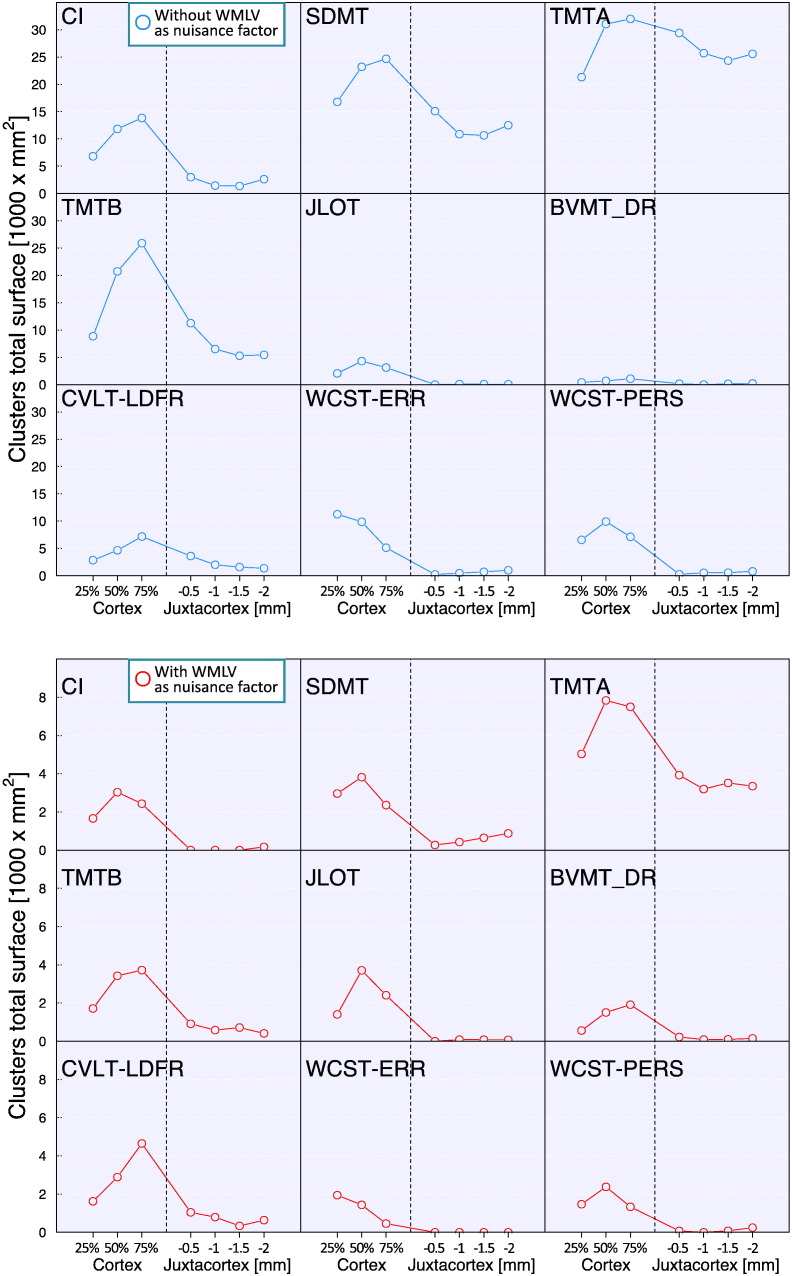
To further explore the cortical depth dependence of the relationship between q-T2* and cognitive impairment, we quantified, for each GLM output, the total surface area of clusters with a significant correlation (clusterwise p < 0.05, corrected for multiple comparisons) between q-T2* and NP tests scores, at 25%, 50%, and 75% depth from the pial surface, and at 0.5, 1, 1.5 and 2 mm below the WM surface (Fig. 3). We found that, for most of the NP tests assessed and CI, the largest surface area of significant clusters was in deep cortical layers (50% and 75% depth from the pial surface). The surface of clusters of correlation between juxta-cortical q-T2* and NP scores was low compared to intracortical q-T2*, except for TMT A.
