Abstract
Aims
The prevalence of left ventricular diastolic dysfunction increases with age, particularly in hypertensive women. We aimed to determine the age‐ and sex‐related differences in diastolic function, and its relation to alterations of cardiac dimensions in a hypertensive population.
Methods and results
We enrolled 479 hypertensive patients with a left ventricular ejection fraction (LVEF) ≥50% (men/women, 267/212) and their echocardiographic parameters regarding LV performance and vascular function were measured. Left atrial volume index (LAVI) and operant diastolic elastance (EdI: E/e′/stroke volume index), but not LV mass index (LVMI), correlated weakly with age in both sexes. The arterial elastance index (EaI) and EdI did not differ significantly between sexes in any of the three age groups (A, <65 years; B, ≥65 years but <75 years; C, age ≥75 years). The EdI indexed to EaI, EdI/EaI = E/e′/(0.9 × systolic blood pressure), was significantly more impaired in women than in men only in group C. There were significant differences in LAVI, LVMI, and EdI/EaI between groups B and C only in women.
Conclusions
Impairment of diastolic function relative to arterial elasticity, EdI/EaI, occurred in elderly hypertensive women and was coincident with the alteration of cardiac dimensions. The coincidence with the changes in diastolic function and the alterations of cardiac dimensions occurred in a different time point between the sexes.
Keywords: Ageing, Cardiac dimension, Diastolic function, Echocardiography, Women
Introduction
Heart failure with a preserved ejection fraction (HFpEF) is an important clinical condition, observed mainly in elderly women. Diastolic dysfunction alone is not pathognomonic of HFpEF, which involves multiple cardiovascular abnormalities.1 In patients with HFpEF, structural or functional alterations contribute to the transition to, and progression of overt heart failure, thus acting as prognostic indicators.2, 3 Patients with HFpEF present a particularly high frequency of concentric left ventricular hypertrophy (LVH), left atrial remodelling, and epicardial volume enlargement. Regression modelling has identified the changes in left heart size, such as greater LVH and atrial dilation, as the parameters that best distinguish HFpEF from hypertensive LVH without heart failure.4 These parameters may be useful in the identification of patients at increased risk of developing incidental heart failure at a preclinical level. However, the difference in diastolic function relative to vascular function between elderly men and women, and its relation to the alteration of cardiac dimensions in hypertensive patients before the onset of heart failure, remain unclear. Accordingly, we aimed to determine the age‐ and sex‐related differences in diastolic function relative to arterial elasticity, a new marker for the diastolic function assessment, amongst a hypertensive population with a preserved left ventricular ejection fraction (LVEF) and no history of heart failure. Furthermore, we tested the hypothesis that impaired diastolic function is coincident with altered cardiac dimension, and that the coincidence occurs in a different time point between the sexes.
Methods
The study population consisted of hypertensive patients referred for clinical assessment in the echo lab of our hospital. The inclusion criteria for this study were as follows: LVEF ≥50% without a history of heart failure and regional abnormality of LV wall motion; normal sinus rhythm at the time of enrollment; an echocardiogram of adequate quality; accurate baseline data on medical history, medications, and biochemical markers; and measurement of blood pressure and heart rate at the time of cardiac echocardiographic examination. Blood pressure was measured by a sphygmomanometer. Patients using antihypertensive drugs or with a clinical history of hypertension were classified as hypertensive. Those with hemodynamically significant valve disease (more‐than‐moderate regurgitation or more‐than‐mild stenosis), history of myocardial infarction, asymmetric hypertrophy of the LV wall (septal/posterior wall thickness ratio ≥1.5), and renal failure [estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2] were excluded. Of 745 consecutive patients examined, 479 patients [267 men, 212 women; mean age, 70 years (range 35–96 years)] were included in the final sample.
The entire investigation conforms with the principles outlined in the Declaration of Helsinki. The study protocol was approved by the institutional review board at our hospital.
Echocardiographic study
All echocardiograms were taken by registered diagnostic cardiac sonographers using the same echocardiographic instrument (Aplio XG, Toshiba, Tokyo, Japan) according to a standardized protocol. All parameters were measured in duplicate and averaged. All echocardiographic measurements were interpreted by the attending physician (T. W.).
