Abstract
Significance: Monocyte and macrophage dysfunction plays a critical role in a wide range of inflammatory disease processes, including obesity, impaired wound healing diabetic complications, and atherosclerosis. Emerging evidence suggests that the earliest events in monocyte or macrophage dysregulation include elevated reactive oxygen species production, thiol modifications, and disruption of redox-sensitive signaling pathways. This review focuses on the current state of research in thiol redox signaling in monocytes and macrophages, including (i) the molecular mechanisms by which reversible protein-S-glutathionylation occurs, (ii) the identification of bona fide S-glutathionylated proteins that occur under physiological conditions, and (iii) how disruptions of thiol redox signaling affect monocyte and macrophage functions and contribute to atherosclerosis. Recent Advances: Recent advances in redox biochemistry and biology as well as redox proteomic techniques have led to the identification of many new thiol redox-regulated proteins and pathways. In addition, major advances have been made in expanding the list of S-glutathionylated proteins and assessing the role that protein-S-glutathionylation and S-glutathionylation-regulating enzymes play in monocyte and macrophage functions, including monocyte transmigration, macrophage polarization, foam cell formation, and macrophage cell death. Critical Issues: Protein-S-glutathionylation/deglutathionylation in monocytes and macrophages has emerged as a new and important signaling paradigm, which provides a molecular basis for the well-established relationship between metabolic disorders, oxidative stress, and cardiovascular diseases. Future Directions: The identification of specific S-glutathionylated proteins as well as the mechanisms that control this post-translational protein modification in monocytes and macrophages will facilitate the development of new preventive and therapeutic strategies to combat atherosclerosis and other metabolic diseases. Antioxid. Redox Signal. 25, 816–835.
Keywords: : macrophage, redox signaling, thiols, atherosclerosis, S-glutathionylation
Introduction
Macrophages are ubiquitous to every tissue of the body. They contribute to normal development, promotion and resolution of inflammation, and tissue homeostasis, which explains why macrophage dysregulation plays a critical role in a wide range of disease processes such as impaired wound healing and chronic inflammatory diseases such as atherosclerosis (120, 224). Macrophages belong to the mononuclear phagocyte system and function as part of the innate immunity to detect and clear invading pathogens as well as dying cells and tissue debris (92). Upon phagocytosing bacteria or tissue debris, macrophages can function in certain lymphoid tissues to process and present antigens to lymphocytes (e.g., T cells or B cells), thereby helping to stimulate an adaptive immune response (46, 210). In addition, macrophages are extremely diverse and adaptive (i.e., plastic) to their microenvironment. Macrophages are potent secretory cells of various cytokines in response to activation by infectious agents, danger signals, and other environmental cues, and these cytokines have paracrine and autocrine effects within the tissue (114).
The classification of macrophage activation states dates back to the 1960s and is, to a large extent, based on the ex vivo response of macrophages to Th1 and Th2 cytokines [reviewed in Martinez and Gordon (137)]. In 2004, Mantovani et al. proposed the classification of activated or polarized macrophages into classical (M1: IFN-γ + TNF-α or LPS) polarization and three different types of alternative (M2) polarization based on the activating agent: M2a referred to IL-4 and IL-13 activation, M2b referred to immune complex activation, and M2c referred to IL-10 activation (136). Since then, several other activating agents have been described in macrophages, including CXCL4 being identified as an M4-polarizing agent (75). This macrophage classification system has been broadly utilized to characterize the heterogeneity of macrophages in atherosclerotic plaques in vivo (74, 198). While the M1 and M2 classification system is inadequate to capture the complexity and diversity of macrophage activation states (151), we will use these terms here for the sake of simplicity and clarity, referring to macrophages with predominantly proinflammatory proatherogenic properties as M1 and macrophage phenotypes with predominantly anti-inflammatory and inflammation-resolving properties as M2.
In addition to the assorted cellular functions, macrophages originate from at least two different sources during different developmental stages as well as homeostatic versus inflammatory conditions. During homeostasis, the majority of tissue-resident macrophage populations, including splenic red pulp macrophages, microglia, Kupffer cells, osteoclasts, Langerhans cells, and large peritoneal macrophages, originate from embryonic yolk sac-derived progenitor cells (54, 55, 79, 177, 231). However, in response to tissue injury, there is a vast infiltration of monocyte-derived macrophages that originate from bone marrow hematopoietic stem cells (125, 198, 237). This recruitment of blood monocytes to discrete anatomical locations, followed by their differentiation into mature macrophages, is a rate-limiting step in multiple physiological processes, including wound repair, pathogen clearance, and replacement of tissue-resident macrophages (43, 80), as well as pathophysiological processes such as obesity and atherosclerosis (147, 219). Interestingly, blood monocytes themselves are categorized into subsets that can be identified based on the expression of certain surface markers, with CD14hi:CD16lo monocytes in humans considered classical (inflammatory) monocytes that contribute to the monocyte-derived macrophage tissue population and CD14lo:CD16hi nonclassical (patrolling) monocytes that survey endothelial integrity (73). The two functionally distinct monocyte subsets have also been identified in the mouse based on the expression of Ly6C, CX3CR1, and CCR2, with Ly6Chi, CCR2+, and CX3CR1lo monocytes identified as the classical subset and Ly6Clo, CCR2−, and CX3CR1hi monocytes as nonclassical monocytes (70). Similar monocyte subsets have now been described in various species, including rats, pigs, cows, horses, and nonhuman primates (236).
The increased production of reactive oxygen species (ROS) in monocytes and macrophages is associated with changes in cell function, activation state, and survival (63). These events are usually associated with a shift in the balance between ROS production and cellular antioxidant systems such as the glutathione-dependent antioxidant system (85, 175). Under conditions that lead to elevated rates of hydrogen peroxide (H2O2) and organic peroxide (ROOH) production, cysteine residues become highly susceptible to thiol oxidation, S-nitrosylation, S-cysteinylation, and S-glutathionylation (77, 82). These thiol modifications, in turn, lead to changes in protein activities and/or localization and thus can act as signaling mechanisms to regulate cell function. Recently, our group has characterized a number of proteins that are S-glutathionylated and contribute to (dys)function of monocytes by enhancing infiltration of monocyte-derived macrophages (100, 101, 163, 207, 208). In this review, we will discuss the current state of research of protein-S-glutathionylation and thiol redox signaling in monocytes and macrophages. We will also discuss how thiol redox-dependent changes in cell functions relate to the progression of inflammatory diseases linked to monocyte and macrophage dysfunction, with a specific emphasis on atherosclerosis.
Protein thiol modifications in monocytes or macrophages
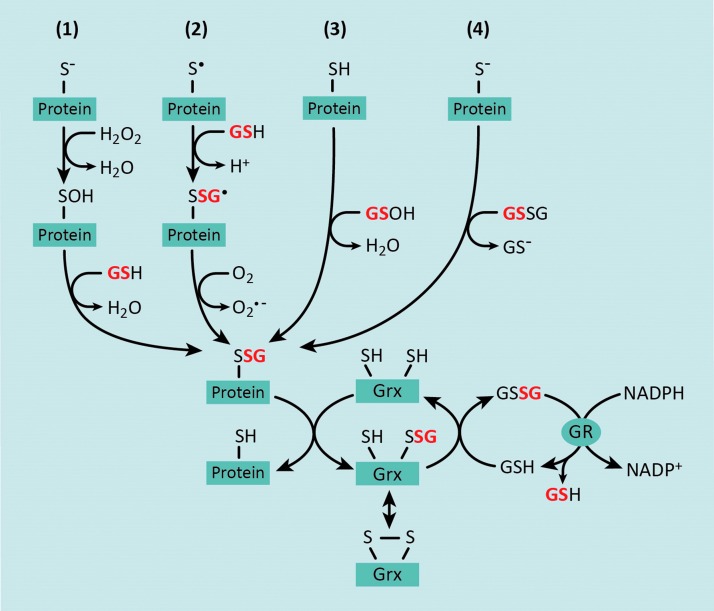
The reactivity of a protein thiol is largely determined by its pKa, whereby lower pKa and more acidic thiols are more easily oxidized (221). Under normal physiological conditions, most protein thiols are in the reduced state due to the highly reductive state of the cytosol (76). However, under conditions of oxidative stress, increased production of H2O2 and ROOH leads to thiol oxidation, producing proteins with sulfenic, sulfinic, and sulfonic acid residues (82, 144) (Fig. 1). In turn, oxidized thiol groups, particularly sulfenic acids, can react with glutathione (GSH) to form mixed disulfides or S-glutathionylated proteins (82) (Fig. 1, #1). Alternatively, deprotonation of protein thiols allows the molecule to exchange GSH with glutathione disulfide (GSSG) to produce an S-glutathionylated protein (82) (Fig. 1, #4). However, only a small proportion of cysteine thiols are deprotonated under physiological pH (82). Furthermore, the intracellular GSH:GSSG surpasses 100 in the absence of overt oxidative stress (72), suggesting that the transfer of the GSH moiety from GSSG to a protein thiol is highly unlikely to be a major intracellular mechanism of S-glutathionylation. A thiyl radical-mediated mechanism for protein-S-glutathionylation has also been proposed, which involves a protein thiyl radical (protein-S•) reacting with GSH and being stabilized by radical transfer to molecular oxygen to form superoxide (O2−•) (186, 218) (Fig. 1, #2). Alternatively, GSH itself can form a sulfenic acid (GS-OH), which can react with protein sulfhydryl residues to generate S-glutathionylated proteins (82, 144) or GSH to form GSSG (Fig. 1, #3). It should be noted that under nitrosative stress, certain protein thiols and GSH can also form S-nitrosothiols, for example, S-nitrosoglutathione (GSNO), which can react with protein thiols to form mixed disulfides, resulting in S-glutathionylation (64). However, a discussion of S-nitrosylated proteins is beyond the scope of this review.
FIG. 1.
Biochemical mechanisms for reversible S-glutathionylation of protein thiols. There are four major mechanisms by which protein thiols can become S-glutathionylated: (1) in the presence of hydrogen peroxide (H2O2), protein thiols are oxidized to highly reactive protein sulfenic acids that can react with the glutathione (GSH); (2) several lines of evidence support a thiyl radical-mediated mechanism for protein-S-glutathionylation, which involves a protein thiyl radical (protein-S·) reacting with GSH and being stabilized by radical transfer to molecular oxygen to form superoxide (O2−•); (3) cellular GSH can react with endogenous reactive oxygen species (ROS) to form a GSH sulfenic acid, thereby facilitating a reaction with a reduced protein thiol; and (4) GSH exchange occurs between glutathione disulfide (GSSG) and a protein thiolate, generating an S-glutathionylated protein and GSH. Protein-S-glutathionylation is reversed by the activity of glutaredoxin (Grx) enzymes. Deglutathionylation and reduction of a target protein involve the transfer of the glutathionyl group onto Grx, which can subsequently be reduced by GSH, resulting in the formation of GSSG. In turn, GSSG is reduced back to two molecules of GSH by glutathione reductase (GR), which uses electrons from NADPH. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The concept that S-glutathionylated proteins could serve as thiol redox signaling proteins was first postulated by Mieyal et al. in 1995 (145). The criteria for S-glutathionylation as a regulatory mechanism are (i) S-glutathionylation occurs at a specific cysteine residue, (ii) S-glutathionylation changes the function of the modified protein, (iii) S-glutathionylation occurs on endogenous proteins in response to a physiological stimulus, (iv) the extent of thiol modification corresponds with the level of the physiological stimulus, and (v) S-glutathionylation is reversible (143). In fact, protein-S-glutathionylation is emerging as a major reversible post-translational cysteine modification that under conditions of prolonged oxidative stress, can lead to the dysregulation of proteins involved in transcription, DNA synthesis, protein turnover, apoptosis, signal transduction, and mitochondrial function (5, 45, 66, 132, 182). The reversal of protein-S-glutathionylation is catalyzed by glutaredoxin (Grx) enzymes whereby Grx catalyzes the transfer of the GSH adduct from substrate proteins and onto its own cysteine residue (Fig. 1). Grx can then return to the reduced state upon donation of the GSH group to another GSH molecule, thereby producing GSSG (Fig. 1). GSSG, in turn, is reduced back to two molecules of GSH by glutathione reductase (GR), which uses electrons from NADPH (Fig. 1).