Fig. 3.
Total surface area of clusters exhibiting a significant correlation between intra- or juxta-cortical quantitative T2* and cognitive results.
Top panel: total surface area of clusters from general linear model (GLM) analyses including age, gender, education and cortical thickness as covariates of no interest.
Bottom panel: total surface of clusters from GLM analyses including age, gender, education, cortical thickness and white matter lesion volume as covariates of no interest.
Dashed vertical lines represent the surface between cortex and white matter.
CI: cognitive index; SDMT: symbol digit modalities test; CVLT LDFR: California verbal learning test long delayed free recall; BVMT - DR: brief visuospatial memory test delayed recall; JLOT: judgment of line orientation test; TMT: trail making test; WCST ERR: Wisconsin card sorting test total errors; WCST PERS: Wisconsin card sorting test total perseverations.
Unlike the rest of the NP battery, the largest clusters of correlation between executive dysfunction (WCST, total number of errors) and q-T2* were located in the outer cortical layers at 25% depth from the pial surface.
3.4. Predictive value of cortical q-T2* for cognitive performance
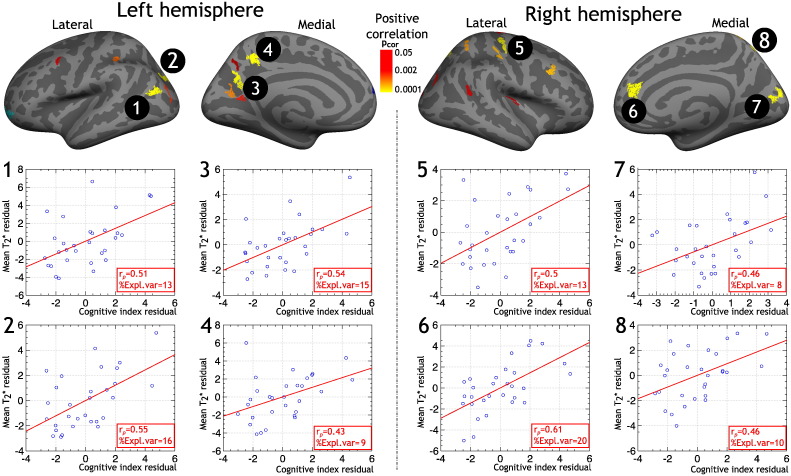
To evaluate the additional explained variance of cortical q-T2* in predicting NP in our MS cohort, independently of demographics and conventional MRI metrics, we specifically analyzed the GLM results of the correlation between CI and q-T2* at 50% depth from the pial surface. We chose to use 50% depth because the total surface of clusters of correlation between q-T2* and CI was the largest at this depth (Fig. 3, bottom panel). For each of the eight clusters exhibiting the lowest clusterwise corrected p-value (< 0.0001), we computed mean CT and mean q-T2* and performed multilinear regression models using CI as dependent variable, and age, gender, education, CT, WMLV and q-T2* as independent variables. The total explained variance (adjusted R-squared) of CI ranged from 52% to 67% (p < 5.10− 4). Adjusted variable plots of partial correlation between CI and q-T2* are presented in Fig. 4. For the eight clusters, q-T2* explained from 8% up to 20% of the variance of CI (depending on the cluster). For reference, mean q-T2* within these clusters in controls and MS subjects is presented in Table 2.
Fig. 4.
Adjusted variable plots of partial correlation between cognitive index (CI) and cortical quantitative T2* at 50% depth from the pial surface in clusters from general linear model (GLM) exhibiting a statistical significance (p < 0.0001). Covariates of no interest included in the model were age, gender, education, cortical thickness and white matter lesion volume. For each cluster are displayed: 1) the partial correlation coefficient (rp) between CI residual and mean T2* residual; 2) the percentage of CI variance explained by T2* (%Expl.var) in addition to the other predictors.
