Abstract
Diabetes mellitus affects millions of people worldwide, and is associated with serious complications that affect nearly all body systems. Because of the severity of this global health concern, there is a great deal of research being performed on alternative treatments and possible cures. Previous treatments for diabetes have included exogenous insulin injection and pancreatic islet transplantations. These treatment methods have several limitations; thus, the use of stem cells in treating diabetes is currently a significant area of research. This review outlines current research on stem cell therapy for diabetes mellitus. Numerous studies have been performed on animals using various types of stem cells, including mesenchymal stem cells and embryonic stem cells. Moreover, results and limitations of animal studies have been confirmed in various clinical trials. Overall, stem cell treatment shows prospective advantages over insulin injections and other current treatment options, and ongoing clinical trials suggest that this therapy may be a viable treatment option for diabetics in the near future.
Keywords: Diabetes, adult stem cells, human trials, spermatogonial stem cells, IPS cells, embryonic stem cells
Introduction
Diabetes mellitus is a group of glucose metabolism disorders characterized by high levels of blood glucose. The disease affects nearly 350 million people worldwide; this number is constantly increasing, and is expected to grow tremendously in the future. Various categories of diabetes exist: type 1 diabetes is an autoimmune disease in which the body’s immune cells attack and damage insulin-producing β cells in the islets of Langerhans of the pancreas, resulting in insulin secretion deficiency. Type 2 diabetes is characterized by insulin resistance at the receptor level and by hyperinsulinemia. Many obese patients are at risk for type 2 diabetes, as massive secretions of insulin not only increase insulin resistance, but also impair the β cell’s insulin-secreting function [1,2].
The long-term hyperglycemia associated with diabetes has severe implications, including blood vessel and nervous system damage, vision complications, cardiovascular disease, and infection [3,4]. Considering the serious complications and enormous associated costs of diabetes, researchers are investigating possible cures [5]. Stem cells are able to replace damaged cells in the body; therefore, they offer a promising treatment to replace the non-functional insulin-producing β cells of the pancreas [2,6]. The purpose of this review is to further explore the current progress of stem cell- derived β cells as a treatment and cure for diabetes mellitus.
Though exogenous insulin therapy, the current treatment for hyperglycemia in diabetics allows patients a degree of control over their blood sugar levels, many diabetics experience the numerous complications of protein glycosylation due to chronically elevated glucose levels. Constant control over insulin administration is critical, as insulin overdose causes hypoglycemia and coma in severe cases, while insulin insufficiency leads to the damaging effects of hyperglycemia in essentially every system of the body. It is clear, therefore, that this standard therapy for diabetics fails to mimic the insulin secretion of healthy β cells. Hence, exogenous insulin is life saving, but not curative [5,7].
For these reasons, many researchers focus their attention on alternative therapies to cure diabetic patients of their insulin deficiency. One potential therapy for patients with type 1 diabetes is infusion of donor islets of Langerhans into the hepatic portal vein. In this procedure, islets containing the insulin-producing β cells are transplanted from a cadaver’s pancreas to the patient. While this method has freed some type 1 diabetics from insulin injection, the donor cells vary in quality and yield results of varying success. Moreover, it is evident that the limited supply of donor islets simply cannot keep up with the increasing number of diabetics in today’s population [8,9]. Because of these limitations, researchers have started to focus on other therapies.
An alternative procedure that has yielded promising results is stem cell therapy, the focus of this discussion. Because pluripotent stem cells represent an unlimited supply of β cells, they are an ideal source for regenerative medicine [2]. Both embryonic stem cells and adult mesenchymal stem cells are being studied for their capacity to differentiate into β cells.
Embryonic stem cells are pluripotent cells that can differentiate into all cell types of the three germ layers and can be isolated from the preimplantation embryo. To promote differentiation into β cells, numerous laboratories have developed protocols that attempt to mimic signaling during embryonic pancreas development. By adding growth factors or inhibitors at times comparable to events occurring in vivo, one can guide differentiation into β cells [10,11]. Mesenchymal (adult) stem cells can also be differentiated into β cells via similar techniques, and they are being investigated due to their easier access and less controversial nature [12]. Additional adult stem cells utilized for diabetes treatment include intrapancreatic autologous bone marrow stem cells, cord blood-derived multipotent stem cells from the umbilical cord, and hematopoietic stem cells found in red bone marrow [13-15].
Various animal and human trials have been conducted with stem cell treatment for diabetics. To assess the effectiveness of treatment, the presence of C-peptide is measured in patients undergoing stem cell treatment. C-peptide is a byproduct of endogenous insulin formation, and its presence in the body indicates insulin secretion from the newly differentiated β cells. Hemoglobin A1c (glycosylated hemoglobin) levels are also used to assess average blood glucose levels over a period of about three months; lower values indicate better blood glucose control [16,17].
Mesenchymal stem cell transplantation
Numerous animal studies have shown that mesenchymal stem cell (MSC) transplantation can be a successful method to treat hyperglycemia associated with type 1 diabetes. Mesenchymal stem cells are multipotent cells that can differentiate into a variety of cell types, and are found in nearly all organs and tissues [18-20]. Because of these properties, as well as the ability to easily retrieve these cells from bone marrow, MSCs are commonly used in tissue repair for a variety of conditions. A study [21] performed in 2014 tested effects of MSC treatment on diabetic rats. Male Wistar rats were divided into five groups: normal control, diabetic control, MSC-treated, supernatant-treated, and MSC- and supernatant-treated. The supernatant in this experiment came from the MSCs that were transplanted in some of the rats. Over the course of several weeks, the MSC- treated diabetic rats had reduced blood glucose levels and higher insulin levels compared to the diabetic control group [21]. Supernatant-treated rats also had reduced blood glucose levels, but to a lower extent. The greatest improvement in glucose and insulin levels was seen in the MSC- and supernatant-treated rats. Immunohistochemical analysis of the pancreatic tissues of the rats revealed that MSC-treated, supernatant-treated, and MSC- and supernatant-treated rats had partially regenerated pancreatic tissues; new and larger islets of Langerhans were seen [21].