Several proteomic approaches have been developed to characterize protein-S-glutathionylation in various cell types, including bacteria, protozoa, plants, and mammalian cells and tissues (29, 52, 65, 98, 127, 153). More recently, our group and others have used proteomic approaches to characterize protein-S-glutathionylation in monocytes and macrophages (148, 187, 209). These proteomic approaches rely on trapping protein thiols in their native redox state by the addition of a thiol-alkylating agent such as N-ethyl maleimide (NEM) (34). After trapping free protein thiols, oxidized thiol residues are labeled directly with chemoselective probes to identify proteins with modified thiol residues. For example, dimedone (5,5-dimethyl-1,3-cyclohexadione) has been used to label and map protein sulfenic acid residues in cells (34), and a biotinylated dimedone analog has been used to affinity purify and identify sulfenated proteins in a proteomic screen using rat cardiac myocytes and heart tissue (30). In addition, GSH can be enzymatically removed from proteins in vitro. The resulting free thiols can then be labeled with specific thiol-reactive probes and analyzed by mass spectrometry to identify proteins with S-glutathionylated thiol residues (3, 88). In monocytes and macrophages, there have been no studies using biotinylated dimedone to identify sulfenated proteins; however, Su et al. recently identified potentially S-glutathionylated proteins in the RAW264.7 macrophage-like cell line by selectively deglutathionylating NEM-treated cell lysates and enriching proteins with a thiol-affinity resin (187). Enriched proteins were then labeled with an isobaric tag (iTRAQ) and examined by mass spectrometry (187). This study identified 265 proteins that are sensitive to S-glutathionylation in response to H2O2 treatment (187). Since the levels of H2O2 in this study were supraphysiological, it remains to be determined whether the proteins identified in this study are bona fide S-glutathionylated proteins under physiological conditions or will meet any of the other criteria set forth by Mieyal et al. for thiol redox signaling (143). In contrast, McGarry et al. have used a tandem mass tag labeling protocol along with MS/MS analysis to identify 724 S-glutathionylated proteins in mouse liver lysates under physiological conditions (139). More recently and more relevant to monocytes and macrophages, our group has used a mass spectrometry-based proteomics approach to identify S-glutathionylated proteins in both the THP-1 monocyte cell line and purified mouse peritoneal macrophages under baseline conditions as well as under conditions of metabolic stress induced by hyperglycemia and high low-density lipoprotein levels (HG+LDL) (209). In this study, we selectively deglutathionylated NEM-treated monocyte or macrophage lysates with recombinant Grx and labeled the reduced thiol residues with iodoacetamide conjugated to desthiobiotin. These labeled proteins were then affinity purified and examined by LC-MS/MS (209). We identified over 100 proteins that were S-glutathionylated under physiological conditions in monocytes or macrophages, many of which showed changes in their S-glutathionylation status in response to metabolic stress (209).
In addition to proteomic screens, there are now a growing number of reports for individual proteins being S-glutathionylated in either monocytes or macrophages under physiological conditions. For example, our group has shown that metabolic stress leads to an increase in global protein-S-glutathionylation levels (207), and we identified β-actin, mitogen-activated protein kinase (MAPK) phosphatase 1 (MKP-1), and 14-3-3ζ as S-glutathionylated proteins in THP-1 monocytes (100, 101, 207). MKP-1 activity is modulated via modification of Cys258, while Cys25 of 14-3-3ζ is crucial for its effects on monocyte chemotaxis (100, 101). In addition to these three proteins, we have recently identified several other S-glutathionylated proteins in metabolically stressed THP-1 monocytes, including 14-3-3γ, S100α (S100 calcium-binding protein A1), histone H3, β-tubulin, PKM (pyruvate kinase muscle isozyme), HSP90β (heat shock protein 90B), HSP60, glutathione peroxidase (GPX), GAPDH (glyceraldehyde-3-phosphate dehydrogenase), cofilin, and ATP5A1 (ATP synthase, α subunit, isoform 1) (209). Rinna et al. showed that (similar to MKP-1) protein tyrosine phosphatase 1B (PTP1B) is S-glutathionylated in a rat alveolar macrophage cell line, NR8383 (168), and PTEN (phosphatase and tensin homolog deleted from chromosome 10) protein has also been identified as an S-glutathionylated protein in these cells (40). NHE1 (Na+-H+ exchanger isoform 1) is S-glutathionylated in primary human monocytes (110), while caspase-1 S-glutathionylation occurs in isolated peritoneal macrophages (141), although it should be noted that S-glutathionylated caspase-1 was only detected in macrophages from mice deficient in the superoxide dismutase 1 (SOD1) enzyme (see section on Prevention or Reversal of Protein Thiol Modifications) (141).
Regulation of Protein Function by S-Glutathionylation
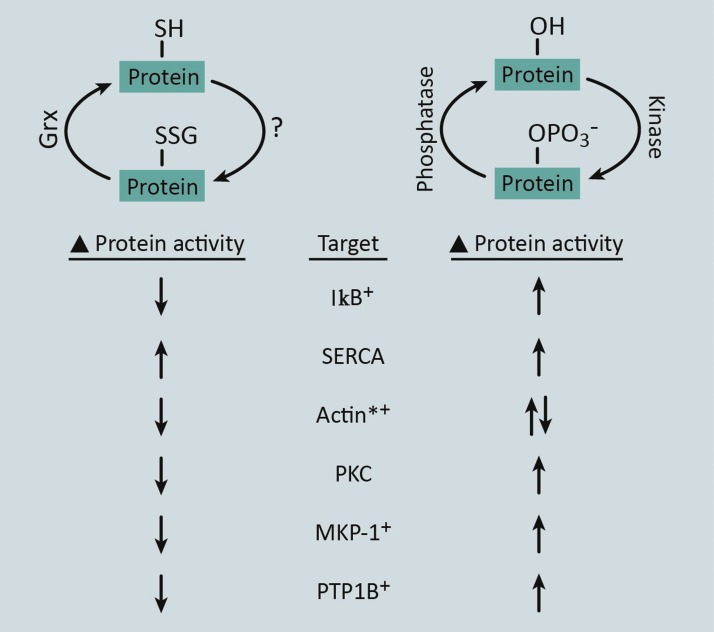
Post-translational modifications (PTMs) of specific protein residues are a widely conserved mechanism for altering protein activity, stability, and/or localization. In signal transduction, phosphorylation of serine, threonine, or tyrosine residues is the primary PTM utilized for orchestrating multidirectional cascades that operate in a highly specific and temporal manner (47). This is made possible by the use of enzymes, namely kinases and phosphatases, which provide the specificity and reversibility of this PTM. Reversible changes in the redox state of specific cysteine thiols by protein-S-glutathionylation also serve as a PTM that affects protein activity or function, and recent studies have aimed at predicting protein-S-glutathionylation motifs in silico (32, 191). Similar to kinase-mediated phosphorylation, transthiolation and thiol oxidation in response to intracellular ROS drive S-glutathionylation of protein thiols at specific residues (Figs. 1 and 2). Alternatively, S-glutathionylation of thiol residues can be reversed by the activity of Grx enzymes, which resembles removal of phosphate by protein phosphatases (Fig. 2).
FIG. 2.
Proteins regulated by both protein-S-glutathionylation and protein phosphorylation. The reversible nature of protein-S-glutathionylation resembles the process of protein phosphorylation. The forward mechanism of protein phosphorylation is driven by kinases, whereas the exact mechanism of protein-S-glutathionylation in intact cells and tissues is not yet fully understood (?). Depending on the system, protein-S-glutathionylation appears to be driven by either nonenzymatic or enzymatic mechanisms. The reversal of phosphorylation is mediated by phosphatase enzymes, whereas S-glutathionylation is reversed by Grx enzymes. In addition, both of these modifications have been reported to alter the activity of the proteins (examples are listed: Target), although via modification of discrete amino acids. *Phosphorylation and glutathionylation of actin result in changes in polymerization state, as opposed to changes in enzymatic activity for the other protein targets. +Proteins for which S-glutathionylation has been reported in monocytes and/or macrophages. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
A number of proteins that are involved in signal transduction (I-κB, PKCα, MKP-1, and PTP1B), cell adhesion (actin), and Ca2+ signaling (SERCA) have been shown to be regulated by both phosphorylation and S-glutathionylation (Fig. 2) (1, 20, 26, 31, 41, 67, 83, 101, 171, 179, 199, 213, 217). In certain cases, phosphorylation or S-glutathionylation of independent residues has similar effects on protein function. For example, phosphorylation or S-glutathionylation of SERCA increases SERCA activity (1, 83). In addition, β-actin S-glutathionylation at Cys374 or β-actin phosphorylation at Tyr53 decreases actin polymerization (171, 199, 213). However, in many cases, phosphorylation or S-glutathionylation of proteins has opposing effects on signal transduction. For instance, S-glutathionylation of inhibitor of nuclear factor-κB (NF-κB), IκB, stabilizes IκB and diminishes NF-κB activity (179), whereas phosphorylation of IκB leads to its proteasomal destruction and enhanced NF-κB transcriptional activation (31). It is therefore conceivable that under certain physiological conditions, these two types of PTMs could mutually regulate the activity or localization of certain proteins given that these modifications are both reversible and exclusive to distinct amino acid residues.
Mechanisms Promoting Protein-S-Glutathionylation
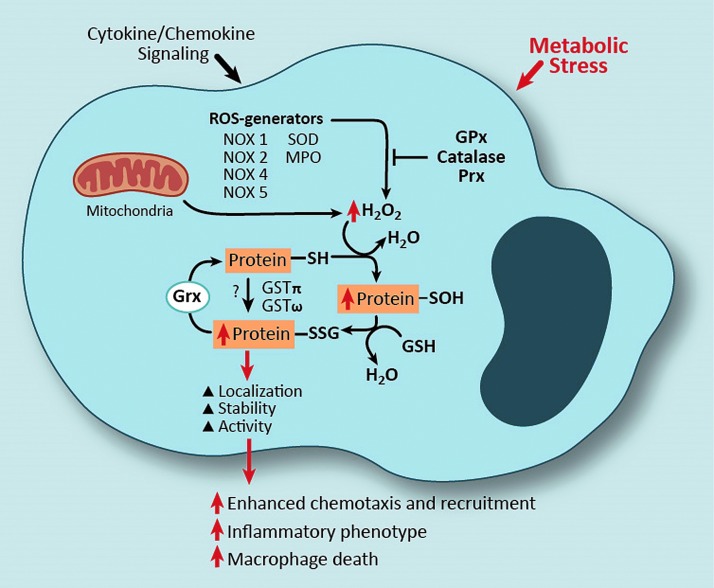
In addition to protein-S-glutathionylation resulting from H2O2-mediated oxidation of reactive thiol residue(s) (Fig. 1), recent studies suggest that certain glutathione S-transferase (GST) enzymes, including GSTπ, can also catalyze protein-S-glutathionylation (see the GSTs linked to protein-S-glutathionylation section). Thus, GST-catalyzed protein-S-glutathionylation requires GST, GSH, and a protein containing a reactive cysteine thiol residue. Like most other cell types, monocytes and macrophages possess the enzymatic repertoire for protein-S-glutathionylation to occur by either of the aforementioned mechanisms, that is, mediated by mitochondrial-generated H2O2, NADPH oxidase (NOX) enzymes or other ROS-generating enzymes, as well as catalyzed by either GSTπ or GSTω (159, 164) (Fig. 3).
FIG. 3.
Mechanisms and consequences of protein-S-glutathionylation in monocytes and macrophages. Both activation by cytokines or chemokines and metabolic stress can result in increased intracellular H2O2 production, which is a product of mitochondria and ROS-generating enzymes. Increased H2O2 production can lead to increased protein-S-glutathionylation in monocytes and macrophages. Alternatively, altered protein-S-glutathionylation has been reported to be mediated by glutathione S-transferase (GST) enzymes in these cell types. Cytokine, chemokine, and metabolic stress-induced protein-S-glutathionylation can be prevented either by the activity of antioxidant enzymes at the level of ROS scavenging or reversed by Grx-mediated deglutathionylation. As a consequence of protein-S-glutathionylation, changes in protein activity, function, and/or localization can lead to monocyte and macrophage dysfunction. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mitochondrial ROS associated with protein-S-glutathionylation
The majority of mitochondrial ROS (mROS) are derived from superoxide (O2−•), which is generated through a one-electron reduction of oxygen (O2) (150). These superoxide anions are efficiently dismutated into H2O2 by mitochondrial SOD, which is expressed at high levels in the mitochondria (150). Thus, H2O2 produced in the mitochondria would have the potential to interact with cysteine thiols of mitochondrial proteins. In addition, H2O2 is able to pass through aquaporin transmembrane proteins (59), which could allow mitochondrial H2O2 to access protein thiols in other subcellular compartments. Aquaporin-2 (AQP2), AQP6, AQP8, and AQP9 are localized to intracellular vesicles (7, 106), with AQP8 and AQP9 shown by electron microscopy to localize to the inner mitochondrial membrane (IMM) (7, 27). AQP9 is expressed in both human monocytes and human monocyte-derived macrophages (44); however, studies to identify an aquaporin protein responsible for H2O2 escape from the mitochondria through the outer mitochondrial membrane (OMM) are lacking thus far. Alternatively, ROS and RNS are thought to exit the mitochondria through the mitochondrial permeability transition pore (mPTP), where there is cross talk between mROS and other pro-oxidant enzymes outside the mitochondria (see “NOXs associated with protein-S-glutathionylation”). The mPTP comprises the voltage-dependent anion channel at the OMM and the adenine nucleotide translocase (ANT) and cyclophilin D (CypD) proteins at the IMM (42). Interestingly, Sloan et al. showed that heart tissue from diabetic rats have more thiol oxidation when compared with nondiabetic animals (183), and thiol cross-linking agents such as diamide increase thiol oxidation of ANT at Cys56 and cause mPTP opening (39).
