Abstract
Significance: With the growing population of baby boomers, there is a great need to determine the effects of advanced age on the function of the immune system. Recent Advances: It is universally accepted that advanced age is associated with a chronic low-grade inflammatory state that is referred to as inflamm-aging, which alters the function of both immune and nonimmune cells. Mononuclear phagocytes play a central role in both the initiation and resolution of inflammation in multiple organ systems and exhibit marked changes in phenotype and function in response to environmental cues, including the low levels of pro-inflammatory mediators seen in the aged. Critical Issues: Although we know a great deal about the function of immune cells in young adults and there is a growing body of literature focusing on aging of the adaptive immune system, much less is known about the impact of age on innate immunity and the critical role of the mononuclear phagocytes in this process. Future Directions: In this article, there is a focus on the tissue-specific monocyte and macrophage subsets and how they are altered in the aged milieu, with the hope that this compilation of observations will spark an expansion of research in the field. Antioxid. Redox Signal. 25, 805–815.
Keywords: : aging, clinical, immunology, infection, inflammation
Introduction
The elderly population is the fastest-growing segment of the U.S. population, with more than 20% of the country projected to be age 65 or older by 2030, compared with 13% in 2010 (70). As the population ages, research examining specific changes in the immune system is relevant not only by sheer number of people affected but also by increasing expense. At age 65, the first year of Medicare eligibility, average annual healthcare charges are less than $5000 per person per year, a figure that more than doubles by age 80 (8). To get an impression of the magnitude of that expense at a national level, the United States treasury department has reported that national healthcare costs due to aging will grow at a rate of ∼2% per year and that government expenditure on healthcare will increase to more than 6% of the potential gross domestic product (GDP) over the next 10 years (88). Government spending on Medicare and Medicaid was 4.8% of the potential GDP in 2010. Not only do aged individuals have worse outcomes after illness or injury, but they also contract infectious diseases such as flu and pneumonia at much higher rates than their younger counterparts (21). For example, during most flu seasons in the United States, an estimated 90% of flu-related deaths and 50% to 60% of flu-related illness occur in people aged 65 or older.
Advanced age is associated with a chronic low-grade inflammatory state that is referred to as inflamm-aging (32). This process is perceived to be responsible for the impaired innate and adaptive immune responses seen in the elderly (34, 80). Although the factors responsible for initiating inflamm-aging have not been fully defined, there are several theories that involve both intrinsic and extrinsic effects on leukocytes and the environment in which they mature and reside. Hallmarks of inflamm-aging include basal levels of pro-inflammatory cytokines interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNFα), which are elevated even in healthy aged individuals (32).
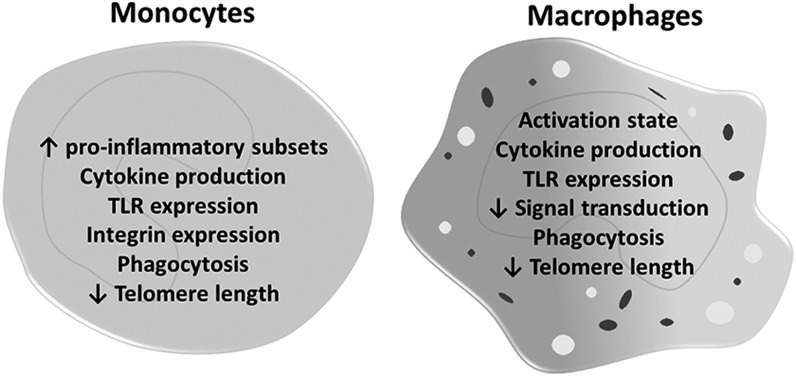
Prevailing theories by which inflamm-aging is initiated are numerous and include chronological age-dependent alterations in the following: (i) post-translationally modified macromolecules, including DNA and proteins that stimulate leukocytes and other cells to secrete pro-inflammatory cytokines; (ii) senescence of immune and nonimmune cells, leading to an increased release of inflammatory mediators via a senescence-associated secretory phenotype; and (iii) increased intestinal permeability, allowing bacteria and bacterial products (e.g., endotoxin) to enter the circulation and change in the bacterial communities or microbiome of the gastrointestinal tract (50, 85). These theories are detailed elsewhere (9, 33) and are beyond the scope of this article. Additionally, the association of aging and inflammation with human diseases, such as atherosclerosis, metabolic syndrome, and osteoporosis, is a well-documented pairing that is also beyond the range of this article but has been recently reviewed (38, 43). The focus of this article is on monocytes and macrophages, key cells in the inflammatory cascade and its regulation (Fig. 1).
FIG. 1.
Macrophage characteristics altered in aging.
The impact of advanced age on macrophage activation and signaling has been examined by several groups, mainly in rodent models of aging [reviewed in Gomez et al. (40)]. Published projects working with human macrophages are rare. Circulating monocytes are the most accessible mononuclear phagocytes, making them and monocyte-derived macrophages the most frequently inspected in human studies, but even those results are sparse and conflicting (90). The bulk of the material presented here will be from animal work, and human data will be highlighted where appropriate.
Regulating Inflammation
Mononuclear phagocytes play a central role in both the initiation and resolution of inflammation. Secreted cytokines are key mediators of these processes (7, 95). In the tissues, macrophages produce various pro-inflammatory cytokines in response to infectious stimuli, including TNFα, IL-1β, IL-6, nitric oxide (NO), reactive oxygen species, and neutrophil chemokines CXCL2 and CXCL8 (human homologs to murine macrophage inflammatory protein 2 (MIP-2), CXCL1, and KC) (20, 45, 63). Prolonged pro-inflammatory activation of macrophages leads to unregulated collateral tissue damage (7, 15, 94). Therefore, by necessity, tissue inflammation is a highly regulated process and mononuclear phagocytes are integral to this process.