The results of the study and the immunohistochemical analysis suggested that the MSCs differentiated into insulin-producing β cells. Thus, the rats treated with MSCs saw a reduction in their blood glucose and an increase in insulin levels. The study also showed that MSCs secreted growth factors, cytokines, and chemokines present in the supernatant could contribute to pancreas cell healing and regeneration [21]; however, there were several limitations to this study and its applicability to treatment of diabetes in humans using MSCs. Firstly, the study only involved male rats; there may be known or unknown sex differences that could lead to different effects in females. Secondly, the study found that a single injection of MSCs was less effective than multiple injections. If stem cell therapy for diabetes were implemented for humans, it may be burdensome and costly to implement a treatment with multiple, ongoing injections. It is also worth noting that during this study, the diabetic control group rats on average lost weight, while the MSC-treated rats on average gained weight. This may be because the MSC-treated rats had better glucose uptake as the stem cells differentiated into insulin-producing cells [21]. This weight gain, when applied to humans, may not be favorable for type 2 diabetes treatment. Type 2 diabetes is largely tied to obesity, and weight gain would not be a favorable outcome of stem cell therapy. However, the weight gain exhibited in this study would be beneficial for newly diagnosed type 1 diabetics who have lost weight due to the cell’s inability to uptake glucose.
In order to induce MSC differentiation into insulin-producing cells in vitro, one laboratory incubated human MSCs with a series of solutions containing transcription factors and signal molecules such as β-fibroblast growth factor, epidermal growth factor, β cellulin, and activin A. The study found that the MSCs differentiated into insulin-producing cells, shown by the presence of proinsulin C-peptide in the solution containing the cells. Furthermore, increasing the concentration of growth factors added to the MSCs increased the percentage of MSCs that differentiated into insulin-producing cells. Many of the cells also migrated and formed spherical islet-like clusters [16].
The above study tested what is referred to as a 2D culture. Another study found that 3D culture may be even more effective at creating insulin-producing cells that closely mimic endogenous β cells. A 3D culture more closely resembles the in vivo environment of β cells by facilitating cell-cell and cell-matrix interactions [22]. A 3D culture was created with fibrin glue, a fibrous protein made of fibrinogen and thrombin and that is involved in blood clotting. The fibrin glue created a scaffold-matrix in which MSCs could differentiate and proliferate. The study had a control group with MSCs placed in serum without any fibrin glue - this was the 2D culture. Both groups received IPC-differentiating factors [22].
By examining the cells using a scanning electron microscopes, researchers found that the MSCs in the 3D culture differentiated into IPCs at a faster rate; the fibrin glue scaffolding created pores along which the MSCs formed continuous sheets. In addition, the MSCs that differentiated into IPCs in the 3D culture were round and more closely resembled β cells, while the MSCs that differentiated into IPCs in the 2D culture were flat and elongated. After transplanting the IPCs from the 3D culture and 2D culture into male Wistar rats, the IPCs from the 3D culture were better able to normalize blood glucose [22].
Another research group also illustrated the ability of MSCs to improve the condition of diabetes by studying the effects of MSCs from bone marrow of albino rats with Alloxan-induced type 1 diabetes. Alloxan results in a significant increase in serum glucose, total cholesterol, triglyceride, and a significant decrease in serum insulin. The study illustrates the ability of rat bone marrow cells to differentiate into functional insulin-producing cells capable of controlling hyperglycemia. Four groups were set up: 7 normal rats injected with saline (control), 7 diabetic rats without treatment, 7 diabetic rats injected with MSCs, and 7 diabetic injected with insulin. After 15 days of MSC injection, fasting blood samples were collected and glucose and serum insulin values were measured. The results of MSCs on serum insulin are shown in Table 1: serum insulin for untreated diabetic mice was significantly decreased when compared to that of the controls. In diabetic animals injected with MSCs and insulin, insulin levels were significantly increased compared to the untreated diabetic group [23].
Table 1.
Serum glucose and insulin in control, diabetic, diabetic + stem cells, and diabetic + insulin treated groups
| Parameter | Normal control | Diabetic | Diabetic + stem cell | Diabetic + insulin |
|---|---|---|---|---|
| Glucose (mg/dl) | 110 ± 6.44 | 371 ± 20.5a | 122 ± 8.58b | 123 ± 5.6b |
| Insulin (ng/ml) | 2.68 ± 0.03 | 0.63 ± 0.06a | 1.67 ± 0.01a,b,c | 2.51 ± 0.076a,b |
Serum glucose and insulin in control, diabetic, diabetic + stem cells, and diabetic + insulin treated groups. Results are expressed as mean ± SE.
Significantly different from control group.
Significantly different from diabetic group.
Significantly different from insulin treated diabetic group [23].
Histopathological findings further support that administration of MSCs in type 1 diabetic animal models could alleviate hyperglycemic symptoms, along with signs of islet regeneration. In the control group, there was no histopathological alteration in both islands of Langerhans cells as endocrine portions and the acini as well as the duct system of the exocrine portion. Untreated diabetic group showed atrophy with pyknosis of the nuclei, associated with focal hemorrhage in between lobules. On the other hand, diabetic mice treated with MSCs as well as insulin showed that most of the islands of Langerhans cells were histologically intact within normal histological diameter [23].
The results of this study demonstrate that MSC infusion could partially reconstruct islet formation and effectively ameliorate hyperglycemia in alloxan-treated rats; therefore, rat bone marrow harbors cells that have the capacity to control hyperglycemia in diabetic rats [23].
Taken altogether, these studies have illustrated the beneficial effects of MSCs in type 1 diabetes which may be related to their capacity to elicit tissue regeneration [24-26] and an even more remarkable but less understood ability to migrate to sites of tissue injury [27-30].
