Abstract
Disulfide bonds could be valuable linkers for a variety of therapeutic applications requiring tunable cleavage between two parts of a molecule (e.g., antibody–drug conjugates). The in vitro linker immolation of β-mercaptoethyl-carbamate disulfides and DNA alkylation properties of associated payloads were investigated to understand the determinant of cell killing potency of anti-CD22 linked pyrrolobenzodiazepine (PBD-dimer) conjugates. Efficient immolation and release of a PBD-dimer with strong DNA alkylation properties were observed following disulfide cleavage of methyl- and cyclobutyl-substituted disulfide linkers. However, the analogous cyclopropyl-containing linker did not immolate, and the associated thiol-containing product was a poor DNA alkylator. As predicted from these in vitro assessments, the related anti-CD22 ADCs showed different target-dependent cell killing activities in WSU-DLCL2 and BJAB cell lines. These results demonstrate how the in vitro immolation models can be used to help design efficacious ADCs.
Keywords: Antibody−drug conjugate, cancer, disulfide linker, linker immolation, pyrrolobenzodiazepine
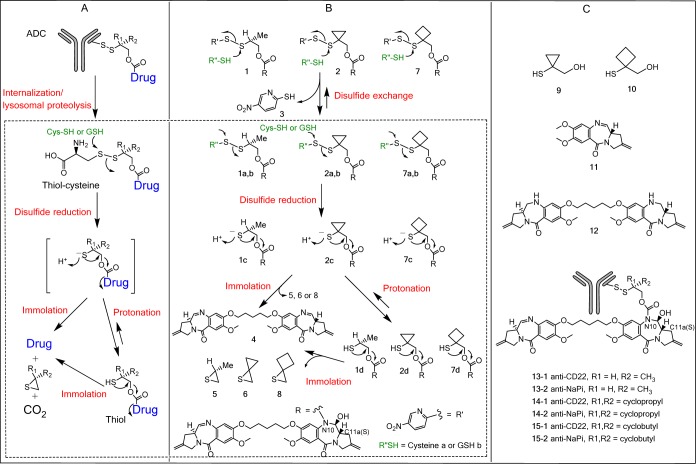
Antibody–drug conjugate (ADC) technology can enable the use of very toxic compounds as potential cancer therapeutics. Such potent cytotoxins include maytansinoid and auristatin-based antimitotic agents along with DNA alkylating entities derived from pyrrolo[2,1-c][1,4]benzodiazepine dimers (PBD dimers).1−6 Several challenges are frequently encountered in the design of ADCs including (1) preventing the premature release of the cytotoxin during in vivo circulation and (2) ensuring that a biologically active form of the cytotoxic payload is released at the desired site of action at an adequate rate. The complex structure of an ADC together with its associated unique dispositional and functional properties requires careful design of every component of the molecule including antibody, conjugation site, linker structure, and a small molecule drug (payload).7−10 The linker determines the mechanism and rate of payload release, which both affect cell killing activity, and is thus a critical part of an ADC.10,11 Cleavable linkers commonly employed in ADC design include the valine-citrulline para-aminobenzyl carbamate (Val-Cit-PABC) present in brentuximab vedotin (Adcetris)12 and disulfide moieties contained in multiple maytansinoid-derived conjugates13 as well as in gemtuzumab ozogamicin (Mylotarg).14 Recently, Pillow et al. reported a self-immolating disulfide linker (β-mercaptoethyl-carbamate, −SCH(R)CH2OCO−) that can be directly attached to antibody cysteine residues and therefore decouples in vivo stability from payload release of associated disulfide linker ADCs in cells.15 This linker is traceless in that, following internalization and lysosomal mAb proteolysis,16 it releases the free drug (payload) in a two-step process involving (1) disulfide cleavage of a thiol-cysteine adduct and (2) subsequent immolation of the resulting thiolate intermediate (Figure 1A). The thiolate could also capture a proton to form a thiol that is also set up for immolation. For payloads that require separation from the linker to properly exert their biological effects, the rate and extent of immolation/release will likely impact the efficacy of corresponding ADCs. In order to further optimize the disulfide stability and release kinetics through substitution adjacent to the linker sulfur, we have studied immolation/release of model compounds containing various β-mercaptoethyl-carbamate disulfide linkers with substitution of proton, methyl, ethyl, dimethyl, cyclopropyl, cyclobutyl, cyclopentanyl, and cyclohexanyl groups. In this report, we provide detailed in vitro studies of immolation/release parameters associated with PBD-dimer-containing β-mercaptoethyl-carbamate disulfide linkers with methyl-, cyclopropyl-, and cyclobutyl-substitutions that showed representative immolation patterns. In addition, we characterized the in vitro oligonucleotide (DNA Oligo) binding/alkylation properties of several related PBD-containing payloads. We subsequently correlated these in vitro measurements with the cell killing potency of related ADCs.