Resolution of inflammation is achieved by several mechanisms that incorporate resident tissue macrophages: (i) removal of pathogens by neutrophils and macrophages; (ii) downregulation of neutrophil chemokines; and (iii) removal of apoptotic neutrophils (4). Efferocytosis is the phagocytosis of dying cells, including neutrophils and bacteria, by professional phagocytes and other cells, a process that downregulates IL-12, TNFα, and NO secretion and upregulates anti-inflammatory IL-10 and transforming growth factor beta (TGFβ) (5, 35, 51, 71, 93). For example, in the lung, macrophage efferocytosis helps facilitate restoration of tissue integrity via macrophage release of epithelial growth factors, platelet-derived growth factor, vascular endothelial growth factor, and hepatocyte growth factor (42, 64). In addition, they release prostaglandin E2 to stimulate endothelial cell migration and promote angiogenesis (17). Migration of macrophages to nearby lymph nodes and the apoptosis of macrophages themselves also help to resolve inflammation (49, 52). Overall, both macrophage efferocytosis and apoptosis are important in the restoration and remodeling of injured tissue; however, dysregulation of anti-inflammatory signals also has consequences. Animal experiments have shown an inadequate pro-inflammatory response and insufficient pathogen clearance after excessive efferocytosis (61). Taken together, the initiation and resolution of inflammation is a narrowly orchestrated process that is dependent on macrophages for coordinating both pro- and anti-inflammatory responses.
Recent research into the resolution of inflammation has uncovered an important role for monocyte- and macrophage-produced lipid signaling molecules that are known as specialized pro-resolving mediators (SPMs). Apart from the prostaglandins and leukotrienes classically characterized as pro-inflammatory lipid mediators, SPMs are a group of ω-3 polyunsaturated fatty acid-derived molecules encompassing families such as lipoxins, protectins, maresins, and the D- or E-series resolvins. Mainly metabolized from docosahexanoic acid and eicosapentaenoic acid, SPMs contribute to the resolution of inflammation by regulating the production of cytokines and chemokines, limiting neutrophil influx, and enhancing the pro-resolving actions of macrophages such as efferocytosis of apoptotic cells and clearance of bacteria and debris [reviewed in Serhan et al. (76)]. Maresins, for example, promote a transition from pro-inflammatory to anti-inflammatory macrophages in an autocrine manner, encouraging resolution of tissue inflammation and wound healing (77, 78). The pro-resolution activities of SPMs do not seem to act in an immunosuppressive manner (69, 74, 83). Animal experiments offer evidence that SPMs may also improve microbial removal and play a protective role against infections, lowering the antibiotic requirements to effectively clear bacterial infections (26, 74). In a murine model of age-associated adipose inflammation (14), low doses (1 nM) of lipoxin-A4, a potent SPM, reduced production of IL-6 and increased IL-10 levels in adipose tissue explants, as well as attenuated in vitro secretion of TNFα and monocyte chemotactic protein 1 (MCP-1) by lipopolysaccharide (LPS)-treated J774 macrophages. Furthermore, studies by Arnardottir et al. (6) demonstrated that aged mice exhibit delayed resolution of acute inflammation in parallel with altered lipid signaling dynamics, finding that aged mice produced lower levels of SPMs and increased quantities of pro-inflammatory prostaglandins and thromboxanes relative to young controls. Remarkably, levels of pro-inflammatory lipid mediators were higher in the aged mice even at baseline, indicating that dysregulated SPM signaling is an inherent factor in age-related immune dysfunction. The authors also employed resolvins D1 and D3 to enhance efferocytosis by macrophages from aged mice and to reduce prolonged inflammation in aged mice, partially compensating for deregulated SPM production. The pro-resolving activities of SPMs, as well as their potent ability to help macrophages control inflammation, make SPMs and their synthetic analogs attractive candidates for immune-modulating therapeutics, with a wide range of applications in experimental models and human disease (28, 75).
Macrophage Activation Nomenclature
Macrophage transition from equilibrium to inflammation and back again is made possible by the remarkable plasticity of macrophages (36, 59). Reversible activation into a pro-inflammatory state depends on factors present within the microenvironment. Classification guidelines of macrophage activation profiles have recently changed. Leading experts in macrophage research provided a consensus classification terminology in 2014 (65). Three recommendations were put forth to describe the activation state of macrophages. First, identify the model (in vitro vs. in vivo) and method of isolation. Second, identify the mediators used to stimulate macrophage activation. Lastly, describe the up- or downregulation of cell-surface or intracellular markers that are associated with mediator-specific induction.
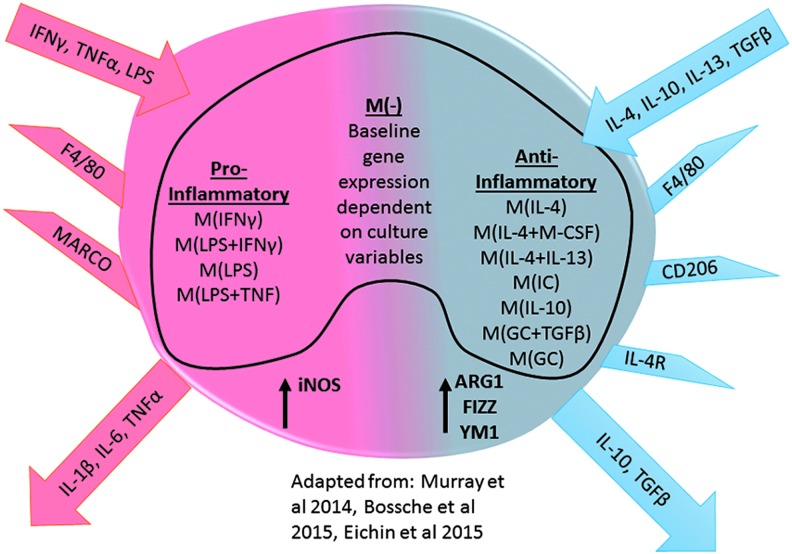
Before these guidelines, most reports generalized macrophages into either a classical, pro-inflammatory M1-activated macrophage or an alternative, anti-inflammatory M2-activated macrophage (this included all postulated subsets of M2: M2a, M2b, and M2c). Figure 2 outlines a generalized classification of M1 and M2 macrophages. M1/pro-inflammatory macrophages are characterized by mediators of activation, including interferon gamma (IFNγ), TNFα, and LPS. These factors activate signal transducer and activator of transcription (STAT) 1 signaling and upregulate a scavenger receptor macrophage receptor with collagenous structure, inducible nitric oxide (iNOS, Nos2), and IL-6 and TNFα gene expression. In contrast, M2/anti-inflammatory macrophages are activated by IL-4, IL-10, IL-13, and TGFβ. These mediators stimulate the STAT3 or STAT6 signaling cascade and upregulate gene expression of mannose receptor CD206, IL-4 receptor, IL-10, TGFβ, arginase 1 (ARG1), resistin-like α (Retnla, Fizz1), and chitinase 3-like 3 (Chi3l3, Ym1) (81). Publications are now starting to utilize this environmental nomenclature to define macrophage populations (29, 89). Thus, the studies reviewed here will attempt to use the terms pro-inflammatory and anti-inflammatory as well as these markers for the general classification of macrophages and to avoid terms such as M1, M2, classical, regulatory, etc.