Embryonic stem cells in animal studies
In addition to mesenchymal stem cells, studies have shown that embryonic stem cells (ESCs) can be used to normalize blood glucose in streptozotocin-induced diabetic mice. One research group generated insulin-producing cells from mouse ESCs by constructing a plasmid containing human insulin gene with selectors and transfecting the plasmid into the stem cells. After selection, cells underwent induced differentiation into an insulin-secreting cell line, and were assayed for insulin secretion. Diabetes was induced in the mice by a single intraperitoneal injection of streptozotocin, a toxin to pancreatic β cells, and diabetes was confirmed by presence of weight loss, polyuria, and blood glucose levels greater than 500 mg/dl. 15 diabetic Swiss albino mice were later implanted with the ESC-derived insulin-secreting cells, and four mice were sham- operated as diabetic controls. Once blood glucose was restored to the physiologic range, blood glucose and weight were monitored every week [31].
Plasma insulin determinations, intraperitoneal glucose tolerance tests, and meal challenge tests were performed in the ESC-implanted, sham operated (diabetic) animals and control (non-diabetic) animals. The results illustrate that ESC-derived insulin-secreting cells maintain a stable in vivo glucose response. Body weight of sham-operated (diabetic) mice was 40% lower 8 weeks after the streptozotocin injection due to the insulin deficiency, while ESC-implanted mice increased in body weight after cell implantation. Moreover, cell implantation led to correction of hyperglycemia within one week, suggesting the ability of the implanted cells to mimic physiologic β cell function in vivo. However, it should be noted that this normalization was reversible in 40% of ES-implanted mice, which became hyperglycemic approximately 12 weeks after implantation, for unknown reasons. Despite being hyperglycemic, however, all of these animals maintained their body weight and had a longer survival than sham-operated diabetic mice [31].
To assess the ability of the transplanted mice to dispose a glucose load, intraperitoneal glucose tolerance tests and meal challenge tests were performed. Sham operated mice showed significantly higher plasma glucose levels than the control non-diabetic mice and mice transplanted with ESCs. Transplanted mice also showed higher plasma glucose levels after 30 minutes of glucose challenge than the control non-diabetic mice. Recovery of normal blood glucose was delayed in transplanted mice (210 minutes) with respect to non-diabetic mice (120 minutes); however, the insulin levels of the transplanted mice were significantly higher than sham-operated diabetic animals [31]. Another study also reported that streptozotocin-induced diabetic rats transplanted with cells transduced with pancreatic duodenal homebox 1 (Pdx-1) under the renal capsule resulted in lowered blood glucose, higher glucose tolerance, smoother fur, and less cataract [32].
The studies illustrate that these easily implanted cells are able to maintain a stable in vivo glucose response in diabetic mice and strongly suggest that therapy with ESCs provides a possible treatment for type 1 diabetes [31].
Human clinical trials in treatment of type 1 diabetes
In 2007, the first clinical trial assessing stem cell transplantation as a viable, safe and effective treatment for type 1 diabetes was reported by Dr. Julio C. Voltarelli and his fellow researchers. The treatment of immunosuppression, or pharmaceutically suppressing the immune system to prevent an immune response against the transplanted cells, followed by autologous nonmyeloablative hematopoietic stem cell transplantation (AHST) in human patients was hypothesized to prevent further loss of insulin-producing β cells and improve β cell function. The sample population consisted of 15 patients who had received a type 1 diabetes diagnosis within six months leading up to the trial. Patients with previous diabetic ketoacidosis were excluded due to potential safety issues in patients with higher risk of complications. The 15 patients were followed anywhere from 7 to 36 months; this presented inconsistencies in follow up. During the follow up, 14 patients became insulin-free for variable lengths of time. At six months post-treatment, mean total C-peptide levels were significantly increased from patients’ baseline values. Additionally, hemoglobin A1c levels were maintained below 7% for 13 of the patients. These results show support for AHST as an effective type 1 diabetes treatment. With regard to morbidity, one patient developed pneumonia and two patients developed late endocrine dysfunction. Due to the involvement of immunosuppression in addition to mobilizing and conditioning stem cells, most patients developed fibrile neutropenia, nausea, vomiting and alopecia. Mortality rate was zero [33]. Although a monumental first human clinical trial, the study presented obvious limitations: it was not randomized, did not have a control group, had a relatively short and inconsistent follow up time, and had a very small sample size.
The same research laboratory performed a follow up study two years later to address some limitations of their previous study [34]. The sample included the original 15 patients and an additional 8 patients, and the study had a longer follow up time of four years. The treatment protocol was the same as the 2007 study, excluding patients with previous ketoacidosis. During the follow up time (7 to 58 months), 20 patients attained insulin independence, and 12 of those patients maintained the insulin independence for 14 to 52 months (mean 32 months). C-peptide levels were significantly increased in all patients, and hemoglobin A1c levels were maintained below 7%. These parameters are indicative of increased insulin production and β cell function, and the prolonged follow up period showed sustained positive results of treatment. Oligospermia was the most frequent complication; in addition, bilateral nosocomial pneumonia was noted in two patients. This study further supports the benefits of stem cell treatment for type 1 diabetes, with most patients achieving insulin independence and sustained glycemic control. Again, however, this study was not a randomized or controlled trial and had a relatively small sample size [34].
Further efforts were made to discover the underlying mechanism of improved β cell function and to address limitations of previous studies. A clinical trial was conducted in 2012 with nine recently diagnosed type 1 diabetic patients. The study focused on the potential benefits of stem cell treatment in order to optimize treatment and target a patient population that would benefit most, considering previous complications. 12 months following AHST, two groups were identified: 6 patients who were no longer dependent on insulin, and 3 patients who were still insulin dependent, though at a reduced dosage. Patients who were insulin independent had significantly higher C-peptide production and demonstrated more AHST-modified genetic events. Each group had distinct associated patterns of top pathways and co-expression networks. The difference in patient responses to treatment could possibly be attributed to these distinct transcriptional events in peripheral blood mononuclear cell. The group’s further immune cell population analysis suggests that improvement of islet function following treatment in newly diagnosed type 1 diabetics could be due to elimination of islet-specific autoreactive T cells [35].
