Abstract
Purpose
Although it is generally accepted that consolidation therapy for neuroblastoma includes irradiation of the primary site and any remaining metaiodobenzylguanidine (MIBG)-avid metastatic sites, limited information has been published regarding the efficacy of this approach.
Methods and Materials
Thirty patients with high-risk neuroblastoma were treated at 1 radiation therapy (RT) department after receiving 5 cycles of induction chemotherapy and resection. All patients had at least a partial response after induction therapy, based upon international neuroblastoma response criteria. The primary sites were treated with 24 to 30 Gy whereas the MIBG-avid metastatic sites were treated with 24 Gy. RT was followed by high-dose chemotherapy with autologous stem cell rescue and 6 months of cis-retinoic acid.
Results
The 5-year progression-free survival (PFS) and overall survival (OS) rates were 48% and 59%, respectively. The 5-year locoregional control at the primary site was 84%. There were no differences in locoregional control according to degree of primary surgical resection. The 5-year local control rate for metastatic sites was 74%. The 5-year PFS rates for patients with 0, 1, 2, and >3 postinduction MIBG sites were 66%, 57%, 20%, and 0% (P<.0001), respectively, whereas 5-year OS rates were 80%, 57%, 50%, and 0%, respectively (P<.0001).
Conclusions
RT to the primary site and postinduction MIBG-positive metastatic sites was associated with 84% and 74% local control, respectively. The number of MIBG-avid sites present after induction chemotherapy and surgery was predictive of progression-free and overall survival.
Introduction
Neuroblastoma is the most common extracranial solid tumor in the pediatric population, with approximately 650 cases diagnosed each year in the United States (1). Half of the patients who receive diagnoses of neuroblastoma will present with high-risk disease, and despite aggressive multimodal therapy, the historical 5-year event-free and overall survival (OS) rates for these children are poor (2, 3). Although the recent addition of immunotherapy to the maintenance phase of treatment has improved event-free and OS for many children, advances in treatment options and delivery methods are still required to improve long-term survival (4).
The current treatment for high-risk neuroblastoma is divided into 3 phases: induction, consolidation, and maintenance. During the first portion of therapy, patients receive induction chemotherapy and undergo surgical resection of their primary lesion. Patients who have had at least a partial response to induction will continue on to consolidation therapy which includes radiation therapy (RT), either before or after receiving high-dose chemotherapy and autologous stem cell transplantation. RT is given to the primary site, and iodine-131-labeled metaiodobenzylguanidine (MIBG)-avid metastatic sites after induction therapy (5). Although a preponderance of reports support the use of RT to the primary disease in high-risk neuroblastoma, there is little published evidence to support its use in the treatment of metastatic disease. The primary purpose of this retrospective study was to determine the local control efficacy of RT to the primary and postinduction MIBG-avid metastatic sites using a RT dose of 24 to 30 Gy in 12 to 15 fractions. A secondary aim of the study was to determine whether the degree of resection had an impact on primary site locoregional control.
Methods and Materials
Eligibility criteria
After the study received institutional review board approval, patients were identified by searching the institutional database at Texas Children's Hospital for those with newly diagnosed high-risk neuroblastoma from January 1, 2006, through June 30, 2011. Additional eligibility criteria for inclusion within this cohort included: (1) no previous neuroblastoma-directed therapy; (2) absence of progression during induction therapy or as evident on postinduction imaging; and (3) follow-up at our institution.