FIG. 2.
Macrophage mediator-based nomenclature. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Advanced Age and Mononuclear Cells
No differences have been found in the number of peripheral blood monocytes in the circulation of elderly subjects compared with their younger counterparts. However, there is an age-dependent shift in the proportion of monocyte subsets and subsequent inflammatory profiles (60, 86). Human monocytes from elderly participants have increased levels of CD14++(high)CD16+ and CD14+(low)CD16+ pro-inflammatory/nonclassical monocytes and decreased levels of CD14+CD16- monocytes (44, 67, 73). However, these monocytes have a markedly attenuated inflammatory response (decreased levels of both IL-6 and TNFα) after TLR1/2 stimulation compared with the younger age group alongside a decreased TLR1 expression (67).
In elderly participants compared with young subjects, Hearps et al. (44) showed that CD11b, an integrin involved in transendothelial migration and important in plaque formation (82), had greater expression on circulating monocytes (91). L-selectin is responsible for leukocyte rolling and adhesion to endothelial cells and is downregulated on monocytes from elderly individuals (27). The altered expression of CD11b and L-selectin may affect monocyte migration and function in elderly individuals. Additionally, Hearps and coworkers demonstrated that TLR4-stimulated monocytes from elderly participants had impaired phagocytosis, shorter telomeres, and elevated intracellular TNF; these results suggest a dysregulation of monocyte function in the elderly. Clinically, the pro-inflammatory nature of elderly monocytes may prove to be a beneficial target to help maintain healthy aging. Recent evidence from a study of Japanese centenarians shows that a pro-inflammatory state, marked by elevated serum C-reactive protein, IL-6, and TNFα, is a significant predictor of longevity over telomere length (3).
Renshaw et al. revealed that defects in the production of cytokines by monocytes and macrophages from aged mice in response to in vitro LPS stimulation stemmed from aberrant expression of Toll-like receptor (TLR) mRNAs (72). We and others have corroborated the observed diminished cytokine release by macrophages from aged mice (11–13, 16, 24, 47, 56, 58). The mechanism responsible for this defect has not been confirmed. However, it seems that chronic exposure to low-level elevations of IL-6, a factor frequently implicated in inflamm-aging, is associated with age-related dampening of the macrophage pro-inflammatory response. In 2010, Gomez et al. published their observations on the role of IL-6 and the behavior of macrophages from aged mice (39). Using young and aged IL-6 knockout mice, in vitro cytokine production by splenic macrophages was tested after LPS stimulation. When IL-6 was present in aged mice, an impaired inflammatory response was seen in these macrophages (lower TNFα, IL-1β, IL-6, and IL-12). However, in the aged IL-6 deficient mouse model by the same group, the aged mice had a stronger inflammatory response compared with young mice. The presence of elevated levels of IL-6 in aged mice did not seem to play a role in the number of macrophages in the splenocyte population or the surface expression of TLR4. Two independent studies from separate groups also failed to show age-dependent differences in cell-surface expression of TLR4 in macrophages from aged mice (11, 23). Human monocyte studies also fail to show a clear age-related difference in TLR expression; factors such as exercise, sex, and comorbidities seem to play a role as well [reviewed in van Duin and Shaw (90)]. Possible explanations for the discrepancy in TLR4 expression could be related to cell populations and purity (resident macrophages vs. peripheral blood mononuclear cells) or methods of assessment (PCR vs. flow cytometry). It should also be noted that although pro-inflammatory stimuli, such as LPS and IFNγ, are the more commonly used in vitro mediators, aged macrophage response to anti-inflammatory activation, for example, IL-4, is also altered (31, 48, 58).
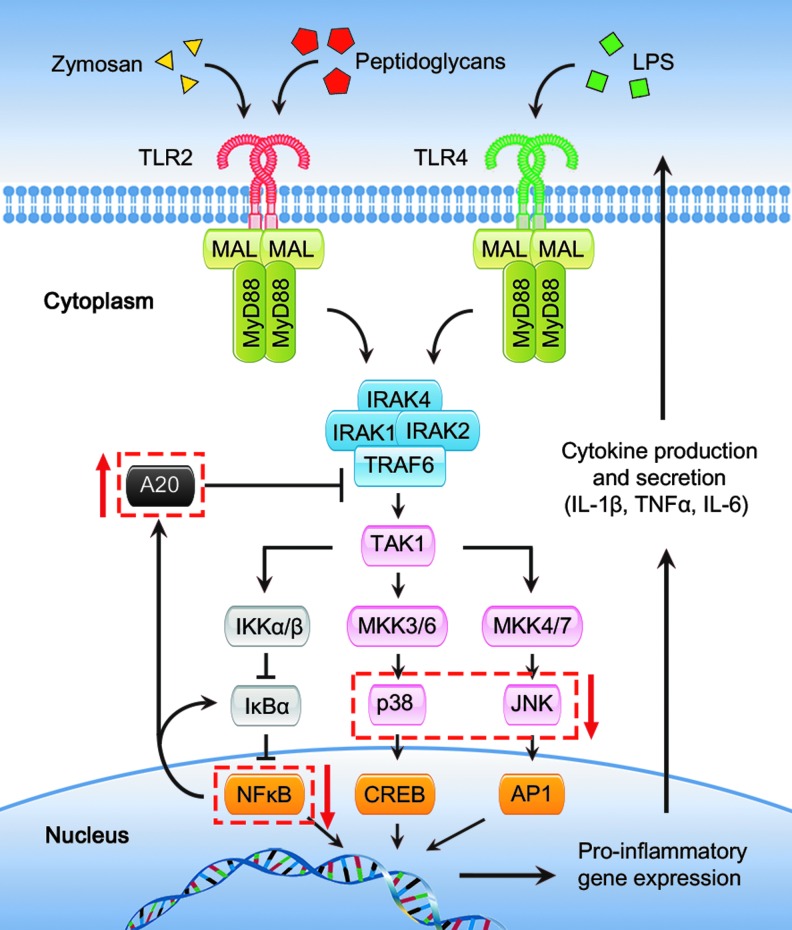
Boehmer et al. demonstrated the relationship between aging and defects in mitogen-activated protein kinase (MAPK) signaling, leading to decreased cytokine production (11–13). A subsequent publication identified that the activation/signaling deficiencies in macrophages from aged mice were limited to the TLR2 and TLR4 signaling pathways. Parallel studies, using alternate means of activating macrophages with IFNγ, failed to show an age-dependent reduction in cytokine production (12). Relative to macrophages from young mice, cells from aged animals also have decreased levels of cytoplasmic p38 and c-Jun N-terminal kinase (JNK) MAPKs (12, 13), which may help explain the inability of macrophages in aged mice to be appropriately activated. A simplified signal transduction model illustrating these findings is included in Figure 3. Work from another group, also using interferon activation, revealed that macrophages from aged mice had a reduction in the activation