Abstract
Dietary protein limitation (PL) is not only beneficial to human health but also applied to minimize nitrogen excretion in livestock production. However, the impact of PL on intestinal physiology is largely unknown. In this study, we identified 5275 quantitative proteins using a porcine model in which pigs suffered PL. A total of 202 proteins |log2 fold-change| > 1 were taken as differentially expressed proteins and subjected to functional and pathway enrichment analysis to reveal proteomic alterations of the jejunal mucosa. Combining with the results of western blotting analysis, we found that protein/carbohydrate digestion, intestinal mucosal tight junction and cell adhesion molecules, and the immune response to foreign antigens were increased in the jejunal mucosa of the pigs upon PL. In contrast, amino acid transport, innate and auto immunity, as well as cell proliferation and apoptosis were reduced. In addition, the expression of functional proteins that involved in DNA replication, transcription and mRNA splicing as well as translation were altered in the jejunal mucosa in response to PL. Furthermore, PL may reduce amino acid transport and cell proliferation through the depression of mTOR pathway. This study provides new insights into the molecular mechanisms underlying the small intestinal response to PL.
Dietary protein limitation (PL) has been shown to exert multiple roles in extending lifespan1, improving chronic kidney disease recovery2 and enhancing stress resistance1,3,4, as well as minimizing environmental pollution arisen from livestock farming by reducing nitrogen excretion5. Nevertheless, whether protein limitation would exert elevated or even negative impacts on intestinal physiological reaction, immune response and protein metabolism is largely unknown.
The small intestine is a major site of protein digestion and amino acid (AA) absorption and metabolism6. Before entering the portal vein, intestinal luminal free AA were metabolized in the enterocytes, which significantly modified absorbed AA profile comparing to dietary AA profile6,7,8. Simultaneously, the small intestine mucosa is also an interactive membrane that allows dietary nitrogen compounds to influence intestinal structure, epithelium renewal, cellular metabolism, mucosal immunity, and genetic modification9,10, which has recently attracted great interest in the field. Thus, further study is required to reveal the proteomic adaptation of the intestinal mucosa membrane in response to PL.
In pigs, growing evidence showed that pigs offered protein-limited diets cannot synthesize sufficient amount of all non-essential amino acids (NEAA) to achieve their maximum growth rate and keep healthy11. However, the influences of NEAA deficiency on intestinal physiological reaction, immune response and AA extra-/intra- cellular metabolism remain unclear. In addition, intestinal mucosal AA transporters have been shown to be able to trigger cellular responses that result in functional adaptations in growth, immune response, and energy expenditure appropriate to the alteration of dietary protein supply12. Nevertheless, whether PL decreases the expression of AA transporters has been inconsistently shown in previous studies13,14.
Therefore, in the present study, we employed a porcine model, an excellent animal model for studying human nutrition and digestion physiology15,16, to investigate the impact of PL on intestinal mucosal proteomics and key physiological functions using the isobaric tags for relative and absolute quantitation (iTRAQ) technique. At the same time, this study is the first report to show high-throughput quantitative proteomic changes in porcine jejunum in response to PL.
Results
Animal performance
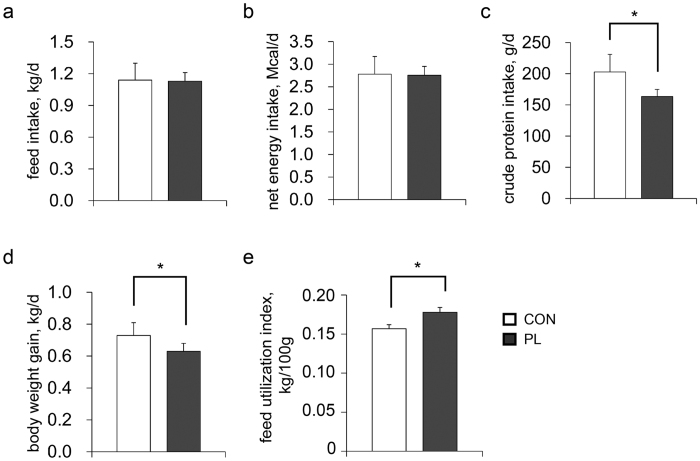
During 28-d PL, we observed that feed intake and net energy intake remained unchanged (p > 0.05), whereas protein intake and body weight gain were decreased (p < 0.05). The feed utilization index was significantly increased in response to PL (p < 0.05) (Fig. 1).
Figure 1. Performance of pigs upon dietary protein limitation.
Feed utilization index was defined as feed intake per 100 g of daily body weight gain. *Significant differences between groups; p < 0.05. n = 8. CON, control group; PL, dietary protein limitation.
Small intestinal proteomic profile
In the current study, we analysed three technical replicates from each sample pools of the control (CON) or PL treatment, respectively, with each sample pool consisting of equiponderant proteins isolated individually from the jejunal mucosae of eight pigs. After quality controls (Supplementary file Figure S1), a total of 5275 quantitative proteins were identified based on 65535 spectra from the jejunal mucosae, and 202 proteins with |log2 fold-change| > 1 between PL and the CON samples were taken as differentially expressed proteins (DEP), with 178 proteins being up-regulated and 24 being down-regulated upon PL. Details of the proteomics are listed in Supplementary Data.
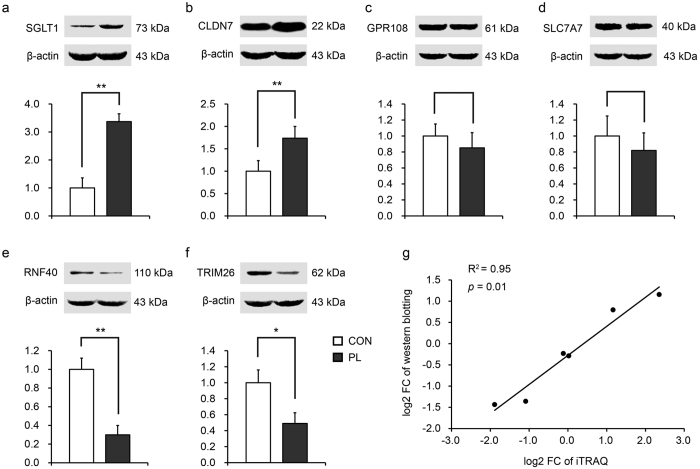
To validate the results of iTRAQ analysis, western blotting was employed to determine the relative abundances of six proteins, including sodium/glucose cotransporter 1 (SGLT1, UniProtKB accession no. F1RLV1_PIG), claudin 7 (CLDN7, UniProtKB accession no. C3VPJ4_PIG), G protein-coupled receptor 108 (GPR108, UniProtKB accession no. F1SCN1_PIG), solute carrier family 7 member 7 (SLC7A7, UniProtKB accession no. A8HG48_PIG), ring finger protein 40 (RNF40, UniProtKB accession no. F1RG77_PIG) and tripartite motif-containing protein 26 (TRIM26, UniProtKB accession no. F1RZ52_PIG). Correlation analysis showed a high consistency between the iTRAQ data and the western blotting data (R2 = 0.95, p = 0.01) (Fig. 2), indicating high reliability of our proteomic workflow.