In addition to elevated production of ROS, including H2O2, mitochondria also contain GSH at a similar concentration to that in the cytosol (167). GSH, which is synthesized in the cytosol, diffuses across the OMM where it is carried across the IMM by different proteins dependent upon the cell type. 2-Oxoglutarate carrier and dicarboxylate carrier were identified as GSH carrier proteins in the liver and kidney, whereas tricarboxylate carrier was identified in brain mitochondria and astrocytes (132, 167). It is still unclear what contribution these two mechanisms play in GSH transport into monocyte or macrophage mitochondria.
Due to the high H2O2 levels and GSH presence, mitochondria have a microenvironment that favors protein-S-glutathionylation reactions (131). In fact, several mitochondrial proteins are known to be targets for S-glutathionylation, including mitochondrial complex 1 subunits, uncoupling protein 2 (UCP2), and UCP3 (131). In addition, the α subunit of ATP synthase is S-glutathionylated in heart tissue from dogs that experience heart failure, and the enzyme is S-glutathionylated in vitro at both Cys244 and Cys294, although under conditions of artificially high oxidative stress (214). Our group has recently shown that the α subunit of ATP synthase undergoes S-glutathionylation in THP-1 monocytes cultured under physiological conditions (5 mM glucose) as well as THP-1 cells cultured under metabolic stress (25 mM glucose +100 μg/ml LDL) (209).
NOXs associated with protein-S-glutathionylation
NOX enzymes are transmembrane protein complexes that transport electrons from NADPH across membranes to reduce oxygen to generate superoxide (O2−) and H2O2. The superoxide (O2−) generated by NOX enzymes can also be further converted to H2O2 either by spontaneous dismutation or catalyzed by superoxide dismutase (SOD). H2O2 can be converted to hydroxyl radicals (.OH) and hydroxyl anions (OH−) by iron-catalyzed reactions or to hypochlorous acid (HOCl) by myeloperoxidase (MPO) (63). To date, seven Nox family members (Nox 1–5 and Duox 1–2) have been characterized and found to differ in their tissue distribution (21), with four of these family members (NOX1, NOX2, NOX3, and NOX4) reported to be expressed in monocytes or macrophages (21, 62, 115, 174). NOX enzyme function is regulated both by subcellular localization and interaction with regulatory proteins that help localize ROS production and activation of specific redox signaling pathways that mediate various cellular functions (211).
NOX2, also known as gp91 phagocytic oxidase (gp91phox), is the most well-characterized catalytic subunit of NOX enzymes in macrophages and is localized to both intracellular and plasma membranes where it directly produces superoxide into the extracellular space or phagolysosome for pathogen killing. The oxidative burst mounted by activation of NOX2 is the major ROS-generating process in macrophages and was originally described by Rossi and Zatti in the early 1960s (170, 232). Activation of NOX2 occurs through a complex series of protein–protein interactions with regulatory proteins that include p22phox, p47phox, p67phox, and the GTPase Rac, which are required for stabilization and translocation (21, 190). NOX1 was the first homolog of NOX2 to be characterized, and the NOX1 and NOX2 proteins display 56% identity (18, 189). The NOX1 protein is localized to endosomes and, similar to NOX2, produces ROS in the form of superoxide (211). Additionally, NOX1 and NOX2 share some regulatory proteins for their functionality (21). NOX1 or NOX3 expression can be induced in RAW264.7 macrophage-like cells in response to TLR agonists, that is, LPS and nonmethylated CpG DNA, as well as RANKL (receptor activator of NF-κB ligand) stimulation (103, 104, 118, 174). In addition, induction of NOX1 expression occurs in response to ethanol exposure in alveolar macrophages (229).
NOX4 expression has been characterized in multiple cell types, including kidney, vascular endothelial cells, and cardiac tissue and cardiomyocytes, with localization to the endoplasmic reticulum (6), mitochondria (23), and nucleus (113). Several reports suggest that NOX4 is also expressed in both human and murine myeloid cells (78, 115, 116). However, there are conflicting reports about which myeloid cells express NOX4 and under what conditions (178). For example, our laboratory previously characterized the inducible nature of NOX4 in human monocyte-derived macrophages stimulated with oxidized LDL (115) and in THP-1 cells exposed to metabolic stress and monocyte chemoattractant protein-1 (MCP-1) (207). NOX4 is also detectable by RT-PCR in mouse bone marrow-derived macrophages and in murine thioglycollate-elicited peritoneal macrophages (data unpublished). Unlike NOX1 and NOX2, NOX4 activation can occur upon interaction with the p22phox regulatory protein only whereby the NOX4-p22phox complex generates primarily H2O2 (21).
Although there have been no definitive studies to address which NOX protein is responsible for changes in global protein-S-glutathionylation, several independent studies have demonstrated that one or more of the NOX proteins are essential for the S-glutathionylation of certain target proteins. For example, a peptide inhibitor of NOX2, Nox2ds-tat, blocks S-glutathionylation of endothelial nitric oxide synthase (eNOS) in human lung microvascular endothelial cells (222), and eNOS S-glutathionylation is significantly diminished in aorta and heart tissue of angiotensin-II-treated NOX2−/− and p47phox−/− mice (112). In isolated triad vesicles (T-tubules attached to sarcoplasmic reticulum vesicles) from skeletal muscle, the NOX inhibitor, apocynin, blocks S-glutathionylation of ryanodine receptor type 1 (RyR1) (87). However, it is uncertain which NOX protein contributes to RyR1 S-glutathionylation as the authors noted that both NOX2 and NOX4 are present in cardiac muscle tissue. In THP-1 monocytes, our group has shown that siRNA directed against NOX4 mitigated the enhanced β-actin-S-glutathionylation induced by metabolic stress (207).
Importantly, studies have shown that cross talk occurs between NOX enzymes and mROS and that this cross talk affects protein-S-glutathionylation. Khan et al. showed that intermittent hypoxia of PC-12 cells leads to S-glutathionylation of both the 75 kD mitochondrial Complex 1 protein (NDUFS1) and the 50 kD mitochondrial Complex 1 protein (NDUFV1), which diminishes Complex 1 function—this increased S-glutathionylation of both proteins is blocked by apocynin or siRNA-mediated depletion of NOX2 (99). Alternatively, mROS, generated by phorbol esters or myxothiazole, activates NOX2 in isolated human neutrophils by enhancing membrane localization of phox regulatory subunits (112). This translocation and NOX activation are blocked by sanglifehrin A (SfA), an inhibitor of the mPTP, and mROS-induced activation of NOX activity is impaired in whole blood from CypD−/− mice (112). To our knowledge, there have been no studies to assess cross talk between mROS and NOX enzymes in monocytes or macrophages.
GSTs linked to protein-S-glutathionylation
GSTs are a class of isoenzymes that catalyze the conjugation of reduced GSH to reactive sulfur groups on various molecules such as xenobiotics (84). GSTs are catalytically active as homo- or heterodimers and are divided into seven classes based on sequence similarity (84, 200). There are increasing amounts of data, suggesting that two GST enzymes, GSTπ and GSTω1, play a role in protein-S-glutathionylation. Both enzymes are expressed in mouse monocytes and macrophages, as well as human monocytes (14).
Although GST has been reported to bind GSH and produces a thiolate anion (GS−) at physiological pH (81), the mechanism for GST-mediated protein-S-glutathionylation is still unclear. GSTπ itself can be S-glutathionylated at Cys47 and Cys101, and mutation of these residues reduces GSTπ activity in vitro (203). Alternatively, GSTπ interacts with both peroxiredoxin 1 (Prx1) and 1-cys peroxiredoxin (1-cysPrx or Prx VI) (105, 135), and GSTπ can S-glutathionylate 1-cys peroxiredoxin (1-cysPrx or Prx VI) in vitro (135). However, the role of this GSTπ S-glutathionylation or the interaction of GSTπ with Prx proteins in facilitating S-glutathionylation of target proteins remains unknown. Nonetheless, mouse embryonic fibroblasts (MEFs) from GSTπ−/− mice that are treated with PABA/NO, a prodrug that releases NO, or a mimetic of oxidized GSH, NOV-002, exhibit lower global protein-S-glutathionylation, including lower levels of β-actin S-glutathionylation, when compared with MEFs from wild-type (WT) mice (203). However, it should be noted that in the absence of nitrosative or oxidative stress, there was very little detectable protein-S-glutathionylation in either WT or GSTπ−/− MEFs (203), suggesting that H2O2-induced protein sulfenic acid residues are still necessary for GST-mediated S-glutathionylation. This notion is supported by data from McGarry et al. showing that very few proteins from liver tissue are differentially S-glutathionylated when comparing GSTπ−/− mice with WT mice (139).
Using a yeast two-hybrid assay, GSTπ was reported to interact with AMP-activated protein kinase (AMPK), an interaction that occurs in vitro and in liver tissue lysates (108). In vitro, GSTπ facilitates the S-glutathionylation of AMPK in the presence of low concentrations of GSH, and this S-glutathionylation enhances AMPK kinase activity independent of AMPK phosphorylation (108). In addition, inhibition of GSTπ in C10 murine alveolar epithelial cells with TLK-199 or siRNA blocks S-glutathionylation of the Fas ligand (FasL) in response to FasL stimulation (10), suggesting that GSTπ plays a role in the FasL autoregulatory loop involving protein-S-glutathionylation. In contrast to GSTπ, GSTω1 catalyzes the removal of GSH from a peptide substrate in vitro, and overexpression of GSTω1 leads to a reduction in protein-S-glutathionylation in T47-D breast cancer cells under normal culture conditions (142). However, overexpression of GSTω1 leads to accelerated protein-S-glutathionylation in response to treatment with GSNO, a result that the authors attributed to increased GSSG levels in the presence GSTω1 (142). In the Raw 264.7 macrophage cell line, aflatoxin B1 treatment increases GSTω1 expression as well as S-glutathionylation of several proteins, a result that is mitigated by siRNA-mediated depletion of GSTω1 in these cells (159). The role that GST proteins play in facilitating protein-S-glutathionylation and how GST-mediated effects on S-glutathionylation are related to ROS-mediated thiol oxidative stress remain open questions.
Additional enzymes linked to protein-S-glutathionylation
In addition to mROS and NOX proteins, MPO is a pro-oxidant enzyme that has been linked to protein-S-glutathionylation. MPO is an enzyme stored in azurophilic granules of phagocytic cells, which is released into the extracellular fluid in the setting of inflammation. The major product of MPO is HOCl, which forms upon its interaction with H2O2 and chloride (Cl−). Although MPO itself has not been directly linked to any S-glutathionylated proteins, Stacey et al. used a redox proteomic assay to show that treatment of HUVECs with HOCl or chloramines results in a number of proteins with oxidized thiol residues (185). Additionally, cyclophilin A is S-glutathionylated at Cys161 in response to chloramines (185). Last, the flavoprotein sulfhydryl oxidase enzyme (QSOX) has been listed as an enzyme with the potential to S-glutathionylate target proteins (144, 161); however, there have been no studies to address this possibility in monocytes and macrophages or other cell types to this point.
Prevention or Reversal of Protein Thiol Modifications
For thiol redox-sensitive signaling pathways to function properly, changes in protein thiols must be controlled and reversible. To maintain thiol redox homeostasis, monocytes and macrophages contain a large array of antioxidant enzymes to prevent or reverse protein-S-glutathionylation, as well as small-molecule ROS scavengers such as ascorbic acid, vitamin E, and GSH. These antioxidant enzymes can be divided into either enzymatic ROS scavengers or deglutathionylating enzymes, that is, enzymes that remove GSH from S-glutathionylated proteins. Intracellular ROS scavengers include SOD, catalase, and substrate-specific peroxidases such as GPX enzymes, while Grx enzymes catalyze deglutathionylation reactions.
Enzymatic ROS scavengers
Superoxide dismutase
Three SODs have been characterized in mammalian cells: cytosolic CuZn-SOD (SOD1), mitochondrial Mn-SOD (SOD2), and extracellular EC-SOD (SOD3). Each of these enzymes catalyzes the dismutation of superoxide O2•− into H2O2 and O2. Although SOD enzymes can generate H2O2 as a by-product, at least one study has shown that increased SOD levels prevent forskolin-induced S-glutathionylation of the Na+-K+ pump β1 protein in isolated rabbit ventricular myocytes (220). Similarly, SOD1 or SOD2 overexpression blocks NO-induced S-glutathionylation of the SERCA protein in smooth muscle cells (1). Interestingly, a recent study reported that SOD2 itself undergoes S-glutathionylation in NRK-52E (normal rat kidney proximal tubular cells) when treated with GSNO, thereby decreasing SOD2 activity (158).
