Abstract
The aim of this paper is to evaluate the treatment effects of recommended drugs and devices on key clinical outcomes for patients with heart failure with reduced ejection fraction (HFREF). Randomized controlled trials (RCTs) listed in the 2012 HF guideline from the European Society of Cardiology as well as the 2013 HF guideline from the American College of Cardiology Foundation and American Heart Association were evaluated for use in the meta‐analysis. RCTs written in English evaluating recommended drugs and devices for the treatment of patients with HFREF were included. Meta‐analyses, based on the outcomes of all‐cause mortality and hospitalization because of HF, were performed with relative risk ratio as the effect size. In the identified 47 RCTs, patients were on average 63 years old and 22% were female. Drugs targeting the renin‐angiotensin‐aldosterone system, beta‐blockers, cardiac resynchronization therapy (CRT), and intracardiac defibrillator devices (ICDs) significantly reduced the risk of death with reductions of 14–19, 23, 20, and 20%, respectively. Drugs targeting the renin‐angiotensin‐aldosterone system, beta‐blockers, digoxin, and CRT significantly reduced the risk of HF hospitalization with reductions of 24–37, 22, 60, and 36%, respectively, while ICDs significantly increased the risk with 34%. Ivabradine showed no significant effects on either outcome. As such, the majority of recommended HFREF treatments offered significant treatment benefits. However, many of the included studies were from the 1990s or earlier, and one must therefore be cautious when extrapolating these results to contemporary patients with HF.
Keywords: HFREF, Recommended HF treatments, Review, Meta‐analysis, Guidelines
Introduction
Past decades have seen a remarkable innovation and progress in the treatment of heart failure (HF). Despite advances in HF treatment, the prognosis for patients with HF with reduced ejection fraction (HFREF), accounting for approximately half of HF cases, resembles that of patients with malignant diseases, with a 1‐year mortality rate of 15–20% and a median survival of 4–5 years from the time of diagnosis.1 Therefore, in addition to developing new treatments, systematic and careful use of existing expertise is crucial. Today, clinicians across the Western world follow similar guidelines for the treatment of HFREF. Many of the studies in these guidelines are from the 1990s or earlier, which begs the question of whether the studies listed in the guidelines remain relevant today.
This systematic review aims to evaluate the effectiveness of current best practice in drug and device therapy for HFREF, by examining the evidence behind leading guidelines from the European Society of Cardiology (ESC)2 and the American College of Cardiology Foundation and American Heart Association (ACCF/AHA).3 This is approached by conducting meta‐analyses on randomized controlled trials (RCTs) referenced in said guidelines and calculating the effect of each treatment on all‐cause mortality and hospitalization because of HF. A sensitivity analysis including additional studies found through a literature search is presented, and it is discussed whether one can reasonably extrapolate the results to contemporary patients with HF.
Methods
Study design
The newest guidelines from the ESC and ACCF/AHA were examined, and all relevant RCTs and their data were extracted and used in the meta‐analyses. For use in a sensitivity analysis, PubMed was systematically searched for additional relevant multicentre RCTs.
Inclusion and exclusion criteria
Only RCTs with a distinct control group mentioned in ESC and ACCF/AHA guidelines were included in the primary meta‐analyses. Only articles written in English were reviewed, and studies had to examine the effects of angiotensin‐converting enzyme inhibitors (ACE inhibitors), angiotensin receptor blockers (ARBs), beta‐blockers, aldosterone receptor antagonists, digoxin, ivabradine, cardiac resynchronization therapy (CRT), or intracardiac defibrillator devices (ICDs) on HFREF. Studies on combination therapy of ACE inhibitors and ARBs were excluded.
Search methods
For use in the sensitivity analysis, additional relevant multicentre studies that met the previously mentioned criteria, but were not referenced in guidelines, were included. These studies were found through a literature search in the PubMed database. Keywords were ‘randomized controlled trial’, ‘heart failure’, ‘multicenter,’ and the individual treatment (full search information in Appendix S1 in Supporting Information). Last search was carried out on 16 December 2014. The searches and extraction of relevant studies and their data were carried out independently by two investigators (MMT, CL), and consensus was found in cases of discrepancies.
Outcomes and data extraction
The primary outcomes collected from the studies were all‐cause mortality and HF hospitalization. Because HF hospitalization can be defined in various ways, and in order to ensure sufficient data for a meaningful analysis, data for surrogate measures of HF hospitalization were used in a number of studies.4, 5, 6, 7, 8, 9 The surrogate measures consisted of ‘worsening heart failure requiring intravenous diuretic therapy or discontinuation of the study’, ‘intravenous furosemide for the control of acute exacerbations or symptoms’, ‘heart failure episode/decompensation’, ‘hospitalization for cardiovascular causes/reasons’, ‘cardiovascular hospitalization’, and ‘severe/resistant heart failure’.
Characteristics of the studies including epidemiological variables, clinical measurements, co‐morbidities, and concurrent medication were gathered at baseline. A priori, they were sample size, follow‐up, age, gender, left ventricular ejection fraction (LVEF), creatinine blood levels, systolic blood pressure, diabetes, ischemic heart disease, atrial fibrillation, and concurrent treatment with ACE inhibitors, beta‐blockers, aldosterone receptor antagonists, digoxin, diuretics, and antiplatelet drugs.
Statistical analysis
Meta‐analyses were performed for each treatment with calculations based on the number of randomized patients and the number of events (deaths and HF hospitalization) in the intervention and control group in each study, with the relative risk ratio (RR) being the calculated effect size.
Higgins et al.10 suggest that I 2 ≥ 50% represents moderate heterogeneity. Accordingly, the random effects model (DerSimonian–Laird) was used whenever I 2 ≥ 50%; otherwise, the fixed effect model (inverse variance) was used. The sensitivity analysis including relevant studies not referenced in the guidelines was calculated in the same way.
