Abstract
AIM
To study the tolerance and the efficiency of FOLFIRINOX in elderly patients diagnosed with colorectal or pancreatic cancer.
METHODS
This retrospective study included elderly patients aged over 70 years of age treated at Georges-Francois Leclerc Center by FOLFIRINOX for histological proved colorectal or pancreatic cancer between January 2009 and January 2015. Chemotheapy regimen consisted of oxaliplatin (85 mg/m2 in over 120 min) followed by leucovorin (400 mg/m2 in over 120 min), with the addition, after 30 min of irinotecan (180 mg/m2 in over 90 min) then 5 fluorouracil (5FU) (400 mg/m2 administred intravenous bolus), followed by 5FU (2400 mg/m2 intraveinous infusion over 46 h) repeated every 2 wk. Geriatric parameters were recorded at the beginning. Toxicities were evaluated with the Common Terminology Criteria for Adverse Events 4.03. Tumor response was evaluated by CT scan. Treatment continued until disease progression, unacceptable toxicities or patient refusal.
RESULTS
Fifty-two patients aged from 70 to 87 years were treated by FOLFIRINOX, 34 had colorectal cancer and 18 had pancreatic cancer. Most of them were in good general condition, 82.7% had a 0-1 performance status and 61.5% had a Charlson Comorbidity Index < 10. The most frequent severe toxicities were neutropenia (17 patients, n = 32.7%) and diarrhea (35 patients n = 67.3%); 10 of the case of neutropenia and 5 of diarrhea registered a grade 4 toxicity. Thirty-nine patients (75%) initially received an adapted dose of chemotherapy. The dosage was adjusted for 26% of patients during the course of treatment. Tumor response evaluated by RECIST criteria showed a controlled disease for 25 patients (48.1%), a stable disease for 13 and a partial response for 12 patients. Time under treatment was higher for colorectal cancer with a median time of 2.44 mo (95%CI: 1.61-3.25). Overall survival was 43.88 mo for colorectal cancer and 12.51 mo for pancreatic cancer. In univariate or multivariate analysis, none of geriatric parameters were linked to overall survival. Only the type of tumor (pancreatic/colorectal) was linked in both analysis.
CONCLUSION
For people over 70 years old, FOLFIRINOX regimen seems to induce manageable toxicities but similar, even higher, median survival rates compared to younger people.
Keywords: Elderly patients, Feasibility treatment, Pancreatic cancer, Colorectal cancer, FOLFIRINOX
Core tip: The incidence of cancer in patients over 70 years old is still increasing, especially for pancreatic and colorectal cancer. Database is missing concerning elderly patients, especially for considered aggressive chemotherapies, like FOLFIRINOX. The aim of this retrospective study was to show the feasibility of a combination chemotherapies (FOLFIRINOX) in an elderly population, by initially adapting the treatment dose, according to the patient’s general condition and comorbidities. We surprisingly observed prolonged survival and manageable toxicity levels.
INTRODUCTION
In 2016, pancreatic cancer is the fourth leading cause of death by cancer in the United States for patients from 60 to 79 years of age. Between 2005 and 2011, the recorded survival rate at 5 years was of 8% in elderly population[1]. In 2012, in France the incidence was of 6690 cases in patients over 70 years old[2]. With an incidence of 134490 new cases per year in the United States, colorectal cancer is the third most common and lethal cancer in 2016[1]. In France, it is also the third most common cancer in the general population with a majority of patients over 70 years old (58% in 2012)[2]. These proportions will probably increase in the future. Recent studies have already proved the efficiency of a triple-drug combination of fluorouracil, oxaliplatin and irinotecan (FOLFIRINOX) in both types of cancer. For metastatic pancreatic cancer a phase 2-3 trial demonstrated a survival advantage when FOLFIRINOX was used as a first-line therapy compared to gemcitabine, but the patients involved were under 76 years old[3]. For colorectal cancer, this chemotherapy regimen has shown efficiency in association alone or in association with bevacizumab or cetuximab as first-line therapy[4-7] and for more advanced stages, refractory to oxaliplatin and irinotecan[8]. It can also be used as a neo-adjuvant treatment for locally advanced or metastatic rectal cancer as part of a clinical trial[9,10].
