Anti-vascular endothelial growth factor (anti-VEGF) injections have revolutionised vision for patients with macular degeneration or diabetic macular oedema. However, the choice of anti-VEGF is controversial. Two drugs licensed for macular oedema are ranibizumab (Lucentis) and aflibercept (Eylea). A third drug, bevacizumab (Avastin), is only licensed for colorectal cancer, and therefore, use in the eye is ‘off label'.1 Yet, all three are effective. Independently supported randomised controlled trials,2, 3 and systematic reviews were unable to find a significant difference in safety or efficacy.4, 5
The cost difference is significant however. Prices, according to the British National Formulary (BNF), are: ranibizumab; £742 per single injection vial, aflibercept; £816 per single injection vial; bevacizumab £242.66 per vial, but in practice each bevacizumab vial is divided into 20 injections, giving a price of £12.13 per injection.6 In each case, these costs are multiplied many times over, because patients require repeated injections.
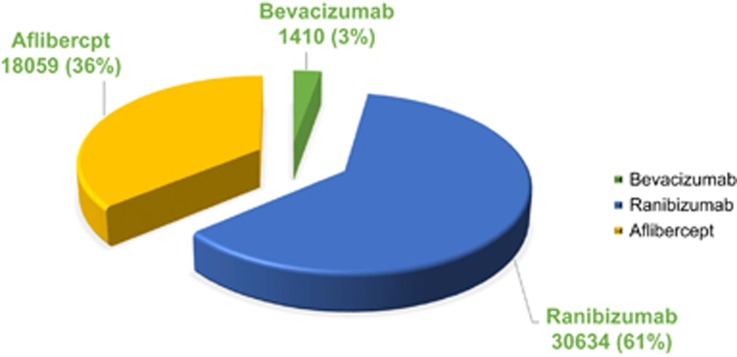
Given the current government's cost-cutting ambitions,7 we hypothesised the majority of injections would use the less expensive drug, bevacizumab. We therefore performed a freedom of information (FOI) request to all UK NHS ophthalmological units for the number of ranibizumab, aflibercept, and bevacizumab injections prescribed during January 2015 (Table 1 and Figure 1).
Table 1. The number of different anti-vascular endothelial growth factor injections and estimated costs assuming British National Formulary prices.
| Ranibizumab £742/injection £890.4 incl. VAT | Aflibercept £816/injection £979.2 incl. VAT | Bevacizumab £12.13/injection £14.56 incl. VAT | Total | |
|---|---|---|---|---|
| Number of injections | 30 634 | 18 059 | 1410 | 50 103 |
| Costs per month | £22 730 428 | £14 736 144 | £17 103 | £37 483 675 |
| Costs per month incl. VAT | £27 276 514 | £17 683 373 | £20 530 | £44 980 416 |
| Estimated costs per year | £272 765 136 | £176 833 728 | £205 240 | £449 804 104 |
| Estimated costs per year incl. VAT | £327 318 163 | £212 200 474 | £246 355 | £539 764 992 |
The price of bevacizumab was calculated by dividing the cost of a 100 mg vial (£242.66) by 20. Non-NHS units were excluded. Monthly costs were determined by multiplying the injection number with the BNF price.6 Annual costs were determined by multiplying this number by 12. A total of 189 FOI requests were sent. The overall response rate was 95.8%. Fifty-three (28.0%) Trusts and Health Boards confirmed that they do not provide intravitreal injection services (49 in England, 3 in Northern Ireland, and 1 in Wales). Two responses were excluded owing to incomplete data.
Figure 1.
The proportions of the different anti-VEGF injections performed in the UK for the month of January 2015.
These results indicate the anti-VEGF injection cost to the NHS. Contrary to the hypothesis, the vast majority (97%) were with the more expensive drugs ranibizumab and aflibercept. If all injections used divided bevacizumab, the estimated cost would be £607 749 (£729 500 incl. VAT) saving the NHS £449 196 354 (£539 035 492 incl. VAT) per year. With 5% unaccounted for, savings may be greater.
These figures assume: first, that procedural costs are the same. Second, that the frequency of injections would remain the same if solely using bevacizumab. (In fact, both aflibercept and bevacizumab have longer pharmacokinetic profiles,8, 9 which should result in clinic visit rate reduction.) Third, that the injection rate for January 2015 is representative of every month.