Location of clusters: 1. inferior parietal; 2. superior parietal; 3. precuneus; 4. precuneus; 5. precentral; 6. superior frontal; 7. cuneus; 8. superior parietal.
Table 2.
Mean q-T2* measurements in controls and patients with MS, extracted from clusters exhibiting a statistically significant correlation (p < 0.0001) between cognitive index (CI) and cortical quantitative T2* at 50% depth from the pial surface in the MS cohort.
| # cluster | Controls | All MS | CIS-RRMS | SPMS |
|---|---|---|---|---|
| 1 | 32.99 (2.47) | 33.13 (3.04) | 32.5 (2.94) | 34.61 (2.89) |
| 2 | 30.13 (1.57) | 30.52 (2.45) | 30.1 (2.51) | 31.5 (2.12)⁎ |
| 3 | 31.64 (2.04) | 32.47 (2.09) | 32.12 (1.96) | 33.24 (2.3)⁎ |
| 4 | 34.41 (2.38) | 34.99 (2.75) | 34.66 (2.95) | 35.75 (2.17)⁎ |
| 5 | 27.8 (1.76) | 28.39 (2.4) | 28.71 (2.2) | 29.96 (2.22)⁎ |
| 6 | 39.48 (2.54) | 40.54 (2.51) | 39.97 (2.58) | 41.88 (1.81)⁎⁎ |
| 7 | 28.67 (1.49) | 29.57 (2.83) | 28.7 (2.26) | 31.61 (3.11)⁎⁎ |
| 8 | 32.07 (2.18) | 32.57 (2.02) | 32.15 (2.12) | 33.57 (1.38)⁎ |
Cluster number refers to Fig. 4.
All q-T2* measurements in bold font are statistically different in SPMS compared to healthy controls (by linear regression including age as a covariate of no interest).
MS = multiple sclerosis; CIS = clinically isolated syndrome; RRMS = relapsing-remitting MS; SPMS = secondary-progressive MS.
p < 0.05.
p < 0.005.
4. Discussion
Our results demonstrate that cortical q-T2* is related to cognitive impairment in MS, independently of WM lesions and cortical atrophy, explaining up to 20% of the variance of cognitive performance. Moreover, regional q-T2* increase mainly in the deepest layers in key cortical regions is associated with cognitive impairment in MS in selective cognitive domains.
The pattern of cognitive impairment observed in our MS cohort was similar to previous reports (Chiaravalloti and DeLuca, 2008, Rocca et al., 2015). Using a global measure of cognitive impairment through PCA, a dimensionality reduction technique, we found a strong correlation between overall cognitive dysfunction and longer q-T2* in several cortical areas that strikingly overlap with regions of the default-mode network (DMN), known to be highly critical for attention and memory (precuneus/posterior cingulate cortex that forms a major hub, medial prefrontal cortex, medial temporal lobe, lateral and inferior parietal cortex). Although most of the clusters of correlation between q-T2* and cognitive impairment were bilaterally located across the cortex, some areas, including the sensorimotor cortex, were more represented in the right hemisphere in our cohort, which may reflect the asymmetry of cortical pathology in our cohort. Previous MRI studies reported resting-state functional connectivity changes or volume decrease associated with MS cognitive impairment in selective cortical DMN areas (Cruz-Gomez et al., 2014, Hawellek et al., 2011, Louapre et al., 2014, Rocca et al., 2010).
Interestingly, by mapping intracortical q-T2* at 7 T on a larger MS cohort that partly overlaps with that of the present study, we previously found that physical disability (EDSS) was associated with intracortical laminar pathology in cortical areas linked to sensorimotor function, mainly the precentral and postcentral gyri (Mainero et al., 2015). These findings highlight that the patterns of correlation between CI and cortical q-T2* was spatially specific, thereby suggesting that it does not merely reflect global disease severity.