To assess the risk of publication bias, funnel plots were constructed using standard error as the measure for study size.
The meta‐analysis calculations were done using the R programming software11 and the R package ‘meta’ version 4.0‐3,12 while the R package ‘metafor’ version 1.4‐013 was used for the funnel plots.
Results
Description of studies
The ESC and ACCF/AHA guidelines included 29 and 41 relevant RCTs, respectively (Table 1). All included studies are listed in Appendix S2 in Supporting Information. A total of 23 studies were mentioned in both guidelines, while six studies were exclusively mentioned in the ESC guideline and 18 studies were exclusively mentioned in the ACCF/AHA guideline, thus totalling 47 unique studies. Two studies included subgroups permitting inclusion in the analysis of two treatments each. Six studies were used for the analysis of ACE inhibitors, four for ARBs, 15 for beta‐blockers, three for aldosterone receptor antagonists, five for digoxin, two for ivabradine, seven for CRT, and seven for ICD therapy. For the mortality analyses, 49 patient populations were included, while only 34 patient populations measured HF hospitalization.
Table 1.
Included RCTs mentioned in the ESC and ACCF/AHA HF guidelines
| ESC 2012 guideline only | Both | ACCF/AHA 2013 guideline only |
|---|---|---|
| TRACE; SENIORS subgroup; BEAUTIFUL; SHIFT; DEFINITE; IRIS | CONSENSUS; SOLVD treatment; SOLVD prevention; SAVE; Val‐HeFT subgroup; CHARM‐Alternative; RALES; EPHESUS; EMPHASIS‐HF; US Carvedilol; CIBIS‐II; MERIT‐HF; BEST; COPERNICUS; DIG; COMPANION; CARE‐HF; REVERSE; MADIT‐CRT; RAFT; MADIT‐II; DINAMIT; SCD‐HeFT | Losartan in Heart Failure; STRETCH; XISHF; MDC; Fisher, Gottlieba; CIBIS; Olsen, Gilbertb; Krum, Sackner‐Bernsteinc; PRECISE; ANZHF; CAPRICORN; CDMR; Milrinone Trial; RADIANCE; PROVED; MIRACLE; MIRACLE ICD II; MADIT |
Please refer to Appendix S2 in Supporting Information for the full list of studies divided by treatment and acronym explanations.
Fisher ML, Gottlieb SS, Plotnick GD, Greenberg NL, Patten RD, Bennett SK, Hamilton BP. Beneficial effects of metoprolol in heart failure associated with coronary artery disease: a randomized trial. Journal of the American College of Cardiology. 1994 Mar 15;23(4):943–950.
Olsen SL, Gilbert EM, Renlund DG, Taylor DO, Yanowitz FD, Bristow MR. Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double‐blind randomized study. Journal of the American College of Cardiology. 1995 May;25(6):1225–1231.
Krum H, Sackner‐Bernstein JD, Goldsmith RL, Kukin ML, Schwartz B, Penn J, Medina N, Yushak M, Horn E, Katz SD, et al. Double‐blind, placebo‐controlled study of the long‐term efficacy of carvedilol in patients with severe chronic heart failure. Circulation. 1995 Sep 15;92(6):1499–1506.
The studies varied in size from 49 to 10 917 patients, averaging 1667 patients per study. Patients were on average 63 years old, and female patients were underrepresented in all studies, making up only 22% on average. Follow‐up ranged from 2.8 to 45.5 months, averaging 18.7 months. Table 2 shows baseline characteristics of the study populations divided into groups by investigated treatment.
Table 2.
Baseline characteristics for the study populations according to the studied treatment
| Treatment | ACE inhibitor | ARB | Aldosterone receptor antagonist | Beta‐blocker |
|---|---|---|---|---|