In the monocytic cell lines, U937 and THP-1, changes in expression of SOD proteins were detected during 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced monocyte-to-macrophage differentiation, whereby TPA treatment reduces SOD1 and SOD2 expression while increasing SOD3 expression in a PKC-dependent manner (97). SOD1 activity is also reduced in rat peritoneal macrophages in response to LPS in a time- and dose-dependent manner and this reduction is blocked by inhibitors of NO synthase (94). In contrast, others have reported that SOD2, but not SOD1, mRNA expression and activity increased in mouse peritoneal macrophages in response to LPS exposure (205). The role of SOD enzymes in protein-S-glutathionylation in monocytes and macrophages or the regulation of SOD by S-glutathionylation in monocytes and macrophages has not been examined in any detail thus far.
Catalase
The catalase enzyme, identified over 100 years ago, is responsible for the metabolism of H2O2 into H2O and O2 in peroxisomes (107, 129). In vitro, SOD and catalase enzymes block RyR2-S-glutathionylation in isolated cardiac SR vesicles (172), and reducing catalase activity in lung extracts enhances oxidation of cysteine residues in the proteasomal regulatory subunits, Rpn1 and Rpn2 (238). Similarly, increased oxidation of AMPKα cysteine residues occurs in lung tissue from mice treated with a pharmacologic inhibitor of catalase, aminotriazole (ATZ), or in lungs from acatalasemic mice (239). In contrast, overexpression of catalase prevents (i) UV light-induced mitochondrial pyruvate dehydrogenase-S-glutathionylation in HeLa cells (91), (ii) p65 NF-κB-S-glutathionylation in an endothelial cell line exposed to two different carbon monoxide (CO) donors, tricarbonyl dichlororuthenium (II) dimer and methylene chloride, and (iii) STAT3-S-glutathionylation in bovine aorta endothelial cells exposed CO donors (226).
Catalase expression and activity in monocytes and macrophages have been explored using human blood monocytes and alveolar macrophages, as well as the THP-1 cell line. Alveolar macrophages, which generate higher ROS levels than blood monocytes, have higher catalase levels than blood monocytes and an inability to activate p38 MAP kinase, which can be overcome by ATZ (28). However, this increased catalase expression may be cell-type dependent since blood monocyte-derived macrophages stimulated with GM-CSF have high catalase levels, similar to alveolar macrophages, whereas monocyte-derived macrophages stimulated with M-CSF do not (109). Similar to alveolar macrophages and GM-CSF-stimulated macrophages, TPA-induced monocyte-to-macrophage differentiation of U937 cells results in decreased catalase expression (225). However, catalase itself enhances monocyte-to-macrophage differentiation of THP-1 cells induced by all-trans retinoic acid (50).
GSH peroxidases
GPxs are a family of enzymes that utilize GSH as an electron donor for the metabolism of H2O2 and lipid peroxides, converting them into water and corresponding alcohols, respectively (25). There are eight GPx enzymes in mammalian cells. These enzymes possess a reactive selenocysteine residue (GPx1–4) or a reactive cysteine residue (GPx6–8) in their catalytic domain that facilitates reactivity—human GPx6 possesses a selenocysteine residue, whereas rodent GPx6 possesses a cysteine residue. Thus, GPx enzymes themselves are S-glutathionylated during the course of their reaction with H2O2 (25). GPx activity is significantly higher in alveolar macrophages when compared with blood monocytes (28); however, the only data linking mammalian GPxs to changes in protein thiol modifications are that increased eNOS-S-glutathionylation within heart tissue occurs in GPx1−/− mice in an age-dependent manner (155).
Protein deglutathionylation
Reversal of protein-S-glutathionylation (i.e., deglutathionylation) is almost exclusively determined by the activity of Grx enzymes, which are members of the thioredoxin superfamily of thiol-disulfide oxidoreductases (35). There are six Grx isoforms expressed in human cells, which are generated from four independent genes. Grx1 and Grx2 both possess a dithiol catalytic domain, although Grx2 isoforms (comprising Grx2a, Grx2b, and Grx2c splice variants) have a slightly modified dithiol domain. Grx3 and Grx5 possess monothiol catalytic domains (124). Grx1 and Grx3 are primarily localized to the cytosol, although Grx1 also localizes to the IMM and the nucleus under certain conditions (124). Grx2a and Grx5 both have mitochondrial signaling peptides, causing mitochondrial localization, whereas Grx2b and Grx2c lack a mitochondrial signal and are localized to the cytosol and the nucleus (124). Expression of each Grx isoform is detected at the mRNA level by oligonucleotide microarray chips in Mac-1+ cells from mouse bone marrow (111) as well as in mouse monocytes and macrophages (14). In addition, all Grx mRNA isoforms are present in human blood monocytes and monocyte-derived macrophages (128, 138, 184). Takashima et al. reported that PMA-induced monocyte-to-macrophage differentiation promotes an increase in Grx1 expression (197); however, the relevance of this increase to physiological conditions is still unclear as human blood monocytes and monocyte-derived macrophages expressed similar Grx1 levels as measured on Affymetrix chips (128, 138, 184). While both Grx1 and Grx2 have been shown to play a role in protein-S-glutathionylation, Grx5 protein plays a role in iron–sulfur cluster transfer in cells (123) and its role, if any, in protein thiol signaling is still unclear. Similarly, the effects of Grx3 on protein-S-glutathionylation remain unknown.
Grx1 is the most well-characterized Grx enzyme, and several proteins undergo Grx1-mediated changes in S-glutathionylation. In vitro, purified Grx1 removes S-glutathione from hepatocyte proteins and reverses the S-glutathionylation-mediated inactivation of PTP1B (20, 96). Alternatively, Grx1 facilitates the in vitro S-glutathionylation of GAPDH, β-actin, and PTP1B in the presence of GSH thiyl radicals (GS•) (186). Under physiological conditions, the Grx1 enzyme shows a strong preference for catalyzing deglutathionylation in the presence of NADPH and GR (124, 144). For example, Grx1−/− mice display increased global protein-S-glutathionylation levels in lung tissue in response to cigarette smoke, to antigen-stimulated allergic inflammation with ovalbumin, or to oropharyngeal exposure to LPS (2, 36, 90). Additionally, MEFs isolated from Grx1−/− mice have elevated protein-S-glutathionylation in response to H2O2 (89), and siRNA-mediated depletion of Grx1 blocks fibroblast growth factor-induced deglutathionylation of β-actin in NIH3T3 cells (213). In lung tissue and mouse tracheal epithelial cells, loss of Grx1, that is, Grx1−/− cells, increases S-glutathionylation and augmentation of FasL in response to Pseudomonas aeruginosa infection, and Grx1 overexpression in lung tissue had the opposite effect on FasL-S-glutathionylation and activity (9). Grx1 similarly regulated FasL-S-glutathionylation and activity in C10 cells in response to FasL itself (8). Interestingly, alveolar macrophages isolated from Grx1−/− mice have similar levels of global protein-S-glutathionylation when compared with isolated alveolar macrophages from WT mice, even when mice are exposed to oropharyngeal LPS (2).
Similar to Grx1, Grx2a selectively deglutathionylates synthetically S-glutathionylated RNase or BSA proteins and deglutathionylates mitochondrial complex 1 proteins in vitro in the presence of high GSH/GSSG ratios (22, 68, 95). However, Grx2a promotes S-glutathionylation of mitochondrial complex 1 proteins and GAPDH in vitro in the presence of low GSH/GSSG ratios (22, 68). Thus, the effect of Grx enzymes on protein-S-glutathionylation depends on the ratio of GSH/GSSG in cells. In vivo, the role of Grx2 is also complex. Grx2−/− mice have elevated levels of global protein-S-glutathionylation as well as increased levels of S-glutathionylated β-actin and two different mitochondrial complex proteins in lens tissue (223), suggesting that Grx2 promotes protein deglutathionylation in this tissue. However, overexpression of Grx2a in mouse heart tissue promotes S-glutathionylation of mitochondrial proteins, especially when animals are treated with the cardiotoxic anticancer drug, doxorubicin (51). Thus, the effect of Grx2 on protein-S-glutathionylation may be cell, tissue, and possibly context dependent, as well as dependent on the level of reduced GSH in the cellular or tissue milieu.
Cross Talk Between Thiol Modifications and Signal Transduction Pathways in Monocytes/Macrophages
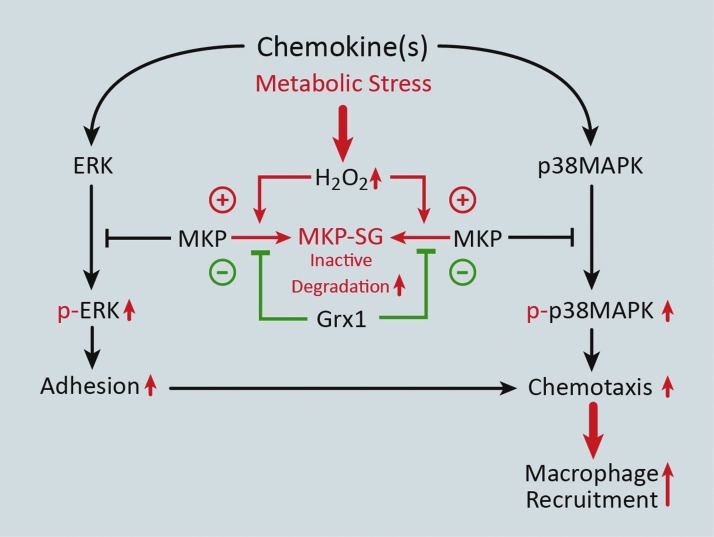
MKPs, which inactivate members of the MAPK signaling pathway, such as ERK and p38, are dual specificity phosphatases (DUSPs) that (like other protein tyrosine phosphatases) contain a conserved C(X)5R motif in their catalytic domain (93). Importantly, the cysteine residue of the C(X)5R motif can be S-glutathionylated in both PTP1B and MKP-1 (20, 101). In macrophages, increased MKP-1 levels are thought to control proliferation and activation in response to M-CSF and LPS, respectively, both of which require ERK 1/2 signaling (38). Our group has shown that in THP-1 monocytes and primary human blood monocytes, oxidative and metabolic stress promotes S-glutathionylation of MKP-1, thereby inhibiting its phosphatase activity and targeting it for proteasomal degradation. Loss of MKP-1 results in hyperactivation of ERK and p38 via increased phosphorylation in response to MCP-1 and increases monocyte adhesion and chemotaxis. Overexpression of Grx1, however, protects MKP-1 from S-glutathionylation-dependent inactivation/degradation and normalizes monocyte adhesion and chemotaxis in response to MCP-1 under conditions of metabolic stress (Fig. 4) (101).
FIG. 4.
Metabolic stress enhances monocyte adhesion and recruitment via S-glutathionylation of mitogen-activated protein kinase phosphatase 1 (MKP-1). Metabolic stress induces H2O2-mediated S-glutathionylation of MKP-1, resulting in MKP-1 inactivation and degradation. As a result, hyperactivation (increased phosphorylation) of both ERK and p38MAPK signaling occurs, which leads to enhanced monocyte adhesion and chemotaxis, respectively. These metabolic stress-induced changes in protein signaling and monocyte migration can be reversed by increased Grx1 expression. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Recently, we have shown that another DUSP family member, Slingshot Homolog 1L (SSH1L), also plays a role in accelerated monocyte chemotaxis caused by metabolic stress. The mechanism by which this occurs is through increased S-glutathionylation and degradation of 14-3-3ζ, a binding partner of SSH1L that maintains SSH1L in an inactive state (102). S-glutathionylation and degradation of 14-3-3ζ result in the release of SSH1L and subsequent dephosphorylation of the Cofilin protein (100). Cofilin is an actin-binding protein that (in its dephosphorylated state) promotes actin depolymerization and actin turnover in response to various extracellular stimuli (146). Thus, dephosphorylation of Cofilin in response to metabolic stress in monocytes results in accelerated chemotaxis (100). Importantly, we showed that overexpression of Grx1 reverses the metabolic stress-induced changes in cofilin phosphorylation (100). The overlap and contribution of S-glutathionylation of these two DUSP proteins, as well as other DUSP proteins, to enhanced monocyte migration induced by metabolic stress are still under investigation.
