Abstract
Object
Anterior skull base meningiomas are frequently associated with changes in personality and behavior. Although such meningiomas often damage ventromedial prefrontal cortex (vmPFC), which is important for higher cognition, the cognitive and behavioral effects of these meningiomas remain poorly understood. Using detailed neuropsychological assessments in a large series of patients, this study examines the cognitive and behavioral effects of meningioma lesions involving vmPFC.
Methods
We reviewed neuropsychology and lesion mapping records of 70 patients who underwent resection of meningiomas. The patients were drawn from the Neurological Patient Registry at the University of Iowa. Patients were sorted into two groups: those with lesions involving vmPFC and those with lesions that did not involve vmPFC. Neuropsychological data pertaining to a comprehensive array of cognitive and behavioral domains were available preoperatively in 20 patients and postoperatively in all 70 patients.
Results
There was no change in basic cognitive functions (e.g. attention, perception, memory, construction and motor performance, language, or executive functions) from the pre-operative to post-operative epochs for vmPFC and non-vmPFC groups. There was a significant decline in the behavioral domain, specifically adaptive function, for both the vmPFC and non-vmPFC groups, and this decline was more pronounced for the vmPFC group. Additionally, postoperative data indicated that the vmPFC group had a specific deficit in value-based decision making, as evidenced by poor performance on the Iowa Gambling Task, compared to the non-vmPFC group. The vmPFC and non-vmPFC groups did not differ postoperatively on other cognitive measures, including intellect, memory, language, and perception.
Conclusions
Lesions of vmPFC resulting from meningiomas are associated with specific deficits in adaptive function and value-based decision making. Meningioma patients showed decline in adaptive function postoperatively, and this decline was especially notable in patients with vmPFC region meningiomas. Early detection and resection of meningiomas of the anterior skull base (involving gyrus rectus) may prevent these deficits.
Keywords: Personality Change, Adaptive Function, Brain Tumor, Neuropsychology, Iowa Gambling Task, Decision Making
Introduction
Anterior skull base meningiomas are frequently associated with changes in personality and behavior10,14,16. Interestingly, despite the frequency of meningiomas affecting personality and behavior, large neuropsychological studies documenting alterations in the cognitive (e.g. perception, memory, executive, and language functions) and behavioral domains (e.g. personal adjustment and emotional function) in these patients are rare. There have been case reports describing personality changes in patients with meningioma21–23, but larger studies specifically describing personality change in anterior skull base meningioma patients are lacking. Some neuropsychological studies have described basic cognitive outcomes in anterior skull base meningioma patients8,18,26. These studies report deficits in cognitive functioning, specifically with executive function, working memory, processing capacity, and motor speed. Documenting outcomes in the behavioral domain in addition to the cognitive domain is crucial, since deficits in the behavioral domain have close relevance to the personality changes frequently associated with anterior skull base meningiomas.
Anterior skull base meningiomas often involve the ventromedial prefrontal cortex (vmPFC), which is implicated in various higher cognitive functions (including value-based decision-making5, moral judgments17) and also in emotional regulation1 and personality2,3. Interestingly, although anterior skull base meningiomas frequently involve (and lesion) vmPFC, the cognitive and behavioral manifestations of anterior skull base meningiomas remain poorly understood26. Several authors have reported minimal change in cognition following surgical resection for meningiomas18,26. However, despite no significant measureable difference in cognitive or behavioral functioning after surgery, many patients do not return to their premorbid level of function18. Such outcomes suggest that meningioma lesions, albeit not entirely caused by surgery itself, can lead to significant morbidity after treatment. The purpose of the current study is to characterize the cognitive and behavioral effects of meningioma lesions involving vmPFC. We hypothesized that meningiomas involving vmPFC would be associated with a specific decline in adaptive functioning, even when other cognitive functions are spared. We addressed this hypothesis by taking advantage of a unique opportunity afforded by the Iowa Neurological Patient Registry, which contains a large number (N = 70) of patients with surgical resection of meningiomas. This dataset allowed us to incorporate two important design features: (1) For patients with pre- and post-surgical neuropsychological assessments (N = 20), we could examine the effects of surgery per se. (2) We could contrast patients with vmPFC meningioma lesions (N = 23) with patients with non-vmPFC meningioma lesions (N = 47).
Methods
Patient Population
The participants for this study (N = 70) were drawn from the Patient Registry in the Division of Cognitive Neuroscience at the University of Iowa. All participants had stable, focal lesions following meningioma resection. Exclusion criteria included dementia, concomitant neurologic disease (e.g., evidence of cerebral infarction), premorbid psychiatric disease, developmental abnormalities, and alcohol or drug abuse. All participants provided informed consent in accordance with the Human Subjects Committee of the University of Iowa. In connection with their enrollment in the Patient Registry, patients were extensively characterized neuropsychologically and neuroanatomically, using standard protocols of the Benton Neuropsychology Laboratory25 and the Human Neuroanatomy and Neuroimaging Laboratory11. All data were collected in the chronic epoch of recovery (i.e., at least 3 months postoperatively). Average age of the participants was 62.1 years (SD = 12.6), average years of education was 13.5 (SD = 2.5). Patients were categorized into two groups: those that have lesions to the vmPFC and those that do not (Figure 1). Patients were placed in the vmPFC group when their lesion included either the left or right gryus rectus. The demographics for participants in the vmPFC group (n=23; 7 men, 16 women) and the non-vmPFC group (n=47; 18 men, 29 women) are listed in Table 1. Independent samples t-tests revealed that participants in the vmPFC group were older at the time of testing postoperatively than those in the non-vmPFC group (t (68) = 3.2, p = 0.002), and were further out from their resection (t (68) = 2.6, p = .01). For patients in the vmPFC group, subjective personality disturbance was the most common presenting complaint and occurred in 74% of patients. In the non-vmPFC group, headache was the most common presenting complaint, occurring in 26% of patients.