| Sample size (n) | 1872.3 (204–4228) | 843.0 (134–2028) | 3677.3 (1663–6632) | 1229.3 (49–3991) |
| Follow‐up (months) | 37.9 (6.0–42.0) | 23.5 (2.8–33.7) | 18.4 (16.0–24.0) | 15.1 (3.3–22.8) |
| Age (years) | 61.1 (56.0–70.5) | 64.8 (60.9–66.6) | 65.3 (64.0–68.6) | 62.7 (49.0–76.0) |
| Male (%) | 82.3 (70.5–88.6) | 69.2 (68.1–85.0) | 73.0 (71.1–77.7) | 77.3 (63.1–96.0) |
| LVEF (%) | 28.1 (24.8–31) | 31.7 (24.2–38.8) | 30.2 (25.4–33.0) | 26.0 (16.3–36.0) |
| Creatinine (µmol/L) | 106.9 (106.08–128.0) | 115.5 | 98.5 (97.2–101.7) | 121.6 (101.0–145.9) |
| SBP (mmHg) | 121.9 (112.5–125.5) | 129.5 (126.0–130.1) | 120.8 (119.0–124.0) | 124.8 (115.6–135.6) |
| Diabetes (%) | 19.0 (13.5–25.8) | 27.0 | 31.8 (31.4–32.0) | 27.4 (12.0–35.6) |
| IHD (%) | 85.8 (63.0–100.0) | 68.7 (64.3–70.9) | 85.4 (54.5–100.0) | 63.2 (0.0–100.0) |
| AF | 7.7 (4.0–50.0) | 25.4 | 30.8 | 15.3 (0.0–34.1) |
| Concurrent treatment with | ||||
| ACE inhibitor (%)a | NA | NA | 85.5 (77.6–94.5) | 92.0 (79.4–97.4) |
| Beta‐blocker (%) | 20.8 (3.0–35.5) | 38.6 (0.4–54.5) | 68.2 (10.5–86.7) | NA |
| Aldosterone receptor antagonist (%) | 7.6 (4.0–52.5) | 17.1 (1.1–23.8) | NA | 15.4 (3.5–32.1) |
| Digoxin (%) | 32.1 (12.5–93.0) | 42.7 (0.0–45.6) | 44.6 (27.0–73.5) | 67.1 (43.3–96.0) |
| Diuretics (%) | 46.7 (16.6–85.5) | 78.4 (59.8–85.5) | 72.5 (60.5–100.0) | 93.9 (75.5–100.0) |
| Antiplatelet (%) | 59.1 (33.4–91.0) | 55.8 (48.9–58.6) | 80.6 (36.5–88.5) | 50.2 (25.9–86.0) |
| Treatment | Digoxin | Ivabradine | CRT | ICD therapy |
| Sample size (n) | 1474.6 (88–6800) | 8737.5 (6558–10 917) | 943.6 (186–1820) | 876.9 (196–1676) |
| Follow‐up (months) | 35.2 (2.8–37.0) | 20.5 (19.0–22.9) | 26.4 (6.0–40.0) | 30.1 (15.6–45.5) |
| Age (years) | 63.2 (59.8–64.0) | 63.4 (60.4–65.2) | 65.4 (62.6–67.3) | 62.6 (58.3–66.7) |
| Male (%) | 77.9 (75.7–85.2) | 80.4 (76.4–82.9) | 76.2 (67.7–89.2) | 76.6 (67.8–93.9) |
| LVEF (%) | 28.4 (25.0–28.5) | 31.1 (29.0–32.4) | 23.5 (20.7–26.7) | 24.5 (21.4–31.8) |
| Creatinine (µmol/L) | NA | NA | 106.1 (106.0–106.1) | 97.2 |
| SBP (mmHg) | 126.0 | 125.6 (121.7–128.0) | 117.5 (110.0–124.6) | 115.6 (112.0–119.0) |
| Diabetes (%) | 28.5 | 34.5 (30.4–37.0) | 30.9 (21.0–41.0) | 32.4 (6.0–42.4) |
| IHD (%) | 70.0 (52.9–70.6) | 88.0 (67.9–100.0) | 56.1 (38.0–66.8) | 73.7 (0.0–100.0) |
| AF | 0.0 | 0.0 | 8.0 (0.0–12.7) | 14.0 (8.6–24.5) |
| Concurrent treatment with | ||||
| ACE inhibitor (%)a | 92.4 (0.0–100.0) | 85.5 (78.6–89.7) | 83.1 (69.5–96.6) | 78.4 (57.5–94.7) |
| Aldosterone receptor antagonist (%) | NA | 39.5 (27.0–60.3) | 38.4 (1.1–56.2) | 34.3 (19.9–55.0) |
| Beta‐blocker (%) | 0.0 (0.0–0.0) | 87.9 (86.9–89.5) | 82.9 (58.5–95.1) | 72.7 (21.0–87.4) |
| Digoxin (%) | NA | 21.8 | 36.6 (25.7–78.5) | 59.8 (41.9–68.1) |
| Diuretics (%) | 82.8 (81.7–100.0) | 68.0 (58.9–83.2) | 84.8 (74.6–99.1) | 82.8 (52.5–95.9) |
| Antiplatelet (%) | NA | 94.1 | 62.9 (51.0–67.1) | 74.1 (56.9–98.3) |
Values shown are weighted means per patient and, in parenthesis, the range of the studies' means.
ACE inhibitor or ARB if no data for ACE inhibitor use specifically.
Treatment effect on mortality
Figure 1 shows the recommended treatments' effect on mortality as found in the primary meta‐analysis. The forest plots for each treatment are shown in Figures S1–S8 in the Supporting Information.
Figure 1.
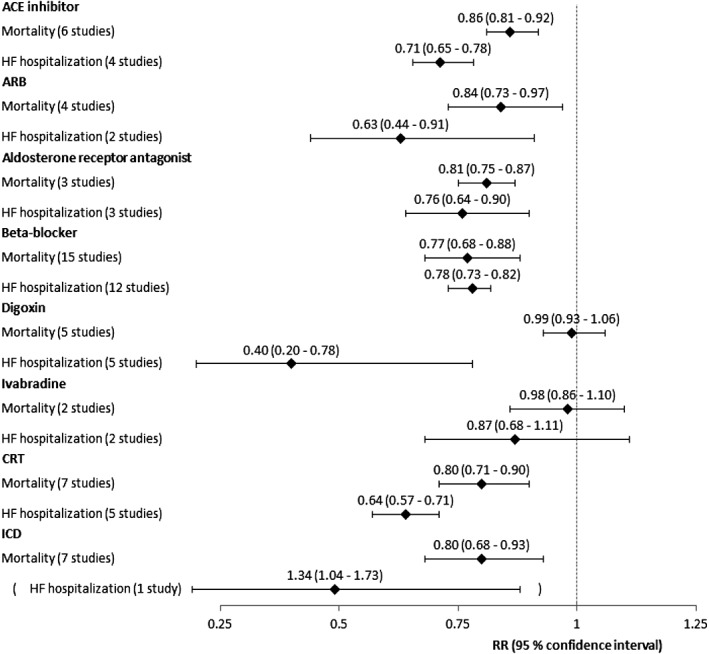
Relative risk ratios for mortality and HF hospitalization for each treatment group, primary meta‐analysis.