In addition to thiol modifications regulating DUSP proteins, recent studies have demonstrated a complex feedback loop between Grx1-regulated protein-S-glutathionylation and the NF-κB signaling pathway in macrophages and other cell types. NF-κB is a transcriptional activator comprising heterodimers of the Rel family of proteins (i.e., p50, p52, cRel, RelA, and RelB), which is activated by the proteasomal destruction of IκB proteins in response to phosphorylation by the IκB kinase (IKK) complex (130). The IKK complex comprises three proteins: IKKα, IKKβ, and NF-κB essential modulator (130). Stimulation of RAW264.7 macrophage-like cells with activators of the NF-κB signaling pathway, including IL-1β, TNFα, and LPS, induces RelA binding to the Grx1 promoter and causes upregulation of Grx1 (4). In addition, oropharyngeal administration of LPS causes Grx1 upregulation in alveolar macrophages (2). In turn, Grx1 has been reported to regulate S-glutathionylation of multiple proteins in the IKK/IκB/NF-κB pathway. Lung tissue and lung homogenates from Grx1−/− mice have elevated global protein-S-glutathionylation and IKKα-S-glutathionylation levels, respectively, in response to cigarette smoke, as well as enhanced NF-κB activity, suggesting that Grx1 inhibits CS-induced IKKα-S-glutathionylation and NF-κB signaling (36). Similarly, siRNA-mediated depletion of Grx1 exacerbates IL-17-induced S-glutathionylation of both IKKα and RelA, thereby enhancing NF-κB signaling in C10 cells (154). In contrast, IKKβ-S-glutathionylation caused by H2O2 decreases IKKβ activity and blocks NF-κB signaling in response to TNFα (166). Furthermore, overexpression of Grx1 blocks these effects, whereas Grx1 siRNA exacerbates the H2O2-mediated inhibition of TNFα (166). These data are similar to data obtained in alveolar macrophages, whereby genetic ablation of Grx1 prevents LPS-induced NF-κB nuclear translocation (2).
Protein Thiol Redox Signaling and Atherosclerosis
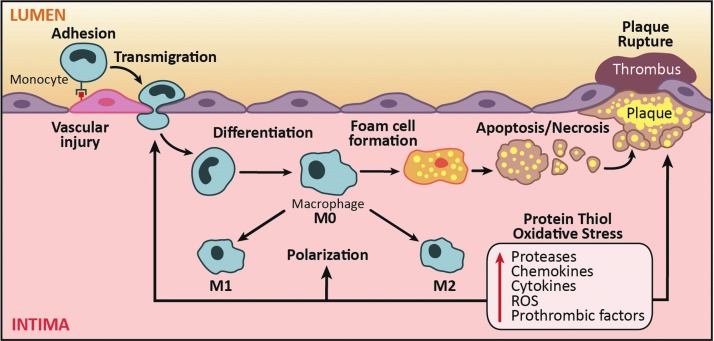
Redox signaling and oxidative stress play key roles in monocyte and macrophage biology in atherogenesis from the formation of the earliest stage lesions, that is, fatty streaks, to development of advanced plaques (121, 122). Subendothelial deposition of lipoproteins in atheroprone regions of the arteries is one of the earliest pathogenic events in atherosclerosis (195). Activation of endothelial cells by modified lipoproteins elicits the influx of monocytes via expression of adhesion molecules into the subendothelial space, which subsequently differentiate to macrophages that can be activated and/or internalize lipoproteins to form lipid-loaded foam cells, a key histological feature of atherosclerotic lesion (195). The death of these macrophages is a major contributor to the development of necrotic core, a well-recognized histological feature of instability in advanced atherosclerotic plaques (Fig. 5).
FIG. 5.
Contribution of protein thiol oxidative stress in monocytes and macrophages to atherogenesis. Development of atherosclerosis is complex and requires monocytes and macrophages at several steps during the pathophysiological process. Upon vascular injury, monocytes adhere to endothelial cells and transmigrate into the intima. Upon entering the subintimal space, monocytes differentiate into macrophages. Activation of macrophages (M0) results in their polarization into either a proinflammatory (M1) or inflammation-resolving (M2) phenotype. Uptake of modified LDL transforms macrophages into lipid-laden foam cells. Ultimately, these foam cells undergo cell death, which exacerbates plaque progression and destabilization. Conditions that promote protein thiol oxidative stress in monocytes have been previously reported to affect chemokine-induced adhesion and migration, processes that control macrophage infiltration into sites of vascular injury and drive atherogenesis and plaque progression. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Role of ROS and protein-S-glutathionylation in monocyte recruitment and differentiation (plaque initiation)
Monocytosis has long been recognized as an independent predictor of ischemic coronary artery disease in humans (156), and studies in hyperlipidemic mouse models have shown that both classical Ly6Chi and nonclassical Ly6Clo monocyte subsets are recruited into the atherosclerotic lesions and contribute to plaque progression (196). Furthermore, high-fat feeding of apoE-deficient mice increases the number of circulating Ly6Chi monocytes and their infiltration into atherosclerotic lesions (192). However, monocyte subsets appear to have different efficiency in entering into atherosclerotic lesions in specific regions of the arteries (162), and the contribution of the major human monocyte subsets, that is, classical CD14hi/CD16lo and nonclassical CD14lo/CD16hi, to the pathogenesis of atherosclerosis and their relative efficiency in recruitment into the atherosclerotic plaques have yet to be determined.
Oxidative stress contributes to various proatherogenic aspects of monocyte recruitment and differentiation. Inhibition of NOX-mediated oxidative stress by apocynin or administration of the antioxidant catalase ameliorates the hyperhomocysteinemia-induced Ly6Chi monocytosis as well as the enhanced recruitment of the inflammatory monocytes into atherosclerotic lesions in apoE-deficient mice (233). Our in vivo studies have confirmed that thiol oxidative stress induced by metabolic stress enhances recruitment of monocyte-derived macrophages into MCP-1-loaded Matrigel plugs and is strongly correlated with atherosclerotic lesion size and macrophage content in aortic root of high-fat diet-fed LDL receptor null (LDL-R−/−) mice (163). We have shown that metabolic stress by hypercholesterolemia and hyperglycemia primes monocytes into a hypermigratory and proatherogenic phenotype, which is dependent on the induction of NOX4-mediated H2O2 production (207). This priming effect is associated with S-glutathionylation of several proteins, including actin (115, 116), MKP-1 (101), and 14-3-3ζ (100), which appear to accelerate monocyte chemotaxis via increasing actin remodeling (207). Prevention of protein-S-glutathionylation by transgenic overexpression of cytoplasmic Grx is sufficient to eliminate the metabolic stress-induced priming effect in monocytes (207). Last, mROS in plaque macrophages has also been shown to accelerate atherogenesis by promoting NF-κB-mediated induction of MCP1, which triggers enhanced influx of monocytes into the aortic root lesions in LDL-R-null mice fed a high-fat diet. Ectopic expression of catalase in the mitochondria of macrophages suppressed monocyte recruitment as well as atherosclerotic progression (216).
Role of ROS and protein-S-glutathionylation in plaque expansion and macrophage activation
Sustained recruitment of monocyte-derived macrophages, as a result of the imbalance between the proinflammatory and inflammation-resolving mechanisms, is a critical pathogenic aspect of atherogenesis (195). Numerous microenvironmental factors, including cholesterol crystals (53), oxidized LDL (OxLDL) (13, 212), and hypoxia (60, 61), as well as cytokines and growth factors [e.g., GM-CSF (188)], can promote a proinflammatory state in macrophages and potentially contribute to the sustained nonresolving inflammation in atherosclerosis. Although the exact role of M1 and M2-polarized macrophages in atherosclerosis has yet to be defined, observational evidence suggests that the relative abundance of M1-polarized macrophages in lesions, in particular, in the unstable region of the plaque, that is, shoulder (19), is associated with plaque vulnerability [recently reviewed in Chinetti-Gbaguidi et al. (33)]. More recently, an additional activation state of macrophages, termed M4 polarization, has been described, which is induced by platelet chemokine CXCL4, and demonstrates a unique transcriptome distinct from M1 and M2 polarization (56, 74, 75). M4-polarized macrophages, defined based on the coexpression of MMP-7 and S100A8, which seems to be exclusive to this activation state, have been identified in human coronary atherosclerotic plaques (56). Additionally, the abundance of M4-polarized macrophages, but not the CXCL4 plasma level, has been associated with plaque vulnerability (57). However, the exact functional role of CXCL4-induced M4 polarization in atherogenesis as well as its signaling mechanisms remains to be defined (74).
The role of ROS and thiol redox signaling in regulation of macrophage polarization state in atherosclerosis is not yet well elucidated. M2-polarized macrophages have been shown to have reduced and delayed ROS production compared with M1-polarized macrophages (160). Downregulation of NOX-2-mediated ROS generation produces a reductive microenvironment within the phagosome, which is critical for the phagocytic activity associated with the tissue repair function of M2-polarized macrophages (17). Additionally, deficiency of p47phox, a scaffolding protein for the NOX complex, renders macrophages hyper-responsive to IL-4-induced M2 polarization (230). Likewise, SOD1 overexpression promotes M2 polarization of alveolar macrophages (86). Together, these ex vivo studies support the significance of attenuated ROS production in development of M2 polarization state, which favors resolution of inflammation.
Role of ROS and protein-S-glutathionylation in macrophage death
In early lesions, macrophage death is rarely detected and occurs mostly through apoptosis, which does not elicit an inflammatory response and has been shown to suppress atherogenesis in mice models by limiting the plaque cellularity (69, 202, 235). However, macrophage death is frequent in advanced lesions, particularly within or in the vicinity of the necrotic core (202, 235), and may be induced by a number of mechanisms, including FasL-mediated cell death, free cholesterol cytotoxicity (24, 58, 227, 228), OxLDL cytotoxicity (11, 12, 215), or endoplasmic reticulum stress (48, 49, 134, 152, 181, 201, 206, 234, 235). Interestingly, FasL-mediated cell death is a central apoptotic mechanism of macrophages (227) and smooth muscle cells in atherosclerosis (71). Binding by FasL as well as caspase-dependent degradation of Grx1 mediates the S-glutathionylation of FasL in lung epithelial cells and promotes the formation of death-inducing complexes and apoptosis (8, 82). However, the role of S-glutathionylation of Fas in the regulation of macrophage apoptosis has not been determined.
Increased oxidative stress in advanced plaques appears to play a key role in macrophage apoptosis both as a direct trigger and as a mediator of ER stress-induced cytotoxicity (193). A multihit model proposed by Tabas et al. suggests that mild ER stress, which is not sufficient to induce apoptosis by itself, reduces the threshold to other proapoptotic stressors and promotes macrophage apoptosis in atherosclerotic lesions (193). ER stress can induce intracellular ROS production through multiple mechanisms. Increased generation of disulfide bonds as a result of ER stress may deplete the reduced GSH to cleave the disulfide bonds (133). ER stress may also enhance mROS formation through induction of oxidative phosphorylation to meet the energy demands associated with ER stress-induced protein modifications (133). Additionally, ER stress may result in calcium leakage from ER, which inhibits complex III and IV and results in enhanced oxygen consumption rate and ROS generation by the electron transport chain (133). ER stress may also induce calcium and calmodulin-dependent protein kinase II-mediated induction of NOX-2, enhancing ROS generation (119). Alternatively, nitrosative stress induces S-glutathionylation of protein disulfide isomerase, thereby activating the unfolded protein response and creating ER stress in cancer cells (204). This ER stress, if left uncorrected, could lead to macrophage death through various mechanisms (173).
Oxidative stress is implicated in OxLDL cytotoxicity, which unlike the native LDL causes a robust increase in intracellular ROS generation as well as protein-S-glutathionylation (37, 149, 165, 215). Lysophosphatidylcholine is a component of oxLDL, which causes macrophage death through NOX-mediated ROS production (157). OxLDL results in depletion of intracellular GSH and caspase-independent macrophage cell death (15), and we reported that siRNA-mediated depletion of Grx1 sensitized macrophages to oxLDL-mediated cytotoxicity (215). We have also reported that OxLDL induces NOX4-mediated ROS generation in human macrophages via the MEK1/ERK pathway and this is required for OxLDL cytotoxicity (115).
Role of ROS and protein-S-glutathionylation in efferocytosis and necroptosis
In contrast to the early lesions, macrophage apoptosis in advanced lesions accentuates necrotic core expansion, recruitment of monocytes, and production of proinflammatory cytokines, all of which are features associated with plaque instability (69, 180, 202, 235). The unfavorable effect of apoptosis in advanced lesions appears to be secondary to the ineffective clearance of the apoptotic cells as a result of either impaired macrophage efferocytosis or the enhanced rate of apoptosis in advanced plaques, which leads to secondary (postapoptotic) necrosis of cells, thereby exaggerating the inflammatory response (69, 176, 193–195). Efferocytosis induces a robust increase in NOX-mediated ROS production and promotes apoptosis of the RAW264.7 cell line (117). Oxidative stress induced by exogenous H2O2 also impairs efferocytosis in human and murine macrophages (140).
