Abstract
In 2010, a panel of Chinese pathologists reported the first expert consensus for the pathological diagnosis of primary liver cancers to address the many contradictions and inconsistencies in the pathological characteristics and diagnostic criteria for PLC. Since then considerable clinicopathological studies have been conducted globally, prompting us to update the practice guidelines for the pathological diagnosis of PLC. In April 18, 2014, a Guideline Committee consisting of 40 specialists from seven Chinese Societies (including Chinese Society of Liver Cancer, Chinese Anti-Cancer Association; Liver Cancer Study Group, Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Pathology, Chinese Anti-Cancer Association; Digestive Disease Group, Chinese Society of Pathology, Chinese Medical Association; Chinese Society of Surgery, Chinese Medical Association; Chinese Society of Clinical Oncology, Chinese Anti-Cancer Association; Pathological Group of Hepatobiliary Tumor and Liver Transplantation, Chinese Society of Pathology, Chinese Medical Association) was created for the formulation of the first guidelines for the standardization of the pathological diagnosis of PLC, mainly focusing on the following topics: gross specimen sampling, concepts and diagnostic criteria of small hepatocellular carcinoma (SHCC), microvascular invasion (MVI), satellite nodules, and immunohistochemical and molecular diagnosis. The present updated guidelines are reflective of current clinicopathological studies, and include a novel 7-point baseline sampling protocol, which stipulate that at least four tissue specimens should be sampled at the junction of the tumor and adjacent liver tissues in a 1:1 ratio at the 12, 3, 6 and 9 o’clock reference positions. For the purposes of molecular pathological examination, at least one specimen should be sampled at the intratumoral zone, but more specimens should be sampled for tumors harboring different textures or colors. Specimens should be sampled at both adjacent and distant peritumoral liver tissues or the tumor margin in order to observe MVI, satellite nodules and dysplastic foci/nodules distributed throughout the background liver tissues. Complete sampling of whole SHCC ≤ 3 cm should be performed to assess its biological behavior, and in clinical practice, therapeutic borders should be also preserved, even in SHCC. The diagnostic criteria of MVI and satellite nodules, immunohistochemical panels, as well as molecular diagnostic principles, such as clonal typing, for recurrent HCC and multinodule HCC were also proposed and recommended. The standardized process of pathological examination is aimed at ensuring the accuracy of pathological PLC diagnoses as well as providing a valuable frame of reference for the clinical assessment of tumor invasive potential, the risk of postoperative recurrence, long-term survival, and the development of individualized treatment regimens. The updated guidelines could ensure the accuracy of pathological diagnoses of PLC, and provide a valuable frame of reference for its clinical assessment.
Keywords: Liver cancer, Hepatocellular carcinoma, Intrahepatic cholangiocarcinoma, Practice guidelines, Pathology, Diagnosis
Core tip: Given the high prevalence of primary liver cancers in China, the present 2015 guidelines were formulated in response to the clinicopathological evidence amassed over the past 5 years. The guidelines included suggestions for a 7-point baseline sampling protocol, updated the definition of small hepatocellular carcinoma (HCC), described a grading system of microvascular invasion for routine pathological diagnosis, and included molecular diagnostic principles, such as the importance of clonal typing for determining the clonal original patterns and therapeutic strategy of postoperative recurrent and multinodule HCC.
INTRODUCTION
Primary liver cancers mainly refer to malignancies that originate from hepatocytes hepatocellular carcinoma (HCC), which account for the majority of PLC, and intrahepatic cholangiocytes intrahepatic cholangiocarcinoma (ICC). PLC ranks as the second leading cause of cancer death worldwide, among which HCC is one of the most lethal malignancies because of its high morbidity and mortality, as well as aggressiveness. An estimated 782500 new PLC cases and 745500 deaths occurred worldwide during 2012, with China alone accounting for about 50% of the total number of cases and deaths[1]. In China, current trends in the crude incidence and mortality of PLC are 28.71/100000 and 26.04/100000, respectively, making it the fourth most common cancer and the second leading cause of cancer related-death[2].
Hepatic pathology is a foundational subject in the field of hepatic surgery, the preferred first-line treatment for PLC. In an effort to ensure the accuracy of pathological diagnosis, a standardized process of pathological examination is required to provide a valuable frame of reference for the clinical assessment of the risk of postoperative recurrence, long-term prognosis, as well as individualized treatment regimens. However, most current practice guidelines for PLC focus on the clinical treatment[3,4]. To our best knowledge, no consensus guidelines for the pathological diagnosis of PLC have ever been published. The lack of such guidelines has led to many contradictions and inconsistencies in the pathological characteristics and diagnostic criteria for PLC. To address this gap, Chinese pathologists developed an expert consensus on the pathological diagnosis of PLC in 2010[5].