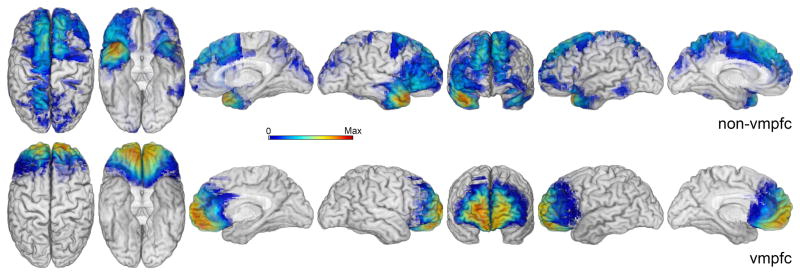
Figure 1.
Lesion maps for non-vmPFC (top panel, N = 32) and vmPFC (bottom panel, N = 19) patient groups. Color scale represents the degree of lesion overlap across patients in each group with red (the right side of color scale) representing maximum lesion overlap (non-vmPFC maximum overlap = 8, vmPFC maximum overlap = 18).
Table 1.
Demographics and presenting symptoms of all participants
| Variable | Mean+SD | Range |
|---|---|---|
| Age | 62.1±12.6 | 30 – 86 |
| Years of Education | 13.5±2.5 | 8 – 20 |
| Chronicity (years) | 5.7± 6.3 | 0.6 – 38.5 |
| Demographics of Participants by Lesion | ||
| vmPFC (n = 23) | non-vmPFC (n = 47) | |
| Variable | Mean + SD | Mean + SD |
| Age | 68.6 ± 9.7 * | 58.9 ± 12.7 |
| Years of Education | 13.2 ± 2.8 | 13.7 ± 2.3 |
| Chronicity (years) | 8.4 ± 8.8 * | 4.3 ± 4.0 |
| Presenting Complaint(s) by Lesion | ||
| vmPFC (n = 23) | non-vmPFC (n = 47) | |
| Personality Disturbance 17 (74%) | Headache 12 (26%) | |
| Anosmia 6 (26%) | Visual Symptoms 9 (19%) | |
| Visual Symptoms 5 (22%) | Personality Disturbance 8(17%) | |
| Memory Impairment 3 (13%) | Seizure 7 (15%) | |
| Confusion 2 (9%) | Motor Disturbance 5 (11%) | |
| Headache 2 (9%) | Language Impairment 5 (11%) | |
| Seizure 2 (9%) | Imbalance 4 (9%) | |
| Imbalance 1 (4%) | Memory Impairment 4 (9%) | |
| Incidental 1 (4%) | Gait Abnormality 2 (4%) | |
| Diplopia 2 (4%) | ||
| Dizziness 2(4%) | ||
| Confusion 2 (4%) | ||
| Vertigo 1 (2%) | ||
| Incidental 1 (2%) | ||
| Sensory Disturbance 3 (6%) | ||
| Hoarse Voice 1 (2%) | ||
| Spatial Disorientation 1(2%) | ||
| Depression 1(2%) | ||
| Eye Swelling 1(2%) | ||
| Syncope 1(2%) | ||
| Incontinence 1(2%) | ||
| Ataxia 1(2%) | ||
| Proptosis 1 (2%) | ||
Abbreviations. SD: Standard Deviation.
A subset of patients (N = 20) were also studied preoperatively. Ten patients with meningioma involving the vmPFC (3 men, 7 women; mean age presurgery 58.9 ± 11.7, postsurgery 68.4 ± 11.2; mean years of education 11.9 ± 2.7) were compared with ten patients with meningioma outside the vmPFC (6 men, 4 women; mean age presurgery 57.0 ± 15.4, postsurgery 61.3 ± 15.0; mean years of education 13.4 ± 2.5). There were no significant differences between the two groups with respect to years of education or age at time of testing both before and after surgery.
Lesion analysis
Lesion mapping procedures were performed as previously described6,7,11,13. In order to analyze the placement of lesions and the overlap of lesions in specific brain areas in a group this size, it is necessary to place all lesions in a common space. Lesions were identified by hypointensity on a T1 sequence MRI or hypodensity on CT. The lesion contour from the lesioned brains is manually warped into a normal template brain, taking into account gyral and sulcal landmarks, using the following steps: (1) a normal brain, the template brain, is reconstructed in three dimensions from thin contiguous MR slices using Brainvox; (2) all major sulci are identified and color-coded in this template brain as well as in the lesion brain; (3) the template brain volume is resliced so as to match the MR slices (or CT slices) of the lesioned brain; (4) the slices in the template brain are matched in orientation and thickness to the lesioned brain taking into consideration the intersection of the slices with the color-coded sulci (a good match can be assured both by inspection of the 2D images as well as by the positioning of the slices seen in the 3D images); (5) once the matching slices have been obtained, the lesion contour on each slice is manually transferred onto the template brain taking into consideration the distance of the lesion contour to the identifiable landmarks, such as gyri and subcortical structures; (6) the collection of transferred traces defines a volume that can be corendered with the template brain; (7) the volumes of several lesions so transferred into the template brain intersect in space and create a complex volume, which can also be corendered with the template brain. The overlaps of lesions in this volume, calculated by the sum of lesions overlapping on any single voxel, can be color-coded. Thus, the final overlap of N lesions can be analyzed in the template brain for areas corresponding to maximal overlaps.
Given the robust literature demonstrating a role for vmPFC cortex in personality and emotional regulation1–3, patients were stratified into two groups based on whether or not their lesion associated with their meningioma involved the vmPFC as described above. Patient demographics for the vmPFC group (n=23; 7 men, 16 women) and the non-vmPFC group (n=47; 18 men, 29 women) are listed in Table 1. Lesion size analysis was performed for all patients with appropriate neuroimaging data (61 patients; 19 from vmPFC group and 32 from non-vmPFC group). For the subjects that had a defined lesion mask, the volume of the mask was calculated using fslstats, a tool provided in FSL's fslutils15. All lesion masks were first binarized so that voxels containing lesion had a value of 1 and voxels outside the lesion had a value of zero. The resultant mask was run through fslstats and the output volume (mm3) for nonzero voxels was calculated.
