Abstract
Aims
In patients with heart failure with reduced ejection fraction (HFrEF) and iron deficiency, treatment with intravenous iron has shown a clinical improvement regardless of anaemic status. Cardiac magnetic resonance (CMR) T2* sequence has shown a potential utility for evaluating myocardial iron deficiency. We aimed to evaluate whether T2* sequence significantly changes after ferric carboximaltose (FCM) administration, and if such changes correlate with changes in left ventricle ejection fraction (LVEF).
Methods and results
In this pilot study, we included eight patients with chronic symptomatic (New York Heart Association II–III) HFrEF and iron deficiency. A CMR, including T2* analysis, was performed before and at a median of 43 days (interquartile range = 35–48) after intravenous FCM administration. Pearson or Spearman correlation coefficient (r) was used for bivariate contrast as appropriate. A partial correlation analysis was performed between ΔLVEF and ΔT2* while controlling for anaemia status at baseline. Anaemia was present in half of patients. After FCM administration, T2* decreased from a median of 39.5 (35.9–48) to 32 ms (32–34.5), P = 0.012. Simultaneously, a borderline increase in median of LVEF [40% (36–44.5) to 48.5% (38.5–53), P = 0.091] was registered. In a bivariate correlational analysis, ΔT2* was highly correlated with ΔLVEF (r = −0.747, P = 0.033). After controlling for anaemia at baseline, the association between ΔT2* and ΔLVEF persisted [r(partial): −0.865, R 2(partial): 0.748, P = 0.012]. A median regression analysis backed‐up these findings.
Conclusions
In a small sample of patients with HFrEF and iron deficiency, myocardial iron repletion assessed by CMR was associated to left ventricular remodelling. Further studies are warranted.
Keywords: Iron deficiency, Intravenous iron, Left ventricular ejection fraction, Systolic heart failure, Magnetic resonance imaging, T2* sequence
Introduction
In patients with heart failure with reduced ejection fraction (HFrEF), treatment with intravenous iron has shown to improve symptoms, functional capacity, and quality of life regardless of anaemic status.1, 2 Experimental studies have shown that iron deficiency (ID) led to structural and functional abnormalities of the heart.3 In humans, a small report showed a reduction in iron content of cardiomyocytes of patients with HFrEF compared with controls.4 More recently, in a small clinical trial of patients with HFrEF, anaemia, and chronic kidney disease, intravenous iron treatment was associated with improved myocardial function and cardiac dimensions.5 Such findings led some authors to postulate that part of the beneficial effect of iron treatment in HFrEF is attributed to myocardial iron repletion.6 Nevertheless, no study so far has evaluated the short‐term effect of intravenous iron therapy on myocardial iron content and its correlation with simultaneous changes in left ventricular (LV) function. Cardiac magnetic resonance (CMR) T2* sequence has emerged as a reliable non‐invasive technique for assessing myocardial iron overload.7, 8 Recently, this technique has also shown a potential utility for evaluating myocardial iron deficiency.9, 10
Aims
We aimed to evaluate whether (i) T2* sequence significantly changes after intravenous iron administration in patients with ID with HFrEF, and (ii) if such changes correlate with simultaneous changes in CMR LV systolic function.
Methods
Study sample
In this observational pilot study, eight patients visited in the heart failure (HF) unit of a third‐level hospital from 29 January 2015 to 26 October 2015 were included. All of them met the following inclusion criteria: (i) ID, defined as serum ferritin <100 µg/L or as ferritin 100–299 µg/L with a transferrin saturation <20%; (ii) New York Heart Association (NYHA) functional class ≥II; (iii) clinical stability during the last 3 months; and (iv) left ventricular ejection fraction (LVEF) <50% assessed by transthoracic echocardiography within the last 3 months. In addition, the patients were excluded if any of the following were documented: (i) severe to moderate primary valve heart disease; (ii) acute coronary syndrome, cardiac surgery, or revascularization within the previous 3 months; and (iii) patients with pacemakers, intracardiac defibrillators, and cardiac resynchronization devices. Informed consent was obtained from every patient, and the study protocol conforms to the Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Protocol
Clinical, laboratory, electrocardiographic, distance walked in 6 minutes (6MWT), Minnesota Living with Heart Failure Questionnaire (MLHFQ), and treatment characteristics were recorded in electronic forms. The patients enrolled underwent a CMR within the first 7 days after ID diagnosis. Following CMR, a single dose of 1000 mg of ferric carboximaltose (FCM) was administered to all patients. A second CMR, blood laboratory, clinical assessment, 6MWT, and MLHFQ were performed at a median of 43 days [interquartile range (IQR) = 35–48] after FCM administration. Anaemia was defined as haemoglobin (Hb) level <12 g/dL in women and <13 g/dL in men.