Drugs targeting the renin‐angiotensin‐aldosterone system significantly reduced the risk of death with risk reductions ranging from 14 to 19%, ACE inhibitors: RR 0.86 [95% confidence interval (CI) 0.81 to 0.92], ARBs: RR 0.84 (CI 0.73 to 0.97), and aldosterone receptor antagonists: RR 0.81 (CI 0.75 to 0.87). Beta‐blockers also significantly reduced this risk by 23% (RR 0.77, CI 0.68 to 0.88). Both digoxin and ivabradine showed no effect on mortality (RR 0.99, CI 0.93 to 1.06, and RR 0.98, CI 0.86 to 1.10, respectively). As for device therapy, both CRT and ICDs reduced the risk of death by 20% (RR 0.80, CI 0.71 to 0.90, and RR 0.80, CI 0.68 to 0.93, respectively).
Treatment effect on HF hospitalization
Figure 1 shows the recommended treatments' effect on HF hospitalization. Forest plots for each treatment are shown in Figures S9–S16 in the Supporting Information.
Drugs targeting the renin‐angiotensin‐aldosterone system significantly reduced the risk of HF hospitalization with risk reductions ranging from 24 to 37%, ACE inhibitors: RR 0.71 (CI 0.65 to 0.78), ARBs: RR 0.63 (CI 0.44 to 0.91), and aldosterone receptor antagonists: RR 0.76 (CI 0.64 to 0.90). Beta‐blockers significantly reduced the risk of death by 22% (RR 0.78, CI 0.73 to 0.82). Based on only two studies each, digoxin significantly reduced the risk of HF hospitalization by 60% (RR 0.40, CI 0.20 to 0.78), and ivabradine showed an insignificant risk reduction of 13% (RR 0.87, CI 0.68 to 1.11). CRT significantly reduced the risk of HF hospitalization by 36% (RR 0.64, CI 0.57 to 0.71). Only one study was found for the analysis of ICDs' effect on HF hospitalization, and this study suggested a significant 34% increase in the risk of HF hospitalization (RR 1.34, CI 1.04 to 1.73).
Sensitivity analysis
The PubMed search gave a total of 1781 articles. The flowchart in Figure 2 summarizes the study selection. An additional 36 multicentre RCTs were found, 2 of which included subgroups permitting inclusion in the analysis of two treatments each.14, 15 The sensitivity analysis thus included an additional 11 studies for ACE inhibitors, 2 for ARBs, 15 for beta‐blockers, 2 for aldosterone receptor antagonists, 1 for digoxin, 1 for ivabradine, 4 for CRT, and 2 for ICD therapy. Refer to Appendix S3 in Supporting Information for the full list of additional studies. A total of 36 additional studies were included in the mortality analysis, and 20 additional studies were used in the HF hospitalization analysis. No additional studies examining the effect of treatment with digoxin and ICDs on HF hospitalization, nor ivabradine on mortality, were found.
Figure 2.
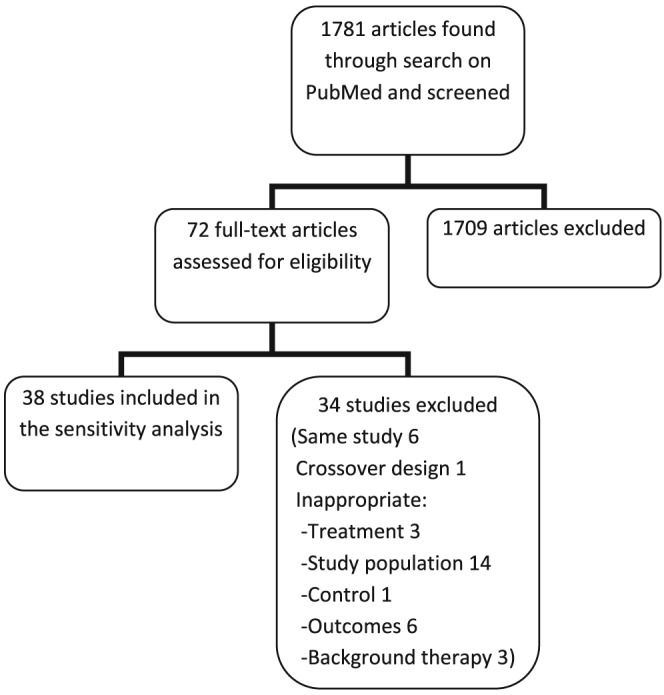
Flowchart depicting the study selection for the sensitivity analysis.
Figure 3 shows the results of the sensitivity analyses with additional studies included. The forest plots for each treatment can be seen in the Supporting Information.
Figure 3.
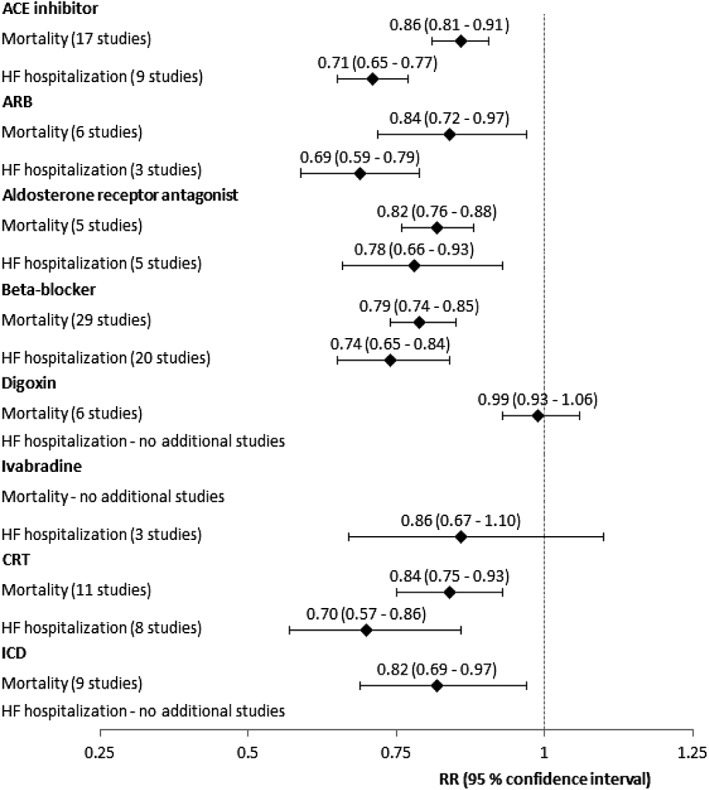
Relative risk ratios for mortality and HF hospitalization for each treatment group, sensitivity analysis.