Figure 2. Validation of iTRAQ data by western blotting with beta-actin as a loading control.
*Significant differences between groups; *p < 0.05, **p < 0.01. n = 8. CON, control group; PL, dietary protein limitation; FC, fold-change.
Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
GO and KEGG enrichment analysis were performed with 202 DEP. In GO analysis, we significantly enriched 307 biological processes terms, 62 molecular functions terms, 56 cellular components terms, respectively (p < 0.05). Moreover, by matching DEP to the KEGG and NCBI BLAST databases, we enriched 35 KEGG pathways (p < 0.05) (Supplementary Data). Based on GO function annotation and KEGG pathway analysis, combining with NCBI and UniProtKB and existing literatures, DEP were classified into five function groups: 1) nutrient digestion, absorption, and metabolism (30.2%, 61 out of 202 DEP); 2) immune function (18.3%, 37 out of 202 DEP); 3) cell renewal (including cell proliferation and apoptosis) (12.4%, 25 out of 202 DEP); 4) regulation of DNA replication and gene expression (17.3%, 35 out of 202 DEP); and 5) others and/or unknown (32.2%, 65 out of 202 DEP). Some DEP participate in multiple biological functions. Table 1 shows the relevant DEP involved in the above key intestinal physiological functions.
Table 1. Intestinal key physiological functions and relevant differentially expressed proteins in the jejunal mucosa of pigs upon dietary protein limitation.
| UniProtKB accession no. | protein name | associated gene name | FC | function |
|---|---|---|---|---|
| Nutrient digestion, absorption, and metabolism | ||||
| up-regulated | ||||
| WAP3_PIG | protein WAP-3 | WAP-3 | 7.26 | peptidase inhibitor |
| C6L245_PIG | putative trypsinogen | TRY | 5.58 | protein digestion and absorption |
| F1RLV1_PIG | sodium/glucose cotransporter 1 | SGLT1 | 5.11 | digestive system process |
| F1SFI5_PIG | histidine-rich glycoprotein | HRG | 3.79 | cysteine-type endopeptidase inhibitor |
| DPEP1_PIG | dipeptidase 1 | DPEP1 | 2.96 | extracellular peptidase |
| down-regulated | ||||
| F1RG77_PIG | ring finger protein 40 | RNF40 | 0.27 | proteolysis involved in cellular protein catabolic process |
| F1SKG5_PIG | putative glycerol kinase 5 | GK5 | 0.32 | citrate cycle; glycerolipid metabolism |
| I3LRA1_PIG | lon peptidase 2 | LONP2 | 0.43 | serine-type endopeptidase activator (cytoplasm) |
| Immunity | ||||
| up-regulated | ||||
| F8J302_PIG | MHC class II antigen | SLA-DQB | 36.57 | immune response |
| GILT_PIG | gamma-interferon-inducible-lysosomal thiol reductase | IFI30 | 5.61 | antigen processing |
| L8AXK3_PIG | IgG heavy chain | IGHG | 4.57 | response to wounding; intestinal immune network for IgA production |
| K7GQR1_PIG | complement factor properdin | CFP | 4.06 | humoral immune and inflammatory response |
| Q9N2H7_PIG | poly-Ig receptor | PIGR | 3.92 | polymeric immunoglobulin receptor |
| down-regulated | ||||
| F1S514_PIG | COMM domain containing 7 | COMMD7 | 0.30 | negative regulation of NF-kappa B transcription factor activity |
| Q0MRZ8_PIG | MHC class I antigen | SLA | 0.41 | antigen processing; allograft rejection |
| C1QA_PIG | complement C1q subcomponent subunit A | C1QA | 0.46 | complement activation; innate immune response |
| H4_PIG | histone H4 | HIST4H4 | 0.47 | autoantigen component |
| F1RZ52_PIG | tripartite motif-containing protein 26 | TRIM26 | 0.47 | innate immune response; negative regulation of viral entry into host cell |
| Cell renewal | ||||
| up-regulated | ||||
| GILT_PIG | gamma-interferon-inducible-lysosomal thiol reductase | IFI30 | 5.61 | negative regulation of cell proliferation |
| F1SFI5_PIG | histidine-rich glycoprotein | HRG | 3.79 | negative regulation of cell growth and proliferation |
| Q9GJV4_PIG | four and a half LIM domains 1 protein isoform C | fhl1C | 3.66 | negative regulation of G1/S and G2/M transition of mitotic cell cycle |
| DPEP1_PIG | dipeptidase 1 | DPEP1 | 2.96 | negative regulation of apoptotic process and cell migration |
| F2Z5K4_PIG | ras homolog family member C | RHOC | 2.47 | negative regulation of programmed cell death |
| down-regulated | ||||
| F1RXX7_PIG | marker of proliferation Ki-67 | MKI67 | 0.34 | a marker of cellular proliferation |
| MEA1_PIG | male-enhanced antigen 1 | MEA1 | 0.45 | may play an important role in spermatogenesis and/or testis development |
| F1SGC0_PIG | FYVE, rhoGEF and PH domain containing 4 | FGD4 | 0.47 | involved in the actin cytoskeleton and cell shape |
| F1S436_PIG | fatty acid 2-hydroxylase | FA2H | 0.48 | regulation of cell proliferation |
| Regulation of DNA replication and gene expression | ||||
| up-regulated | ||||
| F1RM65_PIG | U2 small nuclear RNA auxiliary factor 1-like 4 | U2AF1L4 | 12.93 | pre-mRNA splicing factor |
| Q53DY7_PIG | histone H1.3-like protein | LOC595122 | 3.78 | nucleosome assembly |
| F2Z5E6_PIG | ribosomal protein S5 | RPS5 | 3.58 | RNA binding; regulation of translational fidelity |
| F1RIU9_PIG | coiled-coil-helix-coiled-coil-helix domain containing 2 | CHCHD2 | 3.17 | positive regulation of transcription from RNA polymerase II promoter |
| F1SA98_PIG | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, theta | YWHAQ | 3.06 | negative regulation of transcription |
| down-regulated | ||||
| F1SDG0_PIG | splicing factor 3b subunit 4 | SF3B4 | 0.46 | positive regulation of mRNA splicing |
| H4_PIG | histone H4 | HIST4H4 | 0.47 | nucleosome formation; chromatin organization |
| F1RZ52_PIG | tripartite motif-containing protein 26 | TRIM26 | 0.47 | positive regulation of sequence-specific DNA binding transcription factor activity |
| F1SPG1_PIG | histone H1x | H1FX | 0.49 | nucleosome assembly |
| ROCK2_PIG | rho-associated protein kinase 2 | ROCK2 | 0.50 | positive regulation of centrosome duplication |
FC, fold-change calculated by the abundance of a specific protein upon dietary protein limitation divided by it in control pigs.