The same year, research was expanded in China with a controlled study to include type 1 diabetic patients with diabetic ketoacidosis, unlike previous studies. 13 patients, 10 of which had associated diabetic ketoacidosis, were treated with AHST and followed for 31 to 54 months. Post AHST treatment, the number of CD3+ T cells, B and NK cells were significantly decreased. Autoantibody data suggested that AHST inhibits the responses of these cells in some patients. Overall, 11 of the patients required significantly reduced doses of insulin with decreased levels in glycosylated hemoglobin and increased levels of C-peptide. This study further showed promising results for AHST as a type 1 diabetes treatment and suggests that therapeutic benefits can be inclusive to patients with diabetic ketoacidosis [36].
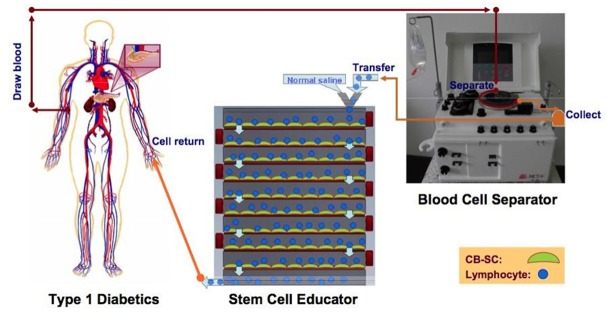
Furthermore, a novel procedure for Stem Cell Educator therapy was developed in which patients’ blood lymphocytes were separated and co-cultured with adherent human cord blood-derived multipotent stem cells, while the blood was circulating through a closed-loop system and ultimately returning to the patient’s circulation [15] (Figure 1). This procedure was tested in 15 patients with type 1 diabetes. 12 of the patients received the Stem Cell Educator therapy with adherent human cord blood-derived multipotent stem cells, and 3 patients received the Stem Cell Educator therapy without the stem cells (the control group). All patients tolerated the treatment well and none suffered harmful effects. This approach did not show the adverse effects of previous immunosuppression and stem cell treatments. In comparison to the control group, patients who received stem cells showed increased baseline and glucose-stimulated C-peptide levels, decreased medial hemoglobin A1c values, and decreased medial doses of insulin [15]. As previously seen, these are indicators of improved insulin production, β cell function, and glycemic control. Although a controlled study, the control group was significantly smaller than the experimental group and the overall patient sample size was small.
Figure 1.
Overview of Stem Cell Educator Therapy. type 1 diabetic participant (left) is connected to a Blood Cell Separator (right) and the Stem Cell Educator (bottom center) to form a closed system. Lymphocytes isolated from the participant by the Blood Cell Separator travel through the Stem Cell Educator where they come in contact with cord blood stem cells attached to the interior surfaces of the device. Educated lymphocytes are returned to the patient’s blood circulation [15]. With permission from The Creative Commons Attribution License 4.0. http://creativecommons.org/licenses/by/4.0/.
Human clinical trials in treatment of type 2 diabetes
Although fewer clinical trials have been conducted in stem cell treatment for type 2 diabetes, some advances have been made in this field as well. In 2008, a clinical trial study combined intrapancreatic autologous stem cell (ASC) infusion therapy with hyperbaric oxygen treatment (HBO) in 25 patients with type 2 diabetes. Plasma glucose and hemoglobin A1c values decreased, and C-peptide was shown to increase during the follow up period. Additionally, oral hypoglycemic drug dosage and injected insulin requirements both decreased post-treatment. BMI remained relatively constant throughout the study, which suggests that physician follow up, diet, exercise, and diabetes management were not the sole factors that caused improvements. This study suggests that ASC in combination with HBO treatment can have significant benefits for patients with type 2 diabetes [14]. The study was not randomized or controlled and had a relatively small sample size, which are marked limitations.
An additional study that investigated combined autologous bone marrow stem cell transplantation with HBO treatment in patients with type 2 diabetes was reported three years later. This study included a larger sample size (31) and followed the patients over a two year period. Post stem cell treatment, all patients showed a significant decrease in mean hemoglobin A1c values within 30 days. C-peptide was shown to significantly increase 90 days post-treatment, but levels were similar to baseline at other points in the follow up. Overall, all patients showed significantly reduced oral hypoglycemic drug dosage and reduced exogenous insulin dosage. The study found that although combined stem cell therapy and HBO treatment in patients with type 2 diabetes can improve glycemic control and reduce insulin/oral hypoglycemic drug dependency, the pancreatic β cell functional improvement may be transient [37]. The study was not randomized or controlled and still had a relatively small sample size.
The following year, a larger controlled clinical trial on bone marrow mononuclear stem cell treatment for type 2 diabetes was published. The sample of 118 patients was separated into experimental and control groups based on willingness to receive stem cell treatment, so the study was not random. The control group (62 patients) received insulin intensification therapy, while the experimental group (52 patients) received autologous bone marrow mononuclear cell implantation; all patients were followed for 36 months. Throughout the follow up time, the experimental group showed decreased mean fasting plasma glucose, while the control group showed increased fasting plasma glucose. This warranted an increase in insulin dosage in the control group, resulting in a non significant difference between the fasting plasma glucose of the two groups. In comparison to the control, the experimental group showing significant and progressive decrease in insulin dosage per day with 18 out of 56 patients achieving insulin independence. There was no significant difference between the two groups’ BMIs. The mean C-peptide and C-peptide/glucose ratio was significantly increased from baseline for the experimental group as well. Furthermore, difference in hemoglobin A1c levels between the two groups was significant at the end of the first year and at subsequent time points [38]. All of these results suggest positive therapeutic effects of bone marrow mononuclear stem cell treatment for type 2 diabetes.