Concerning these cancers, elderly patients are underrepresented in trials, or are selected according to their general condition[11,12]. Therefore, there is a lack of evidence-based data when it comes to an older population. The older cancer population is heterogeneous with differences in co-morbidities, functional statuses, geriatric syndroms and socioeconomic aspects[13]. The G8 instrument was approved as a screening tool to identify older patients who needed a geriatric assessment[11]. When it is carried out, it can influence the decision of oncological treatment. The patient’s biological age should ideally be established through this comprehensive geriatric assessment. Age alone should not be an exclusion criteria for the use of new targeted treatments, especially for metastatic colorectal cancer[14,15]. Few trials had already shown that for selected elderly patients, chemotherapy with 5 fluorouracil (5FU), oxaliplatin or irinotecan is feasible with manageable toxicity levels[16-24]. In this retrospective study we report the tolerance and efficacy of FOLFIRINOX in patients over 70 years old treated in our center.
MATERIALS AND METHODS
Study design
This retrospective study was carried out at Georges-Francois Leclerc Center from January 2009 to January 2015. The use of FOLFIRINOX was evaluated and validated by the local multidisciplinary staff. This protocol has been proposed by the referent oncologist for patients who received FOLFIRINOX after 70 years with locally advanced or metastatic pancreatic cancer, or with metastatic colorectal cancer in first-line treatment, or a more advanced stage. All treatments were validated in multidisciplinary staff. Two patients with rectal cancer who had received FOLFIRINOX as neo-adjuvant treatment as part of a clinical trial were included (GRECCAR 4).
Patients characteristics
The study included patients over 70 years old who received FOLFIRINOX, for locally advanced or metastatic pancreatic or colorectal cancer, whatever the treatment line.
We used 70 years old as cut off, because on retrospective evidence, the incidence of geriatric problems increases sharply after 70 years old in oncologic population[25]. Almost, main oncogeriatric studies, with recommendations from the SIOG, are using the age of 70 years old as cut off for geriatric assessment and developing geriatric screening tools[26,27].
For all patients, cancer was histologically confirmed. Patients had to have adequate bone marrow function (granulocyte count > 1500 per cubic millimeter, hemoglobin > 9.0 g/dL and platelet count > 100000 per cubic meter), liver function [total bilirubin < 3 times the upper limit of normal (ULN) and aspartate/alanine transaminases < 5 times the ULN], and renal function (creatinine < 1.2 mg/dL or creatinine clearance > 50 mL/min).
Exclusion criteria were an uncontrolled infection, pre-existing neuropathy grade ≤ 1, a history of drug hypersensitivity, active concomitant malignancy, and concurrent severe medical conditions.
Treatment
The FOLFIRINOX regimen consisted of oxaliplatin at a dose of 85 mg per square meter, given as a 2-h intravenous infusion, immediately followed by leucovorin at a dose of 400 mg per square meter, given as a 2-h intravenous infusion, with the addition, after 30 min of irinotecan at a dose of 180 mg per square meter, given as 90-min intravenous infusion. This treatment was immediately followed by 5FU at a dose of 400 mg per square meter, administered by intravenous bolus, followed by a continuous intravenous infusion of 2400 mg per square meter over a 46-h period. This sequence was repeted every 2 wk. For metastatic colorectal cancer, chemotherapy could be associated with targeted therapies such as bevacizumab or cetuximab. Dose reductions were based on adverse events that were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. Treatment was temporarily suspended in cases of grade 3/4 hematological toxicity or grade 2 or higher non-hematological toxicity. Once the toxicity level was reduced to grade 1 or below, chemotherapy was continued at a lower dose. The treatment was suspended if the patients experienced further toxicity. Dose re-escalation was not applied in this setting. Treatment continued until disease progression, unacceptable toxicity, or patient refusal. As a reminder, FOLFIRINOX was not necessarily administrated as first line therapy for both types of cancer.
Pretreatment and follow-up evaluation
Pretreatment evaluation included comorbidities (heart/lung/liver), usual medications, ECOG performance status, home help, autonomy, metastatic status, metastatic site (liver/lung/peritoneum/other), tumor marker level (ACE, CA 19-9), albumin levels, the use of a targeted therapy (anti-VEGF/anti-EGFR). Tumor response was evaluated using RECIST criteria. Follow-up evaluation included tumor assessment by thorax abdominal and pelvic CT-Scan, tumor marker levels, loss of autonomy, toxicities. Toxicity was graded according to the CTCAE version 4.03. The loss of autonomy was defined by home helps or a convalescence.