Treatment plan
The chemotherapy treatment scheme consisted of 5 cycles of induction chemotherapy consisting of cisplatin, etoposide, doxorubicin, and cyclophosphamide. Surgical resection of primary disease took place during the induction period but occurred at time points ranging from the completion of the second cycle to the fifth cycle of chemotherapy. The degree of resection of the primary site was assessed with computed tomography (CT) and/or a magnetic resonance imaging MRI scan. A gross total resection (GTR; n = 22 patients) was obtained when there was no residual tumor on postoperative imaging, whereas a subtotal resection (STR; n = 8 patients) was defined as any residual tumor after the primary site tumor on postoperative imaging. Patients were subsequently treated with conformal RT to the primary disease and to any metastatic sites that were positive on MIBG-labeled scan after induction therapy. Following RT, patients were treated with autologous stem cell transplantation, using a preparative regimen consisting of a carboplatin, etoposide (Etopophos), and melphalan. Subsequently, patients with no evidence of disease progression after consolidation were treated with 6 months of maintenance therapy with cis-retinoic acid. Nine patients in this cohort were treated in neuroblastoma-specific immunotherapeutic trials: 4 patients in the Children's Oncology Group (COG) phase 3 study, adding ch14.18, interleukin-2 (IL-2), and sargramostim (a granulocyte macrophage colony-stimulating factor [GM-CSF]) to cis-retinoic acid therapy (4); and 5 patients with an allogeneic neuroblastoma tumor cell vaccine modified to secrete lymphotactin and IL-2 (ClinicalTrials.gov. NCT00703222).
Radiation therapy
The dose of RT to the primary was 24 Gy in 12 fractions for 27 patients, 26 Gy in 13 fractions for 1 patient, and 30 Gy in 15 fractions for 2 patients. Three patients received doses >24 Gy because of concern for residual disease that needed a higher RT dose; however, none of these patients was found to have residual disease on postoperative imaging. All patients who had subtotal resection (n = 8) received 24 Gy in 12 fractions; none of these 8 patients received an RT boost. The dose to sites of metastatic disease was 24 Gy in 12 fractions for all patients. The RT volume for the primary site was the tumor volume prior to surgical resection as defined by CT or MRI with a 1- to 1.5-cm clinical target volume (CTV) expansion and a 0.5-cm expansion for the planning target volume (PTV). Regional lymph node regions that were not involved prior to resection were not included in the CTV. The RT volumes for metastatic sites were the sites visible on the MIBG-labeled scan after induction therapy with a 1- to 2-cm margin for CTV. A maximum number of 3 metastatic sites were irradiated. If a patient had more than 3 metastatic sites visible on the postinduction MIBG-labeled scan, then the 3 most avid sites were irradiated. The number of sites irradiated (primary and metastatic) for the 30 patients was analyzed. A total of 50 sites were irradiated (29 primary and 21 distant sites). One patient did not have a defined primary site and received irradiation only to the postinduction chemotherapy MIBG-avid distant site. Seventeen children had 1 site, 6 had 2 sites, 3 had 3 sites, and another 3 had 4 total sites irradiated. Of the 21 metastatic sites receiving RT, 14 were in bone, 5 in lymph nodes, 1 in the lung, and 1 in the liver.
Statistics
Progression-free survival (PFS) time was calculated as the time from diagnosis to relapse or progression. Overall survival was calculated as the time from diagnosis to death from any cause. SPSS, version 20.0, statistical software (IBM, Armonk, NY) was used. Kaplan-Meier survival curves were created to examine PFS and OS. For a comparison of survival curves, the log-rank test was used.
Results
A total of 30 patients with high-risk neuroblastoma treated from January 2006 to June 2011 met inclusion criteria. There were 15 boys and 15 girls in this cohort, with a median patient age of 32 months (range, 8 months to 19 years) at the time of diagnosis. Six patients had International Neuroblastoma Staging System (INSS) stage III, whereas 24 had stage IV disease. Nineteen of 30 had MycN amplification.