Dr. Bhansali and fellow researchers conducted the most recently reported clinical trials in 2014. The initial study was a randomized, single-blinded, controlled clinical trial investigating the effects of autologous bone marrow-derived stem cell transplantation (ABMSCT) in 21 patients with type 2 diabetes [39]. The follow up study was conducted on 10 patients [13]. The initial study randomly assigned the 21 patients to experimental and control groups. There was a significant difference in insulin dose between the experimental and control groups at 3, 6, and 12 months post-treatment with the experimental group showing a mean 66.7% decrease in insulin requirements at 12 months. The decrease in insulin requirements was shown to be positively and significantly associated with an increase in C-peptide. Changes in hemoglobin A1c levels were not significantly different between the two groups; however, at 12 months, 10 of the 11 experimental group patients reached hemoglobin A levels <7% while only six of the 10 control group patients had done so. These results suggest that ABMSCT treatment has beneficial therapeutic potential for treatment of type 2 diabetes. Patients would need to be followed for a longer time to confirm these results [39]. Additionally, the study involved a relatively small sample size, which is a marked limitation. The follow up study was performed on 10 patients with type 2 diabetes, all of which received ABMSCT and were followed for 15 months. The mean insulin requirement decreased in all patients 6 and 15 months post treatment. Improvements in blood pressure and HDL cholesterol were noted in all patients and the majority of patients displayed decreased insulin requirements by ≥50%. Additionally, quality of life assessments showed improvement in general well-being, and no serious adverse effects of treatment were noted [13]. This follow-up study further supports ABMSCT as an effective treatment option for type 2 diabetes. but had a significantly smaller sample size that was not random or controlled.
Randomized, controlled, double-blind clinical trials on a larger sample population of patients is suggested in the future investigation of stem cell treatments in type 1 and type 2 diabetes.
All human clinical trials discussed are summarized in Table 2; in addition, Table 3 provides information on current ongoing trials that seek participants.
Table 2.
Summary of discussed human clinical trials
| Authors | Year Published | Sample Size | Patients (Sample type) | Study Protocol | Follow-up Duration (time) | Treatment Conclusion |
|---|---|---|---|---|---|---|
| Voltarelli J.C., et al. | 2007 | 15 | Newly type 1 diabeticsa, diagnosed within 6 months | High Dose Immunosuppression and AHSTc | 7-36 months (mean = 18.8) | Treatment showed acceptable toxicity and increased beta cell function in all but 1 ptient and attributed to prolonged insulin dependence. |
| Couri C.E.B., et al. | 2009 | 23 | Newly type 1 diabeticsa, diagnosed within 6 months | High Dose Immunosuppression and AHSTc | 5-58 months (mean = 29.8) | Treatment attributed to increased C-peptide levels with the majority of patients having insulin independence and glycemic control. |
| Estrada E.J., et al. | 2008 | 25 | Type 2 diabetics on metformin or combination of OHAsc | Hyperbaric oxygen treatment combined with ABMSCTb | 12 months | Treatment can induce improvement in metabolic control and insulin requirements. |
| Wang L., et al. | 2011 | 31 | Type 2 diabetics, failure of triple OHA therapy & insulin dependent | Hyperbaric oxygen treatment combined with ABMSCTb | 10 months | Treatment can induce improvement in glycemic control, insulin and/or oral hypoglycemic drug requirements, and beta cell function if only transiently. |
| Zhaog Y., et al. | 2012 | 15 | Type 1 diabeticsa | Stem Cell Educator: immune modulation by human cord blood-derived multipotent stem cells | 40 weeks | Stem Cell Educatory therapy did not show adverse outcomes and a single therapy induced lasting metabolic control improvements, initially reversd autoimmunity and induced regeneration of b cells. |
| Hu J., et al. | 2012 | 118 | Type 2 diabetics on metformin, rosiglitazone and insulin dependent | Exp: ABMSCTb | 33 months | Treatment was concluded to be safe and effective and partially restored the function of b cells and blood glucose homeostasis. |
| Control: insulin intensification treatment | ||||||
| Li L., et al. | 2012 | 13 | Newly type 1 diabeticsa, diagnosed within 6 months | AHSTc | 60 days | Treatment preserved b cell function and help modulate lymphocytes. |
| Zhao, X. et al. | 2012 | 9 | Newly type 1 diabetics1, diagnosed within 6 months | AHSTc | 23 months | Treatment induced improvements in b cell function and a proposed mechanism may be elimination of islet specific autoreactive T cells. Difference in patient response to treatment may be attributed to distinct transcriptional events. |
| Bhansali A., P. Asokumar, et al. | 2014 | 21 | Type 2 diabetics with triple oral antidiabetic drug failure and insulin dependent | ABMSCTb | 12 months | Treatment induces significant decrease in insulin requirements and improvements in C-peptide levels. |
| Bhansali A., V. Upreti, et al. | 2014 | 10 | Type 2 diabetics with >5 year duration of disease with documented triple drug failure, receiving metformin and pioglitazone, and insulin dependent | ABMSCTb | 15 months | Results suggest treatment is effective and maintained for over 15 months without adverse effects. |
T1 diabetics confirmed via clinical findings, hyperglycemia, and positive antibodies against glutamic acid decarboxylase.
ABMSCT: autologous bone marrow stem cell transplantation.
AHST: autologous nonmyeloablative hematopoietic stem cell transplantation.
dOHA: Oral Hypoglycemic Agents.
Table 3.