End‐systolic pressure (Pes) was calculated as systolic pressure × 0.90.5, 6 Stroke volume (SV), calculated from the LV outflow tract diameter and pulsed‐wave Doppler measurement of the blood flow through the tract,7 was indexed to body surface area (BSA) (stroke volume index, SVI = SV/BSA). Effective arterial elastance (Ea) was calculated as Pes/SV and was indexed to the BSA to obtain the effective arterial elastance index (EaI).5 Ea correlates very well with measures of arterial load derived from aortic input impedance data.5 Systemic vascular resistance index (SVRI) was calculated as mean blood pressure/cardiac index × 80.
Measurement of LVEF was performed by the method reported previously.8 Fractional shortening (FS, %) was calculated as [(LVEDD − LVESD) / LVEDD], where LVEDD is the LV end‐diastolic dimension and LVESD is the end‐systolic LV dimension. End‐systolic wall stress (σes, g/cm2) was estimated as (Pes × LVESD × 1.35)/[4 × hes × (1 + hes/LVESD)], where hes is the average of the end‐systolic thicknesses of the septal and posterior walls.9 Instead of end‐systolic elastance, the ratio of FS to σes (stress‐corrected FS) was used as a load‐independent measure of systolic performance.
LV end‐diastolic volume (LVEDV) was calculated by the previously reported method7 and was indexed to the BSA to determine the LVEDV index (LVEDVI). Left atrial volume was measured using the prolate ellipse method and was indexed to the BSA to obtain the left atrial volume index (LAVI). LV mass was measured by the method reported previously8 and was indexed to the BSA to obtain the LV mass index (LVMI). Relative wall thickness was calculated as (2 × posterior wall thickness) / LVEDD. Pulsed‐wave Doppler examination of mitral inflow and Doppler tissue imaging of the mitral annulus were performed in each patient.10, 11 To provide a continuous variable that would reflect diastolic elastance (Ed), E/e′ was examined, where E is the early diastolic mitral inflow velocity and e′ is the early diastolic mitral annular velocity.12, 13 We measured e′ averaged from septal and lateral part of the mitral annulus. Operant Ed was then calculated as E/e′ divided by SV, assuming the absence of significant aortic regurgitation, and was indexed to the BSA to obtain the diastolic elastance index (EdI = E/e′/SVI).
To clarify the age‐ and sex‐related differences in all parameters, our subjects were divided into three age groups (A, age <65 years, n = 133; B, age ≥65 to <75 years, n = 170; C, age ≥75 years, n = 176) in both men and women.
Statistical analysis
Data are reported as mean ± SD. P values are two‐sided, and P < 0.05 was considered statistically significant. Correlations were tested using the Pearson or Spearman coefficient. Between‐sex differences in all participants or each age‐based group were assessed by unpaired t‐test, Wilcoxon rank‐sum test, or χ2 test as appropriate. Differences amongst all three age groups in each sex were assessed by one‐way analysis of variance or Kruskal–Wallis analysis, and differences between pairs of age groups were assessed by post hoc Bonferroni test. We also performed multivariate regression analysis to identify factors associated with diastolic function indexed to arterial elastance by using significant parameters in univariate analysis. All statistical analyses were performed using StatFlex for Windows Version 6 (Artech Co. Ltd., Osaka, Japan).
Results
Demographics and comorbidities
The baseline characteristics of the study participants are provided in Table 1. The haemoglobin level, but not eGFR, was significantly lower in women than in men. The difference in haemoglobin level was also observed for each age group (Table 1). The eGFR and haemoglobin levels decreased with age in both men (r = −0.349, P < 0.001, and r = −0.469, P < 0.001, respectively) and women (r = −0.418, P < 0.001, and r = −0.214, P = 0.002, respectively). No significant differences in any hypertensive medications were observed between men and women in any age group.
Table 1.