of STAT1, relative to cells from younger mice (96). In both sets of studies, the changes in the activation of signaling molecules parallels a reduction in the total protein in cells from aged mice. In the setting of infection, a recent study examining human monocyte-derived macrophage showed altered responses in PI3K-AKT signaling in cells from elderly subjects. Before infection, there were no differences in protein kinase B (AKT) phosphorylation but the baseline AKT phosphorylation in cells from the elderly varied widely, perhaps again pointing to environmental factors such as diet and exercise that can modulate the basal inflammatory state of an elderly individual. Then after exposure to heat-killed bacteria, there was greater activation of AKT in macrophages derived from monocytes obtained from younger volunteers (92). This observation is not consistent with murine splenocyte studies in which an increase in PI3K-AKT activation was seen in splenic macrophages from aged mice. In the latter study, activation with bacterial ligands yielded a decreased cytokine production (30). These divergent observations may reflect species-specific differences, variations in activation parameters or culture conditions. PI3K-AKT signaling is involved in bacterial killing, NO production, and cytokine secretion.
FIG. 3.
TLR2 and TLR4 signaling pathways and aging. This figure illustrates intracellular TLR signaling pathways as discussed in the text of this article. Red arrows and dashed boxes signify components of signal transduction pathways that are found to be involved in age-related monocyte/macrophage dysfunction (11–13, 16, 46). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Recently, Hinojosa et al. (46) focused on the cytosolic suppressor of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and MAPK known as A20. The A20 molecule has been shown to deubiquitinate and inhibit TNF receptor-associated factor 6 (TRAF6), which activates the NFκB and MAPK signaling cascades via interactions with TGFβ-activated kinase 1 (TAK1) (Fig. 3). It was found that A20 is elevated in some of the tissues and macrophages of aged mice, specifically in the lungs and alveolar macrophages. The overexpression of A20 was found to dampen the cytokine response to bacteria but had no effect on the ability of macrophages to phagocytize. This study also describes a potential mechanism by which TNFα contributes to inflamm-aging, since in vitro stimulation of alveolar macrophages with TNFα induced a rise in A20 levels. The authors discussed that the elevated TNFα in this model could be secreted from senescent lung cells, further supporting the thought that multiple factors play a role in regulating cytokine production by macrophages.
When examining phagocytosis in macrophages from aged mice, differences in age and tissue type again play an important role. The ability of peritoneal macrophages from aged mice to phagocytose fluorescent particles was reduced relative to young mice, but bone marrow monocytes and bone marrow-derived macrophages from aged mice had phagocytic ability similar to their younger counterparts. No intrinsic defect in macrophages from aged mice could be found, but it seems that the microenvironment in the peritoneum likely caused the impairment of macrophage function in this model (57). The aged mice had a decreased proportion of macrophages in the resident peritoneal population, as well as increased peritoneal levels of B cell-derived IL-10, leading to compromised macrophage phagocytosis. Takahashi et al. (87) recently showed that in vivo phagocytosis of necrotic cells was attenuated in aged peritoneal macrophages. In this study, the decreased necrotic cell clearance led to prolonged peritoneal inflammation, which may contribute to deleterious clinical outcomes.
Further investigation revealed that the altered behavior of macrophages from aged subjects is not necessarily due to intrinsic aged macrophage defects but the tissue-specific inflamm-aging microenvironment (48, 54, 58). These studies set out to characterize the aged phenotype and, though they are conflicting at times, there are a few common elements. Some inconsistencies can be explained by differences in experimental technique and design. Nevertheless, bone marrow-derived cells seem to be the least affected by the aging microenvironment (Table 1). Mononuclear cells separated directly from aged bone marrow as well as those cultured from harvested bone marrow did not tend to show differences in cytokine production or phagocytosis when compared with young cells (57, 58). This could suggest that, though there is evidence of age-related changes (48, 68), the bone marrow is not as affected by inflamm-aging (58). On the other hand, peritoneal macrophages appear to be the most impaired of tissue-specific types but this has not been well delineated. Differences between publications could be attributed to inconsistencies between resident cells and thioglycolate-elicited macrophages and/or the increasing numbers of peritoneal lymphocytes and their secretions as animals age (22, 25, 54, 57).
Table 1.