Network of small intestinal physiological pathways upon PL
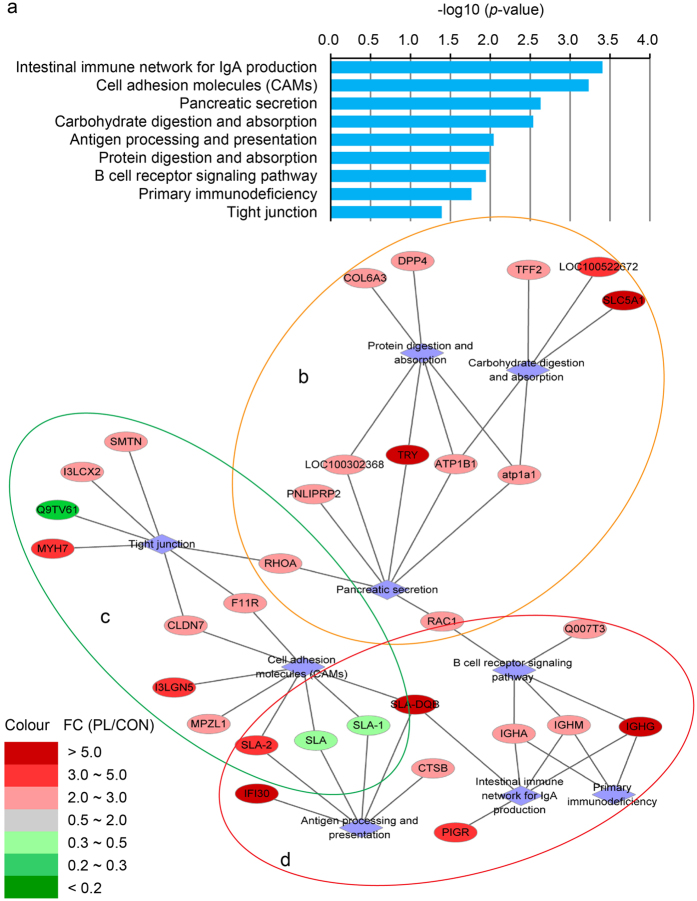
To obtain an overview on proteomic adaptation of the jejunal mucosa in response to PL, we functionally integrated pathways which are known to be typical intestinal physiology-related pathways via the DEP that are involved among nutrient digestion and absorption, intestinal mucosa epithelium structure and micro-milieu, and intestinal immunity (Fig. 3). We observed that ras homolog family member A (RHOA, UniProtKB accession no. I3LVS7_PIG) that acted as a hub to correlate pancreatic secretion with tight junction was significantly up-regulated upon PL. The up-regulation of MHC class I antigen 2 (SLA-2, UniProtKB accession no. V9PRE5_PIG) and MHC II class antigen (SLA-DQB, UniProtKB accession no. F8J302_PIG) accompanied by the down-regulation of SLA (UniProtKB accession no. Q0MRZ8_PIG) and SLA-1 (UniProtKB accession no. Q8SPB9_PIG) linked the cell adhesion molecules with the antigen processing and presentation in response to PL. Simultaneously, the pathway of pancreatic secretion was associated with B cell receptor signaling pathway through the up-regulated ras-related C3 botulinum toxin substrate 1 (RAC1, UniProtKB accession no. I3LFI0_PIG).
Figure 3. Cluster of KEGG pathways and DEP putatively related to intestinal structure and micro-milieu, protein/carbohydrate digestion and absorption, and immunity in the jejunal mucosa.
(a) KEGG pathways, the horizontal line represents -log10 (p-value) for KEGG pathways; DEP putatively related to (b) protein digestion and absorption, (c) intestinal structure integrity, and (d) immunity. (b–d) Networks were visualized by Cytoscape (3.2.0). DEP, differentially expressed proteins; FC, fold-change; PL, dietary protein limitation; CON, control group.
Protein digestion and amino acid transport
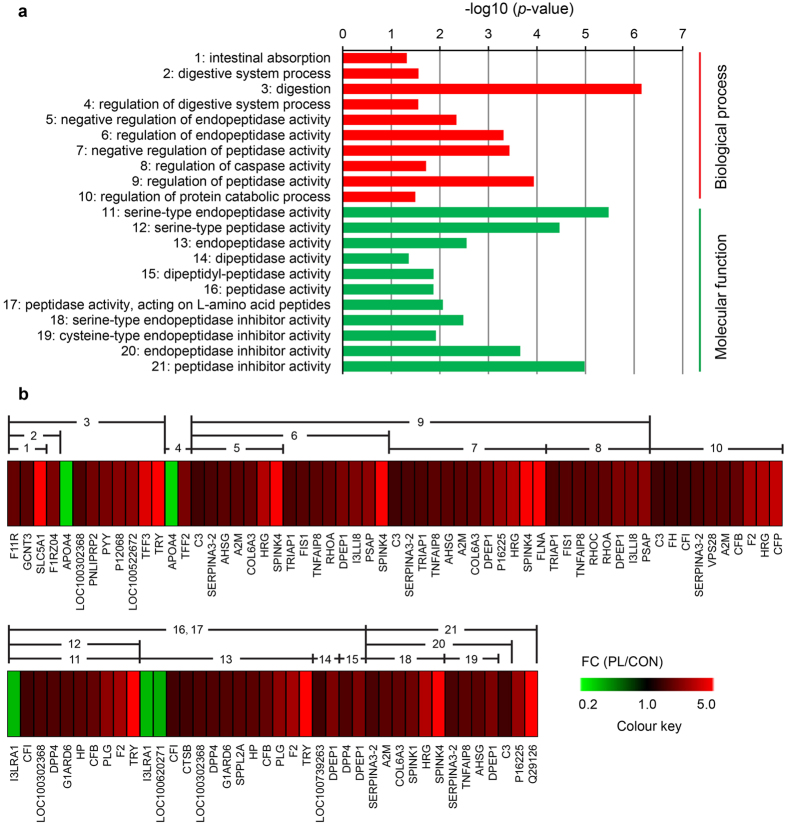
From the significantly enriched GO terms, we clustered 10 biological processes terms, 11 molecular functions terms with regard to protein digestion and transport (Fig. 4), including digestive system process (GO:0022600), regulation of peptidase activity (GO:0052547), peptidase activity (GO:0070011), peptidase inhibitor activity (GO:0030414), intestinal absorption (GO:0050892) and so on.
Figure 4. Cluster of GO annotations and DEP putatively related to protein digestion and absorption in the jejunal mucosa.
