Abstract
Aims: Protein S-glutathionylation, the formation of a mixed disulfide between glutathione and protein thiols, is an oxidative modification that has emerged as a new signaling paradigm, potentially linking oxidative stress to chronic inflammation associated with heart disease, diabetes, cancer, lung disease, and aging. Using a novel, highly sensitive, and selective proteomic approach to identify S-glutathionylated proteins, we tested the hypothesis that monocytes and macrophages sense changes in their microenvironment and respond to metabolic stress by altering their protein thiol S-glutathionylation status. Results: We identified over 130 S-glutathionylated proteins, which were associated with a variety of cellular functions, including metabolism, transcription and translation, protein folding, free radical scavenging, cell motility, and cell death. Over 90% of S-glutathionylated proteins identified in metabolically stressed THP-1 monocytes were also found in hydrogen peroxide (H2O2)-treated cells, suggesting that H2O2 mediates metabolic stress-induced protein S-glutathionylation in monocytes and macrophages. We validated our findings in mouse peritoneal macrophages isolated from both healthy and dyslipidemic atherosclerotic mice and found that 52% of the S-glutathionylated proteins found in THP-1 monocytes were also identified in vivo. Changes in macrophage protein S-glutathionylation induced by dyslipidemia were sexually dimorphic. Innovation: We provide a novel mechanistic link between metabolic (and thiol oxidative) stress, macrophage dysfunction, and chronic inflammatory diseases associated with metabolic disorders. Conclusion: Our data support the concept that changes in the extracellular metabolic microenvironment induce S-glutathionylation of proteins central to macrophage metabolism and a wide array of cellular signaling pathways and functions, which in turn initiate and promote functional and phenotypic changes in macrophages. Antioxid. Redox Signal. 25, 836–851.
Keywords: : macrophage, proteomics, thiols, atherosclerosis, S-glutathionylation
Introduction
Monocytes and macrophages are inflammatory cells central to the innate and adaptive immune system. These cells not only play key roles in host defense (24) and tissue repair (56) but they also contribute to a wide array of pathologies and chronic inflammatory diseases such as atherosclerosis (7) and diabetic complications (40, 66). Common to many monocyte and macrophage functions is the production of reactive oxygen species (ROS) (5, 14, 69), which can range in concentrations from millimolar levels for antimicrobial activity (3, 39) to nanomolar and micromolar concentrations generated locally to target specific protein residues for reversible oxidative modifications observed in signal transduction (1, 5, 13, 60).
Innovation.
We describe a new and sensitive proteomic technique for specific identification of S-glutathionylated proteins in monocytes and macrophages. We show that this reversible post-translational modification occurs on a large number of proteins and may alter expression, activity, and/or degradation of these proteins, thereby affecting a vast array of cellular functions. This newly identified redox-sensitive regulatory network may provide a mechanistic link between metabolic stress and the well-documented changes in the functional phenotype of monocytes and macrophages observed in patients with metabolic disorders. Finally, we provide the first evidence for sexual dimorphism in metabolic stress-induced thiol oxidation in macrophages.
ROS-mediated oxidative modification of protein thiols has been studied extensively for its role in signal transduction and regulation of various cellular processes, including proliferation, transcription and translation, and apoptosis (38). Protein thiol oxidation products include (i) sulfenic, sulfinic, or sulfonic acids, (ii) S-nitrosothiols, (iii) sulfenylamide, (iv) intra- and intermolecular disulfides, and (v) mixed disulfides with low-molecular-weight thiols, including glutathione and cysteine (30, 67). However, protein S-glutathionylation (and deglutathionylation) has emerged as a bona fide signaling mechanism (54, 55) due to the (enzymatically) reversible nature of the protein S-glutathionylation reaction, the specificity and selectivity of cysteine residues modified, and the ability of S-glutathionylation to modulate protein function. To date, only a few S-glutathionylated proteins have been validated as bona fide redox signaling transducers based on five criteria originally outlined by Mieyal et al. (42). We recently identified three proteins critical for monocyte migration that fulfill these criteria: actin (36, 62), mitogen-activated protein kinase phosphatase 1 (MKP-1) (32), and 14-3-3ζ (31). Others reported that phosphatase and tensin homolog deleted from chromosome 10 (PTEN) (11), protein tyrosine phosphatase 1B (PTP1B) (50), Na-H exchanger isoform 1 (NHE1) (33), caspase-1 (41), and paraoxonase 1 (PON1) (52) were found to be S-glutathionylated in monocytes and macrophages of varying lineages (11, 33, 41, 50, 52). These data raise the question whether protein S-glutathionylation is limited to only select proteins and specific pathways or whether this unique post-translational modification may play a broader functional role in monocytes and macrophages. In support of the latter hypothesis, we found that metabolic stress increases global S-glutathionylation in peritoneal macrophages from mice fed a high-fat diet (HFD) (47). Increased S-glutathionylation correlated with functional changes in these macrophages, including the hypersensitization to chemokine-induced adhesion and accelerated monocyte chemotaxis, a phenomenon we termed metabolic priming of monocytes (47, 62).
To identify S-glutathionylated proteins in macrophages exposed to metabolic stress, we developed a novel, specific, and highly sensitive proteomic approach. Previous studies have used global labeling techniques to identify individual S-glutathionylated proteins (10, 15, 27, 37, 38); however, major limitations of these approaches include a lack of sensitivity and the use of either supraphysiological concentrations of oxidants such as hydrogen peroxide (H2O2) or nonphysiological oxidants such as diamide to induce detectable changes in protein S-glutathionylation. The redox proteomic technique we developed is sensitive enough to identify S-glutathionylated proteins in both a human monocytic cell line and in murine peritoneal macrophages and to monitor changes in the level of modification in response to metabolic stress. Our study demonstrates that in monocytic cells, metabolic stress promotes the S-glutathionylation of over 600 proteins, and we provide the first report of global cellular changes in S-glutathionylation in response to (patho)physiological stimuli both in vitro and in vivo. Furthermore, our data suggest that S-glutathionylation of reactive protein thiols may serve as a mechanism for cells to sense and rapidly respond to changes in the extracellular environment by altering activities and/or expression levels of a large number of proteins. This novel mechanistic link between changes in the metabolic environment and global alterations in macrophage signaling pathways and functionalities may help explain the plasticity of macrophages and their adaptability to vastly different and dynamic microenvironments.
Results
Strategy for specific labeling of S-glutathionylated proteins
Current proteomic techniques designed to detect S-glutathionylated proteins lack either the sensitivity and/or the specificity for protein-GSH mixed disulfides. To address both limitations, we designed a new strategy (Fig. 1) that involves a novel enrichment technique based on iodoacetamide conjugated to desthiobiotin (IAM-Desthiobiotin) labeling, followed by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS), to identify and quantify S-glutathionylated proteins.
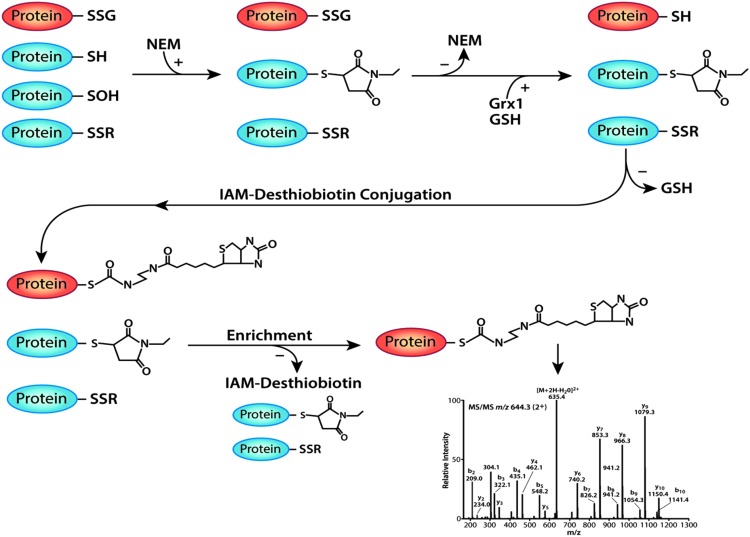
FIG. 1.