Current human clinical trials for stem cell intervention in diabetes treatment
| Phase | Condition | Intervention | Status | Eligibility (age, gender) | Location (s) | NIH ClinicalTrials.gov study title with link |
|---|---|---|---|---|---|---|
| 1/2 | Type 2 diabetes | Procedure: Harvesting and Implantation of Adipose-Derived Stem Cells (ASCs) | Recruiting | 18-80 years, both genders | Miami, FL, USA | Safety and Effects of Autologous Adipose-Derived Stromal Cells Delivers in Patients with type II diabetes |
| Unknown | Diabetes mellitus (patients undergoing hematopoietic stem cell transplant) | Procedure: Assessment of therapy complications Other: laboratory biomarker analysis | Not yet recruiting | 18 years and older, both genders | Nashville, TN, USA | Predicting Development of Diabetes Mellitus in Patients Undergoing Allogeneic Stem Cell Transplant |
| 4 | Type 2 diabetes | Drug: Saxagliptin Drug: placebo | Recruiting | 40 = 70 years, both genders | Washington, DC, USA | Effect of Saxagliptina on EPCs as a Cellular Biomarker for Evaluating Endothelial Dysfunction in Early type 2 diabetes |
| 2 | Type 1 diabetes | Procedure: Immunosuppression and stem cell transplantation | Recruiting | 8-35 years, both genders | Nanjing, Jiangsu, China | Efficacy and Safety Study of Autologous Hematopoietic Stem Cell Transplantation to Treat New Onset type 1 diabetes |
| 2 | Type 1 diabetes | Device: Stem Cell Educator | Recruiting | 14-60 years, both genders | 1. Shijiazhuang, Hebei, China 2. Changsha, Hunan, China 3. Jinan, Shandong, China 4. Oviedo, Asturias, Spain | Stem Cell Educator Therapy in type 1 diabetes |
| 2 | Type 1 diabetes | Biological: Autologous mesenchymal stem cell transplantation | Recruiting | 18-40 years, both genders | Uppsala, Sweden | Mesenchymal Stem Cells to Intervene in the Development of type 1 diabetes: A Blinded Randomized Study |
| 1 | Type 2 diabetes | Biological: Umbilical cord mesenchymal stem cells Biological: Controlled suspension liquid | Recruiting | 20-60 years, both genders | Beijing, China | Mesenchymal Stem Cells to Treat type 2 diabetes (UC-MSCs) |
| 1/2 | Diabetes mellitus, insulin dependent | Biological: Intravenous Mesenchymal stem cell infusion | Recruiting | 12-35 years, both genders | Ribeirão Preto, São Paulo, Brazil | Safety and Efficacy Of Mesenchymal Stem Cells in Newly-diagnosed Type 1 diabetic Patients |
| 2/3 | Type 1 diabetes | Biological: Autologous transplantation | Recruiting | 10-40 years, both genders | Chongqing, China | Autologous Transplantation of Mesenchymal Stem Cells for Treatment of Patients with Onset of type 1 diabetes |
| 1/2 | Diabetes mellitus | Other: Intra thecal transplantation of autologous mono nuclear cells | Recruiting | 18-55 years, both genders | Pune, Maharashtra, India | Study Safety and Efficacy of Bone Marrow Derived Autologous Cells for the Treatment of Diabetes Mellitus (BMACD) |
| 1/2 | Type 2 diabetes | Other: Bone Marrow Mononuclear Cell Transplantation | Recruiting | 30-70 years, both genders | Beijing, China | Autologous Bone Marrow Mononuclear Cell Transplantation in Treating type 2 diabetes Mellitus |
| 1/2 | Type 1 diabetes | Device: Stem Cell Educator | Recruiting | 6-14 years, both genders | Changsha, Hunan, China | Reversal of type 1 diabetes in Children by Stem Cell Educator Therapy |
| 1 | Type 1 diabetes | Genetic: Stem Cell Educator Therapy | Not yet recruiting | 18 years and older, both genders | Hackensack, New Jersey, USA | A Pilot Study of the Therapeutic Potential of Stem Cell Educator Therapy in Type 1 Diabetes |
Saxagliptin is an FDA approved DPP-4 inhibitor prescription medicine DPP-4 inhibitors have been shown to increase Endothelial Progenitor Cells in patients with type 2 diabetes.
(ClinicalTrials.gov A service of the U.S. National Institutes of Health. https://clinicaltrials.gov/).
Challenges
Despite the tremendous progress made in recent years regarding stem cell research, a remaining obstacle is defending the transplanted stem cells from immune attack. Even though mature β cells can be derived from the diabetic’s own stem cells, type 1 diabetes is an autoimmune disease in which the body destroys its own β cells. Like patients receiving transplanted islets from a donor’s pancreas, type 1 diabetics receiving stem cell therapy may have to take immunosuppressant drugs to prevent a response to the new stem cell-derived β cells [9,40]. As referenced in the studies above, with immunosuppression comes various complications, such as pneumonia and oligospermia [34,41,42]. ViaCyte, a regenerative medicine company based in San Diego, recently developed an encapsulated, biocompatible sheath of cells that may be a potential solution to immunosuppression. Though promising results have been found in animal studies, the body tends to form scar tissue around foreign bodies, which may prevent the enclosed cells from receiving nutrients [43]. Despite these challenges and the likely need to take immunosuppressant drugs, many diabetics would choose to take immunosuppressants rather than continue with insulin injections and the potentially life-threatening fluctuations of daily blood glucose, if stem cell therapy is further improved upon and studied in more randomized controlled trials [9].
Another challenge regarding mesenchymal stem cells (MSCs) is the issue of contamination while differentiation and manufacturing takes place in large quantities [44]. It is important to avoid the tumorigenic properties that MSCs can pose, and to keep the integrity of their stem cell-like properties during their production [45,12].
Conclusion
Stem cell therapy used to treat diabetes mellitus clearly has great potential to relieve diabetics of daily insulin injections, and may offer a more permanent solution to controlling high blood sugar levels. Animal studies have suggested that both embryonic and mesenchymal stem cells can be induced to differentiate into insulin-producing cells, and these cells most closely mimic the in vivo β cell regulation of insulin secretion. The increased C-peptide levels and decreased hemoglobin A1c levels in both animal and human trials show that stem cell therapy offers an effective and promising treatment for type 1 and possibly type 2 diabetes. Recent clinical trials have utilized various stem cell therapies for humans including hematopoietic, human cord blood- derived multipotent, intrapancreatic autologous, and bone marrow (mesenchymal) stem cells [47]. Testing these various stem cell therapies on humans has confirmed many of the positive results discovered in animal studies. However, there are some unanswered questions in the studies that are worthy of further research prior to implementing these protocols in humans [48]. For example, in the study with streptozotocin-induced type 1 diabetes, it is unknown why normalization of hyperglycemia was reversed in 40% of embryonic stem cell-implanted mice at 12 weeks post-implantation [31].
Furthermore, although experimental results are positive for ameliorating symptoms of type 1 diabetes, various limitations in the studies conducted thus far call for larger, better-controlled trials. For example, the animal studies conducted used only male rats or mice. Although there is no known data on sex-based differences in type 1 diabetes, there may be a sex-dependent response to stem cell treatment. Moreover, there is difficulty in randomizing human trials. As noted above, most experimental and control groups were based upon patients’ willingness to receive stem cell treatment. More controlled and randomized trials with larger groups are therefore needed to further validate the positive results of stem cell treatment noted thus far. In addition, there should be a comparative study to decide which method and which type of stem cell yields the best outcome for human patients.