We found that the pattern of cortical pathology also reflects impairment in specific cognitive domains. SDMT dysfunction was associated with cortical pathology disseminated across the entire cortical surface, which is in line with functional MRI experiments showing large areas of activation during SDMT execution (Forn et al., 2011). Learning and recall abilities, assessed by CVLT and BVMT, were associated with longer q-T2* in medial and dorsolateral prefrontal cortex, and in precuneus and posterior cingulate cortex. A previous study demonstrated that lesions in posterior medial frontal regions or in the left posterior dorsolateral frontal cortex can determine severe impairment in immediate free recall (Alexander et al., 2003), while another study found a positive correlation between cingulate cortex glutamate levels and visual memory performance in RRMS cases (Muhlert et al., 2014). Visuo-spatial processing is known to rely on parietal (predominantly right) and occipital integrity. We found scattered clusters of correlation between q-T2* and JLOT score in parietal and occipital cortex, however, their prominent location was in the paracentral and medial superior frontal gyri. This location was also relevant for TMT impairment. Interestingly, this cluster mainly co-localizes with supplementary and pre-supplementary motor areas, whose functions, although complex, include spatial attention (Nachev et al., 2008). Moreover, the supplementary eye field, part of the supplementary motor complex, is critical for visual saccades. Pathology in this area could impact performance for visual scanning of several targets as during the TMT. Executive dysfunction affects predominantly later stages and/or progressive forms (Brissart et al., 2013, Calabrese et al., 2015a, Ruet et al., 2013). In our cohort, lower WCST scores correlated with longer q-T2* in the right medial prefrontal cortex, and in parietal areas including precuneus.
In this study we also characterized the relationship between cognitive impairment and q-T2* as a function of cortical depth. We previously demonstrated, in another MS cohort, that, among cortical and subcortical imaging metrics, focal leukocortical lesions detected at 7 T, which extend across deepest cortical layers and subcortical WM, exhibited the greatest explanatory power for predicting impaired performance in different cognitive domains (Nielsen et al., 2013). These findings have been subsequently confirmed in a different MS cohort (Harrison et al., 2015). Histopathological-MR correlative examinations showed, however, that a variable proportion of cortical lesions go undetected at visual examinations of MRI scans, even at ultra high field MRI (Kilsdonk et al., 2016, Pitt et al., 2010). The ability to map in vivo cortical structural integrity as function of cortical depth without being biased by visual detection of cortical lesions could allow us to better understand which patterns of cortical pathology, including those readily detectable at lower field strength, can better explain and predict disease worsening and cognitive dysfunction in MS. We found that most of the cortical clusters of correlation between q-T2* and NP tests scores were localized at 75% depth, except for executive dysfunction, which was associated with cortical pathology mainly located in the outer cortical layers (25% and 50% depth from the pial surface). It is possible that pathology in deepest cortical layers has the greatest impact on those cognitive functions that rely on integrity of large scale cortico-subcortical and cortico-cortical circuits. Executive dysfunction may be associated with the presence of focal intracortical or subpial lesions, or a more diffuse degenerative process origination from outer cortical layers, which could also explain why executive impairment is characteristic of late and/or progressive MS course. Interestingly, in a previous study, we found that neurological disability and disease severity, which are prominent in the progressive stages of the disease, were also associated with pathology located predominantly in the outer cortical layers of the sensorimotor cortex (Mainero et al., 2015).
Some limitations apply to our study. Our findings are based on cross-sectional observations; longitudinal studies are needed to provide a more definite evaluation on the temporal relationship between cortical degeneration as measured by q-T2* and cognitive worsening in MS. Confirmatory studies are also needed to clarify how myelin and iron content independently contribute to q-T2* changes in MS (Yao et al., 2014), both inside and outside focal cortical lesions. Other quantitative contrasts could be combined with q-T2* to increase pathological specificity to cortical pathology (Mangeat et al., 2015).