CMR
All CMR studies (1.5 T unit, Magnetom Sonata, Siemens, Erlangen, Germany) were performed by the same operator. Steady‐state free precession cine sequences at rest were made, and the following were quantified: LV end‐diastolic and end‐systolic volumes (LVEDV and LVESV) (mL/m2), LV end‐systolic diameter, LVEF determined by the Simpson method (%), and LV mass (g/m2) determined by manually outlining the endocardial borders in all short‐axis cine slices.
The basic T2* pulse sequence was a single breath‐hold, multi‐echo, gradient echo T2* sequence. In brief, a six‐element cardiac phased array coil and a chest saturation band were used to image a single 10 mm mid‐ventricular short axis slice at eight echo times from ~2 to ~18 with 2.0 ms increments. Data were acquired every other cardiac cycle.
For T2* analysis, a region of interest was chosen in the mid‐left ventricular septum. The mean signal intensities of region of interest were measured in the series of increasing echo time images to give an exponential decay curve. The monoexponential decay model and the nonlinear curve fitting algorithm were used to fit the curve to obtain T2* measurement. An example of T2* calculation is shown in Figure S1 .
Endpoints
Absolute changes in LVEF (ΔLVEF) (%) were selected as the main endpoint. Secondary endpoints were absolute changes in LV mass (ΔLVmass) (g/m2), LVEDV (ΔLVEDV) (mL/m2), and LVESV (ΔLVESV) (mL/m2). Reverse remodelling was defined as (i) LVEF increase ≥ 15%, or (ii) LVEF increase ≥10% + LV end‐systolic diameter reduction ≥ 20%, or LVESV reduction ≥ 40%.
Statistical analysis
Continuous variables were expressed in alignment with their distribution, and discrete variables were expressed as percentages. The median was always presented with IQR = 25–75 percentiles as a measure of dispersion. The equality of each pair of pre–post observations was tested either with paired t‐test or Wilcoxon matched‐pair signed‐rank test as appropriate. Pearson or Spearman correlation coefficient (r) was used for bivariate contrast as appropriate. A partial correlation analysis was performed between ΔLVEF (dependent variable or Y) and ΔT2* (independent variable or X) while controlling for anaemia status at baseline (X2). Within the same framework, ΔLVmass, ΔLVEDV, and ΔLVESV were contrasted with ΔT2*. The partial correlation coefficient [r(partial)] reflects the degree of correlation between X and Y after removing the effect of X2 on Y and X2 on X. By the same token, the R 2(partial) is interpreted as the proportion of shared variance between Y and X controlling for X2. To add robustness to the correlational analysis, we also tested the relationship between ΔT2* and the median of ΔLVEF by using quantile regression analysis (q = 0.5). In this latter analysis, anaemia at baseline and changes in haemoglobin were used as covariates. A sensibility analysis assessing the relationship between inverse of T2* (R2*) and changes in left ventricular systolic function was also performed.