Despite the challenges associated with stem cell studies and the further research required, the benefits of stem cell treatment outweigh potential complications, and the treatment has the potential to significantly improve the quality of life for millions of diabetics. The advent of adult stem cell research has opened up numerous possibilities and accessibility to treatment, compared to embryonic stem cells. Although both embryonic and adult stem cells have been shown to have the capacity to differentiate into insulin-producing cells, adult stem cells may offer treatment to a wider patient population; not only are adult stem cells more easily accessible, but adult stem cell usage is also less controversial in nature than embryonic stem cell usage. Many patients may feel more comfortable being treated with adult stem cells from their own tissues, and treatment may be faster and more effective via this method.
Overall, monumental strides have been made in stem cell research in recent years, and stem cell therapy offers a promising outcome for both type 1 and type 2 diabetic patients [46]. Progress continues to be made in clinical trials, and stem cell therapy as a standard for treating diabetes will likely be a viable option in the foreseeable future [49,50].
Disclosure of conflict of interest
None.
References
- 1.Lau DC, Teoh H. Current and emerging pharmacotherapies for weight management in prediabetes and diabetes. Can J Diabetes. 2015;5:134–141. doi: 10.1016/j.jcjd.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Wang Y, Li Y, Pei X. Research status and prospect of stem cells in the treatment of diabetes mellitus. Sci China. 2013;56:306–312. doi: 10.1007/s11427-013-4469-1. [DOI] [PubMed] [Google Scholar]
- 3.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2015;15:379–4. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 5.Madsen OD. Stem cells and diabetes treatment. Hagedorn Research Institute. APMIS. 2005;113:858–75. doi: 10.1111/j.1600-0463.2005.apm_418.x. [DOI] [PubMed] [Google Scholar]
- 6.Xu YX, Chen L, Wang R, Hou WK, Lin P, Sun L, Sun Y, Dong QY. Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Med Hypotheses. 2008;71:390–3. doi: 10.1016/j.mehy.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi H. Pancreatic islet transplantation. World J Gastrointest Surg. 2009;1:16–20. doi: 10.4240/wjgs.v1.i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamiolkowski RM, Guo LY, Li YR, Shaffer SM, Naji A. Islet transplantation in diabetes mellitus. Yale J Biol Med. 2012;85:37–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Ledford H. Stem-cell success poses immunity challenge for diabetes. Nature. 2014;514:281. doi: 10.1038/514281a. [DOI] [PubMed] [Google Scholar]
- 10.Mehrfarjam Z, Esmaeili F, Shabani L, Ebrahimie E. Induction of pancreatic beta cell gene expression in mesenchymal stem cells. Cell Biol Int. 2016;40:486–500. doi: 10.1002/cbin.10567. [DOI] [PubMed] [Google Scholar]
- 11.Schiesser JV, Wells JM. Generation of β cells from human pluripotent stem cells: Are we there yet? Ann N Y Acad Sci. 2014;1311:124–137. doi: 10.1111/nyas.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H. MSCs could also transdifferentiate into other cell types including insulin-producing cells under certain circumstances. Discov Med. 2014;17:139–143. [Google Scholar]
- 13.Bhansali A, Upreti V, Walia R, Gupta V, Bhansali S, Sharma RR, Grover S, Marwaha N, Khandelwal N. Efficacy and safety of autologous bone marrow derived hematopoietic stem cell transplantation in patients with type 2 DM: A 15 months follow-up study. Indian J Endocr Metab. 2014;18:838–845. doi: 10.4103/2230-8210.140257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estrada EJ, Valacchi F, Nicora E, Brieva S, Esteve C, Echevarria L, Froud T, Bernetti K, Cayetano SM, Velazquez O, Alejandro R, Ricordi C. Combined Treatment of Intrapancreatic Autologous Bone Marrow Stem Cells and Hyperbaric Oxygen in type 2 diabetes Mellitus. Cell Transplant. 2008;17:1295–1304. doi: 10.3727/096368908787648119. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, Yin Z, Li H, Zhang Y, Diao Y, Li Y, Chen Y, Sun X, Fisk MB, Skidgel R, Holterman M, Prabhakar B, Mazzone T. Reversal of type 1 diabetes via islet b cell regeneration following immune modulation by cord blood-derived multipotent stem cells. BMC Med. 2012;10:1–11. doi: 10.1186/1741-7015-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Czubak P, Bojarska-Junak A, Tabarkiewicz J, Putowski L. A modified method of insulin producing cells’ generation from bone marrow-derived mesenchymal stem cells. J Diabetes Res. 2014;2014:628591. doi: 10.1155/2014/628591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Li F, Gao F, Yang Y, Liu Y, Guo P, Li Y. Transplantation of mesenchymal stem cells improves type 1 diabetes mellitus. Cell Tissue Res. 2016;364:345–55. doi: 10.1007/s00441-015-2330-5. [DOI] [PubMed] [Google Scholar]
- 18.Larijani B, Arjmand B, Ahmadbeigi N, Falahzadeh K, Soleimani M, Sayahpour FA, Aghayan HR. A simple and cost-effective method for isolation and expansion of human fetal pancreas derived mesenchymal stem cells. Arch Iran Med. 2015;18:770–775. [PubMed] [Google Scholar]
- 19.Liu M, Han ZC. Mesenchymal stem cells: Biology and clinical potential in type 1 diabetes therapy. J Cell Mol Med. 2008;12:1155–68. doi: 10.1111/j.1582-4934.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dave S. Mesenchymal stem cells derived in vitro transdifferentiated insulin-producing cells: A new approach to treat type 1 diabetes. Adv Biomed Res. 2014;3:266. doi: 10.4103/2277-9175.148247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aali E, Mirzamohammadi S, Ghaznavi H, Madjd Z, Larijani B, Rayegan S, Sharifi AM. A comparative study of mesenchymal stem cell transplantation with its paracrine effect on control of hyperglycemia in Type 1 diabetic rats. J Diabetes Metab Disord. 2014;13:76. doi: 10.1186/2251-6581-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khorsandi L, Nejad-Dehbashi F, Ahangarpour A, Hashemitabar M. Three-dimensional differentiation of bone marrow-derived mesenchymal stem cells into insulin-producing cells. Tissue Cell. 2015;47:66–72. doi: 10.1016/j.tice.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 23.El-Tantawy WH, Haleem EN. Therapeutic effects of stem cell on hyperglycemia, hyperlipidemia, and oxidative stress in alloxan-treated rats. Mol Cell Biochem. 2014;391:193–200. doi: 10.1007/s11010-014-2002-x. [DOI] [PubMed] [Google Scholar]