Since the development of these guidelines, much progress in the clinical management and pathological assessment of PLC has yielded many new concepts, such as tumor heterogeneity, pathobiological characteristics, molecular classification, personalized therapy and precision medicine, etc. The inclusion of these new concepts has become the cornerstone for the clinical management of PLC, placing greater demands on more stringent criteria and standards for hepatic pathological diagnosis. Therefore, in April 18, 2014, under the guidance of renowned academicians Prof. Wu Meng-Chao, Prof. Tang Zhao-You and Prof. Liu Tong-Hua, a Guideline Committee consisting of 40 specialists (supplementary materials) from Chinese Pathology, Surgery, Hepatology and Oncology Societies was created for the formulation of updated guidelines for the standardization of the pathological diagnosis of PLC.
The principal goals of the Guideline Committee include (1) incorporating the results of worldwide clinicopathological studies in PLC over the past 5 years according to the Evaluation Criteria of Grades of Evidence recommended by the American Association for the Study of Liver Diseases guideline (Table 1)[6]; (2) accepting the comments and suggestions of experts in hepatic pathology, surgery and oncology; (3) responding to the clinical concerns for improving the therapeutic efficacy for PLC; and (4) creating guidelines for the standardized pathological diagnosis of PLC. To meet these goals, the Guideline Committee organized several seminars for guideline formulation, mainly focusing on the following topics: gross specimen sampling, concepts and diagnostic criteria of small HCC (SHCC), microvascular invasion (MVI), satellite nodules, immunohistochemical and molecular diagnosis. The final version of the 2015 guidelines was approved at the last Guideline Committee meeting, which was held in February 1, 2015 in Shanghai, China.
Table 1.
Grades of evidence and classes of recommendations
| Description | |
| Grade of evidence | |
| A | Data derived from multiple randomized, controlled trials or meta-analyses |
| B | Data derived from a single randomized trial or nonrandomized studies |
| C | Evidence based on clinical experience, descriptive studies, and opinion of respected authorities where further research is highly likely to impact confidence on the estimate of clinical effect |
| Class of evidence | |
| I | Conditions for which there is evidence and/or general agreement that a given diagnostic evaluation, procedure, or treatment is beneficial, useful, and effective |
| II | Conditions for which there is conflicting evidence and/or divergence of opinion about the usefulness/efficacy of a diagnostic evaluation, procedure, or treatment |
| IIa | Weight of evidence/opinion is in favor of the usefulness/efficacy |
| IIb | Usefulness/efficacy is less well-established by evidence/opinion |
| III | Conditions for which there is evidence and/or general agreement that a diagnostic evaluation/procedure/treatment is not useful/effective and in some cases, may be harmful |
GENERAL PATHOLOGY
Sample collection, fixation and processing
Peritumoral zones are representative of tumor heterogeneity in that they are rich in highly invasive cells, susceptible to the formation of MVI and satellite nodules and, therefore, more likely to impact liver cancer metastasis, postoperative recurrence and prognosis[7,8]. Therefore, sampling around the periphery of tumor tissues is critical for objectively evaluating the biological behaviors of PLC. Specifically, a 7-point baseline sample collection protocol is recommended (Figure 1). (1) At least four tissue specimens should be sampled at the junction of the tumor and adjacent liver tissues in a 1:1 ratio at the 12 (A), 3 (B), 6 (C) and 9 (D) o’clock positions; (2) for the purpose of molecular pathological examination, at least one specimen should be sampled at the intratumoral zone (E), but more specimens should be sampled for tumors harboring different textures or colors; (3) specimens should be sampled from both adjacent peritumoral liver tissues (F, ≤ 1 cm from the tumor capsule) and distant peritumoral liver tissues (G, > 1 cm from the tumor capsule) or the tumor margin in order to observe MVI, satellite nodules and dysplastic foci/nodules distributed throughout the background liver tissues; and (4) Tissue blocks should be approximately 1.5 cm - 2.0 cm × 1.0 cm × 0.3 cm in size and marked according to their sampling sites.
Figure 1.
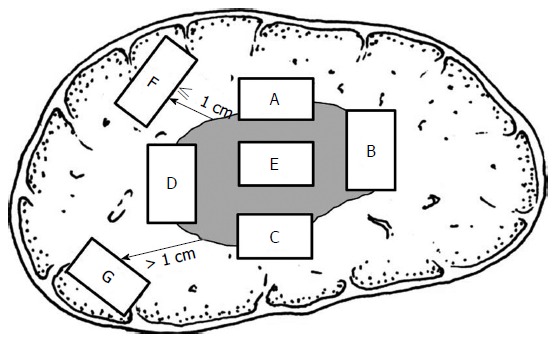
Specimen sampling sites.
Regarding tissue fixation, the following recommendations were made to assure the quality of the tissues for pathological and immunopathological examination[9]. (1) The surgeons should fill an Application Form of Pathological Examination describing the clinical diagnosis, location and type of lesions and number of tissues. The surgical margin, suspected lesions, important vessels and bile duct margin should be marked with a dye or suture by surgeons. Small resected tissues, such as lymph nodes, should be placed into different containers and labeled with corresponding descriptions; (2) to maximally preserve the integrity of intracellular nucleic acids and proteins for avoiding autocytolysis, tumor specimens should be transferred to the Department of Pathology as soon as possible after resection, ideally within 30 min after surgical removal for sectioning and fixation[10]; (3) the fresh specimens should be cut consecutively into 1-cm-thick multiple sections at the maximal diameter; a portion should remain unfixed fresh or cryopreserved for molecular examination; and (4) at room temperature, tissues should be fixed in a neutral formalin solution (v:v, 1:4-5) for 12-24 h and embedded in paraffin. Sections of 5-μm thickness should be cut from each block and stained with hematoxylin and eosin for histological examination[11].