The number of new patients, repeat injections, and cost are likely to escalate with an aging population. Popular belief in 2007 was anti-VEGFs would ‘cure' macular oedema.10 However, clinical experience has shown that continued maintenance injections are required to prevent relapse.11
We are only able to publish BNF prices. Confidential arrangements exist between companies and individual trusts, where drug price is negotiated in ‘Patient Access Schemes', and we are not allowed to expose the true cost. Nevertheless, the evidence indicates a significant saving of using bevacizumab where appropriate.12
So why not a switch towards using bevacizumab?
The answer is simple—it remains unlicensed, and the prescription of ‘off label' drugs is not encouraged by the General Medical Council (GMC), whose guidelines state: ‘Prescribing unlicensed medicines may be necessary where:
There is no suitably licensed medicine that will meet the patient's need…/Or where a suitably licensed medicine that would meet the patient's need is not available'.
We are not making the argument that licensing is unnecessary. Licensing prevents companies marketing drugs without evidence of a drug's safety post ‘thalidomide era'.13 This is regulated in the UK by the Medicines and Healthcare products Regulatory Authority (MHRA). ‘Off label', for example, vials could be divided in the clinic: in a non-regulated environment, there is greater chance of infection, contamination, or incorrect dosage.14
However, for anti-VEGF treatments, the situation is unusual. Arms of the same company produce bevacizumab and ranibizumab. Genentech (San Francisco, CA, USA), a subsidiary of Roche (Basel, Switzerland), markets ranibizumab in the USA and bevacizumab worldwide. Novartis (Basel, Switzerland), a major shareholder in Roche, markets ranibizumab outside the USA. There is no financial incentive for a company producing one drug licensed for macular oedema to license another cheaper drug it already produces.
The ability to change practice rests with advisors and those with the purchasing power. Further, GMC guidance states that clinicians must provide effective treatments based on the best available evidence, and must make good use of resources. A core remit of the National Institute for Health and Care Excellence (NICE) is to ensure NHS treatments are cost effective. The Royal College of Ophthalmologists stated:
‘There is clear evidence that, despite the lack of a licence, Avastin is a safe and effective drug for the treatment of neovascular AMD. The College would therefore welcome an urgent review of this issue by the United Kingdom Health Regulatory Bodies to consider how this unusual situation can be remedied'.15
In February 2015, 120 leaders of clinical commissioning groups wrote to the Health Secretary. The reply was negative:
‘It would not only be unlawful but also against the wider public interest if ministers were to attempt to set aside that licensing system in order purely to cut costs'.16
So how can we retain the benefits of the licensing system, but still ensure the most cost-effective drugs are licensed? One possible solution could be for NICE/MHRA to initiate licensing themselves, thus preserving quality control of bevacizumab preparation, while saving hundreds of millions of pounds of NHS budget.
We believe this is of public interest, for money saved could help many other patients. A paediatric intensive care bed costs £2000/night. The savings could fund 725 beds annually (currently just 431 of these beds exist in England),17 or trastuzumab (Herceptin) for 4945 breast cancer patients,18 or funding for all 53 childrens' hospices in the UK. Naomi House and Jacksplace in the South of England look after 363 children, costing £7 million per year, but only receive 10% of this from NHS England.19
What is the practice elsewhere? In the USA, a market-driven healthcare system, bevacizumab is the most popular anti-VEGF.20 In Holland, bevacizumab is approved as first-line treatment for AMD, and a randomised trial to compare bevacizumab with ranibizumab for diabetic macular oedema is underway.21 In Italy, Roche/Novartis were fined 180 million euros for trying to ‘channel demand toward the much more expensive drug Lucentis, through an artificial distinction between the two products'.22
To conclude, UK regulation has resulted in an unnecessarily expensive choice of anti-VEGF treatment, despite good evidence of safety, efficacy, cost savings, and support from the respected professional bodies for the use of bevacizumab, despite repeated requests to the Government.
The authors declare no conflict of interest.