Programmed necrosis or necroptosis is a recently described and regulated form of cell death, which is mediated by formation of the necrosome complex, containing receptor-interacting protein 3 (RIP3), RIP1, and caspase-8. There is a delicate interplay between the two forms of regulated cell death, necroptosis and apoptosis, which is regulated by the balance between caspase-8 and RIP3 (126). RIP3-dependent cell death contributes to development of advanced atherosclerotic plaques in LDL receptor knockout mice without a significant effect on the development of early lesions (126). Induction of a proinflammatory response by necrotic cells, which are more difficult to clear by efferocytosis compared with apoptotic cells, has been suggested to underlie the proatherogenic role of necroptosis in advanced lesion (126). Proinflammatory stimuli, such as TNF-α and low-dose LPS, have been shown to induce macrophage necroptosis (16, 169), which is mediated by mROS formation (169). The role of ROS in regulation of macrophage necroptosis in atherosclerosis remains unexplored.
Conclusions
While we are still in the early stages of understanding how thiol redox signaling affects monocytes and macrophage function and how dysregulation of thiol redox signaling pathways contributes to disease onset and progression, recent studies suggest that a large number of proteins undergo changes in S-glutathionylation in response to pathophysiological conditions. Further understanding of the mechanisms that control this novel signaling paradigm in monocytes and macrophages, as well as identifying S-glutathionylated proteins that contribute to pathogenesis, is likely to provide novel molecular targets to combat diseases associated with monocyte or macrophage dysfunction, including atherosclerosis.
Funding
Reto Asmis is supported by grants from the National Institutes of Health (RO1 AT006885 and RO1 HL115858).
Abbreviations Used
- AMPK
AMP-activated protein kinase
- ANT
adenine nucleotide translocase
- AQP2
aquaporin-2
- ATZ
aminotriazole
- CO
carbon monoxide
- CypD
cyclophilin D
- DUSP
dual specificity phosphatase
- eNOS
endothelial nitric oxide synthase
- FasL
Fas ligand
- GPX
glutathione peroxidase
- GSNO
S-nitrosoglutathione
- H2O2
hydrogen peroxide
- GR
glutathione reductase
- Grx
glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- HOCl
hypochlorous acid
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- IMM
inner mitochondrial membrane
- MCP-1
monocyte chemoattractant protein-1
- MEFs
mouse embryonic fibroblasts
- MKP-1
mitogen-activated protein kinase phosphatase 1
- MPO
myeloperoxidase
- mPTP
mitochondrial permeability transition pore
- mROS
mitochondrial ROS
- NEM
N-ethyl maleimide
- NF-κB
nuclear factor-κB
- NOX
NADPH oxidase
- OMM
outer mitochondrial membrane
- PTM
post-translational modification
- PTP1B
protein tyrosine phosphatase 1B
- RIP3
receptor-interacting protein 3
- ROS
reactive oxygen species
- RyR1
ryanodine receptor type 1
- SOD
superoxide dismutase
- SSH1L
Slingshot Homolog 1L
- UCP2
uncoupling protein 2
- WT
wild-type
References
- 1.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, and Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Aesif SW, Anathy V, Kuipers I, Guala AS, Reiss JN, Ho YS, and Janssen-Heininger YM. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol 44: 491–499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aesif SW, Janssen-Heininger YM, and Reynaert NL. Protocols for the detection of s-glutathionylated and s-nitrosylated proteins in situ. Methods Enzymol 474: 289–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aesif SW, Kuipers I, van der Velden J, Tully JE, Guala AS, Anathy V, Sheely JI, Reynaert NL, Wouters EF, van der Vliet A, and Janssen-Heininger YM. Activation of the glutaredoxin-1 gene by nuclear factor kappaB enhances signaling. Free Radic Biol Med 51: 1249–1257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen EM. and Mieyal JJ. Protein-thiol oxidation and cell death: regulatory role of glutaredoxins. Antioxid Redox Signal 17: 1748–1763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, and Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Amiry-Moghaddam M, Lindland H, Zelenin S, Roberg BA, Gundersen BB, Petersen P, Rinvik E, Torgner IA, and Ottersen OP. Brain mitochondria contain aquaporin water channels: evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J 19: 1459–1467, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, and Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol 184: 241–252, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anathy V, Aesif SW, Hoffman SM, Bement JL, Guala AS, Lahue KG, Leclair LW, Suratt BT, Cool CD, Wargo MJ, and Janssen-Heininger YM. Glutaredoxin-1 attenuates S-glutathionylation of the death receptor fas and decreases resolution of Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 189: 463–474, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, and Janssen-Heininger YM. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol Cell Biol 32: 3464–3478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asmis R. and Begley JG. Oxidized LDL promotes peroxide-mediated mitochondrial dysfunction and cell death in human macrophages: a caspase-3-independent pathway. Circ Res 92: e20–e29, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Asmis R. and Jelk J. Vitamin E supplementation of human macrophages prevents neither foam cell formation nor increased susceptibility of foam cells to lysis by oxidized LDL. Arterioscler Thromb Vasc Biol 20: 2078–2086, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, and Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104: 210–218, 221p following 218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagger FO, Sasivarevic D, Sohi SH, Laursen LG, Pundhir S, Sonderby CK, Winther O, Rapin N, and Porse BT. BloodSpot: a database of gene expression profiles and transcriptional programs for healthy and malignant haematopoiesis. Nucleic Acids Res 2015. [Epub ahead of print]; DOI: 10.1093/nar/gkv1101 [DOI] [PMC free article] [PubMed]
- 15.Baird SK, Reid L, Hampton MB, and Gieseg SP. OxLDL induced cell death is inhibited by the macrophage synthesised pterin, 7,8-dihydroneopterin, in U937 cells but not THP-1 cells. Biochim Biophys Acta 1745: 361–369, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Baker B, Maitra U, Geng S, and Li L. Molecular and cellular mechanisms responsible for cellular stress and low-grade inflammation induced by a super-low dose of endotoxin. J Biol Chem 289: 16262–16269, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balce DR, Li B, Allan ER, Rybicka JM, Krohn RM, and Yates RM. Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 118: 4199–4208, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, and Krause KH. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science 287: 138–142, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Barlis P, Serruys PW, Devries A, and Regar E. Optical coherence tomography assessment of vulnerable plaque rupture: predilection for the plaque ‘shoulder’. Eur Heart J 29: 2023, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Barrett WC, DeGnore JP, Konig S, Fales HM, Keng YF, Zhang ZY, Yim MB, and Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, and Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem 279: 47939–47951, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Block K, Gorin Y, and Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boullier A, Li Y, Quehenberger O, Palinski W, Tabas I, Witztum JL, and Miller YI. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler Thromb Vasc Biol 26: 1169–1176, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Brigelius-Flohe R. and Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 1830: 3289–3303, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Brondello JM, Pouyssegur J, and McKenzie FR. Reduced MAP kinase phosphatase-1 degradation after p42/p44MAPK-dependent phosphorylation. Science 286: 2514–2517, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Calamita G, Ferri D, Gena P, Liquori GE, Cavalier A, Thomas D, and Svelto M. The inner mitochondrial membrane has aquaporin-8 water channels and is highly permeable to water. J Biol Chem 280: 17149–17153, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Carter AB, Tephly LA, Venkataraman S, Oberley LW, Zhang Y, Buettner GR, Spitz DR, and Hunninghake GW. High levels of catalase and glutathione peroxidase activity dampen H2O2 signaling in human alveolar macrophages. Am J Respir Cell Mol Biol 31: 43–53, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Chardonnet S, Sakr S, Cassier-Chauvat C, Le Marechal P, Chauvat F, Lemaire SD, and Decottignies P. First proteomic study of S-glutathionylation in cyanobacteria. J Proteome Res 14: 59–71, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Charles RL, Schroder E, May G, Free P, Gaffney PR, Wait R, Begum S, Heads RJ, and Eaton P. Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue. Mol Cell Proteomics 6: 1473–1484, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Chen LF. and Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 5: 392–401, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Chen YJ, Lu CT, Huang KY, Wu HY, Chen YJ, and Lee TY. GSHSite: exploiting an iteratively statistical method to identify s-glutathionylation sites with substrate specificity. PLoS One 10: e0118752, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinetti-Gbaguidi G, Colin S, and Staels B. Macrophage subsets in atherosclerosis. Nat Rev Cardiol 12: 10–17, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Chouchani ET, James AM, Fearnley IM, Lilley KS, and Murphy MP. Proteomic approaches to the characterization of protein thiol modification. Curr Opin Chem Biol 15: 120–128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chrestensen CA, Starke DW, and Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem 275: 26556–26565, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Chung S, Sundar IK, Yao H, Ho YS, and Rahman I. Glutaredoxin 1 regulates cigarette smoke-mediated lung inflammation through differential modulation of I{kappa}B kinases in mice: impact on histone acetylation. Am J Physiol Lung Cell Mol Physiol 299: L192–L203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clare K, Hardwick SJ, Carpenter KL, Weeratunge N, and Mitchinson MJ. Toxicity of oxysterols to human monocyte-macrophages. Atherosclerosis 118: 67–75, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Comalada M, Lloberas J, and Celada A. MKP-1: a critical phosphatase in the biology of macrophages controlling the switch between proliferation and activation. Eur J Immunol 42: 1938–1948, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Costantini P, Belzacq AS, Vieira HL, Larochette N, de Pablo MA, Zamzami N, Susin SA, Brenner C, and Kroemer G. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2-independent permeability transition pore opening and apoptosis. Oncogene 19: 307–314, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, and Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282: 2871–2879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dadke S, Kusari A, and Kusari J. Phosphorylation and activation of protein tyrosine phosphatase (PTP) 1B by insulin receptor. Mol Cell Biochem 221: 147–154, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797: 897–906, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Davies LC, Jenkins SJ, Allen JE, and Taylor PR. Tissue-resident macrophages. Nat Immunol 14: 986–995, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Baey A. and Lanzavecchia A. The role of aquaporins in dendritic cell macropinocytosis. J Exp Med 191: 743–748, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demasi M, Netto LE, Silva GM, Hand A, de Oliveira CL, Bicev RN, Gozzo F, Barros MH, Leme JM, and Ohara E. Redox regulation of the proteasome via S-glutathionylation. Redox Biol 2: 44–51, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.den Haan JM. and Martinez-Pomares L. Macrophage heterogeneity in lymphoid tissues. Semin Immunopathol 35: 541–552, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Deribe YL, Pawson T, and Dikic I. Post-translational modifications in signal integration. Nat Struct Mol Biol 17: 666–672, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, and Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol 171: 61–73, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, and Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol 25: 2623–2629, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Ding Q, Jin T, Wang Z, and Chen Y. Catalase potentiates retinoic acid-induced THP-1 monocyte differentiation into macrophage through inhibition of peroxisome proliferator-activated receptor gamma. J Leukoc Biol 81: 1568–1576, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Diotte NM, Xiong Y, Gao J, Chua BH, and Ho YS. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta 1793: 427–438, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Dixon DP, Skipsey M, Grundy NM, and Edwards R. Stress-induced protein S-glutathionylation in Arabidopsis. Plant Physiol 138: 2233–2244, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, and Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, and Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Epelman S, Lavine KJ, and Randolph GJ. Origin and functions of tissue macrophages. Immunity 41: 21–35, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erbel C, Tyka M, Helmes CM, Akhavanpoor M, Rupp G, Domschke G, Linden F, Wolf A, Doesch A, Lasitschka F, Katus HA, and Gleissner CA. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun 21: 255–265, 2015 [DOI] [PubMed] [Google Scholar]
- 57.Erbel C, Wolf A, Lasitschka F, Linden F, Domschke G, Akhavanpoor M, Doesch AO, Katus HA, and Gleissner CA. Prevalence of M4 macrophages within human coronary atherosclerotic plaques is associated with features of plaque instability. Int J Cardiol 186: 219–225, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, and Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol 5: 781–792, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, Di Carli MF, and Libby P. Hypoxia but not inflammation augments glucose uptake in human macrophages: implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-D-glucose positron emission tomography. J Am Coll Cardiol 58: 603–614, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Folco EJ, Sukhova GK, Quillard T, and Libby P. Moderate hypoxia potentiates interleukin-1beta production in activated human macrophages. Circ Res 115: 875–883, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forman HJ. and Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med 166: S4–S8, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Forman HJ. and Torres M. Redox signaling in macrophages. Mol Aspects Med 22: 189–216, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Foster MW, Hess DT, and Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15: 391–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, and Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A 99: 3505–3510, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fratelli M, Goodwin LO, Orom UA, Lombardi S, Tonelli R, Mengozzi M, and Ghezzi P. Gene expression profiling reveals a signaling role of glutathione in redox regulation. Proc Natl Acad Sci U S A 102: 13998–14003, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freeley M, Kelleher D, and Long A. Regulation of Protein Kinase C function by phosphorylation on conserved and non-conserved sites. Cell Signal 23: 753–762, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Gallogly MM, Starke DW, Leonberg AK, Ospina SM, and Mieyal JJ. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles. Biochemistry 47: 11144–11157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, and Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation 119: 1795–1804, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Geissmann F, Jung S, and Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Geng YJ, Henderson LE, Levesque EB, Muszynski M, and Libby P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 17: 2200–2208, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Gilbert HF. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods Enzymol 251: 8–28, 1995 [DOI] [PubMed] [Google Scholar]