(a) The horizontal line represents -log10 (p-value) for GO terms. (b) DEP in each GO terms. Heat-maps were generated by MeV (4.9.0). GO, gene ontology; DEP, differentially expressed proteins; FC, fold-change; PL, dietary protein limitation; CON, control group.
In the current study, we observed several DEP that were involved in the regulation of intestinal proteolysis and peptide hydrolysis, as well as AA transmembrane transport. For instance, according to UniProtKB, complement factor B (CFB, UniProtKB accession no. F1RQW6_PIG) is an endopeptidase. Both CFB and putative trypsinogen (TRY, UniProtKB accession no. C6L245_PIG) exist in extracellular space and play vital roles in proteolysis. Up-regulation of TRY and CFB implies that proteolysis activity in the intestinal mucosa was increased under PL (Table 1; Supplementary Data). Likewise, the up-regulation of some extracellular peptidases such as dipeptidase 1 (DPEP1, UniProtKB accession no. DPEP1_PIG) implies increased peptide hydrolysis. Thus, we surmised that both proteolysis and peptide hydrolysis activities were increased in the small intestinal lumen in response to PL.
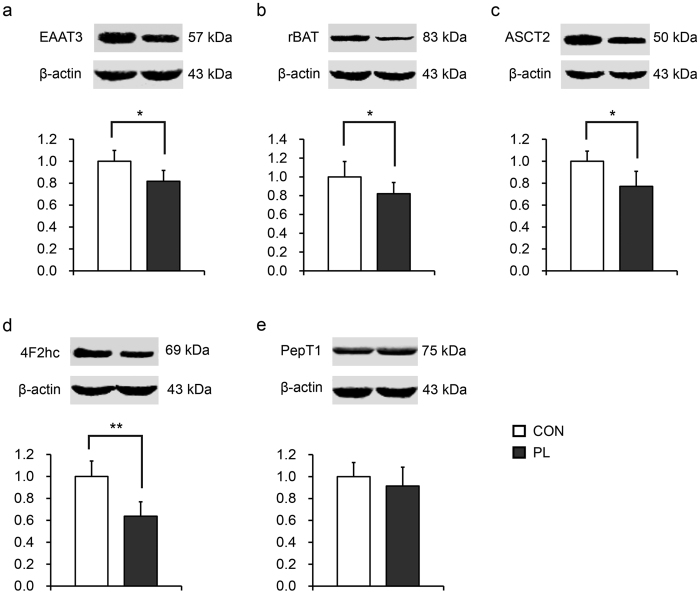
Results obtained from western blotting analysis indicated that the large neutral AA transporter 4F2 cell-surface antigen heavy chain (4F2hc, UniProtKB accession no. I3LB80_PIG) as well as other AA transporters including excitatory amino acid transporter 3 (EAAT3, UniProtKB accession no. C8CAX4_PIG, an anionic AA transporter), neutral amino acid transporter B(0) (ASCT2, UniProtKB accession no. F1RM08_PIG, a neutral AA transporter), and basic amino acid transport protein rBAT (rBAT, UniProtKB accession no. F1S5K2_PIG, a cationic AA transporter), were down-regulated under PL, whereas peptide transporter-1 (PepT1, UniProtKB accession no. B0ZSN9_PIG) remained unchanged between groups (Fig. 5).
Figure 5. Western blotting analysis of the expression of amino acid/peptide transporters in the jejunal mucosa.
*Significant differences between groups; *p < 0.05, **p < 0.01. n = 8. CON, control group; PL, dietary protein limitation.
Protein limitation inhibited phosphorylation of mTOR signalling pathway
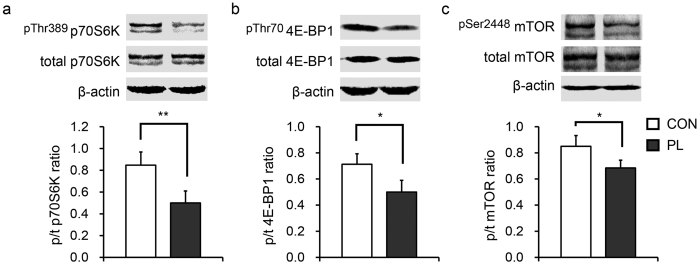
Western blotting analysis showed that PL inhibited the phosphorylation of mammalian target of rapamycin (mTOR, Ser2448), p70-S6 kinase (p70S6K, Thr389), and eukaryotic initiation factor 4E binding protein 1 (4E-BP1, Thr70) (Fig. 6).
Figure 6. Dietary protein limitation inhibits the activity of mTOR signalling pathway.
(a–c) Dietary protein limitation inhibits phosphorylation of p70S6K and 4E-BP1, as well as mTOR itself. *Significant differences between groups; *p < 0.05, **p < 0.01. n = 8. CON, control group; PL, dietary protein limitation.
Adaptations in intestinal mucosa immunity
We significantly enriched some important GO terms and KEGG pathways such as regulation of humoral immune response (GO:0002920), regulation of inflammatory response (GO:0050727), intestinal immune network for IgA production (ko04672), antigen processing and presentation (ko04612), as well as primary immunodeficiency (ko05340) (Fig. 3). Furthermore, we found that 18.3% of DEP were confirmed to be engaged in immunity, indicating that PL significantly leads to alterations in immune function. As shown in Table 1, SLA-DQB and gamma-interferon-inducible-lysosomal thiol reductase (IFI30, UniProtKB accession no. GILT_PIG) were up-regulated in pigs upon PL. SLA-DQB17 and IFI30 had been demonstrated to take part in antigen processing and presentation, moreover, IFI30 plays its role via MHC class I/II18. Similarly, IgG heavy chain (IGHG, UniProtKB accession no. L8AXK3_PIG) and poly-Ig receptor (PIGR, UniProtKB accession no. Q9N2H7_PIG) as well as complement factor properdin (CFP, UniProtKB accession no. K7GQR1_PIG) were also up-regulated upon PL. GO annotation showed that complement C1q subcomponent subunit A (C1QA, UniProtKB accession no. C1QA_PIG) and TRIM2619 are components of innate immune response that were found among the down-regulated DEP. Histone H4 (HIST4H4, UniProtKB accession no. H4_PIG) constitutes an autoantigen in a H3/4 heterodimer form in auto immunity20. NF-kappa B is well-known for its function in immune surveillance, whose inhibitor COMM domain containing 7 (COMMD7, UniProtKB accession no. F1S514_PIG)21 was decreased in our study, which suggests a benefit in immunity under PL. Therefore, for the first time, these results suggest that PL probably increases the immune response to foreign antigens. Moreover, it is likely that innate and auto immunity were depressed upon PL.