Statistical analysis
All patients were followed up until death or the end of data recording (31 January 2015). The time under treatment was defined as the period between the first and last cure of FOLFIRINOX, without progression during this period. Progression free survival was calculated from the date when therapy started to the date of disease progression, and Overall Survival was calculated from the date when therapy started to the date of death. Median follow-up with its 95%CI was calculated using the reverse Kaplan-Meier method. Patient or disease characteristics were examined using the χ2 test or Fisher’s exact test for qualitative variables, and the Student t or Mann-Whitney tests for continuous variables, as appropriate. Survival probabilities were estimated using the Kaplan-Meier method and survival curves were compared using the log-rank test.
Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, United States). All tests were two sided, and P values < 0.05 were considered statistically significant.
RESULTS
Patients characteristics
Between January 2009 and January 2015, a total of 52 patients aged from 70 to 87 years of age were treated at the Department of Medical Oncology, Georges-Francois Leclerc Cancer Center, Dijon, France by FOLFIRINOX, with or without targeted therapy associated, for pancreatic or colorectal cancer. The main demographic and baseline characteristics of patients involved in the study are shown in Table 1.
Table 1.
Patient characteristics n (%)
| Characteristics | Patients |
| Sexe | |
| Male | 34 (67.3) |
| Female | 18 (32.7) |
| Age | |
| < 80 | 9 (17.0) |
| ≥ 80 | 43 (83.0) |
| Tumor location | |
| Colorectal | 34 (65.4) |
| Pancreas | 18 (34.6) |
| Metastasis | |
| ≤ 1 | 26 (50.0) |
| > 1 | 26 (50.0) |
| ECOG performance status | |
| [0-1] | 43 (82.7) |
| [2-3] | 9 (17.3) |
| Comorbidities | |
| No | 15 (28.8) |
| Yes | 37 (71.2) |
| Cardiac comorbidities | |
| No | 40 (76.9) |
| Yes | 12 (23.1) |
| Pulmonary comorbidities | |
| No | 47 (90.4) |
| Yes | 5 (9.6) |
| Hepatic comorbidities | |
| No | 52 (100.0) |
| Yes | 0 (0.0) |
| Albumin level | |
| < 30 g/L | 16 (30.8) |
| ≥ 30 g/L | 21 (40.4) |
| Number of usual drugs | |
| < 3 | 22 (42.3) |
| ≥ 4 | 30 (57.7) |
| Charlson comorbidities index | |
| < 10 | 32 (61.5) |
| ≥ 10 | 20 (38.5) |
| Initial autonomy | |
| No | 3 (5.8) |
| Yes | 49 (94.2) |
The average age was 75, with a median of 74 years of age. The majority of patients had a good general condition, 82.7% (n = 43) were 0-1 performance status and 94.2% (n = 49) had complete autonomy at home. Although 71.2% of patients had comorbidities, the majority of them did not concern vital functions (heart, lung and liver). The Charlson Comorbidity Index (CCI) for general people with a metastatic tumor and without comorbidities is 9 for people aged 70 to 79 years, and 10 for those aged 80 to 89 years. Thirty-two patients (61.5%) had a CCI < 10. The nutritional assessment showed an upper rate of albumin in 30 g/L for 40.4% of patients.
All 52 patients were assessable for toxicity, survival and radiological response using RECIST criteria.
Toxicity and feasibility
A total of 311 cycles of chemotherapy were administrated (median 4.5; range: 1-20). Hematological and non hematological toxicities are listed in Table 2. Any grade 3 or 4 toxicity according to the CTCAE 4.03 was considered severe.
Table 2.
Observed toxicity according Common Terminology Criteria for Adverse Events v 4.03 (n = 52) n (%)
|
CTCAE v 4.03 |
||
| All grades | Severe1 | |
| Hematological | ||
| Anemia | 28 (53.8) | 5 (9.6) |
| Neutropenia | 24 (46.2) | 17 (32.7) |
| Thrombocytopenia | 14 (26.9) | 3 (5.8) |
| Non Hematological | ||
| Diarrhea | 35 (67.3) | 13 (25.0) |
| Nausea and vomiting | 22 (42.3) | 5 (9.6) |
| Asthenia | 49 (94.2) | 5 (9.6) |
| Peripheral Neutropenia | 17 (32.7) | 4 (7.7) |
| Hepatic toxicity | 4 (7.7) | 1 (1.9) |
Grade 3-4 according to the CTCAE version 4.03. CTCAE: Common Terminology Criteria for Adverse Events.