With a median follow-up of 33 months (range, 10 months to 6 years), the 2-year and 5-year PFS rates were 58% and 47%, respectively (Fig. 1A). The 2-year and 5-year OS rates were 65% and 59%, respectively (Fig. 1B). There were no MIBG-avid sites after induction therapy in 14 patients, 1 MIBG site in 7 patients, 2 sites in 5 patients, 3 sites in 2 patients, and 4 sites in 2 patients. The 5-year PFS and OS rates differed significantly based on the number of sites that were MIBG positive after induction therapy. For patients with 0, 1, 2, and >3 positive sites, the 5-year PFS rates were 66%, 57%, 20%, and 0%, respectively (P<.001) (Fig. 2). The 5-year OS rates for patients with 0, 1, 2, and >3 positives sites on MIBG-labeled scan after induction therapy were 80%, 57%, 50%, and 0%, respectively (P<.001).
Fig. 1.
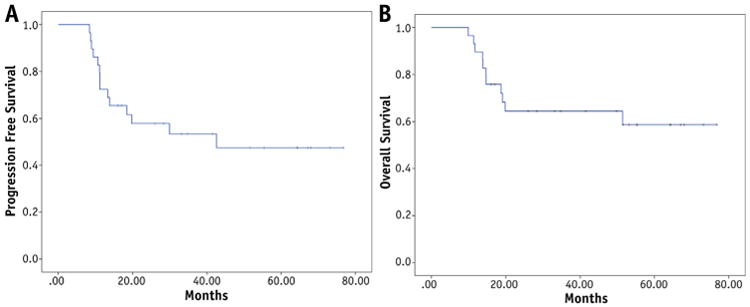
Progression-free survival (A) and overall survival (B) of children with high-risk neuroblastoma treated with radiation therapy to the primary and postinduction MIBG-avid sites.
Fig. 2.
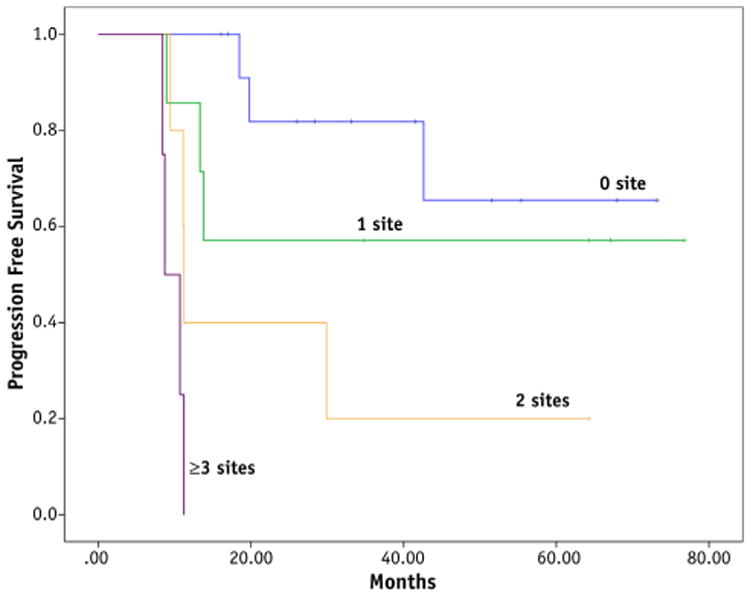
Progression-free survival is shown according to the number of postinduction MIBG-avid sites (P<.0001).
The 5-year locoregional control rate for the primary site was 84%. Primary site locoregional control rates did not differ significantly based on the degree of resection. The 5-year locoregional control rates for the primary site following resection and RT were 85% after GTR and 83% after STR (P = .976) (Fig. 3). All of the primary site failures were in the high-dose region (>95% of prescribed dose); there were no regional nodal failures in nonirradiated areas.
Fig. 3.