Publication bias
Funnel plots for the studies in the primary meta‐analyses can be seen in Supporting Information. All plots exhibited fairly symmetrical inverted funnel shapes indicating that publication bias was not a concern.
Discussion
Summary
In the primary analysis based on RCTs listed in guidelines, it was found that the three drugs affecting the renin‐angiotensin‐aldosterone system, as well as treatment with beta‐blockers and CRT, resulted in significant risk reductions for both mortality and HF hospitalization. In contrast, ivabradine showed insignificant effects on both mortality and HF hospitalization. Digoxin only showed a significant risk reduction for HF hospitalization. While ICDs provided a significant risk reduction in mortality, it appears that the risk of HF hospitalization was significantly increased. However, this must be seen in light of the fact that ICD therapy routinely leads to hospitalization in the event that the device fires.
Sensitivity analysis
After including an additional 36 multicentre RCTs, the sensitivity analysis' results were similar to the ones found in the primary meta‐analysis, suggesting that evidence behind the guidelines is representative of the general literature on the area. However, there were differences to be seen, especially for CRT, where the risk reduction for mortality went from 20 to 16% (RR 0.80, CI 0.71 to 0.90, to RR 0.84, CI 0.75 to 0.93) and for HF hospitalization from 36 to 30% (RR 0.64, CI 0.57 to 0.71, to RR 0.71, CI 0.64 to 0.78). Although these differences were not statistically significant, they suggest that the guidelines may be overestimating the effects of CRT. Furthermore, of the seven studies looking at the effect of CRT on mortality in the primary analysis, only two studies showed significant risk reductions. In the sensitivity analysis, only one of the four additional studies was significant (and actually documented an increase in the risk of death).
Despite only including additional multicentre RCTs in the sensitivity analysis, the 38 additional patient populations were much smaller compared with the studies listed in guidelines; only three included more than 500 patients, vs. 31 of 49 patient populations in the primary meta‐analysis, and averaging 431 patients vs. 1667 in the primary analysis' studies. It makes sense to reference the most powerful studies in guidelines, and this is likely the reason why several of the studies found in the PubMed search were not mentioned in the guidelines.
Comparison with other meta‐analyses
The effect sizes of treatments found in this article are similar to findings in existing systematic reviews and meta‐analyses on ACE inhibitors,16 ARBs,17 aldosterone receptor antagonists,18 beta‐blockers,19 digoxin,20 CRT,21 and ICDs,22 suggesting that the few new RCTs that have been published do not change the general picture of the treatments' effects.
A pooled analysis from 2013, using data from the two major RCTs investigating ivabradine, looked only at a subgroup of patients with HFREF with heart rates ≥70 b.p.m.23 As in this paper's analysis, which included all the patients and not just this subgroup, no significant effect on mortality was found. However, in this subgroup of patients with heart rates ≥70 b.p.m., the trend to a decrease in the risk of HF hospitalization found in this paper (RR 0.87, CI 0.68 to 1.10) became a significant 19% risk reduction (P < 0.001). In accordance with these findings, the ESC guidelines recommend ivabradine for a subset of patients with HFREF with heart rates ≥70 b.p.m.
Differences between studied and contemporary patients with HF and implications
Once the efficacy of a treatment has been established, the relevance of continuing to conduct RCTs investigating the effect of the treatment is doubtful at best and certainly raises ethical concerns. It is therefore not surprising that many of the included studies are from the 1990s or earlier. However, the aging studies can pose a problem when attempting to use their findings to predict the benefits of treatment for contemporary patients. The studied HF patient population in the primary meta‐analysis included more than four times as many men as women, while a cross‐sectional survey following 2042 residents in Olmsted County, Minnesota, from 1997–2000 found that the prevalence of congestive HF was only 1.7 times higher in men.24 The studied patients with HF were on average 63 years old, while the average age of patients with HF in general practices in the UK in 2007 was 77 years.25 In other words, the majority of the studies' recruited patients may not reflect a contemporary HF patient population. This could indicate patient selection but certainly warrants caution when extrapolating results of these studies to contemporary patients with HF, whose characteristics are different and thus may respond differently to treatment, as suggested by the meta‐regression results.
Limitations
The literature search for additional RCTs was restricted to multicentre studies, and although it included thousands of studies, more search terms and additional databases could have been used.
This paper examined the treatment effect for patients with HFREF. As the definition of REF has changed over the years, it was decided to include studies with patients who had an EF ≤ 45%. The Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure study26 mentioned in the ESC guideline included patients with both REF and preserved EF. A paper based on a subgroup of the study including only patients with LVEF < 35% was therefore used instead.9 In Volterrani et al. 15 and Wever et al.,27 REF was not an inclusion criteria, but the patients' mean EF was low, and so the studies were included for analysis. The same decision was made for the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND) study,28 in which approx. 80% of patients had REF. The Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) Trial Study Group29 did not measure EF but instead included only NYHA class IV patients with heart sizes over 600 mL/m2 and 500 mL/m2 for men and women, respectively, and was included. All other included studies mentioned REF as a requirement for patient inclusion, although a few combined it with other measures of congestive HF: REF or increased cardiothoracic ratio,30 REF or cardiomegaly,31 and REF or fractional shortening.32
Although publication bias was assessed, other forms of bias such as selection and ascertainment bias were not.