Figure 1.
(A) Catabolism and payload release mechanism of a disulfide-linked ADC. (B) Disulfide cleavage and immolation of PBD disulfide linker drugs in the presence of cysteine or GSH,. (C) Structures of other PBD analogues and ADCs used in this study. Note: Thiol-cysteine adducts 1a, 2a, and 7a are also proposed intermediates following internalization and lysosomal proteolysis of their corresponding ADCs 13–1, 14–1, and 15–1 in cells; the corresponding thiolates of 1d, 2d, or 7d are proposed intermediates of disulfides 1a, 2a, or 7a following disulfide cleavage; the thiolates 1c, 2c, or 7c could immolate directly to liberate the payload 4 or capture a proton for form thiols 1d, 2d, or 7d; the mechanisms for disulfide cleavage and immolation are similar between in cells and in vitro (dotted-line area). The immolation did not start until the disulfide cleavage therefore was not affected by the leaving groups such as cysteine or nitro-pyridyl group; the corresponding thiol-GSH adducts 1b, 2b, and 7b were identified as major components from incubations with 1, 2, and 7 in the presence of 30 μM GSH.
The pyrrolo[2,1-c][1,4]benzodiazepine (PBD) dimers comprise an important class of DNA alkylators for ADCs and PBD-derived payloads have been used recently in several ADCs entering clinic trials.4,6,17 The PBD dimer molecular structure can influence DNA sequence recognition given that such compounds contain a chiral C11a(S)-center that forms an appropriate conformation to fit in the minor groove of DNA.18−22 The C10–N11 imine moiety in PBDs forms a covalent adduct with a guanine residue in the minor groove of double-stranded DNA with preferred 5′-pu-GA(A/T)TC-Py-3′ sequences (bold = covalent modification site) to produce inter/intrastrand cross-links or monoalkylated products.18,20 One particularly attractive way to connect PBD-dimer payloads to antibodies involves attachment of a self-immolative linker to the N10 nitrogen,4 thereby blocking the C11–N10 imine in the intact conjugates, but generating the C11–N10 imine upon linker cleavage (Figure 1B). In this new linker/payload design, insufficient release of a self-immolative spacer is expected to have a pronounced negative effect on payload efficacy given that the C11–N10 imine is required for DNA alkylation.
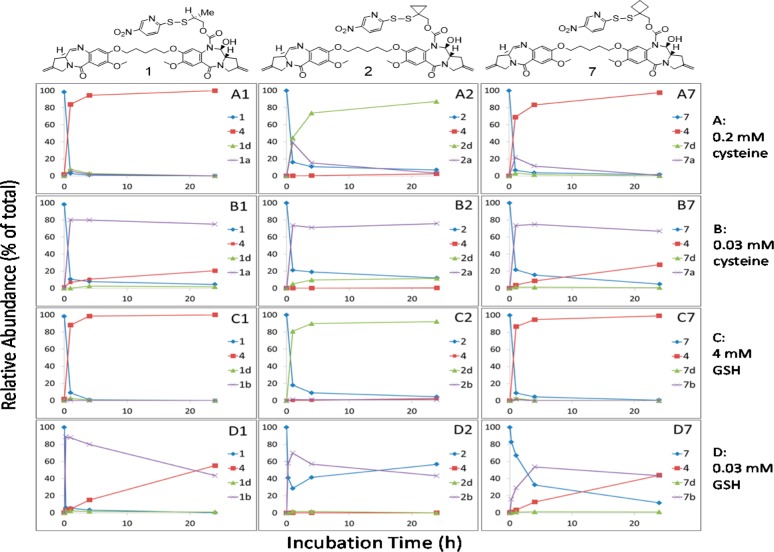
To simplify the studies, we initially investigated disulfide cleavage and immolation using unconjugated small molecule model systems. Accordingly, we performed the experiments with para-nitropyridyl disulfide-containing linker drug analogues 1 and 2 (that were subsequently used to prepare antibody conjugates) (Figure 1B) in the presence of cysteine (0.2 and 0.03 mM) and glutathione (GSH, 4 and 0.03 mM) with the thiol concentrations mimicking those in plasma and cancer cells (unpublished data).23 Under these conditions, the para-nitropyridyl disulfide moieties in these molecules underwent rapid disulfide cleavage with a half-life of minutes (Figure 2, Table S1). Analysis by LC/UV/MS showed that reduction of 1 and 2 gave different products: PBD dimer 4 was obtained as a major product from methyl-containing 1, while the thiol 2d was formed from cyclopropyl-containing 2 (Figure 2A1,A2,C1,C2; Tables S1 and S2) when an excess level of a reducing thiol was present (0.2 mM cysteine or 4 mM GSH). In contrast, when a relatively low concentration of cysteine (0.03 mM) was used (Figure 2B1,B2), thiol-cysteine adducts 1a and 2a were the major and stable products in the 24 h incubations due to consumption of the reducing thiol. Similarly, thiol-GSH adducts 