Neuropsychological Testing
Participants were assessed according to standard protocols of the Benton Neuropsychology Laboratory25. This entails standardized assessment of classically described cognitive domains20: orientation and attention, perception, memory, verbal functions and language skills, construction and motor skills, concept formation and reasoning, executive functions (Table 2). Behavioral domains assessed included: personal adjustment and emotional function and adaptive functions (Table 2). Scores in domains of personal adjustment and emotional function and adaptive functions were determined by a trained neuropsychologist who was blind to the objectives and hypotheses of the study. To assess personal adjustment and emotional function, standardized self-report measures (e.g., MMPI, Beck Depression Inventory) and collateral ratings (e.g., Iowa Scales of Personality Change) were reviewed. To assess adaptive functions, each patient’s history and clinical notes were reviewed with regard to employment, independence, and self-care. Using findings from this chart review, each patient’s personal adjustment and emotional function and adaptive functions were assigned a rating of impairment: 0 = no impairment, 1 = mild impairment, 2 = moderate impairment, 3 = severe impairment by the blinded neuropsychologist who performed the chart review.
Table 2.
Neuropsychological Tests in Each Cognitive and Behavioral Domain
| Orientation and Attention | Concept Formation and Reasoning |
|---|---|
| Orientation | WAIS-Similarities |
| Digit Span | WAIS-Comprehension |
| Letter-Number Sequencing | WAIS-Arithmetic |
| Spatial Span | WAIS-Matrix Reasoning |
| Sentence Repetition | WAIS-Picture Completion |
| Stroop | WAIS-Picture Arrangement |
| Coding | WAIS-Figure Weights |
| Symbol Search | WAIS-Visual Puzzles |
| TMT A | WRAT-Arithmetic |
| Perception | WCST-Categories and Perseverative Errors |
| JoLO | Executive Functions |
| Benton Faces | IGT |
| Hooper | COWA |
| Memory | Category Fluency |
| AVLT-5′ & AVLT-30′ | TMT-B |
| CFT-recall | Personal Adjustment and Emotional Function |
| BVRT-correct | BDI |
| WMS-indices | BAI |
| Verbal Functions and Language Skills | ISPC |
| BNT | MMPI |
| WAIS Vocabulary | Adaptive Functions |
| WAIS Information | Blinded chart review of outcome (e.g. employment, independence, self-care) |
| Token Test | |
| WRAT-Reading | |
| WRAT-Spelling | |
| ICRT | |
| Construction and Motor Performance | |
| CFT-Copy | |
| WAIS-Block Design | |
| WAIS-Object Assembly | |
| Grooved Pegboard |
Abbreviations: TMT A = Trail Making Test A; JoLO = Judgment of Line Orientation; AVLT = Auditory Verbal Learning Test; CFT = Complex Figure Test; BVRT = Benton Visual Retention Test; WMS = Weschler Memory Scale; WAIS = Wechsler Adult Intelligence Scale; WRAT = Wide Range Achievement Test; ICRT = Iowa-Chapman Reading Test; WCST = Wisconsin Card Sorting Test; IGT = Iowa Gambling Task; COWA = Controlled Oral Word Association; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; ISPC = Iowa Scale of Personality Change; MMPI = Minnesota Multiphasic Personality Inventory.
To assess real-life decision making, a subset of patients were tested using the Iowa Gambling Task (IGT)4,5. The IGT is a neuropsychological task designed to evaluate and quantify deficits in real-life decision making during conditions of immediate and delayed reward and punishment4,5. In the task, patients are given $2,000 in virtual money and told to play a game to maximize the amount of money they make. Four card decks are presented: A, B, C, and D, with A and B associated with $100 in immediate reward and C and D associated with $50 in immediate reward. Unpredictably, decks A and B are associated with a large penalty, while C and D have smaller penalties. Thus, patients who pick mostly from A and B will incur a net loss, while patients who pick mostly from C and D will incur a net gain (despite the lower “up front” reward in C and D). In the task, there are 100 trials, analyzed by blocks of 20 (blocks 1 – 5). During the task, patients are not told how many trials they will complete. Patients with vmPFC damage are known to have deficits on the IGT5.
Statistical Analysis
All raw scores were converted to z-scores in accordance with available normative data20. Normalized neuropsychological test data were averaged within each of the aforementioned cognitive domains to obtain a composite z-score.
Results
Comparison of pre- and postoperative cognitive and adaptive functions
Independent-samples t-tests (Bonferroni corrected) were performed to assess group differences (vmPFC versus non-vmPFC) in the change scores from pre- to postoperative testing in the 20 patients with both pre- and postoperative data. These revealed no significant differences between the two groups with respect to any of the cognitive domains (Table 3 top). Similar results were found when looking at individual subtests making up the domains (Table 3 bottom). Both groups showed declines in depressive symptomatology (less depression) postoperatively, as measured by the Beck Depression Inventory, but again no significant differences were found between the two groups.
Table 3.