Conclusions
Both the primary meta‐analysis and the sensitivity analysis found that most recommended HF treatments showed significant treatment effects on patients with HFREF. This suggests both that the recommended treatments are efficacious and that guidelines are based on a representative collection of studies. However, many of the studies are from the 1990s or earlier and include a preponderance of men with a different age distribution than today's average patient with HF, who is now more often female and older. One must therefore still be cautious when using these findings to predict the effect on contemporary patients with HF. Furthermore, individual patient characteristics that might affect treatment efficacy are largely ignored in guidelines, and studies investigating such relationships could prove valuable in clinical practice.
Conflict of interest
None declared.
Supporting information
Appendix S1. List of searches done in the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed).
Appendix S2. List of included RCTs identified in guidelines divided by treatment.
Appendix S3. List of additional multicenter RCTs included for each treatment group in the sensitivity analysis.
Figure S1. ACE inhibitors' effect on mortality, primary meta‐analysis.
Figure S2. ARBs' effect on mortality, primary meta‐analysis.
Figure S3. Aldosterone receptor antagonists' effect on mortality, primary meta‐analysis.
Figure S4. Beta‐blockers' effect on mortality, primary meta‐analysis.
Figure S5. Digoxin's effect on mortality, primary meta‐analysis.
Figure S6. Ivabradine's effect on mortality, primary meta‐analysis.
Figure S7. CRT device therapy's effect on mortality, primary meta‐analysis.
Figure S8. ICD therapy's effect on mortality, primary meta‐analysis.
Figure S9. ACE inhibitors' effect on HF hospitalization, primary meta‐analysis.
Figure S10. ARBs' effect on HF hospitalization, primary meta‐analysis.
Figure S11. Aldosterone receptor antagonists' effect on HF hospitalization, primary meta‐analysis.
Figure S12. Beta‐blockers' effect on HF hospitalization, primary meta‐analysis.
Figure S13. Digoxin's effect on HF hospitalization, primary meta‐analysis.
Figure S14. Ivabradine's effect on HF hospitalization, primary meta‐analysis.
Figure S15. CRT device therapy's effect on HF hospitalization, primary meta‐analysis.
Figure S16. ICD therapy's effect on HF hospitalization, primary analysis.
Figure S17. ACE inhibitors' effect on mortality, sensitivity analysis.
Figure S18. ARBs' effect on mortality, sensitivity analysis.
Figure S19. Beta‐blockers' effect on mortality, sensitivity analysis.
Figure S20. Aldosterone receptor antagonists' effect on mortality, sensitivity analysis.
Figure S21. Digoxin's effect on mortality, sensitivity analysis.
Figure S22. CRT device therapy's effect on mortality, sensitivity analysis.
Figure S23. ICD therapy's effect on mortality, sensitivity analysis.
Figure S24. ACE inhibitors' effect on HF hospitalization, sensitivity analysis.
Figure S25. ARBs' effect on HF hospitalization, sensitivity analysis.
Figure S26. Beta‐blockers's effect on HF hospitalization, sensitivity analysis.
Figure S27. Aldosterone receptor antagonists' effect on HF hospitalization, sensitivity analysis.
Figure S28. Ivabradine's effect on HF hospitalization, sensitivity analysis.
Figure S29. CRT device therapy's effect on HF hospitalization, sensitivity analysis.
Figure S30. Funnel plot for ACE inhibitor studies included in the primary meta‐analysis on mortality.
Figure S31. Funnel plot for ARB studies included in the primary meta‐analysis on mortality.
Figure S32. Funnel plot for aldosterone receptor antagonist studies included in the primary meta‐analysis on mortality.
Figure S33. Funnel plot for beta‐blocker studies included in the primary meta‐analysis on mortality.
Figure S34. Funnel plot for digoxin studies included in the primary meta‐analysis on mortality.
Figure S35. Funnel plot for ivabradine studies included in the primary meta‐analysis on mortality.
Figure S36. Funnel plot for CRT device studies included in the primary meta‐analysis on mortality.
Figure S37. Funnel plot for ICD studies included in the primary meta‐analysis on mortality.
Figure S38. Funnel plot for ACE inhibitor studies included in the primary meta‐analysis on HF hospitalization.
Figure S39. Funnel plot for ARB studies included in the primary meta‐analysis on HF hospitalization.
Figure S40. Funnel plot for aldosterone receptor antagonist studies included in the primary meta‐analysis on HF hospitalization.
Figure S41. Funnel plot for beta‐blocker studies included in the primary meta‐analysis on HF hospitalization.
Figure S42. Funnel plot for digoxin studies included in the primary meta‐analysis on HF hospitalization.
Figure S43. Funnel plot for ivabradine studies included in the primary meta‐analysis on HF hospitalization.
Figure S44. Funnel plot for CRT device studies included in the primary meta‐analysis on HF hospitalization.
Supporting info item
Acknowledgement
Special thanks to Professor John Martin Bland from the University of York, Department of Health Sciences, UK for coming with valuable comments to the manuscript.
Thomsen, M. M. , Lewinter, C. , and Køber, L. (2016) Varying effects of recommended treatments for heart failure with reduced ejection fraction: meta‐analysis of randomized controlled trials in the ESC and ACCF/AHA guidelines. ESC Heart Failure, 3: 235–244. doi: 10.1002/ehf2.12094.