Labeling and identification strategy for S-glutathionylated proteins. Cell lysates, which contain a mixture of protein thiol modifications, including mixed disulfides with glutathione (Protein-SSG), free thiols (Protein-SH), sulfenic acid (Protein-SOH), and intra- or intermolecular disulfides (Protein-SSR), are treated with NEM to alkylate free thiols and sulfenyl groups. NEM is removed and Grx1 and GSH are added to reduce the protein thiol-GSH mixed disulfide (Protein-SSG) to a free protein thiol (Protein-SH). After removal of excess GSH, newly formed free protein thiols are alkylated with IAM-Desthiobiotin. IAM-DES-linked proteins are enriched using streptavidin-coated agarose beads, separated on a 1D SDS-PAGE gel, and processed for liquid chromatography and tandem mass spectrometry. Peptides were identified by comparing the spectra to a human subset of the NCBInr_20130102 database combined with a database containing sequences for common contaminants (see the Materials and Methods section for details). Grx1, glutaredoxin 1; GSH, reduced glutathione; IAM-Desthiobiotin, iodoacetamide conjugated to desthiobiotin; NEM, N-ethylmaleimide; SDS-PAGE; sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
In the first step, all intracellular free thiols and sulfonic acid groups were alkylated by treating intact cells before and during lysis with the cell-permeable alkylating agent, N-ethylmaleimide (NEM). NEM renders thiols unreactive and reduces false-positive signals due to transthiolation and disulfide formation (18) that can occur with free thiols (-SH), sulfenic acid (-SOH), sulfinic acid (-SO2H), and inter- or intramolecular disulfide bonds (-SSR). Second, we used recombinant bacterial glutaredoxin 1 (Grx1), which selectively catalyzes the deglutathionylation of proteins (25), to reduce mixed disulfides between protein thiols and reduced glutathione (GSH) (Fig. 1). Conditions were optimized to ensure a quantitative reduction of all protein thiol-GSH mixed disulfides and maximal labeling of the resulting free thiols (Supplementary Figs. S1A, B, and S2A, B; Supplementary Data are available online at www.liebertpub.com/ars).
To avoid inactivation of Grx1, unreacted NEM was removed from the samples before the addition of the Grx1 enzyme (Fig. 1). In addition, excess GSH was added to the deglutathionylation reaction to recycle the active site of Grx1 and maintain its catalytic activity (19). GSH was removed after completion of the reaction to prevent interference with the thiol-reactive label, IAM-Desthiobiotin (Fig. 1). IAM is more selective for the thiolate anion than NEM and results in lower nonspecific labeling, making IAM-Desthiobiotin a highly specific and selective labeling reagent for the reactive thiols generated after Grx1 reduction. IAM-Desthiobiotin-labeled proteins were enriched using streptavidin-coated agarose beads (Fig. 1). DES weakly interacts with the biotin moiety, allowing for the elution of DES-labeled proteins from streptavidin under mild conditions (28), minimizing protein loss due to denaturation and aggregation. After elution, samples were subjected to mass spectrometry analysis (see the Materials and Methods section for more details).
Protein S-glutathionylation in THP-1 monocytes in response to metabolic stress and H2O2
We showed previously that metabolic stress (and H2O2 treatment) sensitizes monocytes to chemokine activation, converting these cells into a hypermigratory proatherogenic phenotype (32, 62), a process we termed metabolic priming. We went on to demonstrate that metabolic priming is mediated by S-glutathionylation of proteins involved in cell signaling, cytoskeletal remodeling, and cell migration (31, 32, 62), suggesting that protein S-glutathionylation might be a global molecular mechanism by which monocytes and macrophages alter the proteome to respond to cellular stimuli. To test this hypothesis, THP-1 monocytes were cultured in the absence or presence of either metabolic stress (low-density lipoprotein + high D-glucose [LDL+HG]) or thiol oxidative stress (H2O2), and lysates from these cells were analyzed to identify and semiquantify S-glutathionylated proteins.
In three independent labeling experiments, HPLC-ESI-MS/MS analysis identified a total of 636 proteins that were S-glutathionylated in lysates from THP-1 monocytes. With the exception of a single study using diamide as an oxidant (57), this is one of the few studies to identify over 100 S-glutathionylated proteins using a proteomic screen. Due to the surprisingly large number of identified proteins, we focused our subsequent analysis on those 133 S-glutathionylated proteins that were reproducibly identified in each of the three independent experiments (Supplementary Table S1).
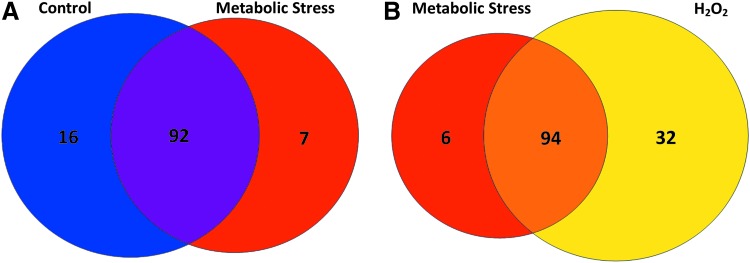
Of these 133 proteins, 115 were detected in either unstressed or metabolically stressed THP-1 monocytes or both (Fig. 2A). Of these 115, 16 proteins (14%) were S-glutathionylated in unstressed THP-1 monocytes, but not in metabolically stressed cells, while only seven proteins (6%) were identified as being detected only in lysates of metabolically stressed THP-1 cells, but not in unstressed cells, that is, de novo S-glutathionylated (Fig. 2A). However, the majority of S-glutathionylated proteins (92 or 81%) were identified by LC-MS/MS under both conditions. These data indicate that most proteins that are S-glutathionylated in monocytes under metabolic stress are also S-glutathionylated to some degree under resting conditions. However, metabolic stress altered the level of S-glutathionylation in many of these proteins. Levels of S-glutathionylation in response to metabolic stress remained unchanged (or inconsistent between experiments) in 63% of the 115 proteins (Supplementary Table S1, “ = ”) and increased in 29% of proteins (Supplementary Table S1, “↑”). Surprisingly, 9% of proteins showed decreased S-glutathionylation levels under metabolic stress (Supplementary Table S1, “↓”). Taken together, the observed changes in protein S-glutathionylation suggest that metabolic stress targets specific proteins for increased S-glutathionylation, yet others for decreased S-glutathionylation. These data support the concept of a global, but highly controlled and specific, response to metabolic stress.
FIG. 2.
S-glutathionylated proteins in THP-1 monocytes. Venn diagrams depicting the relationship between the 133 consistently identified S-glutathionylated proteins in untreated (Control), metabolically stressed, and H2O2-treated THP-1 cells. (A) Comparison between control and metabolically stressed THP-1 cells shows that of 115 proteins, 92 were found in both conditions (purple). Sixteen proteins were found only in control (blue) and 7 were identified only in metabolically stressed THP-1 cells (red) (n = 3). (B) Comparison between metabolically stressed and H2O2-treated THP-1 cells shows that of 132 proteins, 94 were found in both metabolically stressed and H2O2-treated cells (orange). Six proteins were only found in metabolically stressed cells (red) and 32 were found only in H2O2-treated cells (yellow) (n = 3). H2O2, hydrogen peroxide.
When we compared protein S-glutathionylation between metabolically stressed THP-1 monocytes and H2O2-treated cells, we found that the vast majority of the proteins (71%) were S-glutathionylated under both conditions (Fig. 2B), indicating that H2O2 may be the oxidant responsible for mediating S-glutathionylation induced by metabolic stress. Twenty-four percent (32 proteins) of identified proteins were detected in lysates only H2O2 treated and 5% (six proteins) were exclusive to cells exposed to metabolic stress (Fig. 2B). These data suggest that while H2O2 can mimic the effects of metabolic stress (62), high levels of (exogenous) H2O2 target additional proteins that do not appear to be S-glutathionylated in cells exposed to metabolic stress.
Using Ingenuity® Pathway Analysis (IPA®) software and UniProt, we were able to group the 133 proteins (Supplementary Table S1) by specific location (Supplementary Table S2) and function (Supplementary Table S3). The vast majority of the 133 proteins (74%) were predicted to be cytoplasmic, while 14% were predicted to be nuclear (Supplementary Table S2); this distribution did not appear to depend on whether S-glutathionylation of the protein was altered by metabolic stress or not. Of the 24 functions associated with the 133 proteins, the top six functional classes were of oxidoreductases, chaperones, ion-binding proteins, nucleotide-binding proteins, ribonucleoproteins, and translation regulators (Supplementary Table S3).
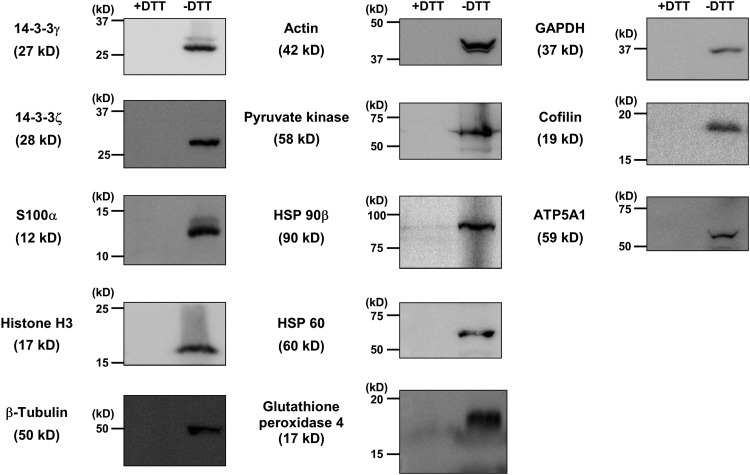
To confirm that IAM-Desthiobiotin-labeled proteins identified with our enrichment technique were S-glutathionylated (Fig. 1), we labeled metabolically primed THP-1 monocytes with biotin-conjugated glutathione ethyl ester (BioGEE, see the Materials and Methods section for details). Thirteen proteins were selected for identification by BioGEE either because others had previously identified them as S-glutathionylated proteins (15, 21, 23, 31, 34, 53, 64) or, in our initial screen, they exhibited consistent and robust S-glutathionylation levels (based on summed intensities of the fragments in the product ion spectra). All 13 proteins selected were detected after BioGEE labeling and streptavidin affinity purification (Fig. 3). Furthermore, these proteins were no longer detectable when lysates were treated with dithiothreitol (DTT), indicating that these proteins were indeed S-glutathionylated in THP-1 monocytes.