Recommendations: (1) Hepatic tumor samples should be collected using the 7-point baseline sampling protocol; (2) the location and number of liver tissues collected should be determined as appropriate according to the size, shape and number of the liver tumors as well as the adjacent liver tissues; (3) because the detection rate of MVI and satellite nodules is related to the extent of adjacent liver tissues, it is necessary to describe the size of the adjacent liver tissues, and the suspected lesions should be sampled after reviewing several sections (C, I); and (4) when the tumor tissue is close to the surgical margin, sampling should be done at the region vertical to the margin closest to the cancer. When the tumor tissue is far away from the surgical margin, sampling should be done parallel to the surgical margin. The status of the surgical margin should be determined using the section with maximal area (C, I).
Description of macroscopic characteristics and clinical significance of SHCC
In the description of general hepatic tissue characteristics, pathologists should emphasize the size, number, color and texture of the tumor, its relationship with blood vessels and the bile duct, tumor capsule, tumor involvement, peripheral liver lesions, type of hepatic cirrhosis, the shortest distance between the tumor and surgical margin, and the status of the surgical margin. For tumor tissues with atypical morphology, the tissues should be photographed. Gross classification of HCC may reference the criteria developed by the Chinese Pathology Working Group for Liver Cancer[12], and the Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2011 Edition) proposed by the Chinese Minister of Health[13], in which a single tumor ≤ 1 cm in diameter is defined as microtumor, and a single tumor from > 1 cm to ≤ 3 cm in diameter is defined as SHCC. According to the classification system proposed by the World Health Organization (WHO), ICCs are classified as mass-forming, periductal-infiltrating, and intraductal growth[14].
Although some studies considered that patient outcomes might not be impacted as tumors reach > 5 cm in size[15], discerning the presence of SHCC is important in the early diagnosis and therapy initiation for patients with liver cancer as it is a key step in the development and progression of HCC. However, the definition of SHCC varies greatly by international criteria - from 2 cm to 5 cm in diameter[15]. Studies indicating that HCC growing near to or larger than 3 cm in diameter is an important turning point in the transformation of a tumor from having relatively benign features to more aggressive behaviors[16,17]. Furthermore, the unique genetic changes in those SHCC ≤ 3 cm in diameter during the early stage have been reported[18,19]. More data indicated that patients with tumors > 3 cm have an increased risk for MVI, satellite nodules, as well as poor prognosis[17,20]. Specifically, the overall postoperative 5-year survival and recurrence-free survival of patients with SHCC ≤ 3 cm are 67.8% and 52%, respectively, which are significantly higher than that of 42.3% and 29.3% in patients with HCC > 3 cm, respectively (P < 0.001)[17,21]. Moreover, up to now, most studies on patients with SHCC ≤ 2 cm are based on multi-center joint studies with long-term data collection because too few surgical cases in a single center exist. At present, there are almost no systematic studies or knowledge based on a large series of cases that describe the pathobiological characteristics of SHCC ≤ 2 cm[16,19,22].
Recommendations: (1) SHCC of ≤ 3 cm is frequently well-differentiated with expansive growth, and has a low risk for MVI and satellite nodules[17], which is suggestive of a relatively benign biological behavior in the progression to malignancy and is the basis of radical therapy. Thus, radical therapy should be initiated at an early stage before the tumor becomes highly invasive (B, I); and (2) in a small number of cases, SHCC may be poorly differentiated, invasive, or containing MVI and satellite nodules, which is indicative of highly malignant behavior. Given the heterogeneity of HCC, complete sampling of SHCC ≤ 3 cm should be performed to assess its biological behavior, and in surgical practice, therapeutic borders should also be preserved, even in SHCC (B, I).
Description of microscopic characteristics
Previous studies have described the analysis of microscopic tissue characteristics that include the following[14,23]: (1) histological types of HCC, including common histological types (e.g., thin trabecular type, thick trabecular type, pseudoglandular type, compact type and fibrolamellar type, etc); (2) HCC cell type (e.g., clear cell type, lipid-rich type, spindle cell type and undifferentiated type, etc); (3) differentiation state of HCC as assessed by the Edmondson-Steiner four-grading system; (4) the area and severity of tumor necrosis (e.g., interventional therapy), lymphocyte infiltration and interstitial fibrosis; (5) adenocarcinoma is the most common histological type of ICC, although it may also present in other special histological and cell types and its differentiation degree can be classified as well, intermediate and poor; (6) tumor growth patterns, including peritumoral invasion, capsule invasion, MVI and satellite nodules; and (7) the presence of chronic liver disease, such as chronic hepatitis or hepatic cirrhosis. Although there are many systems for grading and staging chronic viral hepatitis[24-29], a simple histologic scoring system is recommended for routine pathological diagnosis, such as the Scheuer scoring system, etc. Furthermore, Masson’s trichrome staining and reticular fiber staining can be routinely undertaken to assess the degrees of hepatic fibrosis and lobule reconstruction, respectively[30].