Comparison of patients tested pre- and postoperatively
| vmPFC | Non-vmPFC | |
|---|---|---|
| Cognitive domain | Change Score (SD) | Change Score (SD) |
| Attention | 0.8 + 0.9 | 0.7+ 1.1 |
| Perception | 0.7 + 1.4 | −0.1 ± 1.5 |
| Memory | 0.3 ± 0.8 | 0.7 ± 1.4 |
| Construction and Motor Performance | 0.5 + 1.3 | 0.3 ± 1.4 |
| Verbal Functions and Language Skulls | −0.3 ± 0.6 | 0.7 ± 3.0 |
| Concept Formation and Reasoning | 0.2 ± 0.9 | 0.3 ± 0.8 |
| Executive Functions | 1.3 ± 2.8 | 1.8 ± 3.9 |
| Individual Tests | Change Score (SD) | Change Score (SD) |
| WAIS Verbal IQ | −0.1 ± 0.6 | 0.1 ± 0.1 |
| WAIS Similarities | −0.1 ± 0.8 | −0.1 ± 0.6 |
| WAIS Digit Span | 0.3 ± 0.6 | −0.1 ± 0.9 |
| WAIS Arithmetic | −0.4 ± 0.9 | 0.1 ± 0.4 |
| WAIS Block Design | 0.1 ± 0.7 | −0.1 ± 0.6 |
| WAIS Coding | 1.2 ± 1.4 | 0.1 ± 0.6 |
| BVRT Correct | 0.5 ± 1.3 | 0.6 ± 1.6 |
| BVRT Errors | 0.4 ± 1.4 | 0.8 ± 1.5 |
| AVLT Trial 5 | 0.6 ± 0.9 | −0.1 ± 2.2 |
| AVLT 30’ Delay | −0.1 ± 1.2 | 0.3 ± 1.4 |
| CFT Copy | 1.0 ± 1.0 | 0.9 ± 2.1 |
| CFT Recall | 2.1 ± 1.8 | 1.1 ± 1.1 |
| Boston Naming Test | 0.6 ± 0.4 | 2.8 ± 5.7 |
| COWA | 1.6 ± 2.1 | 0.4 ± 0.7 |
| Facial Recognition Test | 0.1 ± 1.0 | −0.4 ± 1.3 |
| Trail-Making Test A | 3.0 ± 2.3 | 1.9 ± 2.3 |
| Trail-Making Test B | 2.6 ± 2.1 | 2.5 ± 4.5 |
| Pegboard Dominant | 2.0 ± 2.6 | −1.7 ± 1.6 |
| Pegboard Nondominant | 2.7 ± 3.2 | −0.3 ± 2.0 |
| WCST Categories | 1.6 ± 1.3 | 0.3 ± 2.3 |
| WCST Perseverative Errors | 3.9 ± 4.1 | 0.3 ± 2.4 |
| Beck Depression Inventory * | −8.4 ± 6.5 | −12.6 ± 5.6 |
Change scores reflect the change in composite z-scores from pre- to postoperative testing. A positive value indicates relative improvement, whereas a negative value indicates a relative decline. Beck Depression Inventory is a change in the raw score from pre- to postoperative testing. Negative values reflect decline in number of depressive symptoms endorsed. WAIS = Wechsler Adult Intelligence Scale; BVRT = Benton Visual Retention Test; AVLT = Auditory Verbal Learning Test; CFT = Complex Figure Test; COWA = Controlled Oral Word Association; WCST = Wisconsin Card Sorting Test.
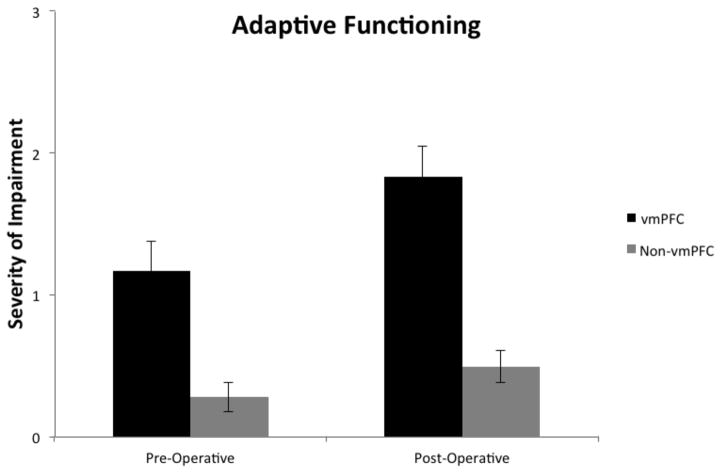
The behavioral domain of adaptive function was assessed in all patients (N = 70) before and after surgery. A 2 x 2 ANOVA was performed to assess differences in adaptive function in the pre- and postoperative epochs as a function of lesion location, where group (vmPFC v. non-vmPFC) and epoch (pre v. post) were the two factors in the ANOVA. This analysis yielded a significant interaction between group and epoch, F(1,68) = 4.50, p = .04, reflecting that the magnitude of postoperative adaptive function decline was greater for the vmPFC group. Both main effects were also significant, reflecting that adaptive function scores in the vmPFC group were lower than in the non-vmPFC group both preoperatively and postoperatively, F(1,68) = 17.42, p < .001, and that there was decline in adaptive function from the pre- to postoperative epochs for both the vmPFC and non-vmPFC groups, F(1,68) = 41.56, p < .001. These data are shown in Figure 2.
Figure 2.
Pre- and postoperative average scores for adaptive functioning in the vmPFC and non-vmPFC groups. Numbers reflect average rating from 0 – 3 (0 = no impairment; 1 = mild impairment; 2 = moderate impairment; 3 = severe impairment).
Postoperative cognitive and behavioral domain performance
Comparison of postoperative performances in each cognitive domain, and individual tests comprising the domains, are summarized in Table 4 and Table 5, respectively. Independent samples t-tests revealed no significant differences (p > .05) between the vmPFC and non-vmPFC groups with respect to any cognitive domain (Table 4) or individual test (Table 5). Given the variability of age and chronicity parameters, a regression analysis was performed to account for age and chronicity. This yielded the same result, namely, no significant difference between the vmPFC and non-vmPFC groups with regard to postoperative impairment in any of the cognitive domains.
Table 4.
Comparison of Cognitive Domains in All Patients
| Cognitive Domain | vmPFC Average Z-Score | Non-vmPFC Average Z-Score |
|---|---|---|
| Attention | 0.0 + 1.1 | 0.0 + 0.8 |
| Perception | 0.2 + 1.1 | −0.2 + 0.9 |
| Memory | 0.2 + 1.3 | 0.0 + 1.0 |
| Construction and Motor Performance | 0.3 + 0.8 | −0.1 + 1.4 |
| Verbal Function and Language Skills | 0.1 + 0.8 | 0.0 + 0.7 |
| Concept Formation and Reasoning | 0.3 + 0.7 | 0.1 + 0.7 |
| Executive Functions | −0.4 + 1.6 | −0.3+ 1.2 |
Table 5.