Unique Age-Dependent Findings in Select Recent Publications
| Species, strain, and age | Major macrophage findings | Additional comments | Reference |
|---|---|---|---|
| Mouse C57BL/6 20–24 months |
In aged mice: ↑ percentage of circulating monocytes and macrophages in the spleen ↓ percentage of macrophages in the peritoneum ↑ number of mitochondria and mitochondrial reactive oxygen species after LPS stimulation ↓ autophagy |
Showed that autophagy deficient macrophages (Atg7 knockout) have similar phenotype to aged macs in their studies | (85) |
| Mouse C57BL/6 18–24 months |
↓ percentage of peritoneal macrophages in aged animals with ↓ phagocytosis of necrotic cells. | Decreased necrotic cell clearance in vivo (peritoneum) lead to elevated peritoneal MIP-2 Prolonged inflammation in aged mice |
(87) |
| Mouse C57BL/6 21 months |
↑ A20 expression in alveolar macrophages from healthy aged mice ↓ NFκB and MAPK signaling A20 can be induced by TNFα but not IL-6. |
Dietary fish oil lowers A20 levels and protects aged mice from Streptococcus pneumoniae infection | (46) |
| Mouse Swiss Albino 12 and 16 months |
↓TLR2 and TLR4 expression in resident peritoneal macrophages from aged mice | (79) | |
| Mouse BALB/c 19–21 months |
Response to infection by alveolar macrophages from aged mice: ↓ TNFα and IL-6 production ↓ NFκB, JNK, and p38 activation ↑ ERK activation |
Aged lung lower levels of IL-6 and IL-1β after infectious challenge | (16) |
| Mouse BALB/c 17–18 months |
↓ number of marginal zone macrophages in the spleen of aged mice No age-dependent difference in phagocytosis |
Anatomical breakdown of the marginal zone with age. | (10) |
| Mouse BALB/c 18–20 months |
↓ splenic macrophages, pro-inflammatory response after LPS stimulation and other pro-inflammatory stimuli ↓ anti-inflammatory response after incubation with IL-4 No difference in pro-and anti-inflammatory phenotype markers between bone marrow-derived macrophages from young and aged mice |
From primary macrophages: global suppression of macrophage function. From bone marrow-derived macrophages: age-dependent differences in macrophage phenotype lost after prolonged cell culture |
(58) |
| Mouse/C57BL/6 and B6.SJL-Ptprca Pepcb/BoyJ 15–20 months |
↓ phagocytosis by peritoneal macrophages from aged mice both in vivo and in vitro. No difference in phagocytosis by bone marrow-derived macrophages or bone marrow monocytes |
(57) |
IL, interleukin; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MIP-2, macrophage inflammatory protein 2; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; TLR, Toll-like receptor; TNFα, tumor necrosis factor alpha.
Additionally, the aged phenotype can be returned to a young phenotype with ex vivo cellular interventions or by in vivo treatments in an animal model. For example, aged macrophage response to stimuli is similar to young macrophage response after removal from the tissue and in vitro treatment with cytokines such as IFNγ. This demonstrates that macrophages from aged mice do not lose their functional plasticity/adaptivity, and it further reveals that altered responses by macrophages from aged mice are due to microenvironmental effects (58, 84). Structural differences in macrophages from aged mice include reduced receptor expression and telomere length, but these changes do not seem to have as dramatic an effect as the cytokine milieu. Finally, and perhaps most clinically relevant, several investigators have found that some of the variances in macrophages from an aged milieu can be abrogated with diet and exercise (2, 37, 46, 54, 62). Exercise-trained older mice had greater resistance to viral infection than the nonexercise group, and their cytokine production also normalized to younger mice levels after LPS stimulation; whereas nonexercising older mice did not show the same change. Exercise has also been shown to enhance TNFα release and antiviral resistance in macrophages from aged mice (53). Hinojosa et al. found that the age-associated elevations in A20 were nullified by supplementing the mouse diet with anti-inflammatory n-3 polyunsaturated fatty acids from fish oil (46).
Responses to systemic stressors such as viral infections and neoplasia are also different in aging. Elsewhere in this Forum, tumor-associated macrophages will be reviewed (Submitted by Jo Van Ginderachten), but it is worth pointing out in this article that there is evidence that the systemic innate immune changes related to neoplasia are different in aged mice compared with young mice (84). For example, Jackaman et al. found that both young and aged mice macrophages were polarized to an anti-inflammatory response when challenged with a tumor. However, aged mouse macrophages made large amounts of IL-4 in response to a neoplastic environment whereas young mice did not, leading to an immunosuppressive tumor microenvironment in the aged (48). After stimulation with herpes simplex virus 1 (HSV-1), peritoneal macrophages from old and middle-aged mice showed better intrinsic viral resistance compared with younger mice. However, alveolar macrophages from middle-aged mice were more effective than those in both young and aged mice. Resident peritoneal macrophages from aged mice also produced less TNFα on HSV-1 stimulation, and alveolar macrophages secreted less IL-12 compared with the younger group, showing both age- and location-related changes in macrophage response. Viral infections are common in the elderly population and typically lead to worse outcomes. Both alveolar and peritoneal resident macrophages seem to have impaired extracellular combat of viral infections and may prove to be important cellular targets for improving clinical outcomes.
Models of aging that involve an in vivo inflammatory challenge (i.e., injury or systemic infection) show that tissue-specific macrophages from aged mice have a heightened inflammatory response and an impaired anti-inflammatory response (31, 55). There are, however, conflicting results from human studies of infection. For example, Verschoor et al. reported that monocyte-derived macrophages from elderly subjects produced less TNFα and IL-6 after exposure to Streptococcus pneumoniae in vitro. This decrease in cytokine production paralleled a decrease in bacterial killing compared with cells derived from young subjects, but there was no difference in phagocytosis (92). Pro-inflammatory responses such as IL-1β, TNFα, and iNOS have all been shown to be upregulated more in aged animals compared with the response seen in younger mice after injury (19, 66). IL-4 receptor expression and cellular anti-inflammatory response to IL-4 treatment are also reduced after exposure to in vivo inflammation.
Yabluchanskiy et al. showed that cardiac outcomes were worse in aged mice but improved if anti-inflammatory macrophages were present (94). Aged animals had elevated plasma levels of matrix metalloproteinase-9 (MMP-9), corresponding to increased left ventricular dilation and worse left ventricular ejection fraction. In a model with gene deletion of MMP-9 expression, mice had improved cardiac function and survival. Importantly, the macrophages isolated from the cardiac scar in the null animals had significantly higher expression of the following tissue-repairing, anti-inflammatory markers: CD163, mannose receptor, TGFβ, and Ym1.