Description of precancerous lesions
The main types of precancerous HCC lesions include the following[23,31,32]: liver cell dysplasia, dysplastic foci, low-grade dysplastic nodule (LGDN), and high-grade dysplastic nodule (HGDN). Liver cell dysplasia refers to either large cellular changes, including increased cellular and nuclear volumes, nuclear pleomorphism, hyperchromatic chromatin and multinucleation, and small cellular changes, including decreased cell volume, increased nuclear-to-cytoplasm ratio with mild pleomorphism and hyperchromasia, but showing crowded nuclei. Dysplastic foci are lesions that are ≤ 1.0 mm in diameter and commonly composed of hepatocytes with small cell changes. LGDN is a nodule mainly comprising large cellular changes without obvious atypia, isolated interstitial arteries, or expansive growth patterns. In contrast, HGDN is composed of small cellular changes with increased atypia, isolated interstitial arteries, and expansive growth. “Nodule-in-nodule” is used to describe a focal malignant lesion occurring within a HGDN. According to the WHO classification system, hepatocellular adenoma (HCA) can be classified into four molecular pathological subtypes, including hepatocyte nuclear factor 1 α-inactivated HCA, β-catenin-activated HCA, inflammatory HCA and unclassified HCA, among which, β-catenin-activated HCA may have a higher risk of malignant transformation[23,31,32].
The main types of precancerous lesions in ICC include biliary intraepithelial neoplasia (BilIN) and intraductal papillary biliary neoplasm (IPBN), as well as others[14]. BilIN is usually graded as BilIN-1 (low-grade lesions), BilIN-2 (intermediate-grade lesions), and BilIN-3 (high-grade lesions or carcinoma in situ), according to the degree of nuclear atypia observed in biliary epithelial cells. IPBN refers to tubular papillary tumors with growth confined to the bile duct lumen. In addition, IPBN may have BilIN with different grades. Along with BilIN and IPBN, other types of precancerous lesions in ICC include mucinous cystic neoplasms and biliary hamartomas harboring a high degree of BilIN, which may also correlate with increased risk of ICC.
Recommendations: It is important to conduct a differential diagnosis between HGDN and well-differentiated SHCC, the latter of which may manifest morphologically as varying degrees of increased cellular density and nuclear-to-cytoplasmic ratio, widened trabecular space, pseudoglandular structures, infiltrative growth, increased MVD as assessed by CD34 staining, higher Ki-67 index, and positive expression of p53 and glypican-3 (GPC-3), etc (B, I).
PATHOLOGICAL DIAGNOSIS OF MVI
MVI is also known as microvascular cancer embolus and refers to the cancer cell nest in vessels lined with endothelial cells. The incidence of MVI in liver cancer patients ranges from 15% to 57.1%[33], which may be partly ascribed to differences in the sample collection protocol and diagnostic criteria between studies. MVI is most frequently found in the small branches of the portal vein in the adjacent liver tissues (including vessels of the cancer capsule) because these vessels are the major ones exiting the tumor, as the portal vein shows disordered hemodynamics[34,35]. Branches of the hepatic vein are the secondary vessels exiting the tumor and may also develop MVI. Occasionally, the hepatic tumor may invade the hepatic artery, bile duct and lymphatic vessels, which should be reported independently[33,36]. To differentiate the vascular nature of the tumor, immunohistochemistry may be performed to examine the expression of CD34 (vascular endothelium), smooth muscle α-actin (vascular smooth muscle), elastic fibers (elastic fiber layer of tiny blood vessel wall) and D2-40 (lymphatic endothelium).
Clinical studies indicate that MVI is related to poor prognosis in patients with HCC, including increased risk for postoperative recurrence and reduced long-term survival. In patients with HCC, a correlation between higher MVI grade and shorter disease-specific survival and recurrence-free survival has been noted[37]. In a systemic review that included 20 observational studies of patients undergoing liver transplantation (LT), the presence of MVI shortened their 3-year disease-free survival [RR = 3.41 (2.05-5.7)] and 3-year overall survival [RR = 2.41 (1.72-3.37)][33]. A similar correlation between MVI and poor prognosis was also observed in patients with SHCC of ≤ 3 cm[38]. Furthermore, Pawlik et al[39] found that the occurrence of MVI was positively correlated with the size of HCC, suggesting that the size and number of tumors are important predictive indices for MVI. Furthermore, Roayaie et al[40] found that vascular smooth muscle involvement of MVI and > 5 MVIs were closely related to postoperative recurrence, and MVI located > 1 cm away from the adjacent liver tissues was associated with postoperative survival[41]. There is also evidence showing that the presence of ≥ 50 loosely suspended cancer cells in MVI is closely related to the prognosis of patients with PLC. In contrast, the presence of < 50 loosely suspended cells in the lumen should be described in the report sheet and may be indicative of a low risk for recurrence[34].