Results
The median (IQR) age was 81 (69–83) years; 62.5% patients were women, 62.5% displayed NYHA III, and 87.5% were of non‐ischemic aetiology. Anaemia was present in half of patients. The median of LVEF, NT‐proBNP, and estimated glomerular filtration rate were 40% (36–45), 3868 pg/mL (1680–4841), and 38 mL/min/1.73 m2 (26–47), respectively. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics
| Variable | n=8 |
|---|---|
| Demographics and medical history | |
| Age, yearsb | 81 (69‐83) |
| Female, n (%) | 5 (62.5) |
| Hypertension, n (%) | 7 (87.5) |
| Diabetes Mellitus, n (%) | 5 (62.5) |
| Dyslipidemia, n (%) | 4 (50) |
| Active smoker, n (%) | 2 (25) |
| Chronic renal disease, n (%) | 5 (62.5) |
| Ischemic heart disease, n (%) | 1 (12.5) |
| NYHA class III, n (%) | 5 (62.5) |
| Atrial fibrillation, n (%) | 4 (50%) |
| Vital signs and physical examination | |
| Systolic blood pressure, mmHgb | 122 ± 14 |
| Diastolic blood pressure, mmHgb | 61 ± 10 |
| Heart rate, bpmb | 75 ± 13 |
| Peripheral edema, n (%) | 4 (50) |
| Laboratory data | |
| Hemoglobin, g/dL | 12.2 ± 1.7 |
| Anemiaa, n (%) | 4 (50) |
| Ferritin, mg/dL | 126 (32‐210) |
| Transferrin saturation, % | 17.7 (15.3‐19.8) |
| Sodium, mEq/Lb | 140 ± 2 |
| Potassium, mg/dLb | 4.5 ± 0.5 |
| Urea, mg/dLb | 106 ± 44 |
| Creatinine, mg/dLb | 1.79 ± 0.62 |
| eGFR, mg/dL/1.73 m2 | 38 (26‐47) |
| CA125, U/mL | 20 (18‐29.5) |
| NT‐proBNP, pg/mL | 3868 (1680‐4841) |
| Cardiac magnetic resonance | |
| LVEF, % | 40 (36‐45) |
| LVEDV, mL/m2 | 105.5 (90‐129) |
| LVESV, mL/m2 | 67 (49‐81) |
| LV mass, g/m2 | 92 (79.5‐96.5) |
| Medical treatment | |
| Diuretics, n (%) | 8 (100) |
| Loop diuretics dose, mg/day | 60 (40, 80) |
| Betablockers, n (%) | 8 (100) |
| Aldosterone blockers, n (%) | 8 (100) |
| ACEI, n (%) | 5 (62.5) |
| ARB, n (%) | 1 (12.5) |
| Statins, n (%) | 6 (75) |
NYHA: New York Heart Association; eGFR: estimated glomerular filtration rate; CA125: antigen carbohydrate 125; NT‐proBNP: amino‐terminal pro‐brain natriuretic peptide; LVEF: left ventricle ejection fraction; LVEDV: left ventricle end‐diastolic volume; LVESV: left ventricle end‐systolic volume; LV: left ventricle; ACEI: angiotensin converting enzyme inhibitors; ARB: angiotensin receptor blockers.
Anemia was defined as hemoglobin lower than 13 mg/dL in men and 12 mg/dL in women.
Values for continuous variables are expressed as median (interquartile range).
Values for continuous variables are expressed as median (interquartile range) unless otherwise specified.
Variable expressed as mean ± standard deviation.
Clinical, laboratory, and CMR changes after intravenous FCM
Clinical, laboratory, and CMR changes after intravenous FCM are shown in Table 2. Briefly, NYHA class improved in 50% of patients, and median 6MWT also showed a significant improvement. There was also an associated significant increase in the median of ferritin [126 µg/L (32 to 210) to 620 µg/L (535–814), P = 0.012] and transferrin saturation [17.7% (15.3–19.8) to 29.6% (28.1–31.4), P = 0.012]. The increase in Hb was borderline significant (12.2 ± 1.7 mg/dL vs. 12.8 ± 1.9 mg/dL, P = 0.088). Regarding CMR parameters, T2* decreased from a median of 39.5 ms (35.9–48) to 32 ms (32–34.5), P = 0.012 (Figure 1 a). Likewise, R2* significantly increased after FCM ( Figure S2 ). Simultaneously, a borderline increase in the median of LVEF [40% (36–44.5) to 48.5% (38.5–53) P = 0.091] and a decrease in the median of LVESV [67 mL/m2 (49–81) to 55 mL/m2 (48–68), P = 0.093] were registered ( Figure S1 ). Reverse remodelling was observed in four patients (50%). No significant changes were found for LV end‐diastolic volumes, LV mass, MLHFQ, or other biomarkers ( Figure S1 and Table S1 ).
Table 2.