Cell proliferation and apoptosis
Considering that 12.4% of DEP found in the jejunal mucosa were involved in cell proliferation and apoptosis, we surmised that the adaptation of cell proliferation and apoptosis occurred in the small intestinal mucosa in respond to PL. We noted that marker of proliferation Ki-67 (MKI67, UniProtKB accession no. F1RXX7_PIG), a well-known endogenous marker of cell proliferation22, was down-regulated under PL. Moreover, fatty acid 2-hydroxylase (FA2H, UniProtKB accession no. F1S436_PIG) which promotes cell proliferation was also down-regulated. In addition, IFI30, which negatively regulates cell proliferation and four and a half LIM domains 1 protein isoform C (fhl1C, UniProtKB accession no. Q9GJV4_PIG), which inhibits the mitotic cell cycle, were both up-regulated under PL. These results illustrated that cell proliferation of the small intestine was retarded.
Several DEP involved in apoptosis were also observed, including DPEP1 and ras homolog family member C (RHOC, UniProtKB accession no. F2Z5K4_PIG). GO annotation reveals that DPEP1 is responsible for the negative regulation of apoptotic process and cell migration, meanwhile, RHOC prevents programmed cell death. Based on the result that DPEP1 and RHOC were up-regulated under PL in this study, we speculated that PL may prevent cell apoptosis.
Regulation of DNA replication and gene expression
17.3% of DEP were observed to take part in the regulation of DNA replication and gene expression. For instance, according to GO annotation, HIST4H4 regulates nucleosome formation, and rho-associated protein kinase 2 (ROCK2, UniProtKB accession no. ROCK2_PIG) positively regulates duplication of centrosome. Given that both HIST4H4 and ROCK2 were down-regulated under PL in this study (Table 1), it is likely that DNA replication was suppressed upon PL.
Both coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2, UniProtKB accession no. F1RIU9_PIG)23 and TRIM2619 are transcription activators, however, CHCHD2 was up-regulated whilst TRIM26 was down-regulated under PL. Additionally, tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein theta (YWHAQ, UniProtKB accession no. F1SA98_PIG), a transcriptional inhibitor24, was up-regulated under PL. U2 small nuclear RNA auxiliary factor 1-like 4 (U2AF1L4, UniProtKB accession no. F1RM65_PIG), a pre-mRNA splicing promoter, was up-regulated while splicing factor 3b subunit 4 (SF3B4, UniProtKB accession no. F1SDG0_PIG), a positive regulator of mRNA splicing was down-regulated (Table 1) upon PL. The complicated alterations of DEP related with transcription and RNA splicing showed that PL exerts a sophisticated role in regulation of transcription. Particularly, ribosomal protein S5 (RPS5, UniProtKB accession no. F2Z5E6_PIG), which plays an important role in maintaining the accuracy of translation in eukaryotes25, was up-regulated, indicating translational fidelity was increased upon PL.
Discussion
Protein limitation not only contributes to human health in terms of longevity extension and insulin sensitivity enhancing3,26 but also reduces excessive nitrogen excretion in farm animal production5. However, previous studies demonstrated that PL impeded individual growth even though all essential amino acid (EAA) were balanced to fulfil requirements of pigs14,27, which may be due to NEAA deficiency28. Recent studies revealed that supplement of NEAA such as glutamic acid, glycine, and proline to protein-limited diets that contain adequate EAA can restore individual growth in pigs28,29. The influence of EAA deficiency on individual nutrient metabolism and physiology has been well elucidated. However, the impacts of NEAA deficiency on intestine physiology remain unknown, which may be the primary problem that clinical nutritionists have to face while PL is applied to improve human health and so on. In the present study, to avoid the interferences arisen from gender and sex hormones on the results, we chose healthy castrated male pigs with 44-day of age as the model animals, which were equivalent to humans during the early childhood. We successfully established a PL porcine model, in which pigs consumed significantly less protein compared to those offered the control diet. Considering the equivalent intake of EAA and net energy between the pigs offered the control and LP diet, we believe that any change observed in the current study was due to PL, that is, dietary NEAA limitation.
The small intestine mucosa provides a dynamic and interactive surface between lumen and the internal milieu of the organism. Therefore, it would embody meaningful sense while proteomic profile of the intestinal mucosa has been altered by PL30.
In the current study, a total of 5275 quantitative proteins were identified in the jejunal mucosa, among which, 202 proteins with |log2 fold-change| > 1 were identified as DEP from pigs under PL. The data of western blotting analysis validated the high reliability of our proteomic analysis. We found that PL remarkably altered the expression of proteins that are involved in a few key physiological functions of the small intestine. For instance, PL influenced protein/carbohydrate digestion and AA transport, intestinal mucosal immune, and cell proliferation and apoptosis, as well as regulation of DNA replication and gene expression.
Under PL, our data suggest that proteolysis (TRY and CFB) and peptide hydrolysis (DPEP1) activities increased in the gut lumen, which demonstrated that protein digestion was enhanced. Furthermore, we also check the protein expression of AA/peptide transporters which reflects the activities of protein absorption. Transporter 4F2hc, located in the basolateral membrane, is a high-affinity bidirectional transporter of large neutral AA such as phenylalanine, tyrosine, leucine, arginine and tryptophan after binding to the light chain AA transporters LAT1/212,31. In the present study, the protein expression of 4F2hc as well as other AA transporters including EAAT3, ASCT2, and rBAT, was down-regulated upon PL, which was consistent with previous study14, suggesting that PL reduced AA transport from the intestinal lumen into enterocytes through the brush-border membrane and further into blood. It has been documented that a protein-limited diet reduces plasma AA concentration32. Therefore, we deduce that increased protein digestion could not remedy the reduction of AA absorption upon PL.
So far, the exact mechanism through which ingested dietary protein mediates small intestinal AA transport remains unclear. Two evolutionarily conserved AA sensing-signalling pathways in mammalian cells are the mTOR and general control non-derepressible (GCN) pathways12. mTOR complex 1 (mTORC1) is a central cell growth controller that integrates a wide array of extracellular and intracellular signals to regulate cell growth33,34. mTORC1 mediates transcription and protein translation, as well as cell growth and proliferation in response to nutrient and growth factor availability, and it does this through the phosphorylation of the key translation regulators p70S6K and 4E-BP133,35. The GCN pathway primarily senses intracellular AA availability and is activated when one or more AA are scarce12,36. However, it has been shown in mice that, unlike the mTOR signalling pathway, GCN2 was dispensable for PL-induced effects3. It has been demonstrated that mTOR play a central role in mediating AA sensing, AA transport, cell growth and proliferation, apoptosis and autophagy, as well as immunity37. Hence we examined the activity of mTOR signalling pathway to understand its role in proteomic adaptation of the intestinal mucosa upon PL. In the current study, our findings indicated that PL inhibits the activity of mTOR signalling pathway in the jejunal mucosa, which was supported by previous studies in rat placenta38, mice liver3, as well as in porcine liver39.