FIG. 3.
Validation of 13 IAM-Desthiobiotin-labeled proteins as S-glutathionylated proteins. THP-1 monocytes were preincubated with BioGEE (250 μM) and subsequently metabolically stressed for 24 h with human LDL plus 20 mM D-glucose (LDL+HG). Before precipitating biotin-labeled proteins with streptavidin–agarose, lysates were preincubated with either phosphate-buffered saline (−DTT) or 10 mM DTT. Precipitates of biotin-labeled proteins were analyzed by Western blots probed with antibodies directed against the proteins of interest (n = 3). BioGEE, biotin-conjugated glutathione ethyl ester; DTT, dithiothreitol; HG, high D-glucose; LDL, low-density lipoprotein.
To assess the contribution of H2O2 to protein S-glutathionylation in monocytes, we examined if overexpression of H2O2-scavenging catalase would block BioGEE conjugation to PKM, β-actin, and histone H3 in THP-1 monocytes. Overexpression of catalase by either adenovirus infection or treatment with polyethylene glycol (PEG)-conjugated catalase resulted in decreased BioGEE labeling of both PKM and β-actin in metabolically stressed cells (Fig. 4). Importantly, BioGEE labeling of both PKM and β-actin was also reduced by catalase overexpression in cells under normal culture conditions in the absence of metabolic stress, indicating that protein S-glutathionylation in resting cells may also require H2O2 (Fig. 4A). These data show that catalase can selectively block protein S-glutathionylation in monocytes and suggest that H2O2 is required for S-glutathionylation of proteins such as PKM and β-actin in THP-1 cells. Interestingly, BioGEE labeling of histone H3 was not affected by catalase overexpression (Fig. 4).
FIG. 4.
Overexpression of catalase selectively prevents metabolic stress-induced S-glutathionylation of pyruvate kinase and actin. (A) THP-1 monocytes were infected for 20 h with an adenoviral vector encoding human catalase. Noninfected cells (NT) or infected cells (Ad-Cat) were then incubated with 250-μM BioGEE and subsequently kept in normal growth media (C) or metabolically stressed for 24 h with human LDL plus 20 mM D-glucose (L + H) for 24 h. WCEs were then used for SAP, and both WCEs and SAPs were analyzed by IB with the indicated antibodies. Representative immunoblots are shown (left), and protein levels of proteins from SAP samples were measured and compared (right). Results are shown as mean ± SE (n = 3). (B) THP-1 monocytes were treated with PEG (P) or PEG-Catalase (P-C) for 20 h, after which cells were preincubated with BioGEE (250 μM) and metabolically primed for 24 h with human LDL and 20 mM D-glucose. Analysis of BioGEE-labeled proteins was performed as described above. Results are shown as mean ± SE (n = 3; right), and representative blots are shown on the left. Dashed lines indicate nonadjacent lanes on the same blot (*p < 0.05, **p < 0.01). IB, immunoblotting; PEG, polyethylene glycol; SAP, streptavidin affinity purification; WCEs, whole cell extracts.
Protein S-glutathionylation can affect expression levels, activity, and/or intracellular localization of proteins (9, 32). We therefore explored whether metabolic stress in THP-1 monocytes altered expression levels of these 13 validated proteins and examined whether these changes could be prevented by overexpressing Grx1. Compared with vehicle-treated cells, metabolically stressed THP-1 monocytes showed a significant increase in the expression of pyruvate kinase and heat shock protein 60 kDa (HSP60) and a significant decrease in 14-3-3ζ protein levels (Fig. 5). None of the other proteins showed any significant change in expression levels. Overexpression of Grx1 completely prevented the changes in protein levels induced by metabolic stress (Fig. 5). These results suggest that metabolic stress-induced S-glutathionylation alters the expression levels of key proteins involved in cell signaling and monocyte metabolism, which may directly impact monocyte functions.
FIG. 5.
Changes in expression levels of pyruvate kinase, HSP60, and 14-3-3ζ in response to metabolic stress are mitigated by protein deglutathionylation. THP-1 monocytes were infected with a doxycycline (Dox)-inducible adenoviral vector carrying the sequence for a Grx1-EGFP fusion protein (pAd-Grx1), and Grx1 expression was induced by treating cells for 24 h with 1 μg/ml doxycycline (Dox). THP-1 monocytes were then treated for 24 h with vehicle or were metabolically stressed with human LDL plus 20 mM D-glucose (L-H). Protein levels were measured by Western blot analysis and results are shown as mean ± SE (n = 4). HSP, heat shock protein.
Protein S-glutathionylation in primary peritoneal macrophages
After validating our labeling strategy in protein S-glutathionylation in THP-1 monocytes, we examined whether protein S-glutathionylation also occurs in peritoneal macrophages of mice exposed to long-term metabolic stress. To our knowledge, this is the first time a proteomic analysis of S-glutathionylated proteins has been applied to primary cells isolated from an animal model mimicking human disease. Both male and female atherosclerotic-prone LDL receptor-null (LDLR−/−) mice were fed an HFD for 10 weeks to induce hyperlipidemia and mild hyperglycemia (48). As a control, LDLR−/− mice received a defined low-fat maintenance diet (MD). Our previous studies in this murine atherosclerosis model showed that peritoneal macrophages isolated from LDLR−/− mice exhibit increased thiol oxidative stress, increased protein S-glutathionylation, and accelerated macrophage chemotactic activity, which all directly correlated with the extent of atherosclerotic lesion formation (48, 62). In the current study, after 10 weeks, male and female mice on the HFD developed hyperlipidemia and mild hyperglycemia, although blood glucose elevations did not reach statistical significance (Supplementary Table S4). In contrast to male mice, HFD-fed females maintained the same body weight and plasma triglyceride levels as MD-fed females, despite developing severe hypercholesterolemia, recapitulating findings from our previous study (48).
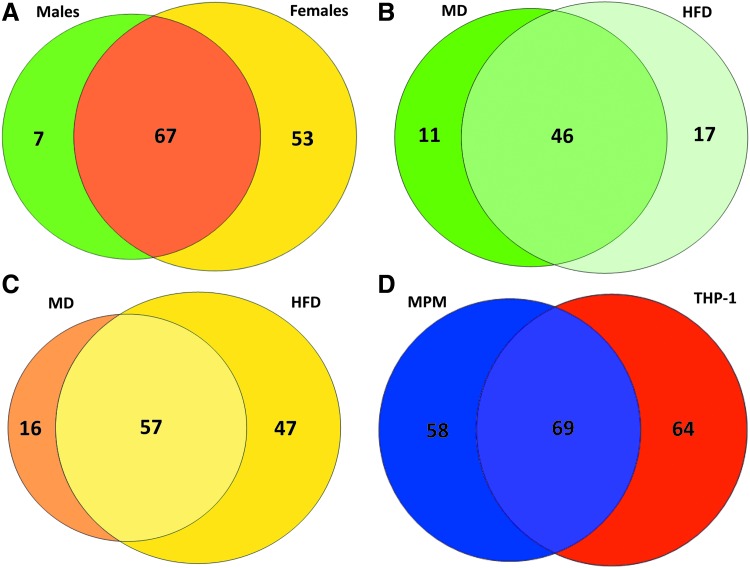
Using IAM-Desthiobiotin-labeled lysates from primary peritoneal macrophages, which had to be pooled by sex and diet due to the low amount of protein, we identified a total of 127 S-glutathionylated proteins in males and females (Supplementary Table S5). Of the 127 IAM-Desthiobiotin-labeled proteins, 67 S-glutathionylated proteins (53%) were found in lysates from both male and female mice (Fig. 6A). Surprisingly, we detected 53 IAM-Desthiobiotin-labeled proteins (42%) in macrophage lysates that were unique to females and not identified in macrophage lysates from male mice. In fact, we detected nearly twice as many IAM-Desthiobiotin-labeled proteins (120) in macrophages from female mice when compared with male mice (74) (Fig. 6A). These data suggest a potential sexual dimorphism in the effect of metabolic stress on protein S-glutathionylation in primary macrophages. As with THP-1 cells, the majority of the proteins (46 or 62%) found in male mice were identified in macrophages from both MD-fed and HFD-fed mice (Fig. 6B). However, 16 of these 46 proteins (35%) showed increased IAM-Desthiobiotin labeling in response to metabolic stress, that is, HFD feeding, 7 (15%) showed decreased IAM-Desthiobiotin labeling, and IAM-Desthiobiotin labeling remained unchanged in 50% of the 46 proteins (Supplementary Table S5). These findings indicate that metabolic stress has divergent effects on the level of S-glutathionylation of these proteins. In macrophages from male mice, 17 of 74 IAM-DES-labeled proteins (23%) were only found in response to HFD, that is, were S-glutathionylated de novo in response to metabolic stress, and only 11 proteins (15%) were found exclusively in macrophages from MD-fed mice (Fig. 6B).