Comparison of individual test scores for vmPFC and non-vmPFC groups in the postoperative epoch
| Individual Tests | vmPFC Z-Score | Non-vmPFC Z-score |
|---|---|---|
| WAIS Verbal IQ | 0.4 ± 0.9 | 0.0 ± 0.9 |
| WAIS Performance IQ | 0.3 ± 0.9 | 0.1 ± 0.9 |
| WAIS Full-Scale IQ | 0.4 ± 1.0 | 0.1 ± 0.9 |
| WAIS Similarities | 0.4 ± 0.9 | 0.1 ± 0.9 |
| WAIS Information | 0.2 ± 1.1 | −0.1 ± 0.9 |
| WAIS Digit Span | 0.2 ± 0.8 | −0.3 ± 0.9 |
| WAIS Arithmetic | 0.4 ± 1.0 | 0.0 ± 1.0 |
| WAIS Block Design | 0.5 ± 0.7 | 0.0 ± 0.9 |
| WAIS Coding | 0.1 ± 1.0 | 0.1 ± 1.0 |
| BVRT Correct | 0.3 ± 1.5 | 0.1 ± 1.0 |
| BVRT Errors | 0.2 ± 1.5 | 0.1 ± 1.2 |
| AVLT Trial 5 | 0.2 ± 1.7 | 0.2 ± 1.4 |
| AVLT 30’ Delay | 0.3 ± 1.6 | 0.3 ± 1.3 |
| CFT Copy | 0.7 ± 1.3 | 0.3 ± 1.4 |
| CFT Recall | 0.1 ± 1.9 | −0.6 ± 1.1 |
| Boston Naming Test | 0.3 ± 1.5 | 0.3 ± 1.0 |
| COWA | 0.3 ± 1.3 | 0.0 ± 1.3 |
| Token Test | 0.6 ± 0.2 | 0.3 ± 0.5 |
| Facial Recognition Test | −0.1 ± 1.2 | −0.3 ± 1.1 |
| Trail-Making Test A | −0.7 ± 3.6 | 0.0 ± 1.6 |
| Trail-Making Test B | −0.7 ± 3.5 | −0.3 ± 2.1 |
| Pegboard Dominant | 0.0 ± 1.4 | −0.9 ± 1.9 |
| Pegboard Nondominant | 0.0 ± 0.8 | −0.5 ± 1.9 |
| WCST Categories | 0.0 ± 1.1 | −0.4 ± 1.7 |
| WCST Perseverative Errors | −0.9 ± 1.7 | −1.0 ± 2.3 |
Abbreviations: TMT A = Trail Making Test A; JoLO = Judgment of Line Orientation; AVLT = Auditory Verbal Learning Test; CFT = Complex Figure Test; BVRT = Benton Visual Retention Test; WMS = Weschler Memory Scale; WAIS = Wechsler Adult Intelligence Scale; WRAT = Wide Range Achievement Test; ICRT = Iowa-Chapman Reading Test; WCST = Wisconsin Card Sorting Test; IGT = Iowa Gambling Task; COWA = Controlled Oral Word Association; BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; ISPC = Iowa Scale of Personality Change; MMPI = Minnesota Multiphasic Personality Inventory.
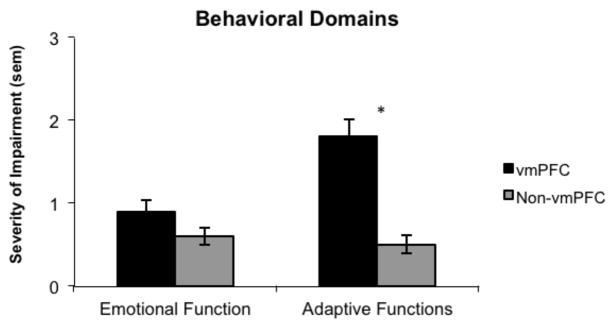
In contrast to the cognitive domains, there was a significant difference between the two groups with respect to the behavioral domain of adaptive functions. Independent samples t-tests revealed that participants in the vmPFC group had a significantly higher rate of impairment than did participants in the non-vmPFC group (t (68) = 5.8, p < 0.001) (Figure 3. No significant differences were found between the groups with respect to emotional function. Similar to the cognitive domains, a regression analysis was performed to account for variability in age and chronicity between the vmPFC and non-vmPFC groups and when accounting for these variables, the vmPFC group still had a significantly higher rate of adaptive function impairment than the non-vmPFC group (p < 0.001).
Figure 3.
Average scores in the behavioral domains for vmPFC and non-vmPFC patients. The adaptive functions score was significant higher for the vmPFC group compared to non-vmPFC group. Numbers reflect average rating from 0 – 3 (0 = no impairment; 1 = mild impairment; 2 = moderate impairment; 3 = severe impairment).
Iowa Gambling Task (IGT) Performance
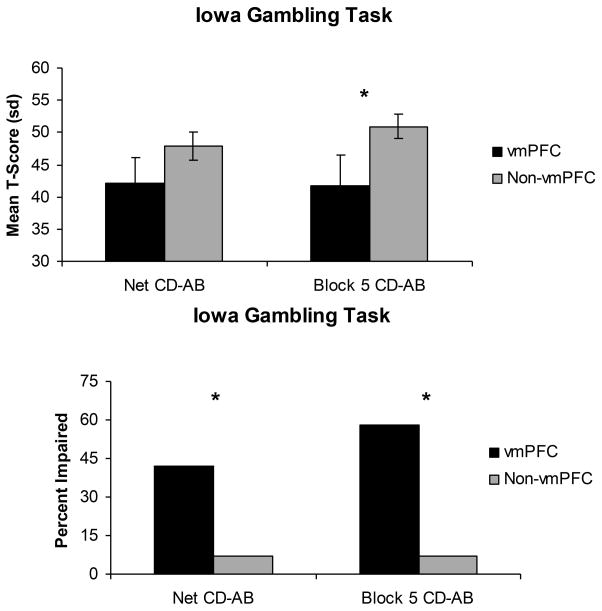
A subset of the participants were administered the Iowa Gambling Task (vmPFC: n = 11; non-vmPFC: n = 15). Participants in the vmPFC group performed significantly worse than non-vmPFC participants during Block 5 (the final 20 card selections) of the task (t (24) = −2.2, p = .03). There was a trend toward significance when comparing the two groups over the entire task of 100 selections (t (24) = −1.9, p = .08) (Figure 4).
Figure 4.