Recommendations
MVI is an independent prognostic marker for HCC patients (A, I); therefore, its presence should be evaluated in all tissue sections and graded according to the risk stratification based upon the number and distribution as follows: M0: no MVI; M1 (low-risk): MVI of < 5 and at ≤ 1 cm away from the adjacent liver tissues; and M2 (high-risk): MVI of > 5 or at > 1 cm away from the adjacent liver tissues (B, I).
PATHOLOGICAL DIAGNOSIS OF SATELLITE NODULES
Satellite nodules refer to the macroscopic or microscopic tumor cell nests located around or near, but separated from the main tumor with similar histological features as observed in the primary tumor. Generally, satellite nodules are derived from MVI. While difficult to distinguish from each other histologically, a diagnosis of satellite nodules is appropriate.
Studies show that the maximum micrometastasis spread distance (MMSD) in the distal area was < 3 cm in 92.3% of HCCs, and the MMSD in the proximal edge was < 1.5 cm in 91.7% of HCCs, suggesting that this area is important for pathological diagnosis and therapy[42]. Lim et al[15] found that the incidence of satellite nodules was 7% and 23% in patients with HCC of < 5 cm and > 5 cm, respectively, indicating that satellite nodules were a factor predicting poor overall survival. Moreover, the presence of satellite nodules is also an important predictor of postoperative recurrence[42]. The presence of MVI and satellite nodules may also provide a reference for the selection of clinical therapeutic modules. For example, Meniconi et al[43] found that in the absence of MVI and satellite nodules in the first resected HCC, a second hepatectomy or radiofrequency ablation for early intrahepatic recurrence predicted a better overall survival as compared to hepatic arterial chemoembolization.
Recommendations
Pathological diagnosis of satellite nodules should include the following pathological parameters[44]: (1) number; (2) distribution and extent; and (3) presence of cancerous nodes in the distant adjacent liver tissues, including multinodular HCC (MNHCC), which may represent either intrahepatic metastases or de novo HCC arising from a polycentric origin. Molecular cloning detection may then be helpful to elucidate the origin of the satellite nodules (B, I).
PROCESSING OF LIVER TISSUES COLLECTED BY LIVER BIOPSY
Regarding the diagnosis of hepatic space-occupying lesions, a 16-gauge puncture needle is usually used to obtain a biopsy specimen containing junctional areas between the tumor and peritumoral zones or one from each zone. A relatively longer biopsy sample is required for the assessment of the degree of hepatic fibrosis or cirrhosis in the setting of chronic viral hepatitis. The appropriate tissue length should be longer than 1.5 cm and fixed with 10% neutral buffered formalin for 1-2 h, and ≥ 6 intermittent and consecutive sliced tissue sections should be placed on each slide for pathological evaluation[45,46].
IMMUNOHISTOCHEMICAL DIAGNOSIS
For HCC, commonly used immunohistochemical markers for diagnosis include hepatocyte paraffin-1 (Hep Par-1), GPC-3, CD34, polyclonal carcinoembryonic antigen (pCEA), CD10, arginase-1, heat shock protein-70, and glutamine synthetase[31,47,48]. Although Hep Par-1, CD10, arginase-1 and pCEA are hepatocyte-specific antigens, they cannot be used to distinguish benign and malignant hepatocellular tumors[47]. For the immunohistochemical diagnosis of ICC, staining with antibodies specific for biliary cytokeratins, such as CK19, CK7 and mucin-1, is commonly employed. The diagnosis of dual phenotype HCC (DPHCC), a new highly aggressive subtype of HCC, is generally characterized by the expression of both HCC and ICC biomarkers[7,49], and the diagnosis of DPHCC can only be made by immunohistochemical detection.
Although some reported biomarkers may aid in the evaluation and prediction of certain biological features of liver cancer, including risk for invasiveness, recurrence and long-term survival[50,51], but further studies are required to confirm their clinical importance in multiple patient populations.
Recommendations
(1) Currently used biomarkers for liver cancer are somewhat imperfect in their diagnostic specificity and sensitivity; thus, a biomarker panel in combination with other tissue-specific markers could represent a useful tool for diagnosis and differential diagnosis between benign and malignant hepatocellular tumors, HCC and ICC, other specific types of hepatic tumors, and primary and metastatic liver cancer (B, I); and (2) although immunohistochemical staining for CD34 does not directly label hepatic parenchymal cells, it is valuable for determining the extent of MVD and examining its unique distribution pattern in different liver tumors. For instance, a diffuse staining pattern is indicative for HCC, a scattered staining pattern for ICC, a patchy staining pattern for HCA, and a cord-like staining pattern for focal nodular hyperplasia, etc (B, I).