1b and 2b were observed as the major products when 1 and 2 were exposed to a low concentration of GSH (0.03 mM) for the same time period (Figure 2D1,D2). These cleavage reactions also formed the para-nitropyridyl thiol 3 as a companion byproduct (Figure 1B). Interestingly, reversible disulfide exchange was observed between para-nitropyridyl thiol 3 and cyclopropyl-containing thiol 2d with a comparable pKa value (4.7–4.8) at 0.03 mM GSH concentration (Figure 2D2). The observation of intermediates 1a and 1d (albeit at low levels) was consistent with the expected mechanism for formation of PBD dimer 4 from 1 that involves (1) cysteine attack on the 2-mercaptoethyl-derived disulfide sulfur atom to form 1a, (2) second cysteine attack on the less hindered sulfur atom of the resulting thiol-cysteine disulfide to afford a thiolate intermediate 1c, and (3) the thiolate 1c immolates to liberate PBD dimer 4 or captures a proton to form the corresponding thiol 1d (Figure 1B). The depicted regiochemistry of initial cysteine attack on 1 is likely influenced by both disulfide electronics and the acidic nitropyridyl thiolate pKa (i.e., this fragment is a good leaving group).24,25 The thiol-cysteine disulfide 1a is identical to the proposed intermediate produced by intracellular catabolism of the corresponding ADC following internalization and lysosomal proteolysis in cells.15 As shown in Figure 2A1,C1 and Table S1, the methyl-containing thiol intermediate 1d could be detected by LC/MS at low levels in the initial phase of the incubations but immolated quickly to afford the PBD dimer 4. However, the corresponding cyclopropyl-containing thiol 2d produced from disulfide 2 did not appreciably immolate and appeared to be stable. Furthermore, the thiirane 5 was detected via LC/MS in the cleavage experiment utilizing 1, while the corresponding thiirane 6 was not identified from studies employing 2 (Figure 1B). Subsequently, the cyclobutyl analogue 7 was prepared. Both 7 and its thiol-cysteine adduct 7a underwent rapid disulfide cleavage followed by efficient immolation of its thiolate 7c and thiol 7d to form thiirane 8 and PBD dimer 4 (Figure 2A7,B7, C7). The cyclobutyl-substituted disulfide in 7 is more stable than the methyl analogue in 1 since greater amounts of starting material 7 were observed relative to 1 in the incubation with a lower concentration of glutathione (0.03 mM) (Figure 2D1,D7). Immolation of cyclobutyl-containing thiol 7d may also be slightly more efficient than the methyl-containing thiol 1d as less 7d was detected than 1d under all incubation conditions (Table S1). Nonimmolation of the cyclopropyl linker compared to efficient immolation of the cyclobutyl or methyl-containing linkers is likely due to multiple factors. For example, the bond angles associated with the cyclopropyl ring are likely to result in increased ring strain in the fused dicyclopropyl thiirane 6 relative to thiiranes 5 and 8 and thereby impede the desired immolation reaction.26 The cyclopropyl ring may also exert an electronic effect (p-orbital character) on the thiolate intermediate, which reduces its nucleophilicity and therefore its ability to cyclize. Consistent with this possibility, a significantly lower pKa value (more acidic) was measured for the cyclopropyl-containing thiol 9 relative to that measured for the cyclobutyl-containing thiol 10 (4.8 versus 9.6, respectively). Collectively, these results demonstrate that minor structural modifications to the disulfide linker can lead to vast differences in the corresponding immolation and release efficiencies.