References
- 1. Roger VL, Go AS, Lloyd‐Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie‐Rosett J, American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics‐‐2011 update: a report from the American Heart Association. Circulation 2011; 123: e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines ESCCfP . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 3. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Writing Committee Members , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 4. Captopril Multicenter Research Group . A placebo‐controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2: 755–763. [DOI] [PubMed] [Google Scholar]
- 5. Cohen‐Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, Merlet P. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. J Nucl Med: Off Publ Soc Nucl Med 2005; 46: 1796–1803. [PubMed] [Google Scholar]
- 6. Krum H, Sackner‐Bernstein JD, Goldsmith RL, Kukin ML, Schwartz B, Penn J, Medina N, Yushak M, Horn E, Katz SD, Levin HR, Neuberg GW, DeLong G, Packer M. Double‐blind, placebo‐controlled study of the long‐term efficacy of carvedilol in patients with severe chronic heart failure. Circulation 1995; 92: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 7. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 8. Packer M, Colucci WS, Sackner‐Bernstein JD, Liang CS, Goldscher DA, Freeman I, Kukin ML, Kinhal V, Udelson JE, Klapholz M, Gottlieb SS, Pearle D, Cody RJ, Gregory JJ, Kantrowitz NE, LeJemtel TH, Young ST, Lukas MA, Shusterman NH. Double‐blind, placebo‐controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial. Prospective Randomized Evaluation of Carvedilol on Symptoms and Exercise. Circulation 1996; 94: 2793–2799. [DOI] [PubMed] [Google Scholar]
- 9. van Veldhuisen DJ, Cohen‐Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole‐Wilson PA, Flather MD, Investigators S. Beta‐blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol 2009; 53: 2150–2158. [DOI] [PubMed] [Google Scholar]
- 10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003 Sep 6; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. R Core Team RFfSC . R: A Language and Environment for Statistical Computing. 2014.
- 12. Schwarzer G. meta: An R Package for Meta‐Analysis. R News 2007; 7: 40–45. [Google Scholar]
- 13. Viechtbauer W. Conducting meta‐analyses in {R} with the {metafor} package. J Statist Software 2010; 36: 1–48. [Google Scholar]
- 14. Remme WJ, Riegger G, Hildebrandt P, Komajda M, Jaarsma W, Bobbio M, Soler‐Soler J, Scherhag A, Lutiger B, Ryden L. The benefits of early combination treatment of carvedilol and an ACE‐inhibitor in mild heart failure and left ventricular systolic dysfunction. The carvedilol and ACE‐inhibitor remodelling mild heart failure evaluation trial (CARMEN). Cardiovasc Drugs ther / Sponsored Intern Soc Cardiovasc Pharmacother 2004; 18: 57–66. [DOI] [PubMed] [Google Scholar]
- 15. Volterrani M, Cice G, Caminiti G, Vitale C, D'Isa S, Perrone Filardi P, Acquistapace F, Marazzi G, Fini M, Rosano GM. Effect of Carvedilol, Ivabradine or their combination on exercise capacity in patients with Heart Failure (the CARVIVA HF trial). Int J Cardiol 2011; 151: 218–224. [DOI] [PubMed] [Google Scholar]
- 16. Flather MD, Yusuf S, Kober L, Pfeffer M, Hall A, Murray G, Torp‐Pedersen C, Ball S, Pogue J, Mosye L, Braunwald E. Long‐term ACE‐inhibitor therapy in patients with heart failure or left‐ventricular dysfunction: a systematic overview of data from individual patients. ACE‐Inhibitor Myocardial Infarction Collaborative Group. Lancet 2000; 355: 1575–1581. [DOI] [PubMed] [Google Scholar]
- 17. Heran BS, Musini VM, Bassett K, Taylor RS, Wright JM. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev 2012. DOI: 10.1002/14651858.CD003040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J 2009; 30: 469–477. [DOI] [PubMed] [Google Scholar]
- 19. Brophy JM, Joseph L, Rouleau JL. Beta‐blockers in congestive heart failure. A Bayesian meta‐analysis. Ann Intern Med 2001; 134: 550–560. [DOI] [PubMed] [Google Scholar]
- 20. Hood WB Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJ. Digitalis for treatment of congestive heart failure in patients in sinus rhythm: a systematic review and meta‐analysis. J Card Fail 2004; 10: 155–164. [DOI] [PubMed] [Google Scholar]
- 21. McAlister FA, Ezekowitz J, Hooton N, Vandermeer B, Spooner C, Dryden DM, Page RL, Hlatky MA, Rowe BH. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. Jama 2007; 297: 2502–2514. [DOI] [PubMed] [Google Scholar]
- 22. Ezekowitz JA, Rowe BH, Dryden DM, Hooton N, Vandermeer B, Spooner C, McAlister FA. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med 2007; 147: 251–262. [DOI] [PubMed] [Google Scholar]
- 23. Fox K, Komajda M, Ford I, Robertson M, Bohm M, Borer JS, Steg PG, Tavazzi L, Tendera M, Ferrari R, Swedberg K. Effect of ivabradine in patients with left‐ventricular systolic dysfunction: a pooled analysis of individual patient data from the BEAUTIFUL and SHIFT trials. Eur Heart J 2013 Aug; 34: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 24. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama 2003; 289: 194–202. [DOI] [PubMed] [Google Scholar]
- 25. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, Borbola J, Cohen‐Solal A, Dumitrascu D, Ferrari R, Lechat P, Soler‐Soler J, Tavazzi L, Spinarova L, Toman J, Bohm M, Anker SD, Thompson SG, Poole‐Wilson PA, Investigators S. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26: 215–225. [DOI] [PubMed] [Google Scholar]
- 27. Wever EF, Hauer RN, van Capelle FL, Tijssen JG, Crijns HJ, Algra A, Wiesfeld AC, Bakker PF, Robles de Medina EO. Randomized study of implantable defibrillator as first‐choice therapy versus conventional strategy in postinfarct sudden death survivors. Circulation 1995; 91: 2195–2203. [DOI] [PubMed] [Google Scholar]
- 28. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43. [DOI] [PubMed] [Google Scholar]
- 29. The CONSENSUS Trial Study Group . Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 30. Crozier I, Ikram H, Awan N, Cleland J, Stephen N, Dickstein K, Frey M, Young J, Klinger G, Makris L, Rucinska E. Losartan in heart failure. Hemodynamic effects and tolerability. Losartan Hemodynamic Study Group. Circulation 1995; 91: 691–697. [DOI] [PubMed] [Google Scholar]
- 31. Gundersen T, Wiklund I, Swedberg K, Amtorp O, Remes J, Nilsson B. Effects of 12 weeks of ramipril treatment on the quality of life in patients with moderate congestive heart failure: results of a placebo‐controlled trial. Ramipril Study Group. Cardiovasc Drugs Ther / Sponsored Intern Soc Cardiovasc Pharmacother 1995; 9: 589–594. [DOI] [PubMed] [Google Scholar]
- 32. Northridge DB, Currie PF, Newby DE, McMurray JJ, Ford M, Boon NA, Dargie HJ. Placebo‐controlled comparison of candoxatril, an orally active neutral endopeptidase inhibitor, and captopril in patients with chronic heart failure. Eur J Heart Fail 1999; 1: 67–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of searches done in the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed).