MOLECULAR PATHOLOGICAL DIAGNOSIS
Development of molecular classification techniques, including the detection of molecular targets and assessment of clonal origin, represents a promising new development in the field. Although many new systems based on molecular typing and predictive biomarkers have been reported in the literature[52], validation of their clinical significance is still required through the use of controlled studies across multiple centers with large sample sizes. Specifically, the selection, detection and clinical significance of molecular targets for targeted drug therapy for PLC are still under investigation, but the results of preliminary clinical trials are worthy of high expectations[53,54]. Regarding risk evaluation of precancerous lesions, molecular identification may be better suited than histopathological evaluation to detect the genomic instability for hepatocarcinogenesis and impact of clinical treatment modalities for patients with precancerous lesions, such as HGDN and HCA[55]. Molecular pathology detection is conducive to optimization and choice of clinical treatment modalities[56].
Postoperative recurrence of HCC (RHCC) seriously restricts the long-term curative effect of HCC treatments. Based on the clonal origin theory, a RHCC may originate from either a monocentric (monoclonal) origin or multicentric (polyclonal) origin. Theoretically, interventional therapy and targeted drug therapy are more suitable for RHCC that originate from residual cancer cells (monoclonal origin) after initial tumor resection, while repeated resections or liver transplantation are more appropriate for RHCC of multicentric origin that resembles a new primary tumor arising from a de novo tumor clone[57]. However, due to long-term “latency” and “dormancy” of residual cancer cells left in the liver after resection, monoclonal recurrence may occur even in the so-called “late period” (> 2 years) after surgical resection, clinically coinciding with a special period for RHCC derived from multicentric origins[58]. Although other researchers have proposed histological criteria for the clonal evaluation of RHCC[59], the accuracy of such morphological criteria requires further validation of the molecular detection.
The clonal origin theory of RHCC is also applicable to MNHCC. Finkelstein et al[60] reported that postoperative survival after LT was significantly better in patients with multicentric MNHCC than in those with monocentric MNHCC, indicating that genotyping has the potential to serve as a reference for LT recipient screening and prognostic evaluation. Gehrau et al[61] also recommended a diagnostic and therapeutic roadmap based on the clonal detection of MNHCC that would assist with patient selection for LT. Specifically, patients with MNHCC derived from multicentric origins could be ranked in the waiting lists to receive a LT, while those patients with monocentric MNHCC would be better suited for interventional treatment or targeted drug therapy using sorafenib[61].
Recommendation
Evaluating the clonal origins of RHCC and MNHCC is vital to developing individualized therapeutic regimens and subsequently improving long-term clinical outcomes. Therefore, assessing the clonal origins of RHCC and MNHCC using molecular cloning methods may provide objective references for the formulation of individualized therapy plans (B, I).
CONCLUSION
A standardized pathological diagnostic process is the first precondition required for a correct pathological diagnosis, scientific clinical decision-making and precision treatment of PLC from the origin. Considering the high prevalence of PLC worldwide, especially in China, we report updated guidelines for pathological diagnosis of PLC, which includes standardized guidelines for specimen fixation, 7-point baseline sampling protocol and examination, a grading system for MVI in a routine pathology diagnosis, and immunohistochemical diagnostic panels, as well as molecular diagnostic principles, such as the importance of clonal typing of RHCC and MNHCC for determining therapeutic strategy and evaluating clinical prognosis.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no conflicts of interest related to this work.
Peer-review started: March 3, 2016
First decision: April 14, 2016
Article in press: August 8, 2016
P- Reviewer: Ercolani G, Lee HC, Liu SM S- Editor: Gong ZM L- Editor: Filpodia E- Editor: Zhang FF
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China, 2009. Chin J Cancer. 2013;32:162–169. doi: 10.5732/cjc.013.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–855. doi: 10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cong WM, Hu XQ, Sun YT, Tan YS, Ji XL, Yun JP, Zhu HG, Guo H, Wang RA, Liu SX, et al. Expert consensus on the scheme of pathological diagnosis of primary liver cancer. Chin Clin Oncol. 2012;1:12. doi: 10.3978/j.issn.2304-3865.2012.06.02. [DOI] [PubMed] [Google Scholar]
- 6.Koh C, Zhao X, Samala N, Sakiani S, Liang TJ, Talwalkar JA. AASLD clinical practice guidelines: a critical review of scientific evidence and evolving recommendations. Hepatology. 2013;58:2142–2152. doi: 10.1002/hep.26578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu XY, Xi T, Lau WY, Dong H, Zhu Z, Shen F, Wu MC, Cong WM. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior. Ann Surg Oncol. 2011;18:2210–2217. doi: 10.1245/s10434-011-1585-7. [DOI] [PubMed] [Google Scholar]