Figure 2.
Relative abundance of disulfide cleavage and immolation products over time. Products were monitored by LC/MS from incubations of linker drugs 1, 2, and 7 in the presence of cysteine (0.2 mM, A1, A2, A7; and 0.03 mM, B1, B2, B7) or glutathione (4 mM, C1, C2, C7; and 0.03 mM, D1, D2, D7) at pH 7.0 and 37 °C. P = starting linker drugs 1 (methyl-), 2 (cyclopropyl-), and 7 (cyclobutyl-). Payload = PBD dimer 4. Thiol-GSH or -cysteine = 1a or 1b (methyl-), 2a or 2b (cyclopropyl-), or 7a or 7b (cyclobutyl). Thiol = 1d, 2d, or 7d.
To complement the linker cleavage and immolation studies, we used a simple in vitro model based on known PBD interactions with DNA Oligos of various lengths and sequences to assess and compare the potential for PBD-containing payloads to alkylate DNA.20,27 As shown in Figure S1, both bis-imine-containing PBD dimer 4 and the corresponding PBD monomer 11 extensively alkylated the tested DNA Oligos20 in this in vitro assessment (Figures S1B,C). In contrast, the cyclopropyl-containing molecule 2d alkylated the DNA Oligos at a minimal level that was similar to that observed for the negative control 12 (the absence of reactive imine moieties in 2d prevents it from alkylating DNA). This result suggests that the structural modification by the cyclopropyl-moiety in 7 prevented the PBD analogue to fit in the DNA minor groove for alkylation although there is still one imine functionality in 2d. Various adducts were formed between different alkylators and the DNA Oligos. As expected, the PBD dimer 4 mainly formed an interstrand cross-link in which the two imine moieties present in 4 separately reacted with guanines19 in each strand of the double-strand DNA Oligo (Figure S1B, insert). In contrast, the PBD monomer 11 formed two types of adducts by independently reacting with the guanine residue in each strand of the DNA Oligo (Figure S1C, insert). The described adduct formation is consistent with observations by others in which 1H NMR methods were used to demonstrate similar binding of PBD compounds in the DNA minor groove.27 The level of DNA Oligo alkylation by PBD dimer 4 appears to correlate with its potent cell-killing activity in BJAB and WSU-DLCL2 (IC50 = ∼20 pM). The inability of compound 2d to alkylate DNA Oligos predicted that ADCs containing the cyclopropyl linker would likely not afford potent cell-killing activity.
We next tested the effects of immolation on cell-killing activities of related ADCs. Anti-CD22 conjugates (LC-K149C-anti-CD22-PBD-dimer) 13-1 (methyl-), 14-1 (cyclopropyl-), and 15-1 (cyclobutyl-) and the corresponding control conjugates (LC-K149C-anti-NaPi2b-PBD-dimer) 13-2, 14-2, and 15-2 were prepared from 1, 2, and 7. The CD22 antigen was chosen for our ADC design because of its high expression on cancers of B-cell origin and relatively low prevalence on non-B cell-related normal cells and tissues.28,29 The methyl- and cyclobutyl-containing conjugates 13-1 and 15-1 showed potent, target-dependent cell-killing activities in two CD22-expressing cell lines (WSU-DLCL2 and BJAB, Table 1, Figure S2, and associated comments). However, the cyclopropyl-containing conjugate 14-1 was significantly (>50-fold) weaker than 13-1 and 15-1 in these experiments and also exhibited almost no potency differences from the corresponding nontarget control conjugate (14-2). These results were consistent with the in vitro data depicted in Figures 2 and S1, which illustrate the inability of the cyclopropyl-containing thiol 2d to both efficiently alkylate DNA Oligos and to release PBD dimer 4 via immolation. The ADC cell data were also consistent with the efficient release of a potent DNA alkylating agent (di-imine 4) from in vitro model systems related to conjugates 13-1 and 15-1 (Figure 2, Table S1). The similarity in BJAB and WSU EC50 values exhibited by these conjugates is in agreement with the potent antiproliferation activity (approximately 20 pM) displayed by the released PBD dimer 4 against both cell lines.