FIG. 6.
S-glutathionylated proteins in murine peritoneal macrophages from normo- and dyslipidemic atherosclerosis-prone mice. Venn diagrams depicting the relationship between S-glutathionylated proteins identified in mouse peritoneal macrophages isolated from male and female LDL receptor-deficient (LDLR−/−) mice fed either MD or HFD for 10 weeks. (A) Of 127 S-glutathionylated proteins identified in murine peritoneal macrophages isolated from males and females fed either MD or HFD, 67 were found in both groups (orange), 7 proteins only in males (green), and 53 exclusively in females (yellow). (B) Of the 74 proteins found in MD-fed and HFD-fed males, 46 proteins were found in both conditions (medium green), but 11 were only found in macrophages from MD-fed mice (dark green) and 17 were found only in HFD-fed mice (light green). (C) Of the 120 proteins identified in female MD-fed and HFD-fed mice, 57 were found in both dietary conditions (light yellow), 16 only in macrophages from MD-fed females (light orange), and 47 were found only in HFD-fed female mice (yellow) (n = 5). (D) The comparison of S-glutathionylated proteins found in MPM and THP-1 cells reveals that 69 proteins were found in both cell types (purple), 58 found in MPM only (blue), and 64 were found exclusively in THP-1 cells (red). MPM, murine peritoneal macrophages; HFD, high-fat diet; MD, maintenance diet.
In contrast, macrophages from female mice showed a much higher level of de novo IAM-Desthiobiotin-labeled proteins, that is, S-glutathionylated proteins only detected in response to HFD (47 proteins or 39% vs. only 23% in males, Fig. 6C). These data strongly suggest that monocytes and macrophages in female mice show a unique spectrum of S-glutathionylated proteins in response to metabolic stress, which may alter macrophage function differently in females compared with male mice. Only selected proteins were found to be S-glutathionylated exclusively in macrophages from MD-fed mice (Fig. 6B, C: males: 15%, females: 13%). Together, these findings also suggest that most proteins that are susceptible to S-glutathionylation in primary (murine) macrophages are already modified under homeostatic conditions and only change their level of modification in response to metabolically stressed environments. This finding is consistent with our results in THP-1 monocytes.
When comparing IAM-Desthiobiotin-labeled proteins identified in mouse peritoneal macrophages with those found in human THP-1 monocytes, 36% of the proteins (69 of 191 proteins) were identified in both cell types, while 30% (58 proteins) were unique to mouse peritoneal macrophages and 34% (64 proteins) were unique to THP-1 cells (Fig. 6D). More importantly, 52% of the IAM-Desthiobiotin-labeled proteins identified in THP-1 monocytes (69 of 133 proteins) were also identified in mouse peritoneal macrophages, while 54% (69 of 127 proteins) of IAM-Desthiobiotin-labeled proteins identified in mouse peritoneal macrophages were also identified in THP-1 monocytes (Fig. 6D). These data suggest that the pattern of thiol oxidation in response to metabolic stress appears to be largely conserved between murine and human monocytic cells.
Identified functional roles of S-glutathionylated proteins
We used IPA and STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) analysis to gain insights into the potential functional consequences of dyslipidemia-induced alterations to the S-glutathionylation status of the macrophage proteome. IPA analysis grouped the 127 IAM-DES-labeled proteins identified in murine peritoneal macrophages by specific function (Supplementary Table S6) and location (Supplementary Tables S7 and S8). Of the 36 functions associated with these 127 proteins, the most abundant functionalities were oxidoreductases, structural proteins, chaperones, ion-binding proteins, nucleotide-binding proteins, and peptidases (Supplementary Table S6), very similar to the main functions associated with S-glutathionylated proteins found in THP-1 monocytes (Supplementary Table S1). As with THP-1 monocytes, the vast majority of the proteins identified in macrophages from both female (76%) and male mice (80%) were located in the cytoplasm, while only 4% IAM-Desthiobiotin-labeled proteins were localized in the nucleus of macrophages from either sex (Supplementary Tables S7 and S8).
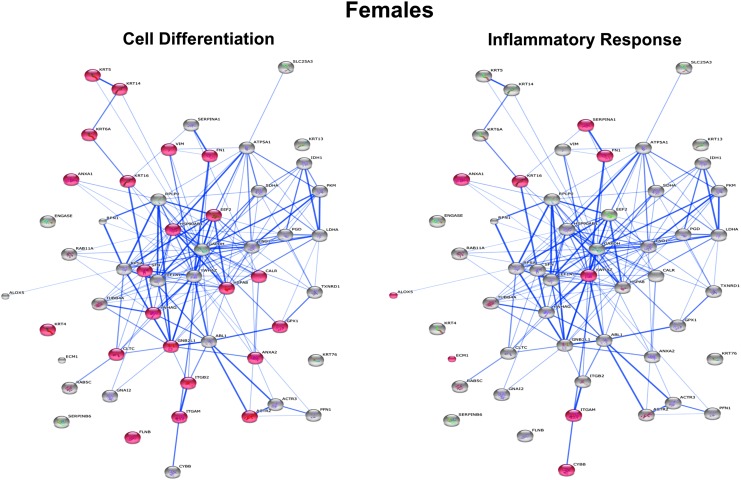
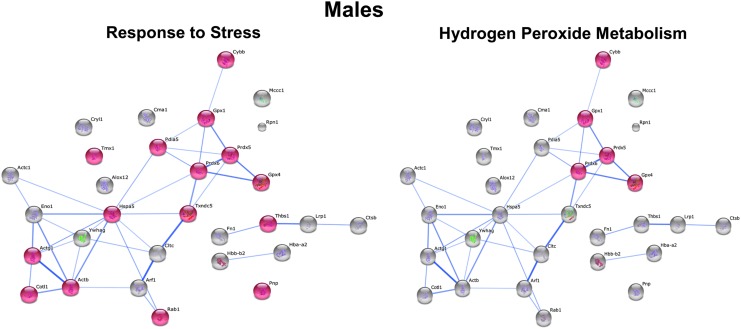
To determine which specific processes in male versus female macrophages would be affected in response to HFD feeding, we performed STRING analysis. The protein network for all S-glutathionylated proteins in female macrophages differs dramatically from that for male macrophages (Supplementary Figs. S3 and S4). The two most significant processes showing the highest enrichment scores for increased protein S-glutathionylation in macrophages of female mice were Cell Differentiation (p = 8.9 × 10−5) and Inflammatory Response (p = 8.8 × 10−4) (Fig. 7), while the two most significant processes for male macrophages were H2O2 Metabolism (p = 3.7 × 10−6) and Response to Stress (p = 6.4 × 10−5) (Fig. 8).
FIG. 7.
Functional cluster and gene ontology of macrophage proteins responding with increased S-glutathionylation to dyslipidemia in female mice. STRING (http://string-db/org) (58) was utilized to identify the most significant processes showing the highest enrichment scores for increased S-glutathionylation in macrophages of female mice. The top two clusters were Cell Differentiation (p = 8.9 × 10−5) and Inflammatory Response (p = 8.8 × 10−4). Clustering of proteins and node distances represent protein interaction confidence based on direct (physical) and indirect (functional) parameters. Confidence scores are generated based on previously published experimental data as well as high-throughput arrays, text mining, genomic annotations, and computational predictions. STRING, Search Tool for the Retrieval of Interacting Genes/Proteins,
FIG. 8.
Functional cluster and gene ontology of macrophage proteins responding with increased S-glutathionylation to dyslipidemia in male mice. STRING (http://string-db/org) (58) was utilized to identify the most significant processes showing the highest enrichment scores for increased S-glutathionylation in macrophages of male mice. The top two clusters were Response to Stress (p = 6.4 × 10−5) and H2O2 Metabolism (p = 3.7 × 10−6). Clustering of proteins and node distances represent protein interaction confidence based on direct (physical) and indirect (functional) parameters. Confidence scores are generated based on previously published experimental data as well as high-throughput arrays, text mining, genomic annotations, and computational predictions.