Future works investigating the physiopathology of multiple sclerosis cognitive impairment using 7 T MRI could also include GM regions outside the cortex, including thalamus, hippocampus and cerebellum.
Funding
This work was supported by a grant of the National MS Society (NMSS 4281-RG-A1 and NMSS RG 4729A2/1), the Claflin Award, and partly by NIH R01NS078322-01-A1, US Army W81XWH-13-1-0122, Shared Instrumentation Grant 1S10RR023043, National Center for Research Resources (NCRR P41-RR14075).
Disclosures
Dr. Louapre was supported by a fellowship from ARSEP foundation.
Mrs. Govindarajan reports no disclosure.
Dr. Gianni' was supported by FISM training fellowship 2012/B/4.
Dr. Madigan reports no disclosure.
Dr. Nielsen reports no disclosure.
Dr. Sloane reports no disclosure.
Dr. Kinkel reports personal fees from Genzyme; a Sanofi Corp., from Biogen Idec, from Novartis, and grants from Accelerated Cure Project, outside the present work.
Dr. Mainero reports no disclosures.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.11.001.
Contributor Information
Céline Louapre, Email: celine.louapre@gmail.com.
Sindhuja T. Govindarajan, Email: sindhuja.tirumalaigovindaraj@stonybrook.edu.
Costanza Giannì, Email: costanza.gia@gmail.com.
Nancy Madigan, Email: nmadigan@bidmc.harvard.edu.
A. Scott Nielsen, Email: a.scott.nielsen@gmail.com.
Jacob A. Sloane, Email: jsloane@bidmc.harvard.edu.
Revere P. Kinkel, Email: rkinkel@ucsd.edu.
Caterina Mainero, Email: caterina@nmr.mgh.harvard.edu.
Appendix A. Supplementary data
Supplementary material
References
- Alexander M.P., Stuss D.T., Fansabedian N. California verbal learning test: performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–1503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- Amato M.P., Bartolozzi M.L., Zipoli V., Portaccio E., Mortilla M., Guidi L., Siracusa G., Sorbi S., Federico A., De Stefano N. Neocortical volume decrease in relapsing-remitting MS patients with mild cognitive impairment. Neurology. 2004;63:89–93. doi: 10.1212/01.wnl.0000129544.79539.d5. [DOI] [PubMed] [Google Scholar]
- Benedict R.H., Bruce J.M., Dwyer M.G., Abdelrahman N., Hussein S., Weinstock-Guttman B., Garg N., Munschauer F., Zivadinov R. Neocortical atrophy, third ventricular width, and cognitive dysfunction in multiple sclerosis. Arch. Neurol. 2006;63:1301–1306. doi: 10.1001/archneur.63.9.1301. [DOI] [PubMed] [Google Scholar]
- Brissart H., Morele E., Baumann C., Perf M.L., Leininger M., Taillemite L., Dillier C., Pittion S., Spitz E., Debouverie M. Cognitive impairment among different clinical courses of multiple sclerosis. Neurol. Res. 2013;35:867–872. doi: 10.1179/1743132813Y.0000000232. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Agosta F., Rinaldi F., Mattisi I., Grossi P., Favaretto A., Atzori M., Bernardi V., Barachino L., Rinaldi L., Perini P., Gallo P., Filippi M. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch. Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Poretto V., Favaretto A., Alessio S., Bernardi V., Romualdi C., Rinaldi F., Perini P., Gallo P. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135:2952–2961. doi: 10.1093/brain/aws246. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Gajofatto A., Gobbin F., Turri G., Richelli S., Matinella A., Oliboni E.S., Benedetti M.D., Monaco S. Late-onset multiple sclerosis presenting with cognitive dysfunction and severe cortical/infratentorial atrophy. Mult. Scler. 2015;21:580–589. doi: 10.1177/1352458514542363. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Magliozzi R., Ciccarelli O., Geurts J.J., Reynolds R., Martin R. Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 2015;16:147–158. doi: 10.1038/nrn3900. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N.D., DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J., Polimeni J.R., Helmer K.G., Benner T., McNab J.A., Wald L.L., Rosen B.R., Mainero C. T(2)* mapping and B(0) orientation-dependence at 7 T reveal cyto- and myeloarchitecture organization of the human cortex. NeuroImage. 2012;60:1006–1014. doi: 10.1016/j.neuroimage.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Gomez A.J., Ventura-Campos N., Belenguer A., Avila C., Forn C. The link between resting-state functional connectivity and cognition in MS patients. Mult. Scler. 2014;20:338–348. doi: 10.1177/1352458513495584. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forn C., Belenguer A., Belloch V., Sanjuan A., Parcet M.A., Avila C. Anatomical and functional differences between the paced auditory serial addition test and the symbol digit modalities test. J. Clin. Exp. Neuropsychol. 2011;33:42–50. doi: 10.1080/13803395.2010.481620. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Polimeni J.R., van der Kouwe A.J., Reuter M., Kober T., Benner T., Fischl B., Wald L.L. Quantitative comparison of cortical surface reconstructions from MP2RAGE and multi-echo MPRAGE data at 3 and 7 T. NeuroImage. 2014;90:60–73. doi: 10.1016/j.neuroimage.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan S.T., Cohen-Adad J., Sormani M.P., Fan A.P., Louapre C., Mainero C. Reproducibility of T2* mapping in the human cerebral cortex in vivo at 7 tesla MRI. J. Magn. Reson. Imaging. 2015;42:290–296. doi: 10.1002/jmri.24789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.M., Roy S., Oh J., Izbudak I., Pham D., Courtney S., Caffo B., Jones C.K., van Zijl P., Calabresi P.A. Association of cortical lesion burden on 7-T magnetic resonance imaging with cognition and disability in multiple sclerosis. JAMA Neurol. 2015;72:1004–1012. doi: 10.1001/jamaneurol.2015.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawellek D.J., Hipp J.F., Lewis C.M., Corbetta M., Engel A.K. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19066–19071. doi: 10.1073/pnas.1110024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilsdonk I.D., Jonkman L.E., Klaver R., van Veluw S.J., Zwanenburg J.J., Kuijer J.P., Pouwels P.J., Twisk J.W., Wattjes M.P., Luijten P.R., Barkhof F., Geurts J.J. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016;139:1472–1481. doi: 10.1093/brain/aww037. [DOI] [PubMed] [Google Scholar]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Louapre C., Perlbarg V., Garcia-Lorenzo D., Urbanski M., Benali H., Assouad R., Galanaud D., Freeman L., Bodini B., Papeix C., Tourbah A., Lubetzki C., Lehericy S., Stankoff B. Brain networks disconnection in early multiple sclerosis cognitive deficits: an anatomofunctional study. Hum. Brain Mapp. 2014;35:4706–4717. doi: 10.1002/hbm.22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Govindarajan S.T., Gianni C., Langkammer C., Sloane J.A., Kinkel R.P., Mainero C. Beyond focal cortical lesions in MS: an in vivo quantitative and spatial imaging study at 7 T. Neurology. 2015;85:1702–1709. doi: 10.1212/WNL.0000000000002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louapre C., Govindarajan S.T., Gianni C., Cohen-Adad J., Gregory M.D., Nielsen A.S., Madigan N., Sloane J.A., Kinkel R.P., Mainero C. Is the relationship between cortical and white matter pathologic changes in multiple sclerosis spatially specific? A multimodal 7-T and 3-T MR imaging study with surface and tract-based analysis. Radiology. 2016;278:524–535. doi: 10.1148/radiol.2015150486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainero C., Louapre C., Govindarajan S.T., Gianni C., Nielsen A.S., Cohen-Adad J., Sloane J., Kinkel R.P. A gradient in cortical pathology in multiple sclerosis by in vivo quantitative 7 T imaging. Brain. 