Table 1. Cell-Killing Activities of Methyl-, Cyclopropyl, and Cyclobutyl-Containing Conjugates 13-1, 14-1, and 15-1 in CD22-Expressing BJAB and WSU-DLCL2 Cell Cultures.
| IC50 (nM) |
||
|---|---|---|
| compd | BJAB | WSU-DLCL2 |
| 13-1, methyl-CD22 | 1.16 ± 0.04 | 1.19 ± 0.11 |
| 13-2, methyl-NaPi | 6.13 ± 0.03 | 13.9 ± 0.02 |
| 14-1, cyclopropyl-CD22 | 85.0 ± 0.06 | 95.7 ± 0.06 |
| 14-2, cyclopropyl-NaPi | 87.8 ± 0.14 | 125 ± 0.04 |
| 15-1, cyclobutyl-CD22 | 0.47 ± 0.04 | 1.50 ± 0.08 |
| 15-2, cyclobutyl-NaPi | 3.84 ± 0.04 | 8.95 ± 0.05 |
In the studies described above, the similar cell killing potency exhibited by conjugates containing the cyclobutyl- and methyl-substituted disulfide linkers closely paralleled the disulfide stability, immolation, payload release, and DNA binding characteristics observed in vitro with the unconjugated linker drugs 1 and 7. More importantly, cyclopropyl substitution prevented linker immolation in vitro following disulfide cleavage, and the resulting thiol product 2d was also shown to poorly alkylate designed DNA oligos. These in vitro results, collectively, explained the poor cell killing activity exhibited by an ADC containing the cyclopropyl-disulfide linker. These correlations indicate that the described in vitro assessments can be good predictors of ADC efficacy, and suggest their future use in routine profiling of unconjugated payloads, model linker drugs, and ADC conjugates prior to conducting in vivo studies. Collectively, these data also illustrate the value of conducting detailed in vitro experiments to understand the ability of a given ADC linker to release an attached payload coupled with the assessment of biological activity of such released entities to support design of efficacious ADCs.
Acknowledgments
The authors would like to thank Drs. Philip Howard, Luke Masterson, and Stephen Gregson from Spirogen, and Leanna Staben and Drs. John Flygare and Rebecca Rowntree from Genentech for their technical contributions. The research of the manuscript was supported by Genentech.
Glossary
ABBREVIATIONS
- ADC
antibody–drug conjugate
- DNA Oligo
deoxyribonucleic acid oligonucleotide
- GSH
glutathione
- LC/MS
high performance liquid chromatography tandem mass spectrometry
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00233.
Two tables, two figures, materials and methods, and synthesis of all compounds used in this study (PDF)
The authors declare no competing financial interest.
This paper was published ASAP on August 26, 2016 with errors in the references. The corrected version was reposted on August 30, 2016.
Supplementary Material
References
- LoRusso P. M.; Weiss D.; Guardino E.; Girish S.; Sliwkowski M. X. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin. Cancer Res. 2011, 17, 6437–6447. 10.1158/1078-0432.CCR-11-0762. [DOI] [PubMed] [Google Scholar]
- Senter P. D.; Sievers E. L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012, 30, 631–637. 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- Chari R. V. J.; Miller M. L.; Widdison W. C. Antibody-drug conjugates: An emerging concept in cancer therapy. Angew. Chem., Int. Ed. 2014, 53, 3796–3827. 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- Saunders L. R.; Bankovich A. J.; Anderson W. C.; Aujay M. A.; Bheddah S.; Black K.; Desai R.; Escarpe P. A.; Hampl J.; Laysang A.; Liu D.; Lopez-Molina J.; Milton M.; Park A.; Pysz M. A.; Shao H.; Slingerland B.; Torgov M.; Williams S. A.; Foord O.; Howard P.; Jassem J.; Badzio A.; Czapiewski P.; Harpole D. H.; Dowlati A.; Massion P. P.; Travis W. D.; Pietanza M. C.; Poirier J. T.; Rudin C. M.; Stull R. A.; Dylla S. J. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M. S. K.; Sanderson R. J.; Gordon K. A.; Andreyka J.; Cerveny C. G.; Yu C.; Lewis T. S.; Meyer D. L.; Zabinski R. F.; Doronina S. O.; Senter P. D.; Law C. L.; Wahl A. F. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J. Biol. Chem. 2006, 281, 10540–10547. 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- Jeffrey S. C.; Burke P. J.; Lyon R. P.; Meyer D. W.; Sussman D.; Anderson M.; Hunter J. H.; Leiske C. I.; Miyamoto J. B.; Nicholas N. D.; Okeley N. M.; Sanderson R. J.; Stone I. J.; Zeng W.; Gregson S. J.; Masterson L.; Tiberghien A. C.; Howard P. W.; Thurston D. E.; Law C. L.; Senter P. D. A potent anti-CD70 antibody-drug conjugate combining a dimeric pyrrolobenzodiazepine drug with site-specific conjugation technology. Bioconjugate Chem. 2013, 24, 1256–1263. 10.1021/bc400217g. [DOI] [PubMed] [Google Scholar]
- Junutula J. R.; Raab H.; Clark S.; Bhakta S.; Leipold D. D.; Weir S.; Chen Y.; Simpson M.; Tsai S. P.; Dennis M. S.; Lu Y.; Meng Y. G.; Ng C.; Yang J.; Lee C. C.; Duenas E.; Gorrell J.; Katta V.; Kim A.; McDorman K.; Flagella K.; Venook R.; Ross S.; Spencer S. D.; Lee Wong W.; Lowman H. B.; Vandlen R.; Sliwkowski M. X.; Scheller R. H.; Polakis P.; Mallet W. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008, 26, 925–932. 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- Junutula J. R.; Flagella K. M.; Graham R. A.; Parsons K. L.; Ha E.; Raab H.; Bhakta S.; Nguyen T.; Dugger D. L.; Li G.; Phillips G. D. L.; Hiraragi H.; Fuji R. N.; Tibbitts J.; Vandlen R.; Spencer S. D.; Scheller R. H.; Polakis P.; Sliwkowski M. X. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target HER2-positive breast cancer. Clin. Cancer Res. 2010, 16, 2–5. 10.1158/1078-0432.CCR-10-0987. [DOI] [PubMed] [Google Scholar]
- Erickson H. K.; Lambert J. M. ADME of antibody-maytansinoid conjugates. AAPS J. 2012, 14, 799–805. 10.1208/s12248-012-9386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombs J. R.; Owen S. C. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. 10.1208/s12248-014-9710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N.; Smith S. W.; Ghone S.; Tomczuk B. Current ADC Linker Chemistry. Pharm. Res. 2015, 32, 3526–3540. 10.1007/s11095-015-1657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronina S. O.; Toki B. E.; Torgov M. Y.; Mendelsohn B. A.; Cerveny C. G.; Chace D. F.; DeBlanc R. L.; Gearing R. P.; Bovee T. D.; Siegall C. B.; Francisco J. A.; Wahl A. F.; Meyer D. L.; Senter P. D. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat. Biotechnol. 2003, 21, 778–784. 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- Kellogg B. A.; Garrett L.; Kovtun Y.; Lai K. C.; Leece B.; Miller M.; Payne G.; Steeves R.; Whiteman K. R.; Widdison W.; Xie H.; Singh R.; Chari R. V. J.; Lambert J. M.; Lutz R. J. Disulfide-linked antibody-maytansinoid conjugates: Optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjugate Chem. 2011, 22, 717–727. 10.1021/bc100480a. [DOI] [PubMed] [Google Scholar]
- Ricart A. D. Antibody-drug conjugates of calicheamicin derivative: Gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin. Cancer Res. 2011, 17, 6417–6427. 10.1158/1078-0432.CCR-11-0486. [DOI] [PubMed] [Google Scholar]
- Pillow T.; Sadowsky J.; Zhang D.; Yu S. F.; Del Rosario G.; Xu K.; He J.; Bhakta S.; Ohri R.; Kozak K. R.; Ha E.; Junutula J. R.; Flygare J. A. Decoupling stability and release in disulfide bonds with antibody-small molecule conjugates. Chem. Sci. 2016, 10.1039/C6SC01831A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. K.; Park P. U.; Widdison W. C.; Kovtun Y. V.; Garrett L. M.; Hoffman K.; Lutz R. J.; Goldmacher V. S.; Blättler W. A. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006, 66, 4426–4433. 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- Kung Sutherland M. S.; Walter R. B.; Jeffrey S. C.; Burke P. J.; Yu C.; Kostner H.; Stone I.; Ryan M. C.; Sussman D.; Lyon R. P.; Zeng W.; Harrington K. H.; Klussman K.; Westendorf L.; Meyer D.; Bernstein I. D.; Senter P. D.; Benjamin D. R.; Drachman J. G.; McEarchern J. A. SGN-CD33A: a novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122, 1455–1463. 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