Appendix S2. List of included RCTs identified in guidelines divided by treatment.
Appendix S3. List of additional multicenter RCTs included for each treatment group in the sensitivity analysis.
Figure S1. ACE inhibitors' effect on mortality, primary meta‐analysis.
Figure S2. ARBs' effect on mortality, primary meta‐analysis.
Figure S3. Aldosterone receptor antagonists' effect on mortality, primary meta‐analysis.
Figure S4. Beta‐blockers' effect on mortality, primary meta‐analysis.
Figure S5. Digoxin's effect on mortality, primary meta‐analysis.
Figure S6. Ivabradine's effect on mortality, primary meta‐analysis.
Figure S7. CRT device therapy's effect on mortality, primary meta‐analysis.
Figure S8. ICD therapy's effect on mortality, primary meta‐analysis.
Figure S9. ACE inhibitors' effect on HF hospitalization, primary meta‐analysis.
Figure S10. ARBs' effect on HF hospitalization, primary meta‐analysis.
Figure S11. Aldosterone receptor antagonists' effect on HF hospitalization, primary meta‐analysis.
Figure S12. Beta‐blockers' effect on HF hospitalization, primary meta‐analysis.
Figure S13. Digoxin's effect on HF hospitalization, primary meta‐analysis.
Figure S14. Ivabradine's effect on HF hospitalization, primary meta‐analysis.
Figure S15. CRT device therapy's effect on HF hospitalization, primary meta‐analysis.
Figure S16. ICD therapy's effect on HF hospitalization, primary analysis.
Figure S17. ACE inhibitors' effect on mortality, sensitivity analysis.
Figure S18. ARBs' effect on mortality, sensitivity analysis.
Figure S19. Beta‐blockers' effect on mortality, sensitivity analysis.
Figure S20. Aldosterone receptor antagonists' effect on mortality, sensitivity analysis.
Figure S21. Digoxin's effect on mortality, sensitivity analysis.
Figure S22. CRT device therapy's effect on mortality, sensitivity analysis.
Figure S23. ICD therapy's effect on mortality, sensitivity analysis.
Figure S24. ACE inhibitors' effect on HF hospitalization, sensitivity analysis.
Figure S25. ARBs' effect on HF hospitalization, sensitivity analysis.
Figure S26. Beta‐blockers's effect on HF hospitalization, sensitivity analysis.
Figure S27. Aldosterone receptor antagonists' effect on HF hospitalization, sensitivity analysis.
Figure S28. Ivabradine's effect on HF hospitalization, sensitivity analysis.
Figure S29. CRT device therapy's effect on HF hospitalization, sensitivity analysis.
Figure S30. Funnel plot for ACE inhibitor studies included in the primary meta‐analysis on mortality.
Figure S31. Funnel plot for ARB studies included in the primary meta‐analysis on mortality.
Figure S32. Funnel plot for aldosterone receptor antagonist studies included in the primary meta‐analysis on mortality.
Figure S33. Funnel plot for beta‐blocker studies included in the primary meta‐analysis on mortality.
Figure S34. Funnel plot for digoxin studies included in the primary meta‐analysis on mortality.
Figure S35. Funnel plot for ivabradine studies included in the primary meta‐analysis on mortality.
Figure S36. Funnel plot for CRT device studies included in the primary meta‐analysis on mortality.
Figure S37. Funnel plot for ICD studies included in the primary meta‐analysis on mortality.
Figure S38. Funnel plot for ACE inhibitor studies included in the primary meta‐analysis on HF hospitalization.
Figure S39. Funnel plot for ARB studies included in the primary meta‐analysis on HF hospitalization.
Figure S40. Funnel plot for aldosterone receptor antagonist studies included in the primary meta‐analysis on HF hospitalization.
Figure S41. Funnel plot for beta‐blocker studies included in the primary meta‐analysis on HF hospitalization.
Figure S42. Funnel plot for digoxin studies included in the primary meta‐analysis on HF hospitalization.
Figure S43. Funnel plot for ivabradine studies included in the primary meta‐analysis on HF hospitalization.
Figure S44. Funnel plot for CRT device studies included in the primary meta‐analysis on HF hospitalization.
Supporting info item