Clinical characteristics, laboratory data, comorbidities, and medications in all participants and the three age‐based groups
| All | Group A: <65 years | Group B: 65 years < age < 75 years | Group C: >75 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Men (n = 267) |
Women (n = 212) |
P value |
Men (n = 85) |
Women (n = 48) |
P value | Men (n = 89) |
Women (n = 81) |
P value |
Men (n = 93) |
Women (n = 83) |
P value | |
| Age, years | 69.4 ± 10.1 | 70.9 ± 10.0 | 0.099 | 57.8 ± 6.4 | 57.1 ± 7.2 | 0.554 | 69.6 ± 2.7 | 69.6 ± 3.0 | 0.956 | 79.8 ± 4.1 | 80.2 ± 4.3 | 0.472 |
| Body mass index, kg/m2 | 23.3 ± 3.5 | 23.5 ± 3.7 | 0.435 | 24.7 ± 2.9 | 24.8 ± 4.2 | 0.917 | 22.7 ± 4.0 | 23.4 ± 3.5 | 0.223 | 22.5 ± 3.0 | 22.9 ± 3.4 | 0.387 |
| eGFR, mL/min/1.73 m2 | 64.7 ± 19.8 | 66.1 ± 21.0 | 0.447 | 73.6 ± 19.6 | 81.0 ± 22.9 | 0.051 | 64.9 ± 19.8 | 66.2 ± 16.2 | 0.627 | 56.3 ± 16.3 | 57.3 ± 19.3 | 0.704 |
| Haemoglobin, g/dL | 13.7 ± 1.8 | 12.6 ± 1.5 | <0.001 | 14.8 ± 1.3 | 12.7 ± 1.7 | <0.001 | 13.7 ± 1.7 | 13.0 ± 1.1 | 0.003 | 12.8 ± 1.8 | 12.1 ± 1.5 | 0.003 |
| Comorbidity, n (%) | ||||||||||||
| Diabetes mellitus | 60 (22) | 43 (20) | 0.320 | 20 (24) | 13 (27) | 0.403 | 21 (24) | 15 (19) | 0.267 | 19 (20) | 15 (18) | 0.419 |
| Hypercholesterolemia | 104 (39) | 99 (47) | 0.054 | 39 (46) | 20 (42) | 0.387 | 32 (36) | 40 (49) | 0.053 | 33 (35) | 39 (47) | 0.081 |
| Medication, n (%) | ||||||||||||
| ACEI/ARB | 164 (61) | 119 (56) | 0.141 | 51 (60) | 32 (67) | 0.282 | 52 (58) | 42 (52) | 0.239 | 61 (65) | 45 (54) | 0.083 |
| BB | 57 (21) | 33 (16) | 0.068 | 21 (25) | 9 (19) | 0.283 | 21 (24) | 12 (15) | 0.105 | 15 (16) | 12 (14) | 0.461 |
| CCB | 153 (57) | 131 (62) | 0.184 | 46 (54) | 26 (54) | 0.570 | 49 (55) | 50 (62) | 0.234 | 58 (62) | 55 (66) | 0.351 |
| Diuretics | 33 (12) | 28 (13) | 0.445 | 8 (9) | 9 (19) | 0.101 | 13 (15) | 5 (6) | 0.062 | 12 (13) | 14 (17) | 0.299 |
ACEI, angiotensin‐converting enzyme inhibitor;
ARB, angiotensin type 1 receptor, blocker;
BB, beta blocker;
CCB, calcium‐channel blocker;
eGFR, estimated glomerular filtration rate.
Age‐ and sex‐related alterations
No differences between sexes in diastolic or systolic blood pressure (SBP) were observed in any age group (Table 2). The correlation between age and EaI was not significant in either men (r = 0.051, P = 0.404) or women (r = −0.014, P = 0.829). There was no difference in EaI between men and women in any age group (Table 2). However, SVRI was significantly lower in women than in men in groups B and C.
Table 2.