Interestingly, we also identified a number of functional clusters for proteins with decreased S-glutathionylation in macrophages from female mice, the top two clusters being Cell Redox Homeostasis (p = 6.1 × 10−6) and H2O2 Metabolism (p = 4.3 × 10−6) (Fig. 9). In contrast, no biological processes showed significant enrichment scores in macrophages from male mice for proteins with decreased S-glutathionylation. Together, these findings suggest that metabolic stress-induced thiol oxidation and protein S-glutathionylation affect not only different proteins but also distinct processes in macrophages from male and female mice. In fact, metabolic stress in male and female macrophages had exactly opposite effects on proteins involved in H2O2 metabolism (increased protein S-glutathionylation in males, but decreased S-glutathionylation in females), which includes proteins involved in antioxidant defense, the detoxification of H2O2, and redox signaling.
FIG. 9.
Functional cluster and gene ontology of macrophage proteins responding with decreased S-glutathionylation to dyslipidemia in female mice. STRING (http://string-db/org) (58) was utilized to identify the most significant processes showing the highest enrichment scores for decreased S-glutathionylation in macrophages of female mice. The top two clusters were Cell Redox Homeostasis (p = 6.1 × 10−6) and H2O2 Metabolism (p = 4.3 × 10−5). No biological processes showed significant enrichment scores in macrophages from male mice for proteins with decreased S-glutathionylation. Clustering of proteins and node distances represent protein interaction confidence based on direct (physical) and indirect (functional) parameters. Confidence scores are generated based on previously published experimental data as well as high-throughput arrays, text mining, genomic annotations, and computational predictions.
Discussion
We have developed a novel, sensitive, and selective technique to identify cellular S-glutathionylated proteins and utilized this labeling technique to examine metabolic stress-induced alterations in the S-glutathionylation state of proteins in monocytes and macrophages. This method allowed us to identify over 600 proteins in THP-1 monocytes and monitor changes in their S-glutathionylation state in response to (patho)physiologically relevant conditions. Furthermore, this technique allowed us to identify S-glutathionylated proteins in lysates of primary macrophages isolated from dyslipidemic and mildly hyperglycemic atherosclerosis-prone mice. To our knowledge, this is the first study identifying over 100 S-glutathionylated proteins in primary cells from an in vivo model of metabolic stress and human chronic inflammatory disease. Previous proteomic studies have typically found fewer than 30 S-glutathionylated proteins, and S-glutathionylation was induced by either supraphysiological concentrations of H2O2 or nonphysiological agents such as diamide (15, 16, 37). Importantly, our report is also the first to identify a potential sexual dimorphism associated with (thiol) oxidative stress in monocytes and macrophages.
There are three main reasons for the unprecedented sensitivity and selectivity of our technique. First, we used cell-permeable NEM to alkylate all free intracellular thiols to minimize both the loss of existing S-glutathionyl residues through transthiolation reactions (18) during cell lysis and any nonspecific IAM-Desthiobiotin labeling. Second, the use of an enzymatic reductant, Grx1, which is highly selective for mixed disulfides between protein thiols and glutathione, ensured that only thiols derived from S-glutathionylated proteins would be conjugation with IAM-Desthiobiotin. Last, we used a highly sensitive thiol-reactive probe coupled to DES rather than a biotin tag to increase the labeling efficiency and to allow for the elution of labeled proteins from the streptavidin-coated beads under mild conditions, thereby increasing the overall yield in IAM-Desthiobiotin-labeled proteins (28). Coupling this highly efficient labeling method to mass spectrometry analysis dramatically increased both selectivity and sensitivity of this proteomic approach. Thirteen proteins were selected for validation based on previous reports of S-glutathionylation and their robust mass spectrometry signal. The validation of these 13 selected IAM-Desthiobiotin-labeled proteins as bona fide S-glutathionylated proteins confirmed that our proteomic technique is not only sensitive and highly selective but also accurate and reliable.
Protein S-glutathionylation occurs under conditions of increased ROS production that leads to oxidation of the thiolate, producing proteins with sulfenic, sulfinic, and sulfonic acid residues (26, 42). These oxidized thiol groups, particularly sulfenic acids, can react with glutathione (GSH) to form mixed disulfides or S-glutathionylated proteins (26). Our data showing that catalase overexpression significantly represses both PKM and β-actin S-glutathionylation suggest that H2O2 mediates the S-glutathionylation of at least some proteins in monocytes. However, histone H3 S-glutathionylation was unaffected by catalase overexpression (Fig. 4). One possibility is that transgenic catalase may only prevent S-glutathionylation of cytosolic protein and was unable to translocate into the cell nucleus to block H3 S-glutathionylation. Alternatively, H3 S-glutathionylation may be regulated by an H2O2-independent mechanism, for example, by glutathione-S-transferase enzymes (26). Nevertheless, our data strongly suggest that at least some proteins in monocytes and macrophages are S-glutathionylated in an H2O2-dependent manner.
One limitation to our approach is inherent to nontargeted mass spectrometry, in that IAM-Desthiobiotin-conjugated peptides were only detected for high-abundance proteins, including actin, actin-related protein 3, GAPDH, HSP60, lactate dehydrogenase, ER60 protein, elongation factor 2, phospholipid glutathione peroxidase, and Grx. Identification of specific cysteine residue(s) labeled was not possible for the vast majority of IAM-Desthiobiotin-tagged proteins due to lack of sufficient sequence coverage. Therefore, future studies will focus on immunoprecipitation of candidate proteins and subsequent targeted tandem MS analysis, which should permit the detection of S-glutathionylated cysteine residue(s) (6, 8). A second more general limitation to this study is the THP-1 monocytic cell line used to validate the labeling technique. These monocytic cells may have limited clinical relevance with regard to metabolic and thiol oxidative stress in human monocytes and macrophages. Although we confirmed our findings in vivo in macrophages from a murine model of diet-induced dyslipidemia and atherosclerosis, validation in human monocytes and patients is still needed.
Originally, S-glutathionylation was considered a protective mechanism by cells to avoid irreversible oxidation of protein thiols and conserve intracellular glutathione during oxidative stress (54, 61). However, our group previously found that S-glutathionylation of MKP-1 not only inactivates this key phosphatase but also results in its proteasomal degradation, demonstrating that monocytes and macrophages respond to changes in their metabolic environment by altering both MKP-1 activity and expression levels (32). Of the 13 S-glutathionylated proteins we selected for validation, metabolic stress induced changes in protein expression levels in three of these proteins: pyruvate kinase, HSP60, and 14-3-3ξ. Furthermore, these metabolic stress-induced changes in protein levels were mitigated by overexpression of Grx1, the thiol transferase that catalyzes the reduction of mixed disulfides between protein thiols and GSH. These data support previous findings from our group and others that protein S-glutathionylation can regulate enzymatic activity as well as expression levels and degradation rates of proteins such as crystalline (72), MKP-1 (32), and 14-3-3ξ (31). Our data suggest that regulation of protein expression, activity, and stability by S-glutathionylation may be common to a much larger number of (redox-sensitive) proteins than previously known.
In silico analyses (IPA) of S-glutathionylated proteins identified in our study suggest that protein S-glutathionylation affects proteins involved in a wide array of cellular functions, including cell adhesion and migration, cell signaling, transcription, translation, and energy metabolism to name only a few. The vast number of proteins and protein functions affected by metabolic stress-induced S-glutathionylation, along with the dramatic changes in global protein S-glutathionylation in response to metabolic stress, suggests a broader role for the redox-dependent post-translational modification in altering the cell's overall functional phenotype.
One rather unexpected discovery from our study is the sexual dimorphism we found with regard to the effect of metabolic disorders on protein S-glutathionylation in murine peritoneal macrophages isolated from dyslipidemic and mildly hyperglycemic mice. While sexual dimorphism is well recognized in atherosclerosis and cardiovascular disease (43, 44), as well as in the immune system (4, 45), to our knowledge, this is the first evidence that metabolic stress-induced thiol oxidation may regulate and modify proteins differently in cells from males and females. While the underlying mechanism is not clear at this time, our findings may help explain the sexual dimorphism reported in numerous mouse models of metabolic diseases, particularly murine models of atherosclerosis (12, 22). Interestingly, male LDLR−/− mice are more prone to diet-induced atherosclerosis than female mice (59), yet the number of S-glutathionylated macrophage proteins we identified was nearly twice as large in females as males. This raises the intriguing possibility that the sexual dimorphic response of macrophages to metabolic stress-induced S-glutathionylation may contribute to relative atheroprotection observed in female LDLR−/− mice. This hypothesis is supported by the observation that the proteins involved in the H2O2 Metabolism cluster (glutathione peroxidases 1 and 4, peroxiredoxins 5 and 6, cytochrome b-245) showed increase S-glutathionylation in male macrophages, whereas in females, the proteins in the same clusters (glutathione peroxidase 4, peroxiredoxins 2, 5, and 6) showed decreased levels of S-glutathionylation in response to metabolic stress. Considering that S-glutathionylation is likely to affect (and inhibit) the activity of these key redox enzymes, altering the overall activity of this functional cluster of antioxidant/redox signaling proteins may have a profound impact on macrophage functions and thus may help explain the relative resistance of female LDLR−/− mice to atherosclerosis.