Models that represent diseases commonly found in the elderly have also shown age-related differences in macrophage activation. Using naturally occurring periodontitis in aged Rhesus monkeys, Gonzalez et al. found that macrophages from healthy aged gingival tissue had increased expression of pro-inflammatory genes compared with young monkeys (41). These genes were then further elevated in the setting of aging and periodontitis. Studies such as these suggest that in certain anatomic locations, healthy aged tissues host a macrophage phenotype that promotes increased inflammation and tissue destruction that is made worse in a diseased state (1, 97).
Conclusions, Opportunities, and Challenges
Research in aging macrophages presents unique challenges. First, defining tissue-specific basal aged phenotypes remains elusive. In areas of the body with constant inflammatory stimulus and exposure to environmental pathogens, the system seems to be heightened with an elevated resting inflammatory state and a subsequent excessive reaction. However, in other areas with limited environmental exposure, the macrophages tend to present an anti-inflammatory phenotype with a subdued reaction to stimulation compared to responses in younger individuals.
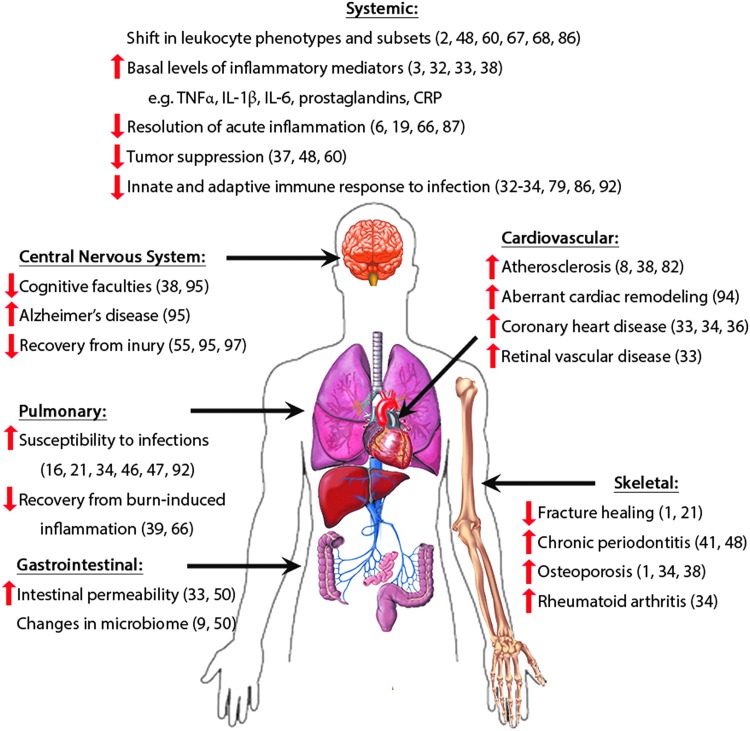
Second, it seems as if the “aged phenotype” is reversible. Once removed from the inflamm-aging microenvironment, the macrophage response can be restored to generate a response similar to the young macrophage activation profiles. This presents therapeutic opportunities that may involve a patient's own macrophages to not only treat acquired diseases but also affect many pathologies and physiological dysfunctions associated with the aging process (Fig. 4) (18).
FIG. 4.
The physiological effects of inflamm-aging. Examples of organ dysfunction and pathologies related to immunosenescence and innate immune dysregulation in the elderly. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Abbreviations Used
- AKT
protein kinase B (PKB)
- AP1
activator protein 1
- ARG1
arginase 1
- Chi3l3
chitinase 3-like 3 (Ym1)
- CREB
cAMP response element binding protein
- CRP
C-reactive protein
- CXCL1
KC
- CXCL2
macrophage inflammatory protein 2 (MIP-2)
- CXCL8
interleukin-8 (IL-8)
- HSV-1
herpes simplex virus 1
- IFNγ
interferon gamma
- IKK
IκB kinase
- IL-10
interleukin-10
- IL-1β
interleukin-1 beta
- IL-4
interleukin-4
- IL-6
interleukin-6
- iNOS
inducible nitric oxide (Nos2)
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAL
MyD88 adaptor-like protein
- MAPK
mitogen-activated protein kinase
- MARCO
macrophage receptor with collagenous structure
- MCP-1
monocyte chemotactic protein 1 (CCL-2)
- MKK
MAPK kinase
- MMP-9
matrix metalloproteinase-9
- MyD88
myeloid differentiation primary response gene 88
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- PI3K
phosphatidylinositide 3 kinase
- Retnla
resistin-like α (Fizz1)
- SPMs
specialized pro-resolving mediators
- STAT1
signal transducer and activator of transcription 1
- TAK1
transforming growth factor beta-activated kinase 1
- TGFβ
transforming growth factor beta
- TLR
Toll-like receptor
- TNFα
tumor necrosis factor alpha
- TRAF6
TNF receptor-associated factor 6
Acknowledgments
The authors would like to thank Brenda J. Curtis, PhD, for her thoughtful editing. This work was supported in part by NIH R01 AG018859 (E.J.K.), R01 GM115257 (E.J.K.), T32 AA012327 (E.J.K.), F31 AA022566 (J.A.S.), and the Dr. Ralph and Marian C. Falk Medical Research Trust (E.J.K.).