Regarding all toxicity grades more than 1/3 the patients suffered from asthenia (94.2%), diarrhea (67.3%), anemia (52.8%), neutropenia (46.2%) and nausea/vomiting (42.3%).
When focusing on severe sides effects, neutropenia (32.7, n = 24) and diarrhea (25%, n = 13) were the most frequent (Table 2).
Concerning treatment administration, initially, a majority of patients had a reduced dose (75%, n = 39), particularly for irinotecan (67.3%, n = 35), bolus of 5FU (25%) and continuous infusion of 5FU (21.1%). Only 7 patients had a dose reduction of oxaliplatin. During treatment, 26.9% of patients had a dose adjustment (n = 14). The treatment was stopped for 20 patients (38.5%) because of an excessive toxicity and for 15 patients (28.8%) due to disease progression. Almost, 25% of patients could benefited from a maintenance therapy (n = 13) after a response or a stabilization of the disease. Most patients died from cancer, and 5 patients are still alive (only patients with colorectal cancer, including 2 with FOLFIRINOX as a neo adjuvant treatment).
Objective tumor response and survival
The assessment of the best tumor response according to RECIST criteria, showed a progression for 21.1% of patients (n = 11), a stable disease for 25% (n = 13), a partial response for 23.1% (n = 12). Only one patient presented a complete response. Accordingly, the objective response rate was 25% and the disease control rate was 50%. A total of 16 patients couldn’t be evaluated because of an early clinical progression or death.
The median time under treatment was 2.62 mo for colorectal cancer and 2.24 mo for pancreatic cancer (Figure 1).
Figure 1.
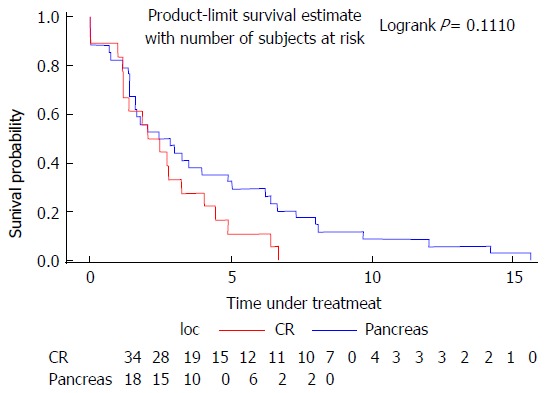
Time under treatment for pancreatic and colorectal cancer.
Overall survival was 43.38 mo (29.64-47.87) for patients with colorectal cancer and 12.51 mo (8.85-17.2) for pancreatic cancer (Figure 2).
Figure 2.
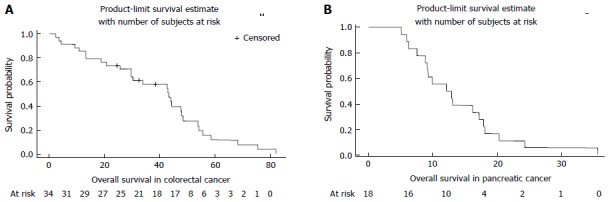
Overall survival for colorectal cancer (A) and pancreatic cancer (B).
In univariate and multivariate analysis, none of geriatric parameters were linked to overall survival or time under treatment (age, comorbidities, autonomy, ECOG performance status, CCI and medication number). Only the type of tumor (pancreas or colorectal) was linked to overall survival in univariate and multivariate analysis, obviously in favor of colorectal cancer (Tables 3 and 4).
Table 3.