Bar graphs depicting IGT performance for vmPFC and non-vmPFC groups as both a mean T-score (top panel) and percent impaired (bottom panel).
Chi-square analysis of task performance revealed a significantly higher proportion of vmPFC participants had impaired performances both on Block 5 (χ2 (1, N = 26) = 5.38, p = .02) and the overall task (χ2 (1, N=26) = 9.67, p = .002). The two groups did not differ significantly with respect to age at time of testing (vmPFC mean = 67.1 ± 6.8; Non-vmPFC mean = 58.1 ± 13.8), lesion chronicity (vmPFC mean = 12.5 years ± 11.0; Non-vmPFC mean = 7.0 years ± 3.4), or years of education (vmPFC mean = 14.9 ± 2.5; Non-vmPFC mean = 14.0 ± 2.6).
Further analysis showed significant correlations between adaptive functions and performance on the IGT. Greater impairment of adaptive functions was significantly correlated with worse performance on the IGT both on Block 5 (r = −.63, p = .001) and the overall task (r = −.57, p = .002). Of the 12 vmPFC participants, seven were impaired on Block 5 of the IFG and all had ratings of moderately or severely impaired adaptive functions. Five were impaired on the overall task and again all had ratings of moderately or severely impaired adaptive function.
Lesion Analysis
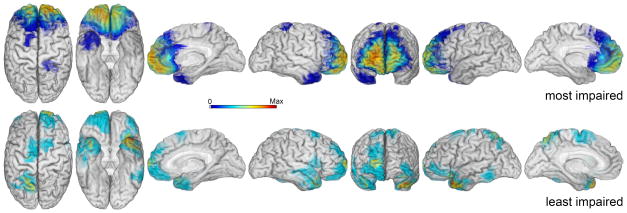
To further examine the effect of lesion location on adaptive functioning, lesion maps were generated for the 15 patients with the worst adaptive functioning scores and the 15 patients with the best adaptive functioning scores who also had available lesion data (13 patients with highest [worst] adaptive functions scores and 14 patients with lowest [best] adaptive functions scores, see Figure 5). Patients with the greatest deficit in adaptive functioning (top panel, Figure 5) had a maximum lesion overlap in vmPFC (max overlap = 12). In comparison, patients with the best adaptive functioning scores had minimal lesion overlap in any particular anatomical region (max overlap = 3).
Figure 5.
Comparison of lesion overlap maps in 15 patients with greatest adaptive function impairment and least adaptive function impairment. For patients with greatest adaptive function impairment, 14 had lesion maps (top panel; N = 14, max overlap = 12). For patients with the least adaptive function impairment, 13 patients had lesion overlap maps (bottom panel; N = 13; max overlap = 3). These lesion overlap maps demonstrate that patients with the greatest deficits in adaptive functioning had similar lesions of vmPFC.
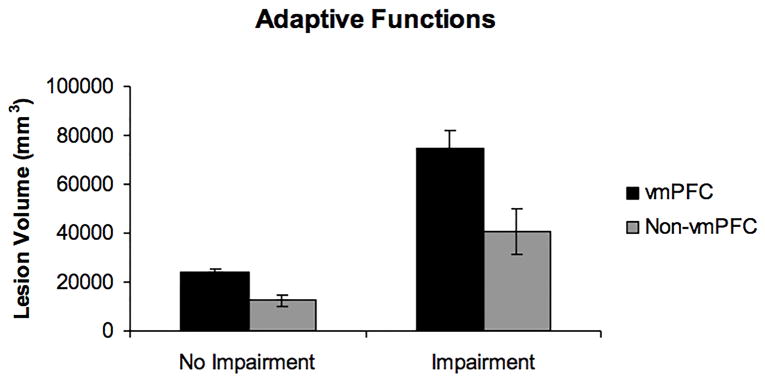
Next, the role of lesion size on cognitive and behavioral outcomes was examined. Regardless of whether patients were in vmPFC or non-vmPFC groups, lesion size was correlated with impairments in attention (r = −0.38, p < 0.003), memory (r = −0.24, p < 0.05), verbal-language function (r = −0.29, p < 0.02), executive function (r = −0.3, p < 0.02), emotional function (r = 0.23, p < 0.05), and adaptive function (r = 0.68, p < 0.001). To further evaluate the role of vmPFC lesion size on adaptive function impairment, lesion size was compared for both impaired and non-impaired patients in both the vmPFC and non-vmPFC groups (Figure 6). When age, chronicity, and lesion size are controlled for in regression analysis, patients in the vmPFC group have significantly worse outcomes in adaptive function (p = 0.003) than patients in the non-vmPFC group, while patients in the non-vmPFC group have worse memory outcomes than the vmPFC group (p = 0.02). When age, chronicity, and lesion size were controlled, none of the other cognitive or behavioral domains showed significantly different levels of impairment between the vmPFC and non-vmPFC groups.
Figure 6.
Bar graph depicting lesion size as a function of adaptive function score in vmPFC and non-vmPFC lesion patients. Lesion size was associated with adaptive functioning deficits in the vmPFC group.
Discussion
These data demonstrate deficits in adaptive functions and real-life decision making (measured by the IGT) in patients with meningioma-induced lesions of vmPFC. Furthermore, we found that decline in adaptive functions from the pre- to postoperative epoch was more pronounced in patients with vmPFC lesions (compared to patients with lesions that did not involve the vmPFC). Previous work has documented associations between frontal meningiomas and personality change14,19 or neuropsychological dysfunction26, but to our knowledge this is the first demonstration of the effects of meningioma lesions involving vmPFC on behavioral functioning in a large group of patients. Additionally, these data demonstrate a deleterious effect of anterior skull base meningioma lesions on value-based decision making, as measured by the Iowa Gambling Task (IGT).