References
- 1.Abdelmagid SM, Barbe MF, and Safadi FF. Role of inflammation in the aging bones. Life Sci 123: 25–34, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Al-Ajmi N, Saretzki G, Miles C, and Spyridopoulos I. Dietary restriction ameliorates haematopoietic ageing independent of telomerase, whilst lack of telomerase and short telomeres exacerbates the ageing phenotype. Exp Gerontol 58: 113–119, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Arai Y, Martin-Ruiz CM, Takayama M, Abe Y, Takebayashi T, Koyasu S, Suematsu M, Hirose N, and von Zglinicki T. Inflammation, but not telomere length, predicts successful aging at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine 2: 1549–1558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, and Muhl H. Macrophages in inflammation and its resolution. Front Immunol 3: 324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariel A. and Serhan CN. New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front Immunol 3: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnardottir HH, Dalli J, Colas RA, Shinohara M, and Serhan CN. Aging delays resolution of acute inflammation in mice: reprogramming the host response with novel nano-proresolving medicines. J Immunol 193: 4235–4244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano K, Takahashi N, Ushiki M, Monya M, Aihara F, Kuboki E, Moriyama S, Iida M, Kitamura H, Qiu CH, Watanabe T, and Tanaka M. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun 6: 7802, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmann JM, DeFina LF, Franzini L, Gao A, Leonard DS, Cooper KH, Berry JD, and Willis BL. Cardiorespiratory fitness in middle age and health care costs in later life. J Am Coll Cardiol 66: 1876–1885, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Biagi E, Candela M, Fairweather-Tait S, Franceschi C, and Brigidi P. Aging of the human metaorganism: the microbial counterpart. Age (Dordr) 34: 247–267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birjandi SZ, Ippolito JA, Ramadorai AK, and Witte PL. Alterations in marginal zone macrophages and marginal zone B cells in old mice. J Immunol 186: 3441–3451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehmer ED, Goral J, Faunce DE, and Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol 75: 342–349, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Boehmer ED, Meehan MJ, Cutro BT, Gomez CR, and Kovacs EJ. Aberrant TLR Signaling in Macrophages from Aged Mice. Schwarzmeier JD. (ed), The 6th International Cytokine Conferences, Bologna, Italy, Monduzzi Editore, 2006, pp. 31–34 [Google Scholar]
- 13.Boehmer ED, Meehan MJ, Cutro BT, and Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev 126: 1305–1313, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Borgeson E, McGillicuddy FC, Harford KA, Corrigan N, Higgins DF, Maderna P, Roche HM, and Godson C. Lipoxin A4 attenuates adipose inflammation. FASEB J 26: 4287–4294, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Bowden DH. The alveolar macrophage. Environ Health Perspect 55: 327–341, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd AR, Shivshankar P, Jiang S, Berton MT, and Orihuela CJ. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol 47: 507–518, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brecht K, Weigert A, Hu J, Popp R, Fisslthaler B, Korff T, Fleming I, Geisslinger G, and Brune B. Macrophages programmed by apoptotic cells promote angiogenesis via prostaglandin E2. FASEB J 25: 2408–2417, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Brown BN, Sicari BM, and Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol 5: 510, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brubaker AL, Palmer JL, and Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: lessons Learned from Rodent Models. Aging Dis 2: 346–360, 2011 [PMC free article] [PubMed] [Google Scholar]
- 20.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, and Herold S. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med 180: 521–532, 2009 [DOI] [PubMed] [Google Scholar]
- 21.CDC. 2105. Healthy Aging. www.cdc.gov/chronicdisease/resources/publications/aag/healthy-aging.htm Accessed July27, 2016
- 22.Cecilio CA, Costa EH, Simioni PU, Gabriel DL, and Tamashiro WM. Aging alters the production of iNOS, arginase and cytokines in murine macrophages. Braz J Med Biol Res 44: 671–681, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Chelvarajan RL, Collins SM, Van Willigen JM, and Bondada S. The unresponsiveness of aged mice to polysaccharide antigens is a result of a defect in macrophage function. J Leukoc Biol 77: 503–512, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Chelvarajan RL, Liu Y, Popa D, Getchell ML, Getchell TV, Stromberg AJ, and Bondada S. Molecular basis of age-associated cytokine dysregulation in LPS-stimulated macrophages. J Leukoc Biol 79: 1314–1327, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Chen Y. and Bradley SF. Aging and eliciting agents: effect on murine peritoneal macrophage monokine bioactivity. Exp Gerontol 28: 145–159, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, and Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484: 524–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Martinis M, Modesti M, and Ginaldi L. Phenotypic and functional changes of circulating monocytes and polymorphonuclear leucocytes from elderly persons. Immunol Cell Biol 82: 415–420, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Dennis EA. and Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol 15: 511–523, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eichin D, Laurila JP, Jalkanen S, and Salmi M. CD73 Activity is Dispensable for the Polarization of M2 Macrophages. PLoS One 10: e0134721, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fallah MP, Chelvarajan RL, Garvy BA, and Bondada S. Role of phosphoinositide 3-kinase-Akt signaling pathway in the age-related cytokine dysregulation in splenic macrophages stimulated via TLR-2 or TLR-4 receptors. Mech Ageing Dev 132: 274–286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenn AM, Henry CJ, Huang Y, Dugan A, and Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav Immun 26: 766–777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, and De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908: 244–254, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Franceschi C. and Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69 Suppl 1: S4–S9, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Frasca D. and Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 17: 7–19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, and Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281: 38376–38384, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Glezeva N, Horgan S, and Baugh JA. Monocyte and macrophage subsets along the continuum to heart failure: misguided heroes or targetable villains? J Mol Cell Cardiol 89(Pt B): 136–145, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Goh J. and Ladiges WC. Exercise enhances wound healing and prevents cancer progression during aging by targeting macrophage polarity. Mech Ageing Dev 139: 41–48, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Goldberg EL. and Dixit VD. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev 265: 63–74, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez CR, Karavitis J, Palmer JL, Faunce DE, Ramirez L, Nomellini V, and Kovacs EJ. Interleukin-6 contributes to age-related alteration of cytokine production by macrophages. Mediators Inflamm 2010: 475139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez CR, Nomellini V, Boehmer ED, and Kovacs EJ. Signal transduction of the aging innate immune system. Curr Immunol Rev 3: 23–30, 2007 [Google Scholar]