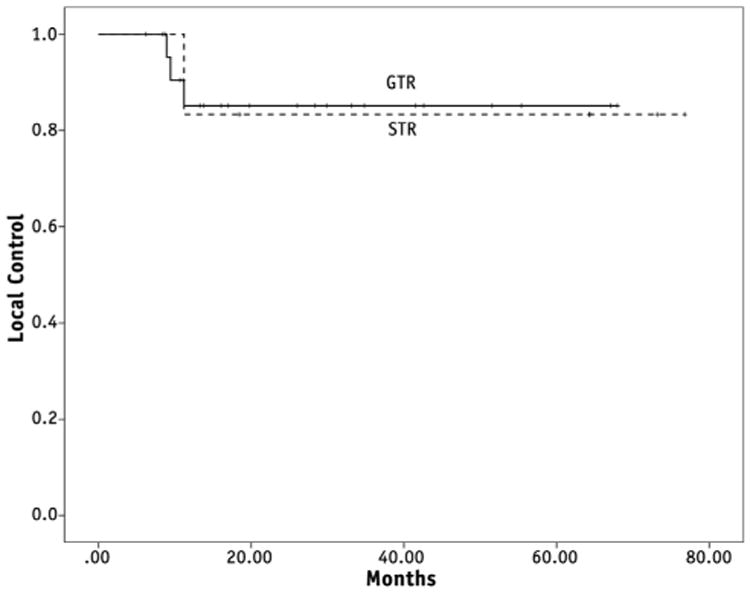
Locoregional control is shown according to degrees of resection (P = .98). GTR = gross total resection; STR = subtotal resection.
There were 23 sites of failure in our patient cohort: 17 in the bone, 3 in lymph nodes (2 regional within the high dose region, 1 distant), 1 in the retroperitoneum, and 1 in the orbit. Upon analysis of the 125 sites that were MIBG-avid prior to any therapy, 19 were found to be a subsequent site of failure. Thus, most of the failures (19 of 23, 82.6%) occurred in sites that were MIBG-positive prior to any therapy. When analyzed by MIBG site positivity at the end of induction, only 4 of the 23 sites of recurrence were MIBG-positive at the end of induction therapy. All of these sites had been treated with RT. Thus, the 5-year local control of irradiated metastatic sites was 74%.
Discussion
Due to the propensity for relapses at the primary site and the efficacy of RT in decreasing locoregional relapse, RT to the primary site is currently a standard component of therapy in high-risk neuroblastoma (6-12). Locoregional control rates in different studies have ranged from 25% to 61% when no RT was used compared to 84% to 100% with systematic use of primary site RT (21-21.6 Gy to the primary site). Some studies have also suggested that a higher dose of RT may be needed for patients who do not achieve a GTR (8-10). A previous German study reported a 3-year event-free survival of 85% in patients with subtotal resection who received a median dose of 36 Gy (30.6-40 Gy). Patients who had a GTR and did not receive primary site RT had a 3-year event-free survival of 61% (8). In the most recent COG high-risk neuroblastoma study (ANBL0532), patients who achieve a GTR were to receive 21.6 Gy, whereas those who had STR were to receive 36 Gy to the primary site, and outcome results from this study are currently pending. In our cohort, patients were treated with 24 to 30 Gy of RT to their primary site, and we found no significant differences in locoregional control rates based on the degree of resection (GTR with 85% and STR with 83% control at 5 years). We also found there were no regional nodal recurrences outside the high-dose region. Some groups have recommended elective treatment for regional nodes and next-echelon lymph nodes without gross involvement in imaging (9-11, 17). Although this is a small retrospective study, our data suggest that elective nodal irradiation does not appear to be necessary in high-risk neuroblastoma, and only nodal disease seen on presurgical imaging need be irradiated. This information may be useful in sparing normal surrounding organs such as the kidneys and liver from unnecessary radiation; however, larger studies are needed to validate this finding.
Irradiation of distant sites that were MIBG-avid after induction therapy resulted in a 74% 5-year local control rate. Sites that were initially MIBG-positive and converted to negative after induction chemotherapy were the most common sites of relapse. Unlike the sites that did not completely respond to induction chemotherapy, these metastatic sites were not irradiated. Thus, either better systemic treatment is needed to ensure that these sites sustain their initial treatment response and/or a more sensitive and specific imaging test is necessary to assess response to therapy.