References
- Lock D. Avastin and Lucentis: a guide through the legal maze. BMJ 2015; 350: h1377. [DOI] [PubMed] [Google Scholar]
- CATT Research Group, Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364: 1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013; 382: 1258–1267. [DOI] [PubMed] [Google Scholar]
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database Syst Rev 2014; 9: CD011230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 2014; 10: CD007419. [DOI] [PubMed] [Google Scholar]
- British National Formulary (online). Available at: https://www.evidence.nhs.uk/formulary/bnf/current/11-eye/118-miscellaneous-ophthalmic-preparations/1182-ocular-diagnostic-and-peri-operative-preparations-and-photodynamic-treatment/subfoveal-choroidal-neovascularisation/aflibercept/eyleahttps://www.evidence.nhs.uk/formulary/bnf/current/8-malignant-disease-and-immunosuppression/81-cytotoxic-drugs/815-other-antineoplastic-drugs/bevacizumab/bevacizumab/avastinhttps://www.evidence.nhs.uk/formulary/bnf/current/11-eye/118-miscellaneous-ophthalmic-preparations/1182-ocular-diagnostic-and-peri-operative-preparations-and-photodynamic-treatment/subfoveal-choroidal-neovascularisation/ranibizumab/lucentis (accessed on 26 October 2015).
- Osborne G, Hands G Public sector efficiency challenge. HM Government. Available at https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/450616/A_message_from_the_Chancellor_and_Chief_Secretary_to_the_Treasury.pdf (accessed on 31 October 2015).
- Bayeur. EYELEA Summary of Product Characteristics. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002392/WC500135815.pdf (accessed on 19 September 2015).
- Lushchyk T, Amarakoon S, Martinez-Ciriano JP, van den Born LI, Baarsma GS, Missotten T. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Acta Ophthalmol 2013; 91: e456–e461. [DOI] [PubMed] [Google Scholar]
- Smith R Eyesight of thousands to be saved after NICE approves drug. The Telegraph 2008. Available at http://www.telegraph.co.uk/news/health/2627245/Eyesight-of-thousands-to-be-saved-after-Nice-approves-drug.html (accessed on 19 September 2015).
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013; 120: 2292–2299. [DOI] [PubMed] [Google Scholar]
- Dakin HA, Wordsworth S, Rogers CA, Abangma G, Raftery J, Harding SP et al. Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial. BMJ Open 2014; 4: e005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier J. Paediatric prescribing: using unlicensed drugs and medicines outside their licensed indications. Br J Clin Pharmacol 1999; 48: 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa M, Messori A. The safety of bevacizumab and ranibizumab in clinical studies. Int Ophthalmol 2015; 35: 157–158. [DOI] [PubMed] [Google Scholar]
- Royal College of Ophthalmologists. Use of Avastin (bevacizumab) in age related macular degeneration 2014. Available at https://www.rcophth.ac.uk/2014/12/use-of-avastin-bevacizumab-in-age-related-macular-degeneration/ (accessed on 28 September 2015).
- Royal National Insitute of Blind People. Government rejects use of avastin. 2015. Available at https://www.rnib.org.uk/government-rejects-use-avastin-england (accessed on 19 September 2015).
- NHS England. Critical care bed capacity. Available at http://www.england.nhs.uk/statistics/statistical-work-areas/critical-care-capacity/critical-care-bed-capacity-and-urgent-operations-cancelled-2015-16-data/ (accessed on 27 October 2015).
- National Institute for Health and Care Excellence. Breast cancer drug costing tens of thousands of pounds more than other treatments ‘unaffordable' for NHS. 2014. Available at https://www.nice.org.uk/news/press-and-media/breast-cancer-drug-costing-tens-of-thousands-of-pounds-more-than-other-treatments-unaffordable-for-nhs (accessed on 28 October 2015).
- Naomi House and Jacksplace. Hospices for children and young adults. Available at http://www.naomihouse.org.uk/about-us and http://apps.charitycommission.gov.uk/Accounts/Ends32/0001002832_AC_20150331_E_C.PDF (accessed on 29 October 2015).
- Curtis LH, Hammill BG, Qualls LG, DiMartino LD, Wang F, Schulman KA et al. Treatment patterns for neovascular age-related macular degeneration: analysis of 284 380 medicare beneficiaries. Am J Ophthalmol 2012; 153: 1116–24.e1. [DOI] [PubMed] [Google Scholar]
- Schauwvlieghe AM, Dijkman G, Hooymans JM, Verbraak FD, Hoyng CB, Dijkgraaf MG et al. Comparing the effectiveness and costs of bevacizumab to ranibizumab in patients with diabetic macular edema: a randomized clinical trial (the BRDME study). BMC Ophthalmol 2015; 15: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly D Italy fines Novartis and Roche in collusion case. New York Times 2014; International Business: B2. Available at http://www.nytimes.com/2014/03/06/business/international/italy-fines-novartis-and-roche-in-collusion-case.html?_r=0 (accessed on 28 October 2015).