In summary, we describe a new and sensitive proteomic technique for the specific identification of S-glutathionylated proteins in monocytes and macrophages. Our findings show that this reversible post-translational modification occurs on a large number of proteins and may alter expression, activity, and/or degradation of these proteins, thereby affecting a vast array of cellular functions. This newly identified redox-sensitive regulatory network may explain how cells respond to changes in their metabolic environment and thus provides a mechanistic link between metabolic stress and the well-documented changes in the functional phenotype of monocytes and macrophages observed in patients with metabolic disorders (7, 17, 65). Applications of this technique to other cell types will provide new insights into their redox-regulated signaling pathways and the responses of these cells to pathophysiological conditions and metabolic diseases.
Materials and Methods
Animals
LDLR−/− mice on a C57BL/6J background (The Jackson Laboratories) were housed and maintained in colony cages on a 12-h light/12-h dark cycle. All studies were performed with the approval of the UTHSCSA Institutional Animal Care and Use Committee. At 18–24 weeks of age, male and female mice were randomized into two groups: MD (AIN-93G, Bio-Serv) or HFD (21% fat wt/wt and 0.15% cholesterol wt/wt; AIN-76A; Bio-Serv). Mice were maintained on their respective diets for 10 weeks. Fasted body weights and blood glucose were assessed at baseline, every 4 weeks during the dietary regimen, and immediately before sacrifice.
Peritoneal macrophage isolation and purification
Peritoneal macrophages were isolated by peritoneal lavage and subsequently purified using negative selection with mouse pan B (B220) and mouse pan T (Thy 1.2) magnetic beads (Dynabeads®). After purification, ∼95% of the cell population was CD68 positive, a monocyte/macrophage lineage marker, as measured by FACS analysis. Purified peritoneal macrophages were washed once with 1 mM NEM in phosphate-buffered saline (PBS) and stored at −80°C until labeling. Purified peritoneal macrophages from five mice of the same sex and dietary regimen were pooled for protein S-glutathionylation labeling and detection.
Cell culture
THP-1 monocytes were cultured at 37°C for 24 h in complete growth media, RPMI 1640 medium (Hyclone and Cellgro®), containing 10% fetal bovine serum, 2% glutamax, 1% sodium pyruvate (Cellgro), 1% penicillin/streptomycin (Cellgro), 1% HEPES, and 0.1% 2-mercaptoethanol. For metabolic priming of THP-1 cells, complete growth media were supplemented with either vehicle or freshly isolated native human LDL (100 μg/ml) plus HG (20 mM) for 20 h. For H2O2 treatment of THP-1 monocytes, cells were treated with 1 mM H2O2 for 15 min.
LDL isolation
LDL was freshly isolated by ultracentrifugation from pooled plasma from healthy blood donors. Freshly isolated LDL was purified by gel filtration chromatography, filter sterilized, and characterized as described previously (2, 68).
Overexpression of Grx1 and catalase
To overexpress human Grx1 in THP-1 monocytes, we used a doxycycline-inducible Tet-On adenoviral system expressing a Grx1-EGFP fusion protein (47, 62). THP-1 monocytes were incubated for 24 h with adenoviruses (multiplicity of infection = 25) in complete growth media. Transgene expression was induced by adding Dox (1 μg/ml; Sigma) for 24 h. For adenoviral delivery of human Catalase, THP-1 cells were infected with adenovirus particles (5 × 105 viral particles/cell) for 20 h. Adenovirus encoding human Catalase was a kind gift from Dr. Markus Bachschmid (University of Boston). For PEG-Catalase overexpression, THP-1 cells were treated with 250-U/ml Catalase-polyethylene glycol (Sigma) or an equal concentration of PEG-Mw 4600 (Sigma) for 20 h.
Labeling of S-glutathionylated proteins
Human monocytic THP-1 cells
After treatment, THP-1 cells were initially washed and incubated with 1× PBS containing 10 mM NEM before lysis. NEM was used for all pre-and postlysis steps to block all reactive thiols that would potentially be labeled by IAM-Desthiobiotin and thus produce false-positive signals. NEM was added prelysis to avoid thiol oxidation during cell lysis and other processing steps and to avoid loss of protein thiol-GSH mixed disulfides due to transthiolation and disulfide formation during sample preparation (18). NEM is a commonly used thiol-alkylating agent because of its high reactivity and specificity for thiols at physiological pH (6.5–7.5); at higher pH (>7.5), NEM reacts with lysines, arginines, histidines, and tyrosines (37, 46) and has been reported to also react with sulfenic acids (49) and possibly with sulfinic acids (29, 49). NEM has proven to be a better overall alkylating agent than IAM based on its ability to react with free thiols and sulfenic acids faster, more efficiently, and within a broader effective pH range (49, 51). Sulfonic acids (-SO3H) and S-nitrosylated thiols (-SNO) appear to be generally unreactive with thiol-alkylating reagents, including NEM and IAM (29, 49). Cells were then lysed using a mild lysis buffer (10 mM NEM, 50 mM Tris/HCl, 150 mM NaCl, 1 mM EDTA, and 0.1% NP-40 with protease inhibitor cocktail [Roche], pH 7.4), and lysates were sonicated and centrifuged to remove cellular debris. Lysates were then passed through a desalting column (GE Healthcare; PD Miditrap G-25) to remove unreacted NEM before the deglutathionylation reaction to avoid inactivation of the Grx1 enzyme (Fig. 1). GSH (2 mM) and recombinant Grx1 (Calbiochem; 25 μg/ml final concentration) were then added to desalted lysates and incubated for 15 min at 37°C. Recombinant bacterial Grx1 enzyme, which selectively catalyzes the deglutathionylation of proteins (25), was added to the lysates to reduce mixed disulfides between protein thiols and GSH (Fig. 1). The Grx1 reaction involves a nucleophilic attack from the N-terminal cysteine to the disulfide between protein thiols and glutathione, forming a disulfide between the N-terminal cysteine and glutathione and thereby reducing the protein thiol (19). The Grx1 enzyme regenerates the N-terminal thiolate anion through a high-affinity nonenzymatic reaction with glutathione, forming glutathione disulfide (GSSG) (19). In the cell, GSSG is subsequently reduced to glutathione by glutathione reductase and its enzymatic cofactor, NADPH. Therefore, excess GSH was added to the deglutathionylation buffer to recycle the active site of Grx1 and maintain its catalytic activity (19). Subsequently, the samples were passed through a desalting column to remove GSH to prevent interference with the thiol-reactive label, IAM-Desthiobiotin (Fig. 1).
To label free thiols, IAM-Desthiobiotin (5 mM, Invitrogen) was added and incubated for 90 min at room temperature (RT) in the dark, pH 7.5. IAM is also a thiol-alkylating agent, but it reacts much slower than NEM. However, IAM is more selective for the thiolate anion and results in lower nonspecific labeling compared with NEM, making IAM-DES a highly specific and selective labeling reagent for the reactive thiols generated after Grx1 reduction. IAM requires a more alkaline pH range (7.5–8.5) for optimum reactivity and reacts more readily with cysteines at their pKa around pH 8.0 (46, 71). At higher pH values and temperatures, IAM can alkylate methionines, lysines, arginines, tyrosines, histidines, and the N-terminal and C-terminal carboxyl (20, 35, 70).
Samples were then passed through a desalting column to remove excess IAM-Desthiobiotin, and proteins binding nonspecifically to the agarose beads were removed using biotin-blocked streptavidin-coated agarose beads (Invitrogen). Lysates were then centrifuged at 13,000 g and supernatant was transferred to a new tube. IAM-Desthiobiotin-labeled proteins were enriched using streptavidin-coated agarose beads (Fig. 1), and labeled proteins were purified from lysates in sequential pulldown steps using 50 μl of fresh streptavidin-coated agarose beads for each step (150 μl total): 2 h of incubation, followed by an overnight (ON) incubation, and a final 6-h incubation. DES binds to streptavidin with a weaker affinity than biotin and, therefore, allows for the elution of DES-labeled proteins from streptavidin under mild conditions (28), minimizing protein loss due to denaturation and aggregation. Beads were pelleted for 1 min at 3000 rpm (∼1000 g) and subsequently washed thrice in lysis buffer before elution of the proteins. Proteins were eluted using 5× sodium dodecyl sulfate (SDS) loading buffer (30 μl) and 20 mM biotin (7.5 μl), combined, and stored at −20°C until further analysis.
Primary murine peritoneal macrophages
Purified murine peritoneal cells were labeled similarly to THP-1 cells (described above), except that due to the limited protein concentration in the primary macrophage samples, the 1D electrophoresis purification step (see below) was omitted and the IAM-Desthiobiotin-labeled proteins were subjected to on-bead digestion immediately before analysis by mass spectrometry as described below.