Univariate and multivariate analysis by subgroup for time under treatment
|
Univariate analysis |
Multivariate analysis |
|||||||
| HR | 95%CI | P vaule | HR | 95%CI | P vaule | |||
| Tumor location | 0.1204 | 0.4288 | ||||||
| CR | Ref | Ref | ||||||
| Pancréas | 1.615 | 0.882 | 2.958 | 1.316 | 0.667 | 2.599 | ||
| Metastatic sites | 0.0478 | 0.1405 | ||||||
| 0-1 | Ref | Ref | ||||||
| ≥ 2 | 0.56 | 0.315 | 0.994 | 0.613 | 0.32 | 1.175 | ||
| Age | 0.2105 | 0.1637 | ||||||
| < 75 | Ref | Ref | ||||||
| ≥ 75 | 0.692 | 0.389 | 1.231 | 0.664 | 0.373 | 1.182 | ||
| Age | 0.371 | |||||||
| < 80 | Ref | |||||||
| ≥ 80 | 1.394 | 0.673 | 2.889 | |||||
| Comorbidities | 0.979 | |||||||
| No | Ref | |||||||
| Yes | 1.008 | 0.549 | 1.851 | |||||
| Neoadjuvant chemotherapy | 0.2339 | |||||||
| No | Ref | |||||||
| Yes | 2.432 | 0.563 | 10.5 | |||||
| Initial autonomy (J0) | 0.9059 | |||||||
| No | Ref | |||||||
| Yes | 0.932 | 0.288 | 3.018 | |||||
| Charlson comorbidities index | 0.7659 | |||||||
| < 10 | Ref | |||||||
| ≥ 10 | 0.915 | 0.511 | 1.639 | |||||
| ECOG performance status | 0.5046 | |||||||
| 0-1 | Ref | |||||||
| ≥ 2 | 1.281 | 0.618 | 2.655 | |||||
| Sexe | 0.7228 | |||||||
| Male | Ref | |||||||
| Female | 0.856 | 0.361 | 2.026 | |||||
| Number of usual drugs | 0.3057 | |||||||
| < 4 | Ref | |||||||
| ≥ 4 | 1.34 | 0.765 | 2.347 | |||||
Table 4.
Univariate and multivariate analysis by subgroup for overall survival
|
Univariate analysis |
Multivariate analysis |
|||||||
| HR | 95%CI | P vaule | HR | 95%CI | P vaule | |||
| Tumor location | < 10-4 | < 10-4 | ||||||
| CR | Ref | Ref | ||||||
| Pancréas | 6.362 | 2.963 | 13.66 | 5.816 | 2.47 | 13.693 | ||
| Metastatic sites | 0.0634 | 0.9874 | ||||||
| 0-1 | Ref | Ref | ||||||
| ≥ 2 | 0.555 | 0.298 | 1.034 | 1.006 | 0.498 | 2.032 | ||
| Age | 0.5721 | 0.7614 | ||||||
| < 75 | Ref | Ref | ||||||
| ≥ 75 | 1.184 | 0.659 | 2.124 | 1.096 | 0.607 | 1.976 | ||
| Age | 0.5365 | 0.274 | ||||||
| < 80 | Ref | Ref | ||||||
| ≥ 80 | 1.277 | 0.588 | 2.774 | 1.611 | 0.686 | 3.786 | ||
| Sexe | 0.1178 | 0.4136 | ||||||
| Male | Ref | Ref | ||||||
| Female | 0.464 | 0.177 | 1.215 | 0.634 | 0.212 | 1.891 | ||
| Comorbidities | 0.0899 | 0.8701 | ||||||
| No | Ref | Ref | ||||||
| Yes | 1.787 | 0.914 | 3.495 | 1.066 | 0.494 | 2.301 | ||
| Initial autonomy | 0.7372 | |||||||
| No | Ref | |||||||
| Yes | 1.277 | 0.306 | 5.336 | |||||
| Charlson comorbidities index | 0.7507 | |||||||
| < 10 | Ref | |||||||
| ≥ 10 | 1.101 | 0.608 | 1.992 | |||||
| ECOG performance status | 0.6426 | |||||||
| 0-1 | Ref | |||||||
| ≥ 2 | 0.833 | 0.385 | 1.802 | |||||
| Number of usual drugs | 0.6116 | |||||||
| < 4 | Ref | |||||||
| ≥ 4 | 1.169 | 0.64 | 2.132 | |||||
DISCUSSION
This retrospective study showed that polychemotherapies considered toxic, like FOLFIRINOX, is feasible in a geriatric population with a good general condition, probably thanks to an initial dose adaptation. Age alone should not be a limiting factor for using this type of treatment. Elderly patients require a global geriatric assessment.
Our study population was healthier than a standard geriatric population with metastatic cancer. Indeed 82.7% of patients had a 0-1 performance status and 61.5% had a CCI < 10 in our study. However, for ethical reasons, a triple-drug combination cannot be given to an unhealthy patient with a performance status > 2.