- Jenkins T. C.; Hurley L. H.; Neidle S.; Thurston D. E. Structure of a covalent DNA minor groove adduct with a pyrrolobenzodiazepine dimer: evidence for sequence-specific interstrand cross-linking. J. Med. Chem. 1994, 37, 4529–4537. 10.1021/jm00052a012. [DOI] [PubMed] [Google Scholar]
- Rahman K. M.; James C. H.; Thurston D. E. Effect of base sequence on the DNA cross-linking properties of pyrrolobenzodiazepine (PBD) dimers. Nucleic Acids Res. 2011, 39, 5800–5812. 10.1093/nar/gkr122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman K. M.; Thompson A. S.; James C. H.; Narayanaswamy M.; Thurston D. E. The pyrrolobenzodiazepine dimer SJG-136 forms sequence-dependent intrastrand DNA cross-links and monoalkylated adducts in addition to interstrand cross-links. J. Am. Chem. Soc. 2009, 131, 13756–13766. 10.1021/ja902986x. [DOI] [PubMed] [Google Scholar]
- Petrusek R. L.; Anderson G. L.; Garner T. F.; Fannin Q. L.; Kaplan D. J.; Zimmer S. G.; Hurley L. H. Pyrrol[1,4]benzodiazepine antibiotics. Proposed structures and characteristics of the in vitro deoxyribonucleic acid adducts of anthramycin, tomaymycin, sibiromycin, and neothramycins A and B. Biochemistry 1981, 20, 1111–1119. 10.1021/bi00508a011. [DOI] [PubMed] [Google Scholar]
- Hartley J. A. The development of pyrrolobenzodiazepines as antitumour agents. Expert Opin. Invest. Drugs 2011, 20, 733–744. 10.1517/13543784.2011.573477. [DOI] [PubMed] [Google Scholar]
- Brülisauer L.; Gauthier M. A.; Leroux J.-C. J. Disulfide-containing parenteral delivery systems and their redox-biological fate. J. Controlled Release 2014, 195, 147–154. 10.1016/j.jconrel.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Singh R.; Whitesides G. M. Thiol-disulfide interchange. Suppl. S. Chem. Sulfur-containing Funct. Groups 1993, 633–658. 10.1002/9780470034408.ch13. [DOI] [Google Scholar]
- Freter R.; Pohl E. R.; Wilson J. M.; Hupe D. J. Role of the central thiol in determining rates of the thiol-disulfide interchange reaction. J. Org. Chem. 1979, 44, 1771–1774. 10.1021/jo01325a005. [DOI] [Google Scholar]
- Jung M. E.; Piizzi G. gem-disubstituent effect: theoretical basis and synthetic applications. Chem. Rev. 2005, 105, 1735–1766. 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
- Thurston D. E.; Vassoler H.; Jackson P. J.; James C. H.; Rahman K. M. Effect of hairpin loop structure on reactivity, sequence preference and adduct orientation of a DNA-interactive pyrrolo[2,1-c][1,4]benzodiazepine (PBD) antitumour agent. Org. Biomol. Chem. 2009, 13, 4031–4040. 10.1039/C4OB02405B. [DOI] [PubMed] [Google Scholar]
- Polson A. G.; Williams M.; Gray A. M.; Fuji R. N.; Poon K. A.; McBride J.; Raab H.; Januario T.; Go M.; Lau J.; Yu S.-F.; Du C.; Fuh F.; Tan C.; Wu Y.; Liang W.-C.; Prabhu S.; Stephan J.-P.; Hongo J.; Dere R. C.; Deng R.; Cullen M.; de Tute R.; Bennett F.; Rawstron A.; Jack A.; Ebens A. Anti-CD22-MCC-DM1: an antibody-drug conjugate with a stable linker for the treatment of non-Hodgkin’s lymphoma. Leukemia 2010, 24, 1566–1573. 10.1038/leu.2010.141. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Yu S. F.; Ma Y.; Xu K.; Dragovich P. S.; Pillow T. H.; Liu L.; Del Rosario G.; He J.; Pei Z.; Sadowsky J. D.; Erickson H. K.; Hop C. E. C. A.; Khojasteh S. C. Chemical structure and concentration of intratumor catabolites determine the efficacy of antibody drug conjugate. Drug Metab. Dispos. 2016, 44, 1517–1523. 10.1124/dmd.116.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.