Between‐sex differences in vascular and ventricular indexes in the three age‐based groups
| Group A: <65 years | Group B: 65 years ≤ age < 75 years | Group C: ≥75 years | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Men (n = 85) |
Women (n = 48) |
P value |
Men (n = 89) |
Women (n = 81) |
P value |
Men (n = 93) |
Women (n = 83) |
P value | |
| Heart rate | 71 ± 12 | 72 ± 12 | 0.729 | 67 ± 13 | 72 ± 10 | 0.006 | 69 ± 11 | 71 ± 11 | 0.239 |
| Systolic blood pressure | 135 ± 18 | 134 ± 15 | 0.705 | 136 ± 17 | 140 ± 18 | 0.117 | 134 ± 17 | 136 ± 16 | 0.307 |
| Diastolic blood pressure | 82 ± 11 | 80 ± 12 | 0.411 | 77 ± 11 | 78 ± 10 | 0.465 | 73 ± 10 | 72 ± 11 | 0.505 |
| SVI | 38.4 ± 8.0 | 39.5 ± 9.3 | 0.484 | 39.8 ± 8.9 | 41.4 ± 9.1 | 0.243 | 38.5 ± 8.5 | 41.9 ± 9.8 | 0.015 |
| SVRI | 3.10 ± 0.82 | 2.95 ± 0.71 | 0.281 | 3.11 ± 0.83 | 2.81 ± 0.74 | 0.014 | 2.97 ± 0.76 | 2.70 ± 0.87 | 0.030 |
| EaI = Pes/SVI | 3.31 ± 0.86 | 3.21 ± 0.78 | 0.527 | 3.23 ± 0.83 | 3.21 ± 0.89 | 0.887 | 3.27 ± 0.77 | 3.12 ± 0.96 | 0.249 |
| LVEF, % | 66 ± 7 | 66 ± 5 | 0.941 | 65 ± 6 | 68 ± 5 | 0.002 | 66 ± 6 | 68 ± 5 | 0.135 |
| FS/sigma ES | 4.2 ± 1.4 | 4.2 ± 1.3 | 0.833 | 4.2 ± 1.3 | 4.3 ± 1.6 | 0.574 | 4.5 ± 1.4 | 4.7 ± 1.9 | 0.250 |
| RWT | 0.42 ± 0.07 | 0.42 ± 0.09 | 0.976 | 0.43 ± 0.07 | 0.42 ± 0.07 | 0.511 | 0.45 ± 0.07 | 0.45 ± 0.09 | 0.895 |
| LVEDVI | 58 ± 11 | 61 ± 19 | 0.351 | 62 ± 13 | 61 ± 12 | 0.672 | 59 ± 12 | 62 ± 14 | 0.102 |
| E/e′ | 10.0 ± 2.9 | 10.5 ± 2.9 | 0.369 | 10.8 ± 3.3 | 10.5 ± 3.0 | 0.473 | 11.7 ± 3.2 | 13.2 ± 4.4 | 0.009 |
EaI, arterial elastance index;
FS, fractional shortening;
LVEDVI, left ventricular end‐diastolic volume index;
LVEF, left ventricular ejection fraction;
Pes, end‐systolic pressure;
RWT, relative wall thickness;
Sigma ES, end‐systolic wall stress;
SVI, stroke volume index;
SVRI, systemic vascular resistance index.
There was no correlation between age and LVEF (r = −0.030, P = 0.614 and r = 0.074, P = 0.280, respectively) in either men or women. However, the stress‐corrected FS (the ratio of FS to σes) was positively but weakly correlated with age in women (r = 0.187, P = 0.006); there was no correlation in men. Stress‐corrected FS was not different between men and women in any age group.
LAVI, but not LVMI and LVEDVI, weakly correlated with age in both men (r = 0.182, P = 0.002) and women (r = 0.190, P = 0.005). No differences were observed in relative wall thickness, LVEDVI, or LAVI between men and women, in any age group (Table 2, Figure 1 A). The LVMI was significantly lower in women than in men only in group B (Figure 1 B). In women there were significant differences between groups B and C, in terms of LAVI (Figure 1 A, P = 0.002) and LVMI (Figure 1 B, P = 0.008), but not LVEDVI. In contrast, in men there were no significant differences amongst the three age groups in terms of LAVI, LVEDVI, or LVMI.
Figure 1.