- 8.Cai SW, Yang SZ, Gao J, Pan K, Chen JY, Wang YL, Wei LX, Dong JH. Prognostic significance of mast cell count following curative resection for pancreatic ductal adenocarcinoma. Surgery. 2011;149:576–584. doi: 10.1016/j.surg.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Bass BP, Engel KB, Greytak SR, Moore HM. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch Pathol Lab Med. 2014;138:1520–1530. doi: 10.5858/arpa.2013-0691-RA. [DOI] [PubMed] [Google Scholar]
- 10.Westra WH, Hruban RH, Phelps TH, Isacson C. Surgical pathology dissection: an illustrated guide. New York: Springer, 2003 [Google Scholar]
- 11.Chinese Medical Association. Normalization of Clinical Technology Operation- Pathology Volume. Beijing: People¡¯s Military Medical Press, 2004 [Google Scholar]
- 12.Ying YY. Pathology of Hepatocellular Carcinoma. In: Tang, ZY. Primary Liver Cancer. Shanghai: Shanghai Science and Technology Press, 1981 In: Tang, ZY, editors. [Google Scholar]
- 13.Ministry of Health of the People’s Republic of China. Guidelines on the diagnosis and treatment of primary liver cancer (2011 Edition) J Clin Hepatol. 2011;27:1141–1159. [Google Scholar]
- 14.Nakanuma Y, Curado MP, Franceschi S, et al. Intrahepatic cholangiocarcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th Ed. Lyon: IARC Press, 2010 In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. [Google Scholar]
- 15.Lim C, Mise Y, Sakamoto Y, Yamamoto S, Shindoh J, Ishizawa T, Aoki T, Hasegawa K, Sugawara Y, Makuuchi M, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg. 2014;38:2910–2918. doi: 10.1007/s00268-014-2704-y. [DOI] [PubMed] [Google Scholar]
- 16.Cong WM, Wu MC. Small hepatocellular carcinoma: current and future approaches. Hepatol Int. 2013;7:805–812. doi: 10.1007/s12072-013-9454-z. [DOI] [PubMed] [Google Scholar]
- 17.Lu XY, Xi T, Lau WY, Dong H, Xian ZH, Yu H, Zhu Z, Shen F, Wu MC, Cong WM. Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol. 2011;137:567–575. doi: 10.1007/s00432-010-0909-5. [DOI] [PubMed] [Google Scholar]
- 18.Moribe T, Iizuka N, Miura T, Kimura N, Tamatsukuri S, Ishitsuka H, Hamamoto Y, Sakamoto K, Tamesa T, Oka M. Methylation of multiple genes as molecular markers for diagnosis of a small, well-differentiated hepatocellular carcinoma. Int J Cancer. 2009;125:388–397. doi: 10.1002/ijc.24394. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Ueno S, Kubo F, Sakoda M, Hiwatashi K, Tateno T, Mataki Y, Maemura K, Shinchi H, Natsugoe S, Aikou T. Efficacy of anatomic resection vs nonanatomic resection for small nodular hepatocellular carcinoma based on gross classification. J Hepatobiliary Pancreat Surg. 2008;15:493–500. doi: 10.1007/s00534-007-1312-8. [DOI] [PubMed] [Google Scholar]
- 21.Wu MC. [Strengthening to diagnosis and treatment of hepatocellular carcinoma] Zhonghua Yixue Zazhi. 2007;87:2089–2091. [PubMed] [Google Scholar]
- 22.Farinati F, Sergio A, Baldan A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnu L, Rapaccini G, Zoli M, Borzio F, et al. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer. 2009;9:33. doi: 10.1186/1471-2407-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theise ND, Curado MP, Franceschi S, et al. Hepatocellular carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th Ed. IARC Press: Lyon, 2010 In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. [Google Scholar]
- 24.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 25.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 26.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 27.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 29.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 30.Cabibi D, Calvaruso V, Giuffrida L, Ingrao S, Balsamo L, Giannone AG, Petta S, Di Marco V. Comparison of Histochemical Staining Methods and Correlation with Transient Elastography in Acute Hepatitis. Pathobiology. 2015;82:48–52. doi: 10.1159/000375264. [DOI] [PubMed] [Google Scholar]
- 31.International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 32.Di Tommaso L, Sangiovanni A, Borzio M, Park YN, Farinati F, Roncalli M. Advanced precancerous lesions in the liver. Best Pract Res Clin Gastroenterol. 2013;27:269–284. doi: 10.1016/j.bpg.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 34.Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Pathologic and radiographic studies of intrahepatic metastasis in hepatocellular carcinoma; the role of efferent vessels. HPB Surg. 1996;10:97–103; discussion 103-104. doi: 10.1155/1996/75210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toyosaka A, Okamoto E, Mitsunobu M, Oriyama T, Nakao N, Miura K. Intrahepatic metastases in hepatocellular carcinoma: evidence for spread via the portal vein as an efferent vessel. Am J Gastroenterol. 1996;91:1610–1615. [PubMed] [Google Scholar]
- 36.Eguchi S, Takatsuki M, Hidaka M, Soyama A, Tomonaga T, Muraoka I, Kanematsu T. Predictor for histological microvascular invasion of hepatocellular carcinoma: a lesson from 229 consecutive cases of curative liver resection. World J Surg. 2010;34:1034–1038. doi: 10.1007/s00268-010-0424-5. [DOI] [PubMed] [Google Scholar]