Changes in patients’ characteristics after FCM administration
| Variable | First assessment | Second assessment | P‐value |
|---|---|---|---|
| Laboratory data | |||
| NT‐proBNP, pg/mL | 3868 (1680‐4841) | 3120.5 (1430‐5788) | 0.674 |
| eGFR, mg/dL/1.73 m2 | 38 (26‐47) | 44 (29‐51) | 0.123 |
| Hemoglobin, g/dLa | 12.2 ± 1.7 | 12.8 ± 1.9 | 0.088 |
| Ferritin, mg/dL | 126 (32‐210) | 620 (535‐814) | 0.012 |
| Transferrin saturation, % | 17.65 (15.3‐19.8) | 29.6 (28.05‐31.35) | 0.012 |
| Vital signs | |||
| SBP, mmHga | 122 ± 14 | 130 ± 24 | 0.134 |
| DBP, mmHga | 61 ± 10 | 69 ± 18 | 0.136 |
| Heart rate, bpm | 72 (65.5‐87) | 68.5 (60‐87) | 0.205 |
| Functional capacity | |||
| NYHA class≥III | 5 (63%) | 1 (13%) | 0.039 |
| 6MWT | 198 (160‐236) | 276 (169‐342) | 0.011 |
| Quality of life | |||
| MLHFQ | 55 (18‐68) | 27 (15‐48) | 0.293 |
FCM: ferric carboximaltose; NT‐proBNP: amino‐terminal pro‐brain natriuretic peptide; eGFR: estimated glomerular filtration rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; 6MWT; 6‐minute walk test; MLHFQ: Minnesota Living with Heart Failure questionnaire
Values for continuous variables are expressed as median (interquartile range) unless otherwise specified.
Variable expressed as mean ± standard deviation.
Figure 1.
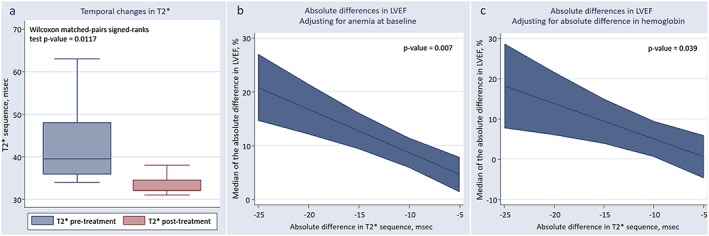
(a) Temporal changes in median of cardiac magnetic resonance T2* sequence after intravenous ferric carboximaltose administration. (b) ΔLVEF and ΔT2*, adjusting for anaemia at baseline. (C) ΔLVEF and ΔT2* adjusting for changes in haemoglobin. CMR, cardiac magnetic resonance; FCM, ferric carboximaltose; ΔLVEF, changes in left ventricular ejection fraction; ΔT2*, changes in T2*.
Absolute changes in T2* and LVEF CMR parameters
In a bivariate correlational analysis, ΔT2* was highly correlated with ΔLVEF (r = −0.747, P = 0.033). After controlling for anaemia at baseline, the association between ΔT2* and ΔLVEF persisted [r(partial) = −0.865, R 2(partial) = 0.748, P = 0.012]. A median regression analysis backed‐up these findings. Indeed, per decrease in 1 unit of ΔT2*, there was an associated increase in the median of LVEF by a factor of 0.81 (P = 0.007), having the sample adjusted by anaemia at baseline (Figure 1 b). The results did not significantly change when absolute difference in pre–post Hb was used instead as covariate (per decrease in 1 unit of ΔT2* and increase in the median of LVEF of 0.88, P = 0.039), as shown in Figure 1 c. Likewise, the correlation analysis also showed a borderline positive correlation between ΔLVEF and ΔR2* (r = 0.65; R 2 = 0.421; P = 0.082); after adjusting for anaemia at baseline, such association became significant (r = 0.795; P = 0.0325)
Absolute changes in T2* and other CMR parameters
ΔT2* was borderline correlated with ΔLVESV (r = 0.663, P = 0.073), but it was not with ΔLVEDV (r = 0.052, P = 0.903) nor ΔLVmass (r = 0.436, P = 0.280). After adjusting for anaemia at baseline, the association between ΔT2* and ΔLVESV became significant [r(partial) = 0.906, R 2(partial) = 0.821, P = 0.005]. Similarly, a quantile regression analysis showed that the associations with ΔLVESV were significant and in the same direction after adjusting either for anaemia at baseline or ΔHb (β‐coefficient = 0.65, P = 0.007 and β‐coefficient = 1.11, P = 0.048, respectively).