Outcome analysis of survival also found that both PFS and OS rates significantly differed based on the number of MIBG-positive sites remaining after induction therapy. None of the patients was alive at 5 years if there were >3 positive sites on the MIBG-labeled scan after induction therapy. This suggests that the standard of care treatment provided to this cohort was not adequate for patients with >3 postinduction MIBG-positive sites. A recent study also showed that the number and extent of MIBG-avid sites in the bone and soft tissue after induction chemotherapy, expressed as a modified Curie score, were predictive for event-free survival in stage IV neuroblastoma (13). Additionally, methods to further decrease tumor burden prior to the start of consolidation, such as targeted radioisotope therapy with treatment doses of Iodine 131-MIBG, are being investigated in cooperative group clinical trials for children with high-risk neuroblastoma (14-16).
In the current COG study, ANBL02P1, children with postinduction MIBG-positive sites were irradiated. If there are more than 4 avid sites, post–stem cell transplantation MIBG is used to determine distant sites that need to be irradiated. It will be interesting to compare our results to the outcomes of the current study to discover whether irradiating more than 2 MIBG-avid sites is beneficial.
In patients who had no postinduction MIBG-positive sites, the 5-year overall and progression-free survival rates were 80% and 66%, respectively. Although this is a very favorable outcome, this is a relatively small number of patients (n = 14). A previous study by Katzenstein et al (17) showed a 4-year event-free survival of 80% for children with no postinduction MIBG-positive sites. Others have reported less favorable outcomes with 3-year overall and event-free survival rates of 50% to 60% and 35% to 55%, respectively (18, 19).
It should be noted that some patients within this cohort were treated with either the COG maintenance protocol using ch14.18, IL-2, and GM-CSF with cis-retinoic acid or an immunotherapeutic allogeneic tumor cell vaccine and that the addition of these therapies may have altered or improved the outcomes for those with minimal residual disease at the end of consolidation (4). However, the fact that two-thirds of our cohort were not treated with immunotherapy lends additional support to the antitumor efficacy of RT for remaining sites of metastatic disease at the end of induction. Overall and event-free survival outcomes seen with this study may also be biased by the fact that all patients included in the analysis were those who had at least a partial response during induction therapy. Nevertheless, this study shows that local control is favorable with the use of RT at both the primary and postinduction MIBG distant sites. Irradiating more than 2 postinduction MIBG-avid lesions does not appear to be beneficial as this indicates a greater burden of disease unlikely to be controlled with the current standard of care as provided in this report.
Conclusions
Irradiation of primary and residual MIBG-avid distant sites provided 5-year local control rates of 84% and 74%, respectively. The number of MIBG-avid sites after induction therapy was predictive of 5-year PFS and OS. Based upon our retrospective cohort data, the current practice of irradiating children with 3 or more MIBG-positive sites remaining after induction chemotherapy appears inadequate given that these patients are most likely to fail in distant sites that initially responded to chemotherapy and were therefore not irradiated during consolidation therapy.
Summary.
Radiation therapy to the primary site and postinduction metaiodobenzylguanidine (MIBG)-positive metastatic sites were associated with favorable local control in children with high-risk neuroblastoma. The number of MIBG-avid sites present after induction chemotherapy and surgery was predictive of progression-free and overall survival.
Acknowledgments
Dr Louis is supported by a Sidney Kimmel Foundation for Cancer Research Translational Science Award and National Institutes of Health grants P01 CA094237 and RO1 CA142636.
Footnotes
This work was presented in part at the 55th Annual Meeting of the American Society for Radiation Oncology, Atlanta, GA, September 22-25, 2013.
Conflict of interest: none.