- 41.Gonzalez OA, Novak MJ, Kirakodu S, Stromberg A, Nagarajan R, Huang CB, Chen KC, Orraca L, Martinez-Gonzalez J, and Ebersole JL. Differential gene expression profiles reflecting macrophage polarization in aging and periodontitis gingival tissues. Immunol Invest 44: 643–664, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, Oslund R, Gelb MH, Lambeau G, Marone G, and Triggiani M. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol 184: 5232–5241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol 294: 102–110, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, and Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11: 867–875, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, Srivastava M, Seeger W, Maus UA, and Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol 177: 1817–1824, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Hinojosa CA, Akula Suresh Babu R, Rahman MM, Fernandes G, Boyd AR, and Orihuela CJ. Elevated A20 contributes to age-dependent macrophage dysfunction in the lungs. Exp Gerontol 54: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinojosa E, Boyd AR, and Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis 200: 546–554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, and Nelson DJ. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 12: 345–357, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, and Henson PM. Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 184: 547–560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim KA, Jeong JJ, Yoo SY, and Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol 16: 9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S, Elkon KB, and Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21: 643–653, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Kirby AC, Coles MC, and Kaye PM. Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol 183: 1983–1989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohut ML. and Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev 10: 6–41, 2004 [PubMed] [Google Scholar]
- 54.Kohut ML, Senchina DS, Madden KS, Martin AE, Felten DL, and Moynihan JA. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp Gerontol 39: 1347–1360, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, and Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol Aging 34: 1397–1411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang S, Domon H, Hosur KB, Wang M, and Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mech Ageing Dev 130: 538–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linehan E, Dombrowski Y, Snoddy R, Fallon PG, Kissenpfennig A, and Fitzgerald DC. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell 13: 699–708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahbub S, Deburghgraeve CR, and Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res 32: 18–26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malyshev I. and Malyshev Y. Current concept and update of the macrophage plasticity concept: intracellular mechanisms of reprogramming and M3 macrophage “switch” phenotype. Biomed Res Int 2015: 341308, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLachlan JA, Serkin CD, Morrey KM, and Bakouche O. Antitumoral properties of aged human monocytes. J Immunol 154: 832–843, 1995 [PubMed] [Google Scholar]
- 61.Medeiros AI, Serezani CH, Lee SP, and Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med 206: 61–68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meneguello-Coutinho M, Caperuto E, Bacurau AV, Chamusca G, Uchida MC, Tibana RA, Pereira GB, Navalta JW, Wasinski F, Cavaglieri CR, Prestes J, Costa Rosa LF, and Bacurau RF. Effects of dietary restriction or swimming on lymphocytes and macrophages functionality from old rats. Immunol Invest 43: 113–122, 2014 [DOI] [PubMed] [Google Scholar]
- 63.Monton C. and Torres A. Lung inflammatory response in pneumonia. Monaldi Arch Chest Dis 53: 56–63, 1998 [PubMed] [Google Scholar]
- 64.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, and Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 24: 608–615, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, and Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nomellini V, Faunce DE, Gomez CR, and Kovacs EJ. An age-associated increase in pulmonary inflammation after burn injury is abrogated by CXCR2 inhibition. J Leukoc Biol 83: 1493–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nyugen J, Agrawal S, Gollapudi S, and Gupta S. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol 30: 806–813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogawa T, Kitagawa M, and Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev 117: 57–68, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, and Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol 188: 4527–4534, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ortman JM, Velkoff VA, and Hogan H. An Aging Nation: The Older Population in the United States. United States Census Bureau, May 2014 [Google Scholar]
- 71.Poon IK, Lucas CD, Rossi AG, and Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 14: 166–180, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, and Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol 169: 4697–4701, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Seidler S, Zimmermann HW, Bartneck M, Trautwein C, and Tacke F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol 11: 30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, and Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 184: 836–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510: 92–101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serhan CN, Chiang N, Dalli J, and Levy BD. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol 7: a016311, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, and Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J 26: 1755–1765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, and Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 206: 15–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharma R, Kapila R, Haq MR, Salingati V, Kapasiya M, and Kapila S. Age-associated aberrations in mouse cellular and humoral immune responses. Aging Clin Exp Res 26: 353–362, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Shaw AC, Goldstein DR, and Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol 13: 875–887, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sica A. and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sotiriou SN, Orlova VV, Al-Fakhri N, Ihanus E, Economopoulou M, Isermann B, Bdeir K, Nawroth PP, Preissner KT, Gahmberg CG, Koschinsky ML, and Chavakis T. Lipoprotein(a) in atherosclerotic plaques recruits inflammatory cells through interaction with Mac-1 integrin. FASEB J 20: 559–561, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, and Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461: 1287–1291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, and Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175: 342–349, 2005 [DOI] [PubMed] [Google Scholar]
- 85.Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, Cerundolo V, and Simon AK. Autophagy controls acquisition of aging features in macrophages. J Innate Immun 7: 375–391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahashi I, Ohmoto E, Aoyama S, Takizawa M, Oda Y, Nonaka K, Nakada H, Yorimitsu S, and Kimura I. Monocyte chemiluminescence and macrophage precursors in the aged. Acta Med Okayama 39: 447–451, 1985 [DOI] [PubMed] [Google Scholar]
- 87.Takahashi R, Totsuka S, Ishigami A, Kobayashi Y, and Nagata K. Attenuated phagocytosis of secondary necrotic neutrophils by macrophages in aged and SMP30 knockout mice. Geriatr Gerontol Int 2015. [Epub ahead of print]; DOI: 10.1111/ggi.12436 [DOI] [PubMed] [Google Scholar]
- 88.Treasury Dot. 2012. The U.S. Economy in Charts. www.treasury.gov/resource-center/data-chart-center/Documents/20120229_EssentialEcon.PDF Accessed July27, 2016
- 89.Van den Bossche J, Laoui D, Naessens T, Smits HH, Hokke CH, Stijlemans B, Grooten J, De Baetselier P, and Van Ginderachter JA. E-cadherin expression in macrophages dampens their inflammatory responsiveness in vitro, but does not modulate M2-regulated pathologies in vivo. Sci Rep 5: 12599, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Duin D. and Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc 55: 1438–1444, 2007 [DOI] [PubMed] [Google Scholar]
- 91.van Royen N, Hoefer I, Bottinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, and Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res 92: 218–225, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Verschoor CP, Johnstone J, Loeb M, Bramson JL, and Bowdish DM. Anti-pneumococcal deficits of monocyte-derived macrophages from the advanced-age, frail elderly and related impairments in PI3K-AKT signaling. Hum Immunol 75: 1192–1196, 2014 [DOI] [PubMed] [Google Scholar]
- 93.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, and Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature 390: 350–351, 1997 [DOI] [PubMed] [Google Scholar]
- 94.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, and Lindsey ML. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci 71: 475–483, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, and Ransohoff RM. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med 211: 1533–1549, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoon P, Keylock KT, Hartman ME, Freund GG, and Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev 125: 137–143, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Zhang B, Bailey WM, Braun KJ, and Gensel JC. Age decreases macrophage IL-10 expression: implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol 273: 83–91, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]