A phase 2-3 French trial that compared gemcitabine to FOLFIRINOX for metastatic pancreatic cancer, had already demonstrated the efficiency of this combination[3]. But the patients in the study were younger and had a good performance status. In this trial, overall survival was 11.1 months in FOLFIRINOX group (95%CI: 9.0-13.1). This results are comparable to the results of our study with an overall survival of 12.51 mo. Another phase 3 trial, this time comparing gemcitabine to gemcitabine plus nab-paclitaxel in pancreatic cancer, showed a median of overall survival of 8.5 mo (95%CI: 7.9-9.5) for nab-paclitaxel group, which was better than the gemcitabine alone group, knowing that the median age in this group was 62[28]. These results are in favor of administrating FOLFIRINOX in pancreatic cancer, even for elderly patients. Concerning colorectal cancer, we could observe a median overall survival of 43.38 mo (95%CI: 29.64-47.87), which is clearly higher than in other trials, involving younger patients[4-6,29,30]. ASCO 2016 presented the first results of the phase 2 multicentric french trial METHEP-2, which tends to confirm the superiority of FOLFIRINOX associated to biotherapies versus a bi-chemotherapy for colorectal cancer with initially unresectable liver metastases[7]. Median overall survival was of 36 mo for bi-chemotherapy, and hasn’t been reached for FOLFIRINOX group. Time under treatment for colorectal cancer in our study was low (2.62 mo), contrasting with high overall survival rates. We could suspect that either colorectal cancer progresses slowly or that tumor response obtained with FOLFIRINOX induces a prolonged control of the disease.
In important trials[4-6,31,32], most common severe toxicities (grade ≥ 3) with this chemotherapy were neutropenia and diarrhea. In our geriatric population, severe neutropenia rates were similar to the rates observed in the literature data for younger patients, with approximatively a third of patients who underwent neutropenia (32.7%). Seventy-five percent of patients had a reduction in the dosage of chemotherapy from the start: 67.3% for irinotecan and 25% for bolus of 5FU. It is thanks to this dose reduction that our geriatric population did not suffer more of neutropenia than a younger population. Systematically using GCSF, could clearly reduce the proportion of severe neutropenia rates. The only severe toxicity that was more frequent than in other studies, was diarrhea. Indeed 25% of our population (n = 13) was concerned. Systematically prescribing antidiarrheal treatments should be recommended.
Our study limits were the retrospective and monocentric plan. However, there was little missing data for patients. Also, we could consider that 52 patients is a poor recruitment, but regarding the characteristics of the population we studied (the elderly people with pancreatic/colorectal cancer, able to receive FOLFIRINOX), these numbers are clearly acceptable. Finally, carrying out a study including both pancreatic and colorectal cancer, which are two different types of cancer when it comes to evolution and prognosis, can be questionable at first. But the primary objective of our study was to evaluate the feasibility of FOLFIRINOX in an elderly population, whatever the primary tumor.
In conclusion, FOLFIRINOX is a triple-drug combination feasible in geriatric population, over 70 years of age, with manageable toxicity levels and interesting rates of overall survival, especially for colorectal cancer, compared to a younger population. Currently, a phase 2 French trial, PAMELA-70, is trying to confirm these results by evaluating the efficiency and tolerance of dose adjusted FOLFIRINOX in elderly patients with a metastatic pancreatic cancer[33].
COMMENTS
Background
FOLFIRINOX regimen is an effective chemotherapy for locally advanced or metastatic pancreatic and colorectal cancer. However, datas are lacking concerning the use of this chemotherapy for elderly people.
Research frontiers
Most studies demonstrating the efficacy of FOLFIRINOX didn’t include elderly patients, aged more than 70 or 75 years old. We treated patients aged more than 70 years old, usually with an initial dose adaptation.
Innovations and breakthroughs
The median overall survival was 43.88 mo for colorectal cancer and 12.51 mo for pancreatic cancer in this geriatric population, similar, even higher, compared to younger people. Only a third (32.7%) had severe neutropenia and a quarter had severe diarrhea.
Applications
This study submit the possibility of using FOLFIRINOX for elderly people aged more than 70 years old by paying attention to main severe toxicities, as neutropenia and diarrhea.