References
- 1.Goodman M, Gurney J, Smith M. Sympathetic nervous system tumors. In: Ries L, Smith M, Gurney J, editors. Cancer Incidence and Survival Among Children and Adolescents United States SEER program 1975-1995. Bethesda, MD: National Cancer Institute; 1999. pp. 65–72. [Google Scholar]
- 2.Park JR, Eggert A, Caron H. Neuroblastoma: Biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24:65–86. doi: 10.1016/j.hoc.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Berthold F, Hero B, Kremens B, et al. Long-term results and risk profiles of patients in five consecutive trials (1979-1997) with stage 4 neuroblastoma over 1 year of age. Cancer Lett. 2003;197:11–17. doi: 10.1016/s0304-3835(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 4.Al Yu, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isoretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging and response to treatment. Prog Clin Biol Res. 1994;385:363–369. [PubMed] [Google Scholar]
- 6.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiation therapy, autologous bone marrow transplantation, and 13-cis-retinoic acid Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 7.Haas-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: A Children's Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 8.Simon T, Hero B, Bongartz R, et al. Intensified external-beam radiation therapy improves the outcome of stage 4 neuroblastoma in children >1 year with residual local disease. Strahlenther Onkol. 2006;182:389–394. doi: 10.1007/s00066-006-1498-8. [DOI] [PubMed] [Google Scholar]
- 9.Wolden SL, Gollamudi SV, Kushner BH, et al. Local control with multimodality therapy for stage 4 neuroblastoma. Int J Radiat Oncol Biol Phys. 2000;46:969–974. doi: 10.1016/s0360-3016(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 10.Pai Panandiker AS, Beltran C, Billups CA, et al. Intensity modulated radiation therapy provides excellent local control in high-risk abdominal neuroblastoma. Pediatr Blood Cancer. 2013;60:761–765. doi: 10.1002/pbc.24350. [DOI] [PubMed] [Google Scholar]
- 11.Gatcombe HG, Marcus RB, Jr, Katzenstein HM, et al. Excellent local control from radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2009;74:1549–1554. doi: 10.1016/j.ijrobp.2008.10.069. [DOI] [PubMed] [Google Scholar]
- 12.Marcus KJ, Shamberger R, Litman H, et al. Primary tumor control in patients with stage 3/4 unfavorable neuroblastoma treated with tandem double autologous stem cell transplants. J Pediatr Hematol Oncol. 2003;25:934–940. doi: 10.1097/00043426-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the Children's Oncology Group. J Nucl Med. 2013;54:541–548. doi: 10.2967/jnumed.112.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung NK, Miraldi FD. Iodine 131 labeled GD2 monoclonal antibody in the diagnosis and therapy of human neuroblastoma. Prog Clin Biol Res. 1988;271:595–604. [PubMed] [Google Scholar]
- 15.Cheung NK, Yeh SD, Gulati S, et al. [131]I-3F8: Clinical validation of imaging studies and therapeutic applications. Prog Clin Biol Res. 1991;366:409–415. [PubMed] [Google Scholar]
- 16.Matthay KK, DeSantes K, Hasegawa B, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 17.Katzenstein HM, Cohn SL, Shore RM, et al. Scintigraphic response by 123I-metaiodobenzylguanidine scan correlates with event-free survival in high-risk neuroblastoma. J Clin Oncol. 2004;22:3909–3915. doi: 10.1200/JCO.2004.07.144. [DOI] [PubMed] [Google Scholar]
- 18.Frappaz D, Bonneu A, Chauvot P, et al. Metaiodobenzylguanidine assessment of metastatic neuroblastoma: Observer dependency and chemosensitivity evaluation. Med Pediatr Oncol. 2000;34:237–241. doi: 10.1002/(sici)1096-911x(200004)34:4<237::aid-mpo1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Simon T, Hero B, et al. The prognostic impact of functional imaging with 123I-mIBG in patients with stage-4 neuroblastoma > 1 year of age on a high-risk treatment protocol: Results of the German Neuroblastoma Trial NB97. Eur J Cancer. 2008;44:1552–1558. doi: 10.1016/j.ejca.2008.03.013. [DOI] [PubMed] [Google Scholar]