Discussion
This is the first study reporting a significant decrease in myocardial T2* sequence after intravenous FCM administration. In addition, we found a significant association between T2* changes and short‐term improvement in LVEF and LVESV in a small group of elderly patients with chronic HFrEF of predominant non‐ischemic aetiology and ID. Interestingly, the association persisted independent of Hb changes.
During the last decade, ID has become a well‐recognized therapeutic target in HFrEF.1, 2, 11 Two large randomized placebo‐controlled trials have shown that intravenous iron administration was associated to improvement in symptoms, functional capacity, quality of life, and reduction in HF hospitalizations.1, 2 However, the pathophysiological mechanisms underlying the effect of iron administration in HF remain poorly understood, because clinical benefits have been observed irrespective of anaemia status.1, 2 Iron is a chemical element that plays a central role in the oxidative metabolism and muscle oxygen storage and transport.12
Despite the crucial role of iron in the physiology of cardiovascular system, this element remains understudied outside of the erythropoietic system. Experimental studies have shown that ID induced cardiac dysfunction13 and cardiac hypertrophy, characterized by aberrant mitochondrial and irregular sarcomere organization.14
Given previous evidence, it seems plausible to speculate that ID may play a causative role in the pathophysiology of HF beyond anaemia.4, 6, 11 Recently, Toblli et al., in a small randomized trial in patients with HFrEF, ID, and chronic kidney disease, showed that iron sucrose administration translated into a significant 6‐month improvement in LVEF by echocardiography estimation (6.6 ± 3.8%).5 More recently, findings from a cohort of 232 patients undergoing renal transplantation showed an increase of LVEF, particularly notorious in those with systolic dysfunction pretransplantation.15 Because these changes were strongly and positively related to haemoglobin changes following transplantation, the authors speculated a potential causal link between ID‐anaemia and HF progression in patients with chronic renal failure.15 Interestingly, all patients included in the present study displayed concomitant renal dysfunction. In summary, our results corroborate the prior findings and add new evidence, suggesting that myocardial iron repletion may explain, at least partially, the beneficial effects of intravenous iron administration observed in prior studies.
There are important limitations that need to be acknowledged. First, this is small single centre observational study using one magnetic resonance imaging system. However, we believe that eight patients in a pre–post testing design provide enough statistical power to detect the obtained effect size in T2* with an alpha of 0.05. Second, T2* is a well‐established sequence for quantification iron overload, but the reliability for assessing ID remains more debatable. Third, the magnitude of CMR changes may be explained in part because of the inherent variability of the technique. Further studies aiming to evaluate the reproducibility of T2* measurements are necessarily warranted.
Conclusions
In a small sample of patients with HFrEF, ID, and renal dysfunction, myocardial iron repletion, as assessed by CMR, was associated to left ventricular remodelling. Further studies are needed.
Funding
This work was supported in part by grants from Instituto de Salud Carlos III and FEDER, Red de Investigación Cardiovascular, Programa 7 (RD12/0042/0010) and PIE15/00013.
Supporting information
Figure S1. Example of T2* measurement before and after intravenous carboximaltose administration.
Figure S2. Cardiac magnetic resonance changes after intravenous carboximaltose administration. R2*: Inverse of T2*LVEF: left ventricle ejection fraction; LVEDV: left ventricle end‐diastolic volume; LVESV: left ventricle end‐systolic volume; LVM: left ventricular mass.
Table S1. Changes in patients' characteristics after FCM administration.
Supporting info item
Supporting info item
Supporting info item
Núñez, J. , Monmeneu, J. V. , Mollar, A. , Núñez, E. , Bodí, V. , Miñana, G. , García‐Blas, S. , Santas, E. , Agüero, J. , Chorro, F. J. , Sanchis, J. , and López‐Lereu, M. P. (2016) Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Failure, 3: 293–298. doi: 10.1002/ehf2.12101.