Several studies have examined neuropsychological outcomes in meningioma patients8,18,26. Dijkstra and colleagues studied 89 patients who underwent resection of WHO I meningiomas and reported significant impairment in executive functioning, verbal memory, information processing capacity, psychomotor speed, and working memory8. In this study, patients with skull base meningiomas were more likely to have impairment of verbal memory, information processing capacity, and psychomotor speed compared to convexity meningioma patients8. Tucha et al. studied 54 patients with frontal meningiomas and found preoperative impairments in working memory, fluency functions, tonic alertness, processing speed, and flexibility, with a reported improvement in attentional functions postoperatively26. In the present study of 70 meningioma patients, we replicated the finding of little change in cognitive function between the pre and postoperative epochs. However, despite no significant difference between pre and postoperative cognitive function, our patients manifested a significant decline in adaptive function postoperatively. This decline occurred in both the vmPFC and non-vmPFC groups, but was especially pronounced for patients in the vmPFC group. One interpretation for the adaptive function decline in both vmPFC and non-vmPFC groups is that neurosurgical intervention for meningioma has an effect on adaptive functions independent of meningioma location. This interpretation is consistent with previous studies showing postoperative decline in quality of life measures18. Interestingly, for patients in the vmPFC group there was greater preoperative impairment in adaptive function, along with greater decline in adaptive function between the pre and postoperative epoch (Figure 2). The vmPFC plays an important role in the maintenance of adaptive functions, which could help explain why vmPFC meningiomas are associated with greater preoperative adaptive function impairment. The greater magnitude of adaptive function decline postsurgically in the vmPFC group could be related to either: 1) relatively more neurosurgical trauma associated with resection of vmPFC region meningiomas (i.e. retraction injury) or 2) that patients with preoperative adaptive function deficits have even greater difficulty coping with neurosurgical intervention, thereby leading to an even greater increase in postoperative adaptive function impairment. It is important to note that despite postoperative impairment in adaptive function, symptoms of depression (as measured by the Beck Depression Inventory) actually decreased postoperatively, and this was true in both the vmPFC and non-vmPFC groups.
In addition to the effect of vmPFC meningiomas on adaptive function, we also demonstrate deficits in value-based decision making for patients with vmPFC meningiomas. This is not surprising given that the vmPFC plays a role in various higher cognitive functions, including value-based decision-making5 (also moral judgments17, emotional regulation1, and personality2,3). Interestingly, we found that impairment on the IGT was associated with adaptive function impairment. Thus, it is possible that some of the adaptive function impairment occurs secondary to impaired decision making.
The mechanism of postoperative encephalomalacia (i.e. the meningioma lesion) following meningioma resection, and the pathophysiology behind the associated neuropsychological deficits, is likely a combination of local tissue ischemia (due to the meningioma itself) and neurosurgical trauma12,24. Tomasello and colleagues documented postoperative lesion volumes in a series of patients with giant olfactory groove meningiomas and found encephalomalacia cavities smaller than the meningioma itself24. In terms of neurosurgical trauma as a cause of postoperative encephalomalacia, approaches to resect large anterior skull base meningiomas may involve brain retraction24 or brain transgression12, both of which could cause postoperative encephalomalacia. Meningioma growth (preoperatively) itself likely plays a substantial role in the genesis of postoperative encephalomalacia in addition to neurosurgical trauma. Using MR imaging, Domingo and colleagues reported decreased cerebral blood volume and increased lactate as far as 4 cm from meningiomas suggesting tissue ischemia outside of the meningioma itself9. Localized brain ischemia surrounding the meningioma may lead to cell death and concomitant encephalomalacia. Thus, meningiomas in the absence of neurosurgical intervention can cause significant disruption of surrounding brain tissue (and associated functions), and this disruption can potentially be worsened by neurosurgical trauma. Despite the effects of neurosurgical trauma and the meningioma itself, previous work has shown that cognitive functions (as measured by neuropsychological tests) typically remain stable or improve after neurosurgical resection26. Interestingly however, our results demonstrate a marked postsurgical decline in adaptive function in meningioma patients, in the presence of stable performance on cognitive testing. This decline in adaptive functions was present in patients with both vmPFC and non-vmPFC meningioma lesions, but was especially notable in patients with vmPFC lesions.
As noted in the Introduction, personality changes have been observed in many patients with frontal meningiomas. Interestingly, Krupp et al. reported that 19% of meningioma patients did not return to their premorbid work level. It is possible that changes in the cognitive domain contribute to the alteration of personality and decreased return to work in meningioma patients, but our results suggest that it is more likely that factors within the behavioral domain (e.g. emotional function and adaptive function) are what play a key role in personality and work outcomes. We examined personality directly by measuring scores on the Iowa Scale of Personality Change as a component of emotional function (see Table 2). Though we did not find significant impairment in emotional function in our patients with vmPFC lesions, there was a trend towards impairment to emotional function in vmPFC lesioned patients (Fig. 2). The addition of one or two subjects to our study group could potentially make emotional function impairment in the vmPFC group a significant finding in our study. Our results demonstrate robust impairment in adaptive function, which is potentially related to the decreased return to work reported by other studies18.
Given the propensity of anterior skull base meningiomas to involve (and lesion) vmPFC24 (e.g. gyrus rectus and orbitofrontal cortex), it is not surprising that anterior skull base meningiomas frequently present with the subjective complaint of personality change21,26. Previously, bilateral vmPFC lesions have been associated with acquired personality change, with specific deficits in dampening of emotional experience, poorly modulated emotional reactions, and defective social decision making1.
Since most meningiomas are relatively slow growing, it is possible that brain reorganization occurs in tandem with meningioma growth and that the cognitive deficits seen in meningioma patients with vmPFC lesions are less pronounced than patients who have more abrupt lesion onset. To determine how the rate of lesion development effects neuropsychological outcome would require comparison of slow growing lesions (e.g. WHO I meningiomas) to rapidly acquired lesions (e.g. a frontal contusion).