1D electrophoresis
Before gel loading, streptavidin-coated agarose beads with IAM-Desthiobiotin-labeled proteins were thawed and incubated at RT for 30 min and then boiled for 5 min. To determine the labeling and pulldown efficiency, 20 μl of sample was loaded onto a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel, separated by gel electrophoresis, and transferred to a polyvinylidene difluoride membrane. Membranes were blocked in 1× TBS containing 5% bovine serum albumin and incubated with either an antibiotin–horseradish peroxidase (HRP) antibody or streptavidin-HRP (ON, 4°C, 1:100; Cell Signaling). Blots were then developed using an enhanced chemiluminescence kit (Pierce).
For mass spectrometry analysis, 40 μl of IAM-Desthiobiotin-labeled proteins (one-third of the final volume eluted from the streptavidin-coated agarose beads) was loaded onto a 12% gel and allowed to run 1 cm into the separating region. The gel was fixed for 30 min in 10% methanol and 7% acetic acid and stained for 1h with Coomassie Blue (8.5% phosphoric acid, 5% methanol, 0.12% Coomassie Blue G-250, 0.132% ammonium sulfate). The gel was destained using the fixative solution until background was sufficiently removed and then imaged on the GS-800 calibrated densitometer (Bio-Rad). The gel was stored at 4°C in water until further processing.
Mass spectrometry
THP-1 cells
Briefly, after destaining, each lane of the 12% SDS-PAGE gels was divided into seven slices. Proteins in each slice were digested with trypsin (Promega), and the digests were analyzed by capillary HPLC-ESI-MS/MS on a Thermo Fisher LTQ Orbitrap Velos mass spectrometer. Online HPLC separation of the digests was accomplished with an Eksigent/AB Sciex NanoLC-Ultra 2-D HPLC system. Precursor ions were acquired in the Orbitrap in centroid mode at 60,000 resolution (m/z 400); data-dependent, collision-induced dissociation spectra of the six most intense ions in the precursor scan were acquired at the same time in the linear trap. Mascot (version 2.4.1; Matrix Science) was used to search the spectra searched against the human subset of the NCBInr_20130102 database combined with a database containing sequences for common contaminants. Postprocessing of the Mascot results (including determination of probabilities of peptide assignments and protein identifications) was accomplished with Scaffold (Proteome Software). Further protein functionality analysis was performed using Ingenuity Software program (IPA, Qiagen; www.qiagen.com/ingenuity), Universal Protein Resource (UniProt, 2013–2015, www.uniprot.org) (63), and STRING (http://string-db/org) (58) to cluster proteins into biological processes (58).
Peritoneal macrophages
Samples were subjected to on-streptavidin bead trypsin digestion. HPLC-ESI-MS/MS analysis of the digests was conducted as described above.
The Scaffold results were exported to Excel using the following parameters: (i) quantitative value based on the summed intensities of the fragments in the product ion spectra (called total ion current in scaffold) without normalization; (ii) 99% protein threshold; (iii) 95% peptide threshold; and (iv) one peptide minimum. The summed product ion current value provides an indication of the relative abundance of the peptide and is used for semiquantitative analysis. The ratios of summed product ion currents for proteins found in THP-1 cells treated with LDL+HG to the untreated cells were used to detect the differences between the two conditions. The result categories are increased if the ratio was equal to or greater than 1.5 in at least two experiments; decreased if the ratio was equal to or less than 0.5; no clear pattern/no difference if the ratio was between 0.5–1.5 or in fewer than two experiments; and no data if values were only found in H2O2-treated samples. When a protein was not detected in one of the paired samples, a value 0.1 was used to permit ratio calculations. For analysis of THP-1 cells, keratin, trypsin, and nonspecifically bound proteins were removed from consideration, while keratin was kept for the primary cell analysis.
Validation of protein S-glutathionylation by Western blot analysis
THP-1 monocytes were preincubated for 1 h in complete growth media containing 250 μM BioGEE (Life Technologies) (41), and then treated with LDL+HG for 20 h. Cells were washed with ice-cold PBS and lysed in RIPA lysis buffer (50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate) supplemented with protease inhibitors and 10 mM NEM to block further thiol oxidation. To confirm the specificity of BioGEE labeling of S-glutathionylated proteins, one aliquot of the lysates (500 μg protein) was reduced using 10 mM DTT for 20 min at 65°C before incubation with streptavidin-conjugated agarose (Invitrogen). Lysates were incubated for 1 h at 4°C with streptavidin-conjugated agarose beads. Beads were then rinsed thrice with lysis buffer. S-glutathionylated proteins were released from the beads with SDS sample buffer containing 10 mM DTT. Samples were then separated by 10% or 12% SDS-PAGE, and levels of BioGEE-labeled protein were evaluated by Western blot analysis using the following antibodies: 14–3-3δ/ζ, 14-3-3γ, β-tubulin, GAPDH, HSP90β, protein S100α, and pyruvate kinase (all from Pierce/Thermo Scientific); ATP synthase subunit alpha (ATP5A1), glutathione peroxidase 4, and histone H3 (all from Abcam); and β-actin, cofilin, and HSP60 (all from Santa Cruz). Proteins were detected by chemiluminescence on a KODAK Image Station 4000MM.
Statistics
Data were analyzed using Student's t-test or ANOVA for multigroup comparisons. ANOVA and post hoc analyses were performed using the least significant difference method (SigmaStat; Systat Software). All data are presented as mean ± SE of at least three independent experiments unless stated otherwise. Results were considered statistically significant at the p < 0.05 level.
Supplementary Material
Abbreviations Used
- BioGEE
biotin-conjugated glutathione ethyl ester
- DTT
dithiothreitol
- Grx
glutaredoxin
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- HFD
high-fat diet
- HG
high D-glucose
- HPLC-ESI-MS/MS
high-performance liquid chromatography–electrospray ionization tandem mass spectrometry
- HRP
horseradish peroxidase
- HSP
heat shock protein
- IAM-Desthiobiotin
iodoacetamide conjugated to desthiobiotin
- IB
immunoblotting
- IPA
Ingenuity Pathway Analysis
- LDL
low-density lipoprotein
- LDLR−/−
LDL receptor-deficient
- MD
maintenance diet
- MKP-1
mitogen-activated protein kinase phosphatase-1
- NEM
N-ethylmaleimide
- Nox4
NADPH oxidase 4
- ON
overnight
- PBS
phosphate-buffered saline
- PEG
polyethylene glycol
- PKM
pyruvate kinase M
- PSSG
protein–glutathione mixed disulfide
- ROS
reactive oxygen species
- RT
room temperature
- SAP
streptavidin affinity purification
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- WCEs
whole cell extracts
Acknowledgments
This work was supported by grants to R.A. from the NIH (HL-70963 and HL115858) and the AHA (0855011F). S.U. was supported by a fellowship from the Translational Science Training (TST) Across Disciplines program at the University of Texas Health Science Center at San Antonio, with funding provided by the University of Texas System's Graduate Programs Initiative. H.S.K was supported by a grant from the National Research Foundation of Korea (NRF-2014R1A5A2009392). This work received computational support from Computational System Biology Core, funded by the National Institute on Minority Health and Health Disparities (G12MD007591) from the National Institutes of Health. The authors would like to thank Kevin Hakala, the former Proteomics Technical Directors of the Institutional Mass Spectrometry Core Laboratory at UTHSCSA, and Ana Carrera for their technical assistance. Mass spectrometry analyses were conducted in the proteomic component of the UTHSCSA Mass Spectrometry Laboratory, supported by UTHSCSA and NIH grant 1S10RR031586-01 (STW). The authors would also like to thank Dr. Markus Bachschmid, Boston University, for providing adenoviruses carrying catalase or EGFP.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Antunes F. and Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 475: 121–126, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Asmis R. and Jelk J. Large variations in human foam cell formation in individuals: a fully autologous in vitro assay based on the quantitative analysis of cellular neutral lipids. Atherosclerosis 148: 243–253, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bouman A, Heineman MJ, and Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 11: 411–423, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Brune B, Dehne N, Grossmann N, Jung M, Namgaladze D, Schmid T, von Knethen A, and Weigert A. Redox control of inflammation in macrophages. Antioxid Redox Signal 19: 595–637, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukowski MR, Bucklin C, and Picklo MJ. Quantitation of protein S-glutathionylation by liquid chromatography–tandem mass spectrometry: correction for contaminating glutathione and glutathione disulfide. Anal Biochem 469: 54–64, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Chawla A, Nguyen KD, and Goh Y. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11: 738–749, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou CC, Chiang BY, Lin JC, Pan KT, Lin CH, and Khoo KH. Characteristic tandem mass spectral features under various collision chemistries for site-specific identification of protein S-glutathionylation. J Am Soc Mass Spectrom 26: 120–132, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Clavreul N, Adachi T, Pimental DR, Ido Y, Schoneich C, and Cohen RA. S-Glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. FASEB J. 20: 518–520, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Clavreul N, Dauly C, Huang H, Sethuraman M, McComb ME, Costello CE, and Cohen RA. Detection and identification of S-glutathiolated proteins in endothelial cells exposed to oxidants by a biotin-labeling liquid chromatography and MS method. J Am Soc Mass Spectrom 17: 121S, 2006 [Google Scholar]