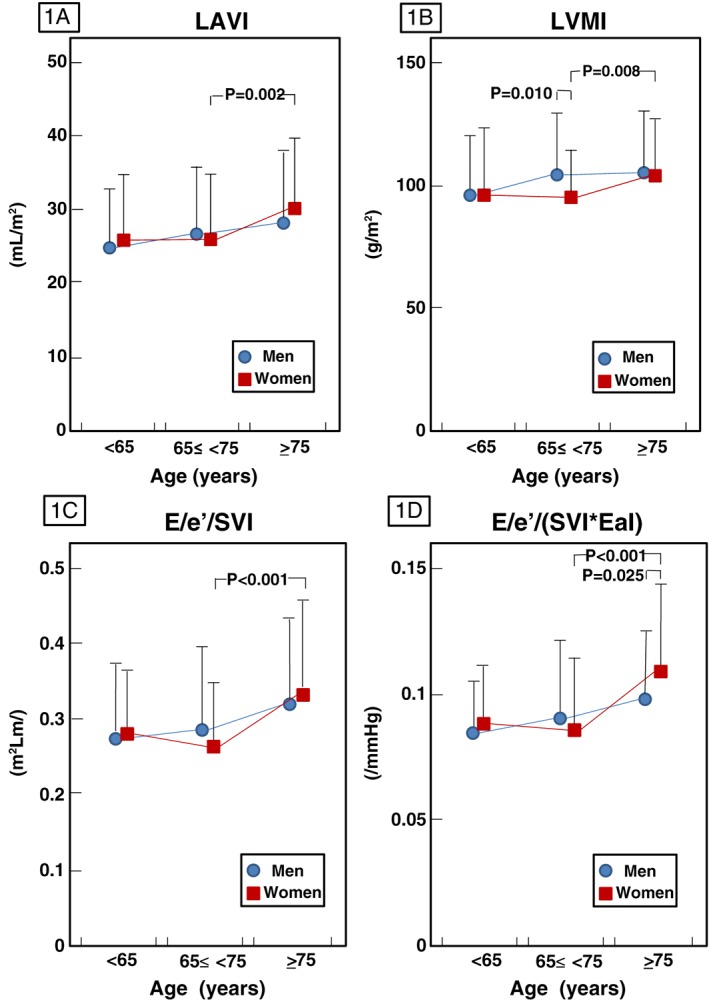
Age‐ and sex‐related differences in cardiac dimensions (left atrial volume index, LAVI, 1A; left ventricular mass index, LVMI, 1B) and diastolic function [EdI = E/e′/stroke volume index (E/e′/SVI) 1C; EdI/EaI = E/e′/(SVI*EaI), 1D]. Data are mean ± SD. Between‐sex differences in each age‐based group were assessed by unpaired t‐test or Wilcoxon rank‐sum test. Differences between pairs of age groups were assessed by post hoc Bonferroni test following the assessment of differences amongst all three age groups in each sex by one‐way analysis of variance or Kruskal–Wallis analysis.
Diastolic function indexed to vascular function
The E/e′ and operant diastolic elastance index (EdI = E/e′/SVI, used to reflect diastolic function) were positively but weakly correlated with age in both men (r = 0.260, P < 0.001, and r = 0.230, P = 0.001, respectively) and women (r = 0.280, P < 0.001, and r = 0.222, P = 0.001, respectively). No difference was observed in chamber stiffness (E/e′/LVEDDI, data not shown) or EdI (Figure 1 C) between men and women in any age group, although E/e′ was significantly higher in women than in men in group C only (P = 0.004, Table 2). The operant diastolic elastance indexed to arterial elastance, EdI/EaI = E/e′/0.9SBP, increased with age in both sexes (men, r = 0.251, P < 0.001; women, r = 0.264, P < 0.001), and was significantly higher in women than in men only in group C (P = 0.025) (Figure 1 D). Similar results with regard to EdI/EaI were obtained when we analysed the data in untreated hypertensive patients of group C (data not shown). Similar to the differences observed in cardiac dimensions such as LAVI and LVMI (Figure 1 A, 1 B), in women, this diastolic index differed significantly between groups B and C (Figure 1 D). The age‐linked change in EdI/EaI occurred over a different time point between sexes, similar to the alteration of cardiac dimension, LAVI or LVMI, in each sex. There was no significant difference in the slopes of regression lines for the relationship with age and LAVI, LVMI, or EdI/EaI between sexes. However, in patients aged over 65 years (groups B and C), the slopes were different in the relationship with age and LVMI (P = 0.039) or EdI/EaI (P = 0.090) between the sexes (Figures 2 A,B).
Figure 2.