2015;138:932–945. doi: 10.1093/brain/awv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat G., Govindarajan S.T., Mainero C., Cohen-Adad J. Multivariate combination of magnetization transfer, T* and B0 orientation to study the myelo-architecture of the in vivo human cortex. NeuroImage. 2015;119:89–102. doi: 10.1016/j.neuroimage.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgen K., Sammer G., Courtney S.M., Wolters T., Melchior H., Blecker C.R., Oschmann P., Kaps M., Vaitl D. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. NeuroImage. 2006;30:891–898. doi: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Muhlert N., Atzori M., De Vita E., Thomas D.L., Samson R.S., Wheeler-Kingshott C.A., Geurts J.J., Miller D.H., Thompson A.J., Ciccarelli O. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J. Neurol. Neurosurg. Psychiatry. 2014;85:833–839. doi: 10.1136/jnnp-2013-306662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nelson F., Datta S., Garcia N., Rozario N.L., Perez F., Cutter G., Narayana P.A., Wolinsky J.S. Intracortical lesions by 3 T magnetic resonance imaging and correlation with cognitive impairment in multiple sclerosis. Mult. Scler. 2011;17:1122–1129. doi: 10.1177/1352458511405561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen A.S., Kinkel R.P., Madigan N., Tinelli E., Benner T., Mainero C. Contribution of cortical lesion subtypes at 7 T MRI to physical and cognitive performance in MS. Neurology. 2013;81:641–649. doi: 10.1212/WNL.0b013e3182a08ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini U., Bozzali M., Spano B., Cercignani M., Serra L., Basile B., Mannu R., Caltagirone C., De Luca J. Exploration of the relationships between regional grey matter atrophy and cognition in multiple sclerosis. Brain Imaging Behav. 2014;8:378–386. doi: 10.1007/s11682-012-9170-7. [DOI] [PubMed] [Google Scholar]
- Pitt D., Boster A., Pei W., Wohleb E., Jasne A., Zachariah C.R., Rammohan K., Knopp M.V., Schmalbrock P. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch. Neurol. 2010;67:812–818. doi: 10.1001/archneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- Portaccio E., Amato M.P., Bartolozzi M.L., Zipoli V., Mortilla M., Guidi L., Siracusa G., Sorbi S., Federico A., De Stefano N. Neocortical volume decrease in relapsing-remitting multiple sclerosis with mild cognitive impairment. J. Neurol. Sci. 2006;245:195–199. doi: 10.1016/j.jns.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Riccitelli G., Rocca M.A., Pagani E., Rodegher M.E., Rossi P., Falini A., Comi G., Filippi M. Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum. Brain Mapp. 2011;32:1535–1543. doi: 10.1002/hbm.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Amato M.P., De Stefano N., Enzinger C., Geurts J.J., Penner I.K., Rovira A., Sumowski J.F., Valsasina P., Filippi M., Group M.S. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14:302–317. doi: 10.1016/S1474-4422(14)70250-9. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Absinta M., Riccitelli G., Rodegher M.E., Misci P., Rossi P., Falini A., Comi G., Filippi M. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- Ruet A., Deloire M., Charre-Morin J., Hamel D., Brochet B. Cognitive impairment differs between primary progressive and relapsing-remitting MS. Neurology. 2013;80:1501–1508. doi: 10.1212/WNL.0b013e31828cf82f. [DOI] [PubMed] [Google Scholar]
- Sbardella E., Petsas N., Tona F., Prosperini L., Raz E., Pace G., Pozzilli C., Pantano P. Assessing the correlation between grey and white matter damage with motor and cognitive impairment in multiple sclerosis patients. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao B., Hametner S., van Gelderen P., Merkle H., Chen C., Lassmann H., Duyn J.H., Bagnato F. 7 tesla magnetic resonance imaging to detect cortical pathology in multiple sclerosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material