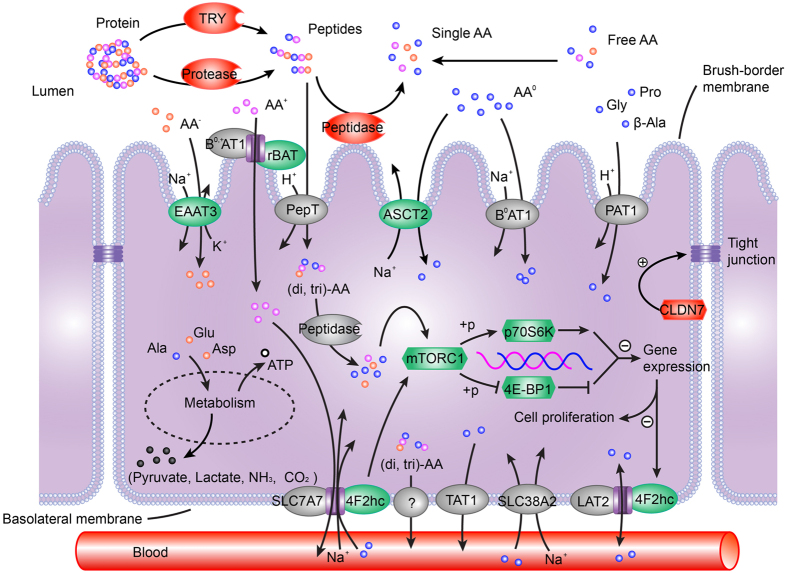
Certain AA transporters, 4F2hc and SLC38A2 for instance, may have dual receptor-transporter functions not only act as AA carriers but also as AA sensors12,34. Thus these AA transporters act directly as the initiating sensors for intracellular AA and mTOR signalling pathway activation40. Not only large neutral AA including EAA such as leucine but also certain NEAA such as glutamine are key stimulants of mTOR signalling pathway activation12,41. Therefore, based on the results of our study, we speculate that PL reduced AA transport and further resulted in extracellular NEAA deficiency, which was sensed by their corresponding AA transporters. This sensing decreased expression of these AA transporters themselves, and thus led to the lack of intracellular NEAA, which subsequently suppressed the mTOR signalling pathway. Finally, the suppressed mTOR signalling pathway would in turn influence a series of biological functions, including the reduction of AA transporter expressions, gene expression as well as intestinal epithelium proliferation and cell growth. A model for mediation of protein digestion and absorption and the activity of mTOR signalling pathway induced by PL is presented in Fig. 7.
Figure 7. Putative model of mediation of intestinal protein digestion and amino acid transport and mTOR signalling pathway upon protein limitation.
Red nodes represent up-regulated proteins, green nodes represent down-regulated proteins upon protein limitation, and grey nodes represent unchanged or undetected proteins in the present study. This model was visualized by Adobe Illustrator (CS5).
Based on the enriched functions involved in cell proliferation and apoptosis, we speculate that PL might inhibit cell proliferation and apoptosis, which had been observed in other cells upon protein restriction3,42. Moreover, our speculation on cell proliferation can also be underpinned by the inhibited mTOR signalling pathway upon PL in the present study. In addition, CLDN7 plays an important role in controlling intestinal epithelial tight junctions and epithelium differentiation, and its expression increases as epithelial cells differentiate along the intestinal crypte-luminal axis43. The tight junction is relevant to extracellular signal perception and the intestinal structure integrity44. We observed that CLDN7 expression was increased in the jejunal mucosa, suggesting that tight junctions were enhanced and epithelium differentiation was promoted upon PL.
To the best of our knowledge, very few studies reveal that proteomic alteration correlated with the regulation of DNA replication and RNA transcription in the intestinal mucosa induced by PL. It had been reported that consumption of high-fat diet elevates mouse small intestine mucosa proteins that are involved in transcription, RNA processing, and in the synthesis, modification, and processing of protein30. Interestingly, we found that a few DEP have been implicated in DNA replication, transcription and RNA splicing as well as translation fidelity upon PL, even the alterations of DEP that mediate transcription and RNA splicing were complicated, implying that PL has a meaningful but complex effect on intestinal gene expression pattern.
Overall, by employing a porcine model, this study provides the first evidence illustrating the proteomic alteration of the small intestine mucosa in response to PL, suggesting PL exerts profound impacts on small intestine physiology. Our findings indicate that PL increases protein digestion but depresses AA transport. Intestinal innate and auto immune, as well as cell proliferation and apoptosis were mitigated upon PL while the immune response to foreign antigens is enhanced. Our study threw new light on how PL influences intestinal physiological functions, in particular AA transport, intestinal mucosa structure and micro-milieu, as well as intestinal immunity. Thereinto, the mTOR signalling pathway seems to play a central role in mediating intestinal physiological functions upon PL through sensing AA supply.
Methods
Feeding trial and tissue harvest
The animal experiment was carried out in accordance with the Chinese Guidelines for Animal Welfare and Experimental Protocol, and was approved by the Animal Care and Use Committee of Sichuan Agricultural University. In this study, 16 44-day old of Duroc × Landrace × Yorkshire crossbred castrated male pigs with an initial body weight of 13.5 ± 0.5 kg were selected from a commercial herd. The pigs were kept individually in the same nursery house for a 3-d adaptation period followed by a 28-d trial period.
Castrated male pigs were randomly assigned by initial body weight to alternative dietary treatments (PL vs. CON; n = 8). Dietary crude protein (nitrogen content × 6.25) content was 18% in CON according to the nutrient recommendation45; whilst crude protein content in PL was 14%. Pigs were allowed ad libitum access to feed and water. Ingredient composition and nutrient content of the experimental diets are presented in Supplementary file Table S1. Experimental diets were balanced in standardized ileal digestible EAA, net energy, fibre, and electrolytes to meet NRC (2012) nutrient recommendations45.
Feed intake and body weight gain were measured individually, while net energy and crude protein intake and the feed utilization index were calculated based on measured data. Net energy or crude protein intake is the dietary net energy or crude protein content multiplied by feed intake, and the feed utilization index was defined as feed intake per 100 g of daily body weight gain. At the end of the feeding trial, castrated male pigs were weighed after 12 h-fasting and then sacrificed by exsanguination post-anaesthesia. Mucosae from the middle jejunal segment were gently scraped. For sampling consistently, an experienced technician harvested the jejunal mucosae throughout the sampling. And then, the mucosal scraps were snap-frozen in liquid nitrogen and stored at −80 °C for further analysis.
Animal performance data (n = 8) were analysed by one-way analysis of variance with each animal as an experimental unit using SAS software (v9.1). Data were expressed as the means and standard error of mean, p < 0.05 was considered statistically significant.