- 73.Ginhoux F. and Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14: 392–404, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Gleissner CA. Macrophage Phenotype Modulation by CXCL4 in Atherosclerosis. Front Physiol 3: 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gleissner CA, Shaked I, Little KM, and Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol 184: 4810–4818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Go YM. and Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 1780: 1273–1290, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Go YM. and Jones DP. The redox proteome. J Biol Chem 288: 26512–26520, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goettsch C, Babelova A, Trummer O, Erben RG, Rauner M, Rammelt S, Weissmann N, Weinberger V, Benkhoff S, Kampschulte M, Obermayer-Pietsch B, Hofbauer LC, Brandes RP, and Schroder K. NADPH oxidase 4 limits bone mass by promoting osteoclastogenesis. J Clin Invest 123: 4731–4738, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, and Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518: 547–551, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gordon S. and Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Graminski GF, Kubo Y, and Armstrong RN. Spectroscopic and kinetic evidence for the thiolate anion of glutathione at the active site of glutathione S-transferase. Biochemistry 28: 3562–3568, 1989 [DOI] [PubMed] [Google Scholar]
- 82.Grek CL, Zhang J, Manevich Y, Townsend DM, and Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem 288: 26497–26504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gustavsson M, Verardi R, Mullen DG, Mote KR, Traaseth NJ, Gopinath T, and Veglia G. Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban. Proc Natl Acad Sci U S A 110: 17338–17343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayes JD, Flanagan JU, and Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Hayes JD. and McLellan LI. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31: 273–300, 1999 [DOI] [PubMed] [Google Scholar]
- 86.He C, Ryan AJ, Murthy S, and Carter AB. Accelerated development of pulmonary fibrosis via Cu,Zn-superoxide dismutase-induced alternative activation of macrophages. J Biol Chem 288: 20745–20757, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hidalgo C, Sanchez G, Barrientos G, and Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem 281: 26473–26482, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Hill BG, Ramana KV, Cai J, Bhatnagar A, and Srivastava SK. Measurement and identification of S-glutathiolated proteins. Methods Enzymol 473: 179–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho YS, Xiong Y, Ho DS, Gao J, Chua BH, Pai H, and Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med 43: 1299–1312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffman SM, Tully JE, Lahue KG, Anathy V, Nolin JD, Guala AS, van der Velden JL, Ho YS, Aliyeva M, Daphtary N, Lundblad LK, Irvin CG, and Janssen-Heininger YM. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am J Physiol Lung Cell Mol Physiol 303: L528–L538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu B, Allina J, Bai J, Kesar V, and Odin JA. Catalase and estradiol inhibit mitochondrial protein S-glutathionylation. Mol Cell Biochem 367: 51–58, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol 18: 49–53, 2006 [DOI] [PubMed] [Google Scholar]
- 93.Jeffrey KL, Camps M, Rommel C, and Mackay CR. Targeting dual-specificity phosphatases: manipulating MAP kinase signalling and immune responses. Nat Rev Drug Discov 6: 391–403, 2007 [DOI] [PubMed] [Google Scholar]
- 94.Joe B. and Lokesh BR. Studies on the inactivation of superoxide dismutase activity by nitric oxide from rat peritoneal macrophages. Mol Cell Biochem 168: 87–93, 1997 [DOI] [PubMed] [Google Scholar]
- 95.Johansson C, Lillig CH, and Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem 279: 7537–7543, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Jung CH. and Thomas JA. S-glutathiolated hepatocyte proteins and insulin disulfides as substrates for reduction by glutaredoxin, thioredoxin, protein disulfide isomerase, and glutathione. Arch Biochem Biophys 335: 61–72, 1996 [DOI] [PubMed] [Google Scholar]
- 97.Kamiya T, Makino J, Hara H, Inagaki N, and Adachi T. Extracellular-superoxide dismutase expression during monocytic differentiation of U937 cells. J Cell Biochem 112: 244–255, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Kehr S, Jortzik E, Delahunty C, Yates JR, 3rd, Rahlfs S, and Becker K. Protein S-glutathionylation in malaria parasites. Antioxid Redox Signal 15: 2855–2865, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, and Prabhakar NR. NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14: 533–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim HS, Ullevig SL, Nguyen HN, Vanegas D, and Asmis R. Redox regulation of 14–3-3zeta controls monocyte migration. Arterioscler Thromb Vasc Biol 34: 1514–1521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim HS, Ullevig SL, Zamora D, Lee CF, and Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc Natl Acad Sci U S A 109: E2803–E2812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim JS, Huang TY, and Bokoch GM. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol Biol Cell 20: 2650–2660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim JS, Yeo S, Shin DG, Bae YS, Lee JJ, Chin BR, Lee CH, and Baek SH. Glycogen synthase kinase 3beta and beta-catenin pathway is involved in toll-like receptor 4-mediated NADPH oxidase 1 expression in macrophages. FEBS J 277: 2830–2837, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Kim SY, Lee JG, Cho WS, Cho KH, Sakong J, Kim JR, Chin BR, and Baek SH. Role of NADPH oxidase-2 in lipopolysaccharide-induced matrix metalloproteinase expression and cell migration. Immunol Cell Biol 88: 197–204, 2010 [DOI] [PubMed] [Google Scholar]
- 105.Kim YJ, Lee WS, Ip C, Chae HZ, Park EM, and Park YM. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res 66: 7136–7142, 2006 [DOI] [PubMed] [Google Scholar]
- 106.King LS, Kozono D, and Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol 5: 687–698, 2004 [DOI] [PubMed] [Google Scholar]
- 107.Kirkman HN. and Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci 32: 44–50, 2007 [DOI] [PubMed] [Google Scholar]
- 108.Klaus A, Zorman S, Berthier A, Polge C, Ramirez S, Michelland S, Seve M, Vertommen D, Rider M, Lentze N, Auerbach D, and Schlattner U. Glutathione S-transferases interact with AMP-activated protein kinase: evidence for S-glutathionylation and activation in vitro. PLoS One 8: e62497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Komuro I, Keicho N, Iwamoto A, and Akagawa KS. Human alveolar macrophages and granulocyte-macrophage colony-stimulating factor-induced monocyte-derived macrophages are resistant to H2O2 via their high basal and inducible levels of catalase activity. J Biol Chem 276: 24360–24364, 2001 [DOI] [PubMed] [Google Scholar]
- 110.Konstantinidis D, Paletas K, Koliakos G, and Kaloyianni M. The ambiguous role of the Na+-H+ exchanger isoform 1 (NHE1) in leptin-induced oxidative stress in human monocytes. Cell Stress Chaperones 14: 591–601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Konuma T, Nakamura S, Miyagi S, Negishi M, Chiba T, Oguro H, Yuan J, Mochizuki-Kashio M, Ichikawa H, Miyoshi H, Vidal M, and Iwama A. Forced expression of the histone demethylase Fbxl10 maintains self-renewing hematopoietic stem cells. Exp Hematol 39: 697–709 e695, 2011 [DOI] [PubMed] [Google Scholar]
- 112.Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T, and Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 20: 247–266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, and Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005 [DOI] [PubMed] [Google Scholar]
- 114.Laskin DL. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem Res Toxicol 22: 1376–1385, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee CF, Qiao M, Schroder K, Zhao Q, and Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee CF, Ullevig S, Kim HS, and Asmis R. Regulation of monocyte adhesion and migration by Nox4. PLoS One 8: e66964, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HN. and Surh YJ. Resolvin D1-mediated NOX2 inactivation rescues macrophages undertaking efferocytosis from oxidative stress-induced apoptosis. Biochem Pharmacol 86: 759–769, 2013 [DOI] [PubMed] [Google Scholar]
- 118.Lee JG, Lim EJ, Park DW, Lee SH, Kim JR, and Baek SH. A combination of Lox-1 and Nox1 regulates TLR9-mediated foam cell formation. Cell Signal 20: 2266–2275, 2008 [DOI] [PubMed] [Google Scholar]
- 119.Li G, Scull C, Ozcan L, and Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191: 1113–1125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002 [DOI] [PubMed] [Google Scholar]
- 121.Libby P. and Hansson GK. Inflammation and immunity in diseases of the arterial tree: players and layers. Circ Res 116: 307–311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Libby P, Tabas I, Fredman G, and Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res 114: 1867–1879, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lill R, Hoffmann B, Molik S, Pierik AJ, Rietzschel N, Stehling O, Uzarska MA, Webert H, Wilbrecht C, and Muhlenhoff U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim Biophys Acta 1823: 1491–1508, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Lillig CH, Berndt C, and Holmgren A. Glutaredoxin systems. Biochim Biophys Acta 1780: 1304–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 125.Lim AK. and Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012: 146154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, and Han J. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep 3: 200–210, 2013 [DOI] [PubMed] [Google Scholar]
- 127.Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, von Lowenhielm HB, Holmgren A, and Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys 406: 229–240, 2002 [DOI] [PubMed] [Google Scholar]
- 128.Liu H, Shi B, Huang CC, Eksarko P, and Pope RM. Transcriptional diversity during monocyte to macrophage differentiation. Immunol Lett 117: 70–80, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Loew O. A new enzyme of general occurrence in organismis. Science 11: 701–702, 1900 [DOI] [PubMed] [Google Scholar]
- 130.Luedde T. and Schwabe RF. NF-kappaB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 8: 108–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mailloux RJ, McBride SL, and Harper ME. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem Sci 38: 592–602, 2013 [DOI] [PubMed] [Google Scholar]
- 132.Mailloux RJ. and Willmore WG. S-glutathionylation reactions in mitochondrial function and disease. Front Cell Dev Biol 2: 68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Malhotra JD. and Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9: 2277–2293, 2007 [DOI] [PubMed] [Google Scholar]
- 134.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, and Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105: 18525–18530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Manevich Y, Feinstein SI, and Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A 101: 3780–3785, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, and Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25: 677–686, 2004 [DOI] [PubMed] [Google Scholar]
- 137.Martinez FO. and Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6: 13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martinez FO, Gordon S, Locati M, and Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177: 7303–7311, 2006 [DOI] [PubMed] [Google Scholar]
- 139.McGarry DJ, Chen W, Chakravarty P, Lamont DL, Wolf CR, and Henderson CJ. Proteome-wide identification and quantification of S-glutathionylation targets in mouse liver. Biochem J 469: 25–32, 2015 [DOI] [PubMed] [Google Scholar]
- 140.McPhillips K, Janssen WJ, Ghosh M, Byrne A, Gardai S, Remigio L, Bratton DL, Kang JL, and Henson P. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. J Immunol 178: 8117–8126, 2007 [DOI] [PubMed] [Google Scholar]
- 141.Meissner F, Molawi K, and Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol 9: 866–872, 2008 [DOI] [PubMed] [Google Scholar]
- 142.Menon D. and Board PG. A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J Biol Chem 288: 25769–25779, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mieyal JJ. and Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: focus on s-glutathionylation. Antioxid Redox Signal 16: 471–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, and Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mieyal JJ, Srinivasan U, Starke DW, Gravina SA, and Mieyal PA. Glutathionyl specificity of the thioltransferases: mechanistic and physiological implications. In: Biothiols in Health and Disease, edited by Packer L. and Cadenas E. New York: Marcel Dekker, Inc., 1995, pp. 305–372 [Google Scholar]
- 146.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal 25: 457–469, 2013 [DOI] [PubMed] [Google Scholar]
- 147.Moore KJ, Sheedy FJ, and Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13: 709–721, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mullen L, Seavill M, Hammouz R, Bottazzi B, Chan P, Vaudry D, and Ghezzi P. Development of ‘Redox Arrays’ for identifying novel glutathionylated proteins in the secretome. Sci Rep 5: 14630, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Muller C, Bandemer J, Vindis C, Camare C, Mucher E, Gueraud F, Larroque-Cardoso P, Bernis C, Auge N, Salvayre R, and Negre-Salvayre A. Protein disulfide isomerase modification and inhibition contribute to ER stress and apoptosis induced by oxidized low density lipoproteins. Antioxid Redox Signal 18: 731–742, 2013 [DOI] [PubMed] [Google Scholar]
- 150.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, and Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, and Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116: 1226–1233, 2007 [DOI] [PubMed] [Google Scholar]