- 11.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, and Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282: 2871–2879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daugherty A, Lu H, Howatt DA, and Rateri DL. Modes of defining atherosclerosis in mouse models: relative merits and evolving standards. Methods Mol Biol 573: 1–15, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman HJ, Maiorino M, and Ursini F. Signaling functions of reactive oxygen species. Biochemistry 49: 835–842, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, and Ghezzi P. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A 99: 3505–3510, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fratelli M, Demol H, Puype M, Casagrande S, Villa P, Eberini I, Vandekerckhove J, Gianazza E, and Ghezzi P. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics 3: 1154–1161, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallogly M. and Mieyal J. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol 7: 381–391, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gallogly M, Starke D, and Mieyal J. Mechanistic and kinetic details of catalysis of thiol-disulfide exhange by glutaredoxins and mechanisms of regulation. Antioxid Redox Signal 11: 1059–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvani M, Hamdan M, Herbert B, and Righetti PG. Alkylation kinetics of proteins in preparation for two-dimensional maps: a matrix assisted laser desorption/ionization-mass spectrometry investigation. Electrophoresis 22: 2058–2065, 2001 [DOI] [PubMed] [Google Scholar]
- 21.García-Giménez, Luis J, Olaso G, Hake SB, Bönisch C, Wiedemann SM, Markovic J, Dasí F, Gimeno A, Pérez-Quilis C, and Palacios O. Histone H3 glutathionylation in proliferating mammalian cells destabilizes nucleosomal structure. Antioxid Redox Signal 19: 1305–1320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Getz GS. and Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol 32: 1104–1115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goch G, Vdovenko S, Kozłowska H, and Bierzyñski A. Affinity of S100A1 protein for calcium increases dramatically upon glutathionylation. FEBS J 272: 2557–2565, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Gordon S. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Gravina SA. and Mieyal JJ. Thioltransferase is a specific glutathionyl mixed-disulfide oxidoreductase. Biochemistry 32: 3368–3376, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Grek CL, Zhang J, Manevich Y, Townsend DM, and Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem 288: 26497–26504, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill BG, Ramana KV, Cai J, Bhatnagar A, and Srivastava SK. Measurement and identification of S-glutathiolated proteins. Methods Enzymol 473: 179–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch JD, Eslamizar L, Filanoski BJ, Malekzadeh N, Haughland RP, Beechem JM, and Haugland RP. Easily reversible desthiobiotin binding to streptavidin, avidin, and other biotin-binding proteins: uses for protein labeling, detection, and isolation. Anal Biochem 308: 343–357, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Jeong J, Jung Y, Na S, Jeong J, Lee E, Kim MS, Choi S, Shin DH, Paek E, Lee HY, and Lee KJ. Novel oxidative modifications in redox-active cysteine residues. Mol Cell Proteomics 10: M110-000513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295: C849–C868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HS, Ullevig SL, Nguyen HN, Vanegas D, and Asmis R. Redox regulation of 14-3-3zeta controls monocyte migration. Arterioscler Thromb Vasc Biol 34: 1514–1521, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HS, Ullevig SL, Zamora D, Lee CF, and Asmis R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc Natl Acad Sci U S A 109: E2803–E2812, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konstantinidis D, Paletas K, Koliakos G, and Kaloyianni M. The ambiguous role of the Na+–H+ exchanger isoform 1 (NHE1) in leptin-induced oxidative stress in human monocytes. Cell Stress Chaperones 14: 591–601, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landino LM, Robinson SH, Skreslet TE, and Cabral DM. Redox modulation of tau and microtubule-associated protein-2 by the glutathione/glutaredoxin reductase system. Biochem Biophys Res Commun 323: 112–117, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Lapko VN, Smith DL, and Smith JB. Identification of an artifact in the mass spectrometry of proteins derivatized with iodoacetamide. J Mass Spectrom 35: 572–575, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Lee CF, Ullevig SL, Kim HS, and Asmis R. Regulation of moncoyte adhesion and migration by Nox4. PLoS One 8: e66964, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lind C, Gerdes R, Hamnell Y, Schuppe-Koistinen I, Brockenhuus von Lowenhielm H, Homgren A, and Cotgreave IA. Identification of S-glutathionylated cellular proteins during oxidative stress and constitutive metabolism by affinity purification and proteomic analysis. Arch Biochem Biophys 406: 229–240, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Lindahl M, Mata-Cabana A, and Kieselbach T. The Disulfide proteome and other reactive cysteine proteomes: analysis and functional significance. Antioxid Redox Signal 14: 2581–2641, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Maxeiner H, Husemann J, Thomas CA, Loike JD, Khoury JE, and Silverstein SC. Complementary roles for scavenger receptor A and CD36 of human monocyte–derived macrophages in adhesion to surfaces coated with oxidized low-density lipoproteins and in secretion of H2O2. J Exp Med 188: 2257–2265, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNelis JC. and Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity 41: 36–48, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Meissner F, Molawi K, and Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol 9: 866–872, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Mieyal J, Gallogly M, Qanungo S, Sabens E, and Shelton M. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller VM. Family Matters: sexual dimorphism in cardiovascular disease. Lancet 379: 873–875, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, and Illig T. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet 7: e1002215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ober C, Loisel DA, and Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet 9: 911–922, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pretzer E. and Wiktorowicz JE. Saturation fluorescence labeling of proteins for proteomic analyses. Anal Biochem 374: 250–262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao M, Kisgati M, Cholewa JM, Zhu W, Smart EJ, Sulistio MS, and Asmis R. Increased expression of glutathione reductase in macrophages decreases atherosclerotic lesion formation in low-density lipoprotein receptor deficient mice. Arterioscler Thromb Vasc Biol 27: 1375–1382, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Qiao M, Zhao Q, Lee CF, Tannock LR, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, and Asmis R. Thiol oxidative stress induced by metabolic disorders amplifies macrophage chemotactic responses and accelerates atherogenesis and kidney injury in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 29: 1779–1786, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reisz JA, Bechtold E, King SB, Poole LB, and Furdui CM. Thiol-blocking electrophiles interfere with labeling and detection of protein sulfenic acids. FEBS J 280: 6150–6161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinna A, Torres M, and Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med 41: 86–91, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogers L, Leinweber BL, and Smith CV. Detection of reversible protein thiol modifcations in tissues. Anal Biochem 358: 171–184, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Rozenberg O. and Aviram M. S-glutathionylation regulates HDL-associated paraoxonase 1 (PON1) activity. Biochem Biophys Res Commun 351: 492–498, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Sakai J, Li J, Subramanian KK, Mondal S, Bajrami B, Hattori H, Jia Y, Dickinson BC, Zhong J, and Ye K. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 37: 1037–1049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schafer FQ. and Buetiner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Shelton MD, Chock PB, and Mieyal JJ. Glutaredoxin: role in reversible protein S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7: 348–366, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Shi C. and Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su D, Gaffrey MJ, Guo J, Hatchell KE, Chu RK, Clauss TR, Aldrich JT, Wu S, Purvine S, Camp DG, Smith RD, Thrall BD, and Qian WJ. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free Radic Biol Med 67: 460–470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, and Tsafou KP. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43: D447–D452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tangirala RK, Rubin EM, and Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. J Lipid Res 36: 2320–2328, 1995 [PubMed] [Google Scholar]
- 60.Thannickal VJ. and Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Thomas JA, Poland B, and Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys 319: 1–9, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.UniProtConsortium. UniProt: a hub for protein information. Nucleic Acids Res 43: D204–D212, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang SB, Foster DB, Rucker J, O'Rourke B, Kass DA, and Van Eyk JE. Redox regulation of mitochondrial ATP synthase implications for cardiac resynchronization therapy. Circ Res 109: 750–757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wellen KE. and Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winterbourn CC. and Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Wintergerst ES, Jelk J, Rahner C, and Asmis R. Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. Eur J Biochem 267: 6050–6059, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Wu QJ, Xiang YB, Yang G, Li HL, Lan Q, Gao YT, Zheng W, Shu XO, and Fowke JH. Vitamin E intake and the lung cancer risk among female nonsmokers: a report from the Shanghai women's health study. Int J Cancer 136: 610–617, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Z. and Attygalle AB. LC/MS Characterization of undesired products formed during iodoacetamide derivatization of sulfhydryl groups of peptides. J Mass Spectrom 42: 233–243, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Ying J, Clavreul N, Sethuraman M, Adachi T, and Cohen RA. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radic Biol Med 43: 1099–1108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zetterberg M, Zhang X, Taylor A, Liu B, Liang JJ, and Shang F. Glutathiolation enhances the degradation of γC-crystallin in lens and reticulocyte lysates, partially via the ubiquitin-proteasome pathway. Invest Ophthalmol Vis Sci 47: 3467–3473, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.