Protein extraction and 8-plex iTRAQ labelling
Individual mucosal scrapings were ground in liquid nitrogen and then lysed in a solution containing 200 μl of L3 buffer (50 mM Tris-Cl, pH 8, 8 M urea, 2 M thiourea, 2 M EDTA, 1× protease inhibitors cocktails), 800 μl of ice-cold acetone, and 10 mM dithiothreitol. The suspensions were incubated at −20 °C for 2 h. After centrifugation at 13,000× g for 20 min at 4 °C, the precipitated pellets were resuspended in 800 μl of ice-cold acetone and 10 mM dithiothreitol. The suspensions were further centrifuged at 13,000× g for 20 min at 4 °C to collect the precipitated pellets, then vacuum dried. The dried precipitated pellets were dissolved in 200 μl of L3 buffer. Subsequently, total protein concentration was determined using the Bradford assay.
Equiponderant proteins from each animal were pooled based on groups, and then, each pooled sample was divided into three equal parts (named CON1~3 and PL1~3, respectively) as technical triplicates. Subsequently, an aliquot of 100 μg of protein from each fraction was reduced, alkylated, and digested with trypsin according to the manufacturer’s protocol (Applied Biosystems, Framingham, MA, USA). Samples were iTRAQ labelled as following: CON1, 113; CON2, 114; CON3, 115; PL1, 116; PL2, 117; PL3, 118. The labelled peptides were then mixed and vacuum dried.
2D-RPLC fractionation and MS/MS analysis
The LC-MS/MS (Triple TOF 5600 plus, AB SCIEX, Framingham, USA) analysis was performed with the modification as described by Ding et al.46. Briefly, the iTRAQ labelled peptide mixture was reconstituted in reverse phase liquid chromatography (RPLC) mobile phases buffer A (20 mM ammonium formate in 2% acetonitrile, pH 10.0) and subsequently fractionated using a high pH C18 high-performance liquid chromatography system (Dinoex Ultimate 3000 BioRS, Thermo Fisher, Waltham, MA, USA) equipped with Durashell-C18 reverse phase column (5 μm particle size, 100 Å pore size, 4.6-mm diameter × 250-mm length). The fractionation was performed for 50 min at a flow rate of 1 ml/min. Eluted fractions were monitored by measuring the absorbance at a wavelength of 214 nm. Each fraction was desalted with a Strata X C18 column (Phenomenex, Torrance, CA, USA). Samples were vacuum dried and then reconstituted in 15 μl of buffer (0.1% formic acid, 2% acetonitrile in Milli-Q water). Fractions from the above high pH C18 RPLC were further separated by a 90 min linear gradient of 5% to 76.4% acetonitrile in 0.1% formic acid at a flow rate of 350 nl/min. The MS scan range was 350–1250 m/z for 0.25 s scanning. Subsequently, MS/MS scans were performed for 0.04 s by applying a spray voltage of 2300 V. The dynamic exclusion for MS/MS was set at 12 s. The MIAPE reporting guidelines are followed throughout this work47.
Protein identification and quantification
Protein identification and quantification for iTRAQ experiments were performed using ProteinPilot (v4.0.8085) software (Supplementary file Table S2). The LC-MS/MS data were searched against the UniProtKB (sus scrofa). To minimize the false discovery rate, a threshold for protein identification was applied, with the confident value ≥95% (amount to the confident value “unused ProtScore” ≥1.3 in ProteinPilot software), and at least one unique peptide was considered for protein identification. Proteins that were quantified with |log2 fold-change| > 1 were considered to be DEP. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository48 with the dataset identifier PXD004069.
GO and KEGG enrichment analysis
We matched our proteomic data to GO and KEGG enrichments by matching Blast2GO software or KEGG database to the NCBI database. Fisher Exact test was used to identify the significantly enriched GO terms or KEGG pathways in the DEP compared to the proteomic background. The formula was the following:
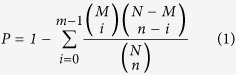 |
where, N was the number of all identified proteins with GO or KEGG annotation; n was the number of DEP in N; M was the number of all identified proteins that were annotated to the specific GO term or KEGG pathway; m was the number of DEP in M. The p < 0.05 were considered as the threshold for significant GO or KEGG enrichment.
Western blotting
The relative abundances of proteins selected for verification (SGLT1, CLDN7, GPR108, SLC7A7, RNF40, TRIM26), AA transporters (EAAT3, rBAT, ASCT2, 4F2hc), PepT1, as well as mTOR signalling pathway members (mTOR, p-mTOR, p70S6K, p-p70S6K, 4E-BP1, p-4E-BP1) were determined by western blotting analysis using the protein samples from all individual animals (n = 8). Beta-actin was run as a loading control. Equal amounts of samples (80 μg protein per lane), together with a pre-stained protein ladder (Thermo Fisher, Rockford, IL, USA), were subjected to SDS-PAGE electrophoresis, subsequently, proteins were electrotransfered onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) and then blocked in 5% (w/v) bovine serum albumin at room temperature for 1 h. The membranes were then incubated against corresponding primary antibodies (Supplementary file Table S3) at room temperature for 1 h and then at 4 °C overnight. After three washes, the membranes were incubated with DyLight 800-labeled secondary antibodies (KPL, Gaithersburg, MD, USA). Finally, band intensities were detected with the Odyssey Clx (4647 Superior Street, LI-COR Biotechnology, Lincoln, NE, USA) and quantified using AlphaImager software (2200), then analysed by Mann-Whitney U test using SPSS software (v20). The correlation between the western blotting and iTRAQ data were assessed using PROC CORR procedure of SAS (9.1).
Additional Information
How to cite this article: Qin, C. et al. A proteomic adaptation of small intestinal mucosa in response to dietary protein limitation. Sci. Rep. 6, 36888; doi: 10.1038/srep36888 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was financially supported by the National Key Basic Research Program of China (2013CB127302, 2012CB124702) and 111 Project (B16044).
Footnotes
Author Contributions J.Y. obtained financial support and conceived and oversaw this study. J.Y. and L.C. designed the animal experiment. C.Q., K.Q., W.S. and L.C. performed the animal experiment and generated the tissue samples. C.Q., N.J., X.Z. and H.S. prepared testing sample and work with the analysis of proteomic and western blotting analysis. C.Q. and H.Z. worked with the bioinformatics analysis. J.Y. and C.Q. wrote the manuscript with the contribution from the other authors. All authors read and approved the final manuscript.