Terminology
FOLFIRINOX regimen consisted of oxaliplatin at a dose of 85 mg per square meter, given as a 2-h intravenous infusion, immediately followed by leucovorin at a dose of 400 mg per square meter, given as a 2-h intravenous infusion, with the addition, after 30 min of irinotecan at a dose of 180 mg per squar meter, given as 90-min intravenous infusion. This treatment was immediately followed by 5 fluorouracil at a dose of 400 mg per square meter, administred by intravenous bolus, followed by a continuous intravenous infusion of 2400 mg per square meter over 46-h period.
Peer-review
The manuscript by Guion-Dusserre and colleagues analyzes FOLFIRINOX in elderly patients with pancreatic (PDAC) and colorectal (CRC) cancer. This retrospective study included patients over 70 years and 52 patients were treated by FOLFIRINOX, 34 had CRC and 18 had PDAC. The authors show that FOLFIRINOX toxicities were manageable and that median survival rates were comparably good. This is a well written and clinical interesting and relevant study.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by the local insitutional review board.
Informed consent statement: I declare that all patients give written inform consent to participate in clinical research before receiving any therapy.
Conflict-of-interest statement: All authors declare no conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: July 1, 2016
First decision: July 29, 2016
Article in press: September 28, 2016
P- Reviewer: Kleeff J, Lakatos PL, Yoshitomi H S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Binder-Foucard F, Belot A, Delafosse P, Remontet L, Woronoff A-S, Bossard N. Estimation nationale de l’incidence et de la mortalité par cancer en France entre 1980 et 2012. Partie 1 – Tumeurs solides. Saint-Maurice (Fra): Institut de veille sanitaire 2013; 112 [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 5.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–1618. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 6.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, Mezi S, Tomasello G, Ronzoni M, Zaniboni A, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 7.Ychou M. FOLFIRINOX combined to targeted therapy according RAS status for colorectal cancer patients with liver metastases initially non-resectable: A phase II randomized Study—Prodige 14 – ACCORD 21 (METHEP-2), an unicancer GI trial. J Clin Oncol. 2016;Abstract 3512:2009-012813-22. [Google Scholar]
- 8.Chaix M, Vincent J, Lorgis V, Ghiringhelli F. FOLFIRINOX bevacizumab is a promising therapy for chemorefractory metastatic colorectal cancer. Oncology. 2014;87:148–158. doi: 10.1159/000361031. [DOI] [PubMed] [Google Scholar]
- 9.Institut du Cancer de Montpellier - Val d’Aurelle. Multicenter Phase 2 Trial: a Tailored Strategy for Locally Advanced Rectal Carcinoma (GRECCAR4), 2011. Available from: https://clinicaltrials.gov/ct2/show/NCT01333709?term=NCT01333709&rank=1.
- 10.Bachet JB, Lucidarme O, Taïeb J, Maillard E, Levache CB, Raoul JL, Lecomte T, Hebbar M, Brocard F, Pernot S, et al. FOLFIRINOX as induction treatment in rectal cancer patients with synchronous metastases (RCSM): Results of the FFCD 1102 phase II trial. J Clin Oncol. 2016;34 Suppl:abtract 3513. [Google Scholar]
- 11.Ruegsegger DR, Lerude SD, Lyle D. Electron-beam arc therapy using a high energy betatron. Radiology. 1979;133:483–489. doi: 10.1148/133.2.483. [DOI] [PubMed] [Google Scholar]
- 12.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, Falandry C, Artz A, Brain E, Colloca G, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decoster L, Van Puyvelde K, Mohile S, Wedding U, Basso U, Colloca G, Rostoft S, Overcash J, Wildiers H, Steer C, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations†. Ann Oncol. 2015;26:288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 14.Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D, Köhne CH, Rostoft S, Lemmens V, Mitry E, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2015;26:463–476. doi: 10.1093/annonc/mdu253. [DOI] [PubMed] [Google Scholar]
- 15.Köhne CH, Folprecht G, Goldberg RM, Mitry E, Rougier P. Chemotherapy in elderly patients with colorectal cancer. Oncologist. 2008;13:390–402. doi: 10.1634/theoncologist.2007-0043. [DOI] [PubMed] [Google Scholar]