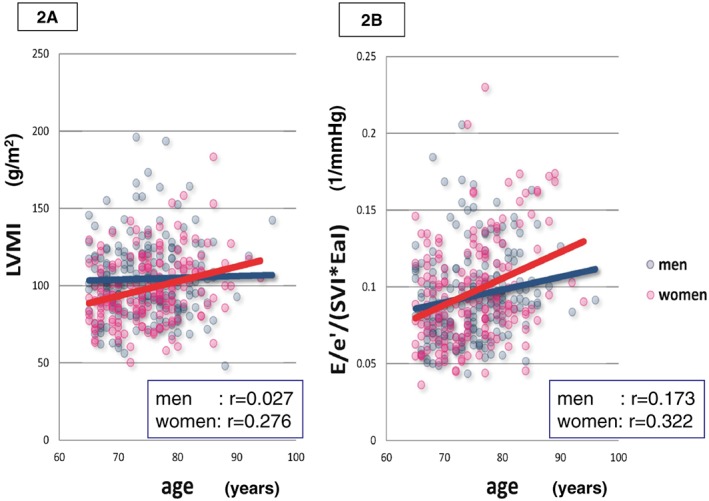
Correlation between age and left ventricular mass index (LVMI, 2A, men, y = 96.0 + 0.113x, r = 0.027, P = 0.710; women, y = 27.1 + 0.945x, r = 0.276, P < 0.001) or EdI/EaI = E/e′/(SVI*EaI) (2B, men, y = 0.0322 + 0.00082x, r = 0.173, P = 0.019; women, y = −0.0322 + 0.00172x, r = 0.322, P < 0.001) in men (blue) and women (red) of patients aged over 65 years. The slopes of the regression lines were different between sexes in both correlations (P = 0.039 and P = 0.090, respectively).
When multivariate regression analysis was performed by using significant parameters in univariate analysis, EdI/EaI was independently associated with age (P = 0.002) and LAVI (P < 0.001) in men, but with age (P = 0.036), eGFR (P = 0.035), heart rate (P = 0.003), and LAVI (P < 0.001) in women (Table 3). In both men and women, the correlation between EdI/EaI and LAVI was moderate (r = 0.336, P < 0.001 and r = 0.398, P < 0.001, respectively) and that between EdI/EaI and LVMI was weak (r = 0.162, P = 0.007 and r = 0.199, P = 0.003, respectively).
Table 3.
Regression analysis for diastolic elastance indexed to arterial elastance, EdI/EaI, in men and women
| Men | Women | |||||
|---|---|---|---|---|---|---|
| P value | P value | |||||
| r value | Univariate | Multivariate | r value | Univariate | Multivariate | |
| Age | 0.251 | <0.001 | 0.002 | 0.264 | <0.001 | 0.036 |
| Body mass index | −0.076 | 0.212 | — | 0.038 | 0.580 | — |
| eGFR | −0.159 | 0.008 | 0.740 | −0.286 | <0.001 | 0.035 |
| Haemoglobin | −0.118 | 0.054 | — | −0.212 | 0.001 | 0.935 |
| Diabetes mellitus | −0.018 | 0.768 | — | 0.042 | 0.536 | — |
| Hypercholesterolemia | 0.028 | 0.647 | — | 0.140 | 0.040 | 0.133 |
| Heart rate | −0.095 | 0.117 | — | −0.283 | <0.001 | 0.003 |
| LVEF | −0.123 | 0.043 | 0.083 | 0.063 | 0.357 | — |
| RWT | 0.102 | 0.096 | — | 0.124 | 0.070 | — |
| LAVI | 0.336 | <0.001 | <0.001 | 0.398 | <0.001 | <0.001 |
| LVEDVI | −0.047 | 0.438 | — | 0.100 | 0.145 | — |
| LVMI | 0.162 | 0.007 | 0.864 | 0.199 | 0.003 | 0.426 |
eGFR, estimated glomerular filtration rate;
LAVI, left atrial volume index;
LVEDVI, left ventricular end‐diastolic volume index;
LVEF, left ventricular ejection fraction;
LVMI, left ventricular mass index;
RWT, relative wall thickness.
Discussion
Diastolic function indexed to arterial elastance
It has been reported that the Ea increases with age14 and is higher in women than in men.14, 15 In the present study, however, no differences were observed in the Ea when indexed to BSA (EaI) between sexes in any age group.