References
- Gallinetti J., Harputlugil E. & Mitchell J. R. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 449, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J. H., Ming W. K., Lin W. A. & Tarn Y. H. Early supplemented low-protein diet restriction for chronic kidney disease patients in Taiwan - A cost-effectiveness analysis. Clin Nephrol. 84, 189–196 (2015). [DOI] [PubMed] [Google Scholar]
- Harputlugil E. et al. The TSC complex is required for the benefits of dietary protein restriction on stress resistance in vivo. Cell Rep. 8, 1160–1170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L. T. et al. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J. Nutr. 145, 1717–1727 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C. et al. Influences of dietary protein sources and crude protein levels on intracellular free amino acid profile in the longissimus dorsi muscle of finishing gilts. J Anim Sci Biotechno. 6, 52 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in nutrition and health. Amino Acids 45, 407–411 (2013). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs. J. Nutr. 137, 1442–1446 (2007). [DOI] [PubMed] [Google Scholar]
- Wang W. et al. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 144, 1540–1548 (2014). [DOI] [PubMed] [Google Scholar]
- Sanderson I. & Naik S. Dietary regulation of intestinal gene expression. Annu Rev Nutr. 20, 311–338 (2000). [DOI] [PubMed] [Google Scholar]
- Ecknauer R., Sircar B. & Johnson L. R. Effect of dietary bulk on small intestinal morphology and cell renewal in the rat. Gastroenterology 81, 781–786 (1981). [PubMed] [Google Scholar]
- Wu G. Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J. Anim Sci Biotechno. 5, 1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M. Role of amino acid transporters in amino acid sensing. Am J Clin Nutr. 99, 223S–230S (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A. et al. Low-protein amino acid-supplemented diets for growing pigs: effect on expression of amino acid transporters, serum concentration, performance, and carcass composition. J. Anim Sci. 93, 2154–2164 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang S. et al. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45, 1191–1205 (2013). [DOI] [PubMed] [Google Scholar]
- Miller E. & Ullrey D. The pig as a model for human nutrition. Annu Rev Nutr. 7, 361–382 (1987). [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Zabielski R., Hammon H. M. & Metges C. C. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev. 23, 4–22 (2010). [DOI] [PubMed] [Google Scholar]
- Lunney J. K., Ho C.-S., Wysocki M. & Smith D. M. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Dev Comp Immunol. 33, 362–374 (2009). [DOI] [PubMed] [Google Scholar]
- Goldstein O. G. et al. Gamma-IFN-inducible-lysosomal thiol reductase modulates acidic proteases and HLA class II antigen processing in melanoma. Cancer Immunol Immun. 57, 1461–1470 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil P. D. et al. TRIM protein-mediated regulation of inflammatory and innate immune signaling and its association with antiretroviral activity. J. Virol. 87, 257–272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir A. R. & Moinuddin. Glycoxidation of histone proteins in autoimmune disorders. Clinica Chimica Acta. 450, 25–30 (2015). [DOI] [PubMed] [Google Scholar]
- Burstein E. et al. COMMD proteins, a novel family of structural and functional homologs of MURR1. J. Biol Chem. 280, 22222–22232 (2005). [DOI] [PubMed] [Google Scholar]
- Urruticoechea A., Smith I. E. & Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 23, 7212–7220 (2005). [DOI] [PubMed] [Google Scholar]
- Aras S. et al. Oxygen-dependent expression of cytochrome c oxidase subunit 4-2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2. Nucleic Acids Res, gks1454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla G., Howald C., Antonarakis S. E. & Reymond A. The subcellular localization of the ChoRE-binding protein, encoded by the Williams-Beuren syndrome critical region gene 14, is regulated by 14-3-3. Hum Mol Genet. 13, 1505–1514 (2004). [DOI] [PubMed] [Google Scholar]
- Tsuboi M. et al. EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. Mol Cell 35, 502–510 (2009). [DOI] [PubMed] [Google Scholar]
- Xiao F. et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 60, 746–756 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R., Lewis A., Miller P. & Chen H. Growth performance, diet apparent digestibility, and plasma metabolite concentrations of barrows fed corn-soybean meal diets or low-protein, amino acid-supplemented diets at different feeding level. J. Anim Sci. 80, 644–653 (2002). [DOI] [PubMed] [Google Scholar]
- Gloaguen M., Floc’h L., Corrent E., Primot Y. & van Milgen J. The use of free amino acids allows formulating very low crude protein diets for piglets. J. Anim Sci. 92, 637–644 (2014). [DOI] [PubMed] [Google Scholar]
- Powell S., Bidner T., Payne R. & Southern L. Growth performance of 20-to 50-kilogram pigs fed low-crude-protein diets supplemented with histidine, cystine, glycine, glutamic acid, or arginine. J. Anim Sci. 89, 3643–3650 (2011). [DOI] [PubMed] [Google Scholar]
- Wisniewski J. R., Friedrich A., Keller T., Mann M. & Koepsell H. The impact of high-fat diet on metabolism and immune defense in small intestine mucosa. J. Proteome Res. 14, 353–365 (2015). [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 88, 249–286 (2008). [DOI] [PubMed] [Google Scholar]
- Figueroa J. L., Lewis A. J., Miller P. S., Fischer R. L. & Diedrichsen R. M. Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J. Anim Sci. 81, 1529–1537 (2003). [DOI] [PubMed] [Google Scholar]
- Fingar D. C. & Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23, 3151–3171 (2004). [DOI] [PubMed] [Google Scholar]
- Kim J. & Guan K. L. Amino acid signaling in TOR activation. Annu Rev Biochem. 80, 1001–1032 (2011). [DOI] [PubMed] [Google Scholar]
- Dowling R. J. et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science 328, 1172–1176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan G., Eylul H. & James R. M. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 449, 1–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. M. & Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Bio. 10, 307–318 (2009). [DOI] [PubMed] [Google Scholar]
- Rosario F. J. et al. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 152, 1119–1129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D. et al. Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem. 20, 544–552 (2009). [DOI] [PubMed] [Google Scholar]
- Broer S. & Palacin M. The role of amino acid transporters in inherited and acquired diseases. Biochem J. 436, 193–211 (2011). [DOI] [PubMed] [Google Scholar]
- Jewell J. L. et al. Differential regulation of mTORC1 by leucine and glutamine. Science 347, 194–198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Godoy M. A. et al. Effects of protein restriction during gestation and lactation on cell proliferation in the hippocampus and subventricular zone: Functional implications. Protein restriction alters hippocampal/SVZ cell proliferation. Brain Res. 1496, 10–27 (2013). [DOI] [PubMed] [Google Scholar]
- Farkas A. E. et al. HNF4α regulates claudin-7 protein expression during intestinal epithelial differentiation. Am J Pathol. 185, 2206–2218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K. & Balda M. S. Signalling to and from tight junctions. Nat Rev Mol Cell Bio. 4, 225–237 (2003). [DOI] [PubMed] [Google Scholar]
- NRC. Nutrient requirements of swine. 11 edn, (Natl Acad Press, 2012). [Google Scholar]
- Ding C. et al. A fast workflow for identification and quantification of proteomes. Mol Cell Proteomics. 12, 2370–2380 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. F. et al. The minimum information about a proteomics experiment (MIAPE). Nat Biotechnol. 25, 887–893 (2007). [DOI] [PubMed] [Google Scholar]
- Vizcaino J. A. et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–456 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.