- 16.Seymour MT, Thompson LC, Wasan HS, Middleton G, Brewster AE, Shepherd SF, O’Mahony MS, Maughan TS, Parmar M, Langley RE. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–1759. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aparicio T, Lavau-Denes S, Phelip JM, Maillard E, Jouve JL, Gargot D, Gasmi M, Locher C, Adhoute X, Michel P, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02) Ann Oncol. 2016;27:121–127. doi: 10.1093/annonc/mdv491. [DOI] [PubMed] [Google Scholar]
- 18.Aparicio T, Desramé J, Lecomte T, Mitry E, Belloc J, Etienney I, Montembault S, Vayre L, Locher C, Ezenfis J, et al. Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly. Br J Cancer. 2003;89:1439–1444. doi: 10.1038/sj.bjc.6601310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popescu RA, Norman A, Ross PJ, Parikh B, Cunningham D. Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol. 1999;17:2412–2418. doi: 10.1200/JCO.1999.17.8.2412. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, Rothenberg ML, Green E, Sargent DJ. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 21.Folprecht G, Cunningham D, Ross P, Glimelius B, Di Costanzo F, Wils J, Scheithauer W, Rougier P, Aranda E, Hecker H, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol. 2004;15:1330–1338. doi: 10.1093/annonc/mdh344. [DOI] [PubMed] [Google Scholar]
- 22.Sastre J, Marcuello E, Masutti B, Navarro M, Gil S, Antón A, Abad A, Aranda E, Maurel J, Valladares M, et al. Irinotecan in combination with fluorouracil in a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. J Clin Oncol. 2005;23:3545–3551. doi: 10.1200/JCO.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Souglakos J, Pallis A, Kakolyris S, Mavroudis D, Androulakis N, Kouroussis C, Agelaki S, Xenidis N, Milaki G, Georgoulias V. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology. 2005;69:384–390. doi: 10.1159/000089992. [DOI] [PubMed] [Google Scholar]
- 24.Rosati G, Cordio S, Tucci A, Blanco G, Bordonaro R, Reggiardo G, Manzione L. Phase II trial of oxaliplatin and tegafur/uracil and oral folinic acid for advanced or metastatic colorectal cancer in elderly patients. Oncology. 2005;69:122–129. doi: 10.1159/000087814. [DOI] [PubMed] [Google Scholar]
- 25.Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 26.Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sørbye L, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–252. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Tapia C, Canoui-Poitrine F, Bastuji-Garin S, Soubeyran P, Mathoulin-Pelissier S, Tournigand C, Paillaud E, Laurent M, Audureau E. Optimizing the G8 Screening Tool for Older Patients With Cancer: Diagnostic Performance and Validation of a Six-Item Version. Oncologist. 2016;21:188–195. doi: 10.1634/theoncologist.2015-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souglakos J, Androulakis N, Syrigos K, Polyzos A, Ziras N, Athanasiadis A, Kakolyris S, Tsousis S, Kouroussis Ch, Vamvakas L, Kalykaki A, Samonis G, Mavroudis D, Georgoulias V. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG) Br J Cancer. 2006;94:798–805. doi: 10.1038/sj.bjc.6603011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masi G, Cupini S, Marcucci L, Cerri E, Loupakis F, Allegrini G, Brunetti IM, Pfanner E, Viti M, Goletti O, et al. Treatment with 5-fluorouracil/folinic acid, oxaliplatin, and irinotecan enables surgical resection of metastases in patients with initially unresectable metastatic colorectal cancer. Ann Surg Oncol. 2006;13:58–65. doi: 10.1245/ASO.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 31.Assaf E, Verlinde-Carvalho M, Delbaldo C, Grenier J, Sellam Z, Pouessel D, Bouaita L, Baumgaertner I, Sobhani I, Tayar C, et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011;80:301–306. doi: 10.1159/000329803. [DOI] [PubMed] [Google Scholar]
- 32.Ychou M, Viret F, Kramar A, Desseigne F, Mitry E, Guimbaud R, Delpero JR, Rivoire M, Quénet F, Portier G, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharmacol. 2008;62:195–201. doi: 10.1007/s00280-007-0588-3. [DOI] [PubMed] [Google Scholar]
- 33.Institut de Cancérologie de l’Ouest. Phase 2 trial: Efficacy and Tolerance Evaluation in FOLFIRINOX Dose Adjusted in Elderly Patients with a Metastatic Pancreatic Cancer (PAMELA70), 2014. Available from: https://clinicaltrials.gov/ct2/show/NCT02143219.