As an index for diastolic function, the diagnostic value of deceleration time or E/A ratio, is limited. The correlation of E/e′ with pulmonary capillary wedge pressure is well established in patients with systolic dysfunction,12, 13 but is very sensitive to changes in LV load. There is insufficient evidence to support that E/e′ can reliably estimate LV diastolic dysfunction in preserved EF.16 It is also well known that pulmonary capillary wedge pressure and diastolic function are not same. A recent longitudinal study showed that LV diastolic stiffness increases over time in humans, particularly in women.17 A previous report using E/e′/SV to calculate Ed found that Ed increased with age and that it was higher in women than in men,18 although the study did not index Ed to BSA. In agreement with that study, the operant Ed indexed to BSA (EdI = E/e′/SVI) increased with age in both men and women in the present study. However, there was no difference in the EdI between men and women in any age group, indicating that the EdI may not be a suitable index for differentiation of diastolic function between sexes.
Given that EdI may increase because of vascular changes, we examined the relationships amongst diastolic function, age, sex, and vascular function. Diastolic elastance indexed to arterial elastance, calculated as EdI/EaI = E/e′/0.9SBP, may be a suitable index to determine between‐sex differences with alterations in diastolic function with age. Furthermore, it is important to note that impaired diastolic function independent of arterial elasticity occurred over a different time point in men and women; only in women, this diastolic index differed significantly between groups B and C. In patients aged over 65 years (groups B and C), the slopes were insignificantly different in the relationship with age and EdI/EaI between sexes (P = 0.090). The reason for the insignificance may be because of small number of patients and/or a relatively larger standard deviation in the slopes.
Relationship between diastolic function and cardiac dimensions
The index of cardiac dimension may help identify preclinical patients at increased risk of developing incidental heart failure.4 It has previously been reported that LV diastolic dysfunction does not negatively affect clinical parameters when it is not associated with changes in the index of left cardiac structural remodelling, based on LVH magnitude and atrial dilation.19 Increased LA volume is an independent predictor of heart failure and worsened mortality in previously asymptomatic elderly subjects with a preserved ejection fraction.20
In the present study, LAVI and LVMI were significantly higher amongst the oldest age group than amongst younger age groups in women, but not in men. Importantly, the changes in the two indexes, EdI/EaI and LAVI or LVMI, coincidently occurred in a different time point between sexes. In women, alteration in cardiac dimensions leading to hemodynamic changes occurs very late in life; in men, on the other hand, such alteration gradually occurs, resulting in the gradual impairment of diastolic function. EdI/EaI was independently associated with heart rate and renal function in addition to age and LAVI in women, but not in men, by multivariate analysis. The reduction of heart rate and/or renal function may be a possible mechanism for the exacerbation of diastolic function, leading to the occurrence of incidental heart failure in elderly women. Clarification of renal function‐ and heart rate‐related roles for the impaired diastolic function may imply a novel clue to identify the mechanism by which elderly hypertensive women are prone to exhibit HFpEF. It is plausible that the change in diastolic function independent of arterial elasticity is because of cardiac response for the alteration of cardiac structure, and that the imbalance between these changes in the elderly women may contribute to the occurrence of HFpEF. A large‐scale prospective study is needed to clarify the differences in the manner in which dynamic and static parameters of vascular and ventricular function including EdI/EaI and heart rate change with age, and the differences in the course of age‐linked alterations of cardiac dimensions and renal function between sexes.
Study limitations
Our statistical analysis may have been biassed because of the modest sample size; a larger data set might reveal differences where we were unable to find any. Our study is also limited by its referral nature as a retrospective database study. Treated and hypertensives receiving no treatment may be different in the evolution of cardiac and vascular function and anatomy with age. We did not evaluate the duration of hypertension or the extent of medication usage, all of which may affect left cardiac performance and diastolic dysfunction, and we did not evaluate right cardiac performance or the effects of exercise or stress. In addition, we did not obtain data on clinical symptoms. Participation bias may also have affected the observed results. Patients with coronary artery disease were not excluded unless they had a history of myocardial infarction, and our participants were not representative of patients with HFpEF because they had not experienced heart failure. These issues limited the applicability of our results with regard to assessing the contribution of altered vascular and ventricular function and changes in cardiac dimensions to the pathogenesis of HFpEF in very elderly women, although increases in vascular and ventricular stiffness may predispose to heart failure.