- 153.Newman SF, Sultana R, Perluigi M, Coccia R, Cai J, Pierce WM, Klein JB, Turner DM, and Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer's disease inferior parietal lobule, a proteomics approach. J Neurosci Res 85: 1506–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 154.Nolin JD, Tully JE, Hoffman SM, Guala AS, van der Velden JL, Poynter ME, van der Vliet A, Anathy V, and Janssen-Heininger YM. The glutaredoxin/S-glutathionylation axis regulates interleukin-17A-induced proinflammatory responses in lung epithelial cells in association with S-glutathionylation of nuclear factor kappaB family proteins. Free Radic Biol Med 73: 143–153, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oelze M, Kroller-Schon S, Steven S, Lubos E, Doppler C, Hausding M, Tobias S, Brochhausen C, Li H, Torzewski M, Wenzel P, Bachschmid M, Lackner KJ, Schulz E, Munzel T, and Daiber A. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension 63: 390–396, 2014 [DOI] [PubMed] [Google Scholar]
- 156.Olivares R, Ducimetiere P, and Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol 137: 49–53, 1993 [DOI] [PubMed] [Google Scholar]
- 157.Park CH, Kim MR, Han JM, Jeong TS, and Sok DE. Lysophosphatidylcholine exhibits selective cytotoxicity, accompanied by ROS formation, in RAW 264.7 macrophages. Lipids 44: 425–435, 2009 [DOI] [PubMed] [Google Scholar]
- 158.Patil NK, Saba H, and MacMillan-Crow LA. Effect of S-nitrosoglutathione on renal mitochondrial function: a new mechanism for reversible regulation of manganese superoxide dismutase activity? Free Radic Biol Med 56: 54–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paul S, Jakhar R, Bhardwaj M, and Kang SC. Glutathione-S-transferase omega 1 (GSTO1-1) acts as mediator of signaling pathways involved in aflatoxin B1-induced apoptosis-autophagy crosstalk in macrophages. Free Radic Biol Med 89: 1218–1230, 2015 [DOI] [PubMed] [Google Scholar]
- 160.Pelegrin P. and Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 28: 2114–2127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pimentel D, Haeussler DJ, Matsui R, Burgoyne JR, Cohen RA, and Bachschmid MM. Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system. Antioxid Redox Signal 16: 524–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, and Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest 121: 2025–2036, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Qiao M, Zhao Q, Lee CF, Tannock LR, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, and Asmis R. Thiol oxidative stress induced by metabolic disorders amplifies macrophage chemotactic responses and accelerates atherogenesis and kidney injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 29: 1779–1786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Raghunathan VK, Ellis EM, and Grant MH. Response to chronic exposure to hexavalent chromium in human monocytes. Toxicol In Vitro 23: 647–652, 2009 [DOI] [PubMed] [Google Scholar]
- 165.Reid VC. and Mitchinson MJ. Toxicity of oxidised low density lipoprotein towards mouse peritoneal macrophages in vitro. Atherosclerosis 98: 17–24, 1993 [DOI] [PubMed] [Google Scholar]
- 166.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, and Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A 103: 13086–13091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ribas V, Garcia-Ruiz C, and Fernandez-Checa JC. Glutathione and mitochondria. Front Pharmacol 5: 151, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rinna A, Torres M, and Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med 41: 86–91, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roca FJ. and Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153: 521–534, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rossi F, Zatti M, and Patriarca P. H2O2 prodution during NADPH oxidation by the granule fraction of phagocytosing polymorphonuclear leucocytes. Biochim Biophys Acta 184: 201–203, 1969 [DOI] [PubMed] [Google Scholar]
- 171.Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, Ye K, Chang CJ, Ho YS, Zhou J, and Luo HR. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 37: 1037–1049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sanchez G, Pedrozo Z, Domenech RJ, Hidalgo C, and Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J Mol Cell Cardiol 39: 982–991, 2005 [DOI] [PubMed] [Google Scholar]
- 173.Sano R. and Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta 1833: 3460–3470, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sasaki H, Yamamoto H, Tominaga K, Masuda K, Kawai T, Teshima-Kondo S, and Rokutan K. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J Med Invest 56: 33–41, 2009 [DOI] [PubMed] [Google Scholar]
- 175.Schieber M. and Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, and Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol 25: 1256–1261, 2005 [DOI] [PubMed] [Google Scholar]
- 177.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, and Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90, 2012 [DOI] [PubMed] [Google Scholar]
- 178.Schurmann C, Rezende F, Kruse C, Yasar Y, Lowe O, Fork C, van de Sluis B, Bremer R, Weissmann N, Shah AM, Jo H, Brandes RP, and Schroder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur Heart J 36: 3447–3456, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Seidel P, Roth M, Ge Q, Merfort I, S'Ng CT, and Ammit AJ. IkappaBalpha glutathionylation and reduced histone H3 phosphorylation inhibit eotaxin and RANTES. Eur Respir J 38: 1444–1452, 2011 [DOI] [PubMed] [Google Scholar]
- 180.Seimon T. and Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res 50 Suppl: S382–S387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, and Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 12: 467–482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shelton MD, Chock PB, and Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7: 348–366, 2005 [DOI] [PubMed] [Google Scholar]
- 183.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, and Brown DA. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol 52: 1009–1018, 2012 [DOI] [PubMed] [Google Scholar]
- 184.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, and Allavena P. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol 185: 642–652, 2010 [DOI] [PubMed] [Google Scholar]
- 185.Stacey MM, Cuddihy SL, Hampton MB, and Winterbourn CC. Protein thiol oxidation and formation of S-glutathionylated cyclophilin A in cells exposed to chloramines and hypochlorous acid. Arch Biochem Biophys 527: 45–54, 2012 [DOI] [PubMed] [Google Scholar]
- 186.Starke DW, Chock PB, and Mieyal JJ. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem 278: 14607–14613, 2003 [DOI] [PubMed] [Google Scholar]
- 187.Su D, Gaffrey MJ, Guo J, Hatchell KE, Chu RK, Clauss TR, Aldrich JT, Wu S, Purvine S, Camp DG, Smith RD, Thrall BD, and Qian WJ. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free Radic Biol Med 67: 460–470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Subramanian M, Thorp E, and Tabas I. Identification of a non-growth factor role for GM-CSF in advanced atherosclerosis: promotion of macrophage apoptosis and plaque necrosis through IL-23 signaling. Circ Res 116: e13–e24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, and Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 190.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249–3277, 2008 [DOI] [PubMed] [Google Scholar]
- 191.Sun C, Shi ZZ, Zhou X, Chen L, and Zhao XM. Prediction of S-glutathionylation sites based on protein sequences. PLoS One 8: e55512, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, and Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117: 195–205, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tabas I. Macrophage apoptosis in atherosclerosis: consequences on plaque progression and the role of endoplasmic reticulum stress. Antioxid Redox Signal 11: 2333–2339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol 10: 36–46, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tabas I, Garcia-Cardena G, and Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209: 13–22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, and Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117: 185–194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takashima Y, Hirota K, Nakamura H, Nakamura T, Akiyama K, Cheng FS, Maeda M, and Yodoi J. Differential expression of glutaredoxin and thioredoxin during monocytic differentiation. Immunol Lett 68: 397–401, 1999 [DOI] [PubMed] [Google Scholar]
- 198.Tavakoli S. and Asmis R. Reactive oxygen species and thiol redox signaling in the macrophage biology of atherosclerosis. Antioxid Redox Signal 17: 1785–1795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Terman JR. and Kashina A. Post-translational modification and regulation of actin. Curr Opin Cell Biol 25: 30–38, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tew KD. and Townsend DM. Glutathione-s-transferases as determinants of cell survival and death. Antioxid Redox Signal 17: 1728–1737, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, and Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab 9: 474–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thorp E, Li Y, Bao L, Yao PM, Kuriakose G, Rong J, Fisher EA, and Tabas I. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe−/− mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol 29: 169–172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, and Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem 284: 436–445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Townsend DM, Manevich Y, He L, Xiong Y, Bowers RR, Jr., Hutchens S, and Tew KD. Nitrosative stress-induced s-glutathionylation of protein disulfide isomerase leads to activation of the unfolded protein response. Cancer Res 69: 7626–7634, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsan MF, Clark RN, Goyert SM, and White JE. Induction of TNF-alpha and MnSOD by endotoxin: role of membrane CD14 and Toll-like receptor-4. Am J Physiol Cell Physiol 280: C1422–C1430, 2001 [DOI] [PubMed] [Google Scholar]
- 206.Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, and Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol 30: 1925–1932, 2010 [DOI] [PubMed] [Google Scholar]
- 207.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ullevig SL, Kim HS, Nguyen HN, Hambright WS, Robles AJ, Tavakoli S, and Asmis R. Ursolic acid protects monocytes against metabolic stress-induced priming and dysfunction by preventing the induction of Nox4. Redox Biol 2: 259–266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ullevig SL, Kim HS, Short JD, Tavakoli S, Weintraub ST, Downs K, and Asmis R. Protein S-glutathionylation mediates macrophage responses to metabolic cues from the extracellular environment. Antioxid Redox Signal 25: 836–851, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol 2: 395–428, 1984 [DOI] [PubMed] [Google Scholar]
- 211.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 11: 1289–1299, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, and Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis 214: 345–349, 2011 [DOI] [PubMed] [Google Scholar]
- 213.Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, and Chock PB. Stable and controllable RNA interference: investigating the physiological function of glutathionylated actin. Proc Natl Acad Sci U S A 100: 5103–5106, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang SB, Foster DB, Rucker J, O'Rourke B, Kass DA, and Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res 109: 750–757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang Y, Qiao M, Mieyal JJ, Asmis LM, and Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized low-density lipoprotein-induced cell injury in human macrophages: role of glutathione reductase and glutaredoxin. Free Radic Biol Med 41: 775–785, 2006 [DOI] [PubMed] [Google Scholar]
- 216.Wang Y, Wang GZ, Rabinovitch PS, and Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ Res 114: 421–433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ward NE, Stewart JR, Ioannides CG, and O'Brian CA. Oxidant-induced S-glutathiolation inactivates protein kinase C-alpha (PKC-alpha): a potential mechanism of PKC isozyme regulation. Biochemistry 39: 10319–10329, 2000 [DOI] [PubMed] [Google Scholar]
- 218.Wardman P. and von Sonntag C. Kinetic factors that control the fate of thiyl radicals in cells. Methods Enzymol 251: 31–45, 1995 [DOI] [PubMed] [Google Scholar]
- 219.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.White CN, Liu CC, Garcia A, Hamilton EJ, Chia KK, Figtree GA, and Rasmussen HH. Activation of cAMP-dependent signaling induces oxidative modification of the cardiac Na+-K+ pump and inhibits its activity. J Biol Chem 285: 13712–13720, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Winterbourn CC. and Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 222.Wu F, Szczepaniak WS, Shiva S, Liu H, Wang Y, Wang L, Wang Y, Kelley EE, Chen AF, Gladwin MT, and McVerry BJ. Nox2-dependent glutathionylation of endothelial NOS leads to uncoupled superoxide production and endothelial barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol 307: L987–L997, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wu H, Yu Y, David L, Ho YS, and Lou MF. Glutaredoxin 2 (Grx2) gene deletion induces early onset of age-dependent cataracts in mice. J Biol Chem 289: 36125–36139, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wynn TA, Chawla A, and Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yamamoto T, Sakaguchi N, Hachiya M, Nakayama F, Yamakawa M, and Akashi M. Role of catalase in monocytic differentiation of U937 cells by TPA: hydrogen peroxide as a second messenger. Leukemia 23: 761–769, 2009 [DOI] [PubMed] [Google Scholar]
- 226.Yang YC, Huang YT, Hsieh CW, Yang PM, and Wung BS. Carbon monoxide induces heme oxygenase-1 to modulate STAT3 activation in endothelial cells via S-glutathionylation. PLoS One 9: e100677, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yao PM. and Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J Biol Chem 275: 23807–23813, 2000 [DOI] [PubMed] [Google Scholar]
- 228.Yao PM. and Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem 276: 42468–42476, 2001 [DOI] [PubMed] [Google Scholar]
- 229.Yeligar SM, Harris FL, Hart CM, and Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol 188: 3648–3657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yi L, Liu Q, Orandle MS, Sadiq-Ali S, Koontz SM, Choi U, Torres-Velez FJ, and Jackson SH. p47(phox) directs murine macrophage cell fate decisions. Am J Pathol 180: 1049–1058, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, and Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zatti M, Rossi F, and Patriarca P. The H2O2-production by polymorphonuclear leukocytes during phagocytosis. Experientia 24: 669–670, 1968 [DOI] [PubMed] [Google Scholar]
- 233.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, and Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation 120: 1893–1902, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhou AX. and Tabas I. The UPR in atherosclerosis. Semin Immunopathol 35: 321–332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhou J, Lhotak S, Hilditch BA, and Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 111: 1814–1821, 2005 [DOI] [PubMed] [Google Scholar]
- 236.Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol 289: 135–139, 2014 [DOI] [PubMed] [Google Scholar]
- 237.Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, and Varol C. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol 193: 344–353, 2014 [DOI] [PubMed] [Google Scholar]
- 238.Zmijewski JW, Banerjee S, and Abraham E. S-glutathionylation of the Rpn2 regulatory subunit inhibits 26 S proteasomal function. J Biol Chem 284: 22213–22221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, and Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]