- 37.Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, Satani M, Yamada S, Okamura S, Hori M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–1009. doi: 10.1245/s10434-013-3376-9. [DOI] [PubMed] [Google Scholar]
- 38.Du M, Chen L, Zhao J, Tian F, Zeng H, Tan Y, Sun H, Zhou J, Ji Y. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer. 2014;14:38. doi: 10.1186/1471-2407-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 40.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iguchi T, Shirabe K, Aishima S, Wang H, Fujita N, Ninomiya M, Yamashita Y, Ikegami T, Uchiyama H, Yoshizumi T, et al. New Pathologic Stratification of Microvascular Invasion in Hepatocellular Carcinoma: Predicting Prognosis After Living-donor Liver Transplantation. Transplantation. 2015;99:1236–1242. doi: 10.1097/TP.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 42.Shi M, Zhang C, Feng K, Zhang Y, Chen M, Guo R, Lin X, Li J. [Micrometastasis distribution in liver tissue surrounding hepatocellular carcinoma] Zhonghua Zhongliu Zazhi. 2002;24:257–260. [PubMed] [Google Scholar]
- 43.Meniconi RL, Komatsu S, Perdigao F, Boëlle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery. 2015;157:454–462. doi: 10.1016/j.surg.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Okusaka T, Okada S, Ueno H, Ikeda M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Fukushima N, Sakamoto M. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer. 2002;95:1931–1937. doi: 10.1002/cncr.10892. [DOI] [PubMed] [Google Scholar]
- 45.Hu XQ. [Histopathological staging and assessment of liver fibrosis in chronic hepatitis patients] Zhonghua Ganzangbing Zazhi. 2008;16:169–170. [PubMed] [Google Scholar]
- 46.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 47.Cong WM, Dong H, Xian ZH. Tumors of the liver and intrahepatic bile ducts. In: Wu BQ, Liu YF. Immunohistochemistry for Diagnostic Pathology, Beijing Science and Technology Press: Beijing, 2013 [Google Scholar]
- 48.Ordóñez NG. Arginase-1 is a novel immunohistochemical marker of hepatocellular differentiation. Adv Anat Pathol. 2014;21:285–290. doi: 10.1097/PAP.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 49.Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, Katoonizadeh A, Wouters J, van Kempen LC, Durnez A, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63:674–685. doi: 10.1136/gutjnl-2012-304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia L, Huang W, Tian D, Zhu H, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:600–612. doi: 10.1016/j.jhep.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 51.Jin GZ, Yu WL, Dong H, Zhou WP, Gu YJ, Yu H, Yu H, Lu XY, Xian ZH, Liu YK, et al. SUOX is a promising diagnostic and prognostic biomarker for hepatocellular carcinoma. J Hepatol. 2013;59:510–517. doi: 10.1016/j.jhep.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 52.Roessler S, Budhu A, Wang XW. Deciphering cancer heterogeneity: the biological space. Front Cell Dev Biol. 2014;2:12. doi: 10.3389/fcell.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, Ye QH, Qin LX. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874–1885. doi: 10.1002/hep.26941. [DOI] [PubMed] [Google Scholar]
- 54.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liau SS, Qureshi MS, Praseedom R, Huguet E. Molecular pathogenesis of hepatic adenomas and its implications for surgical management. J Gastrointest Surg. 2013;17:1869–1882. doi: 10.1007/s11605-013-2274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cong W, Wu M. [Liver cancer: new strategy of molecular pathological diagnosis and new mode of clinical therapy] Zhonghua Yixue Zazhi. 2014;94:1521–1523. [PubMed] [Google Scholar]
- 57.Wang B, Xia CY, Lau WY, Lu XY, Dong H, Yu WL, Jin GZ, Cong WM, Wu MC. Determination of clonal origin of recurrent hepatocellular carcinoma for personalized therapy and outcomes evaluation: a new strategy for hepatic surgery. J Am Coll Surg. 2013;217:1054–1062. doi: 10.1016/j.jamcollsurg.2013.07.402. [DOI] [PubMed] [Google Scholar]
- 58.Zhu Y, Gu Y, Lu X, Cong W. [The clonal characteristics of late recurrent hepatocellular carcinoma after resection: a study of 2 cases] Zhonghua Zhongliu Zazhi. 2014;36:450–452. [PubMed] [Google Scholar]
- 59.Matsumoto Y, Fujii H, Matsuda M, Kono H. Multicentric occurrence of hepatocellular carcinoma: diagnosis and clinical significance. J Hepatobiliary Pancreat Surg. 2001;8:435–440. doi: 10.1007/s005340100006. [DOI] [PubMed] [Google Scholar]
- 60.Finkelstein SD, Marsh W, Demetris AJ, Swalsky PA, Sasatomi E, Bonham A, Subotin M, Dvorchik I. Microdissection-based allelotyping discriminates de novo tumor from intrahepatic spread in hepatocellular carcinoma. Hepatology. 2003;37:871–879. doi: 10.1053/jhep.2003.50134. [DOI] [PubMed] [Google Scholar]
- 61.Gehrau R, Mas V, Archer KJ, Maluf D. Molecular classification and clonal differentiation of hepatocellular carcinoma: the step forward for patient selection for liver transplantation. Expert Rev Gastroenterol Hepatol. 2011;5:539–552. doi: 10.1586/egh.11.48. [DOI] [PubMed] [Google Scholar]