Conclusions
In an elderly hypertensive population, LV diastolic function indexed to the arterial elastance, EdI/EaI = E/e′/0.9SBP, was impaired in women compared with that in men, whereas there were no differences in systolic function and relative wall thickness. The age‐linked change in LV diastolic function independent of arterial elasticity was coincident with the alteration of cardiac dimensions in each sex and the coincidence occurred over a different time point between men and women.
Conflict of interest
None declared.
Funding
None.
Hoshida, S. , Shinoda, Y. , Ikeoka, K. , Fukuoka, H. , Inui, H. , and Watanabe, T. (2016) Age‐ and sex‐related differences in diastolic function and cardiac dimensions in a hypertensive population. ESC Heart Failure, 3: 270–277. doi: 10.1002/ehf2.12097.
References
- 1. Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab‐Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD, Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circ Heart Fail 2010; 3: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease: insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol 2009; 54: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE; for the I‐PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 2011; 124: 2491–2501. [DOI] [PubMed] [Google Scholar]
- 4. Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferrici L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community. J Am Coll Cardiol 2007; 49: 198–207. [DOI] [PubMed] [Google Scholar]
- 5. Kelly RP, Ting C‐T, Yang T‐M, Liu C‐P, Maughan WL, Chang M‐S, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992; 86: 513–521. [DOI] [PubMed] [Google Scholar]
- 6. Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single‐beat determination of left ventricular end‐systolic elastance in humans. J Am Coll Cardiol 2001; 38: 2028–2034. [DOI] [PubMed] [Google Scholar]
- 7. Oh JK, Seward JB, Tajik AJ. The Echo Manual, 2nd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. [Google Scholar]
- 8. Hoshida S, Shinoda Y, Inui H, Hosoi R, Teranishi F, Asaoka N, Sugitani T, Hamasaki T. Difference in left ventricular mass index between hypertensive patients with and without renal artery stenosis by propensity score analysis. J Clin Hypertens 2014; 16: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borow KM, Green LH, Grossman W, Braunwald E. Left ventricular end‐systolic stress‐shortening and stress–length relations in human: normal values and sensitivity to inotropic state. Am J Cardiol 1982; 50: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 10. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography in the clinician's Rosetta stone. J Am Coll Cardiol 1997; 30: 8–18. [DOI] [PubMed] [Google Scholar]
- 11. Redfield MM, Jacobson SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202. [DOI] [PubMed] [Google Scholar]
- 12. Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997; 30: 474–480. [DOI] [PubMed] [Google Scholar]
- 13. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. The clinical utility of Doppler echocardiography and tissue Doppler imaging in estimation of left ventricular filling pressures: a comparative simultaneous Doppler‐catheterization study. Circulation 2000; 102: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Study. Hypertension 2004; 43: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 15. Smulyan H, Asmar RG, Rudnicki A, London GM, Safar ME. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol 2001; 37: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 16. Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic accuracy of tissue Doppler index E/e′ for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: A systematic review and meta‐analysis. J Am Heart Assoc 2016; 5: pii: e002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community‐based study. Circ Heart Fail 2013; 6: 944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Redfield MM, Jacobson SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age‐ and gender‐related ventricular‐vascular stiffening. A community‐based study. Circulation 2005; 112: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 19. Germing A, Gotzmann M, Schikowski T, Vierkőtter A, Ranft U, Műgge A. Diastolic dysfunction without abnormalities in left ventricular geometry does not affect quality of life in elderly women. Exp Clin Cardiol 2011; 16: 37–39. [PMC free article] [PubMed] [Google Scholar]
- 20. Takemoto Y, Barnes ME, Seward JB, Lester SJ, Appleton CA, Gersh BJ, Bailey KR, Tsang TS. Usefulness of left atrial volume in predicting first congestive heart failure in patients >65 years of age with well‐preserved left ventricular systolic function. Am J Cardiol 2005; 96: 832–836. [DOI] [PubMed] [Google Scholar]