- 24.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657–64. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Bittira B, Shum-Tim D, Al-Khaldi A, Chiu RC. Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur J Cardiothorac Surg. 2003;24:393–8. doi: 10.1016/s1010-7940(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie T, Flake A. Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis. 2001;27:1–4. doi: 10.1006/bcmd.2001.0424. [DOI] [PubMed] [Google Scholar]
- 29.Pittenger M, Martin B. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 30.Wu GD, Nolta JA, Jin YS, Barr ML, Yu H, Starnes VA, Cramer DV. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75:679–85. doi: 10.1097/01.TP.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 31.Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–62. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 32.Lin G, Wang G, Liu G, Yang LJ, Chang LJ, Lue TF, Lin CS. Treatment of Type 1 Diabetes With Adipose Tissue-Derived Stem Cells Expressing Pancreatic Duodenal Homeobox 1. Stem Cells Dev. 2009;18:1399–1406. doi: 10.1089/scd.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simões BP, Foss MC, Squiers E, Burt RK. Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed type 1 diabetes Mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 34.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simões BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC. C-Peptide Levels and Insulin Independence Following Autologous Nonmyeloablative Hematopoietic Stem Cell Transplantation in Newly Diagnosed type 1 diabetes Mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Ye L, Hu J, Tang W, Liu R, Yang M, Hong J, Wang W, Ning G, Gu W. Acute Response of Peripheral Blood Cell to Autologous Hematopoietic Stem Cell Transplantation in Type 1 diabetic Patient. PLoS One. 2012;7:e31887. doi: 10.1371/journal.pone.0031887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Shen S, Ouyang J, Hu Y, Hu L, Cui W, Zhang N, Zhuge YZ, Chen B, Xu J, Zhu D. Autologous Hematopoietic Stem Cell Transplantation Modulates Immunocompetent Cells and Improves b-Cell Function in Chinese Patients with New Onset of type 1 diabetes. J Clin Endocr Metab. 2012;97:1729–1736. doi: 10.1210/jc.2011-2188. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Zhao S, Mao H, Zhou L, Wang ZJ, Wang HX. Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chin Med J. 2011;124:3622–3628. [PubMed] [Google Scholar]
- 38.Hu J, Li C, Wang L, Zhang X, Zhang M, Gao H, Yu X, Wang F, Zhao W, Yan S, Wang Y. Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J. 2012;59:1031–1039. doi: 10.1507/endocrj.ej12-0092. [DOI] [PubMed] [Google Scholar]
- 39.Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, Sachdeva N, Sharma RR, Marwaha N, Khandelwal N. Efficacy and Safety of Autologous Bone Marrow-Derived Stem Cell Transplantation in Patients With type 2 diabetes Mellitus: A Randomized Placebo-Controlled Study. Cell Transplant. 2014;23:1075–1085. doi: 10.3727/096368913X665576. [DOI] [PubMed] [Google Scholar]
- 40.Ehlers MR, Rigby MR. Targeting memory T cells in type 1 diabetes. Curr Diab Rep. 2015;15:84. doi: 10.1007/s11892-015-0659-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis IC, Randell J, Davis SN. Immunotherapies currently in development for the treatment of type 1 diabetes. Expert Opin Investig Drugs. 2015;24:1331–1341. doi: 10.1517/13543784.2015.1075973. [DOI] [PubMed] [Google Scholar]
- 42.Vudattu NK, Herold KC. Treatment of new onset type 1 diabetes with teplizumab: successes and pitfalls in development. Expert Opin Biol Ther. 2014;14:377–385. doi: 10.1517/14712598.2014.881797. [DOI] [PubMed] [Google Scholar]
- 43.Swartzlander MD, Blakney AK, Amer LD, Hankenson KD, Kyriakides TR, Bryant SJ. Immunomodulation by mesenchymal stem cells combats the foreign body response to cell-laden synthetic hydrogels. Biomaterials. 2015;41:79–88. doi: 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higuchi A, Kao SH, Ling QD, Chen YM, Li HF, Alarfaj AA, Munusamy MA, Murugan K, Chang SC, Lee HC, Hsu ST, Kumar SS, Umezawa A. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci Rep. 2015;14:18136. doi: 10.1038/srep18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Zhong W, Yuan J, Yan C, Hu S, Tong Y, Mao Y, Hu T, Zhang B, Song G. Involvement of WNT/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015;6:42276–89. doi: 10.18632/oncotarget.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhabra P, Brayman KL. Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med. 2013;2:328–36. doi: 10.5966/sctm.2012-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chatterjee S, Davies MJ. Current management of diabetes mellitus and future directions in care. Postgrad Med J. 2015;91:612–21. doi: 10.1136/postgradmedj-2014-133200. [DOI] [PubMed] [Google Scholar]
- 48.Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal stem cells: rising concerns over their application in treatment of type one diabetes mellitus. J Diabetes Res. 2015;2015:675103. doi: 10.1155/2015/675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Girlovanu M, Susman S, Soritau O, Rus-Ciuca D, Melincovici C, Constantin AM, Mihu CM. Stem cells-biological update and cell therapy progress. Clujul Med. 2015;88:265–71. doi: 10.15386/cjmed-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov A service of the U.S. National Institutes of Health. https://clinicaltrials.gov. Accessed 4 March 2015.