A potential limitation of this study is that our patient population is limited to patients who underwent neuropsychological evaluation in conjunction with neurosurgical treatment. It is possible that a large number of meningioma patients who undergo neurosurgical resection are not referred for neuropsychological evaluation. Patients referred for neuropsychological evaluation typically have more noticeable cognitive deficits or behavioral changes associated with the discovery or resection of the meningioma. For this reason, it is possible that the cohort of patients in our study has more pronounced cognitive or behavioral deficits than the typical meningioma patient. With that possibility acknowledged, our results show relative stability of the majority of cognitive functions across the pre- and postoperative epochs, consistent with other studies that had different enrollment protocols8,26. It is possible that patients referred for neuropsychological evaluation (and included in this study) are at greater risk for adaptive function impairment, but since the characteristics of pre- and postoperative cognitive change are the same in our study as in other meningioma studies, this possibility seems less likely. An additional limitation of our study is the relatively small sample size of patients who had both pre- and postoperative neuropsychological data. While it is important to acknowledge this limitation in regard to the pre- and postoperative comparisons, the main objective of this study was to examine the effect of meningioma lesions in the vmPFC, which is well-established by examination of postoperative neuropsychological tests in a large number of patients (N = 70) with detailed neuroanatomical lesion maps.
Conclusion
Lesions of vmPFC resulting from meningiomas are associated with specific deficits in adaptive function and value-based decision making, despite no major dysfunction in basic cognitive abilities. Despite improvement in depression symptoms, meningioma patients with or without vmPFC involvement showed decline in adaptive function postoperatively, and this decline was especially notable in patients with vmPFC region meningiomas. Early detection and resection of meningiomas of the anterior skull base (involving gyrus rectus) may prevent these deficits.
Acknowledgments
Funding: T.A. is supported by a grant through the National Institutes of Health (NIH F32-NS087664). Also supported in part by a McDonnell Foundation Collaborative Award to D.T. (#220020387)
Footnotes
A manuscript was submitted as an abstract to the American Association of Neurological Surgeons annual meeting containing portions of the data presented in this manuscript.
References
- 1.Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. J Int Neuropsychol Soc. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- 2.Barrash J, Asp E, Markon K, Manzel K, Anderson SW, Tranel D. Dimensions of personality disturbance after focal brain damage: investigation with the Iowa Scales of Personality Change. J Clin Exp Neuropsychol. 2011;33:833–852. doi: 10.1080/13803395.2011.561300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9:159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154. [DOI] [PubMed] [Google Scholar]
- 5.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123( Pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 6.Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Arch Neurol. 1992;49:137–143. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- 7.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra M, van Nieuwenhuizen D, Stalpers LJ, Wumkes M, Waagemans M, Vandertop WP, et al. Late neurocognitive sequelae in patients with WHO grade I meningioma. J Neurol Neurosurg Psychiatry. 2009;80:910–915. doi: 10.1136/jnnp.2007.138925. [DOI] [PubMed] [Google Scholar]
- 9.Domingo Z, Rowe G, Blamire AM, Cadoux-Hudson TA. Role of ischaemia in the genesis of oedema surrounding meningiomas assessed using magnetic resonance imaging and spectroscopy. Br J Neurosurg. 1998;12:414–418. doi: 10.1080/02688699844600. [DOI] [PubMed] [Google Scholar]
- 10.Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- 11.Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- 12.Gazzeri R, Galarza M, Gazzeri G. Giant olfactory groove meningioma: ophthalmological and cognitive outcome after bifrontal microsurgical approach. Acta Neurochir (Wien) 2008;150:1117–1125. doi: 10.1007/s00701-008-0142-z. discussion 1126. [DOI] [PubMed] [Google Scholar]
- 13.Glascher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, et al. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci U S A. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunter R, Blackwood W, Bull J. Three cases of frontal meningiomas presenting psychiatrically. Br Med J. 1968;3:9–16. doi: 10.1136/bmj.3.5609.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Kalkanis SN, Quinones-Hinojosa A, Buzney E, Ribaudo HJ, Black PM. Quality of life following surgery for intracranial meningiomas at Brigham and Women's Hospital: a study of 164 patients using a modification of the functional assessment of cancer therapy-brain questionnaire. J Neurooncol. 2000;48:233–241. doi: 10.1023/a:1006476604338. [DOI] [PubMed] [Google Scholar]
- 17.Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krupp W, Klein C, Koschny R, Holland H, Seifert V, Meixensberger J. Assessment of neuropsychological parameters and quality of life to evaluate outcome in patients with surgically treated supratentorial meningiomas. Neurosurgery. 2009;64:40–47. doi: 10.1227/01.NEU.0000336330.75381.39. discussion 47. [DOI] [PubMed] [Google Scholar]
- 19.Lampl Y, Barak Y, Achiron A, Sarova-Pinchas I. Intracranial meningiomas: correlation of peritumoral edema and psychiatric disturbances. Psychiatry Res. 1995;58:177–180. doi: 10.1016/0165-1781(95)02586-l. [DOI] [PubMed] [Google Scholar]
- 20.Lezak MD. Neuropsychological assessment. 5. Oxford ; New York: Oxford University Press; 2012. [Google Scholar]
- 21.Nakazato Y, Hirato J. A 29-year-old woman who presented with personality change and a tumor in the frontal skull base. Neuropathology. 2005;25:395–397. doi: 10.1111/j.1440-1789.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 22.Okhovat A. A 46-year-old woman with a history of personality change, headache, and olfactory hallucination. Iran J Neurol. 2013;12:77–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Pizzoni C, Sarandria C, Pierangeli E. Clear-cell meningioma of the anterior cranial fossa. Case report and review of the literature. J Neurosurg Sci. 2009;53:113–117. [PubMed] [Google Scholar]
- 24.Tomasello F, Angileri FF, Grasso G, Granata F, De Ponte FS, Alafaci C. Giant olfactory groove meningiomas: extent of frontal lobes damage and long-term outcome after the pterional approach. World Neurosurg. 2011;76:311–317. doi: 10.1016/j.wneu.2011.03.021. discussion 255–318. [DOI] [PubMed] [Google Scholar]
- 25.Tranel D, Hathaway-Nepple J, Anderson SW. Impaired behavior on real-world tasks following damage to the ventromedial prefrontal cortex. J Clin Exp Neuropsychol. 2007;29:319–332. doi: 10.1080/13803390600701376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucha O, Smely C, Preier M, Becker G, Paul GM, Lange KW. Preoperative and postoperative cognitive functioning in patients with frontal meningiomas. J Neurosurg. 2003;98:21–31. doi: 10.3171/jns.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]