References
- 1. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P, Trial Investigators FAIR‐HF. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–48. [DOI] [PubMed] [Google Scholar]
- 2. Filippatos G, Farmakis D, Colet JC, Dickstein K, Lüscher TF, Willenheimer R, Parissis J, Gaudesius G, Mori C, von Eisenhart RB, Greenlaw N, Ford I, Ponikowski P, Anker SD. Intravenous ferric carboxymaltose in iron‐deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR‐HF trial. Eur J Heart Fail 2013; 15: 1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong F, Zhang X, Culver B, ChewHGJr KRO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (London) 2005; 109: 277–286. [DOI] [PubMed] [Google Scholar]
- 4. Maeder MT, Khammy O, Remedios C, Kaye DM. Myocardial and systemic iron depletion in heart failure implications for anemia accompanying heart failure. J Am Coll Cardiol 2011; 58: 474–80. [DOI] [PubMed] [Google Scholar]
- 5. Toblli JE, Di Gennaro F, Rivas C. Changes in echocardiographic parameters in iron deficiency patients with heart failure and chronic kidney disease treated with intravenous iron. Heart Lung Circ 2015; 24: 686–95. [DOI] [PubMed] [Google Scholar]
- 6. McMurray J, Ponikowski P. Heart failure not enough pump iron? J Am Coll Cardiol 2011; 58: 481–2. [DOI] [PubMed] [Google Scholar]
- 7. Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN, Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2‐star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001; 22: 2171–9. [DOI] [PubMed] [Google Scholar]
- 8. Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, Sheppard MN, Porter JB, Walker JM, Wood JC, Galanello R, Forni G, Catani G, Matta G, Fucharoen S, Fleming A, House MJ, Black G, Firmin DN, St Pierre TG, Pennell DJ. On T2* magnetic resonance and cardiac iron. Circulation 2011; 123: 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagao M, Matsuo Y, Kamitani T, Yonezawa M, Yamasaki Y, Kawanami S, Abe K, Mukai Y, Higo T, Yabuuchi H, Takemura A, Yoshiura T, Sunagawa K, Honda H. Quantification of myocardial iron deficiency in nonischemic heart failure by cardiac T2* magnetic resonance imaging. Am J Cardiol 2014; 113: 1024–30. [DOI] [PubMed] [Google Scholar]
- 10. Nagao M, Baba S, Yonezawa M, Yamasaki Y, Kamitani T, Isoda T, Kawanami S, Maruoka Y, Kitamura Y, Abe K, Higo T, Sunagawa K, Honda H. Prediction of adverse cardiac events in dilated cardiomyopathy using cardiac T2* MRI and MIBG scintigraphy. Int J Cardiovasc Imaging 2015; 31: 399–407. [DOI] [PubMed] [Google Scholar]
- 11. Cohen‐Solal A, Leclercq C, Deray G, Lasocki S, Zambrowski JJ, Mebazaa A, de Groote P, Damy T, Galinier M. Iron deficiency: an emerging therapeutic target in heart failure. Heart 2014; 100: 1414–20. [DOI] [PubMed] [Google Scholar]
- 12. Sawicki KT, Chang HC, Ardehali H. Role of heme in cardiovascular physiology and disease. J Am Heart Assoc 2015; 4: e001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldstein D, Felzen B, Youdim M, Lotan R, Binah O. Experimental iron deficiency in rats: mechanical and electrophysiological alterations in the cardiac muscle. Clin Sci (Lond) 1996; 91: 233–9. [DOI] [PubMed] [Google Scholar]
- 14. Dong F, Zhang X, Culver B, Chew HG Jr, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond) 2005; 109: 277–86. [DOI] [PubMed] [Google Scholar]
- 15. Hawwa N, Shrestha K, Hammadah M, Yeo PS, Fatica R, Tang WH. Reverse remodeling and prognosis following kidney transplantation in contemporary patients with cardiac dysfunction. J Am Coll Cardiol 2015; 66: 1779–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example of T2* measurement before and after intravenous carboximaltose administration.
Figure S2. Cardiac magnetic resonance changes after intravenous carboximaltose administration. R2*: Inverse of T2*LVEF: left ventricle ejection fraction; LVEDV: left ventricle end‐diastolic volume; LVESV: left ventricle end‐systolic volume; LVM: left ventricular mass.
Table S1. Changes in patients' characteristics after FCM administration.
Supporting info item
Supporting info item
Supporting info item


