Abstract
Prior research has demonstrated that individuals exposed to trauma are subject to impaired autonomic function. We sought to determine if heart rate variability (HRV), a marker of autonomic function, differed across wake, rest, and sleep periods as a function of posttraumatic stress disorder (PTSD) symptom severity. A sample of young adults (N = 209), 95 of whom met full criteria for current PTSD based upon the Clinician Administered PTSD Scale (CAPS), were evaluated for approximately 24-hours using actigraphy and electrocardiogram. Actigraphy findings were categorized as active, rest, and sleep. In multilevel modeling, individuals with high PTSD symptom severity exhibited lower high-frequency HRV than individuals with low PTSD symptom severity during periods of sleep, t(1083) = 2.20, p = .028, Cohen’s d = 0.12, but not during periods of activity, t(1083) = 1.34, p = .499, d = 0.05, or rest, t(1083) = 1.34, p = .180, d = 0.09. Our findings extended those of prior studies to suggest that, those with elevated PTSD symptoms may not only have decreased parasympathetic control during sleep, but relative to periods of wake and rest, sleep may represent a state increased vulnerability for decreased parasympathetic cardiac control. Individuals with elevated PTSD symptoms may require screening for early detection of cardiovascular disease.
Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and consequent autonomic nervous system dysfunction are established features of posttraumatic stress disorder (PTSD) and may contribute to the increased prevalence of cardiovascular disease within this population (Wentworth, 2013). Impaired neurobiological regulation of the stress response among those with PTSD is thought to contribute to the development and maintenance of this autonomic imbalance, which is characterized by a hypoactive parasympathetic nervous system (PSNS) and hyperactive sympathetic nervous system (SNS; Blechert, Michael, Grossman, Lajtman, & Wilhelm, 2007). Behaviors characteristic of PTSD, such as chronic sleep disturbance, have also been associated with decreased PSNS (Bonnet & Arand, 2010; Dennis, et al., 2014) and may be part of the pathophysiological pathway contributing to the excess risk of cardiovascular morbidity and mortality in PTSD (Bigger, et al., 1993; Thayer, Yamamoto, & Brosschot, 2010).
Heart rate variability (HRV) provides a non-invasive means of estimating PSNS (Task Force, 1996) and is also marker of increased risk of incident heart disease and mortality (Decker et al, 2000; Rodrigues et al, 2010). Specifically, frequency domain analysis of the variability of HR extracts two components of HRV that are related to autonomic nervous system control: a high-frequency component which is secondary to the impact of respiratory influences on PSNS control of HR, and a low-frequency component which reflects inputs from both the SNS and the PSNS. Reduced high-frequency HRV has been found in individuals diagnosed with PTSD under both laboratory and ambulatory settings (Shah et al, 2013; Dennis et al, 2014), although this has not been found by all (Bertram et al, 2014).
Ambulatory electrocardiogram (ECG) monitoring is well suited to measure HRV in individuals over an extended time period (i.e., 24-hour) and provides a nonobtrusive tool to study HRV changes across sleep-wake cycles. Because those with PTSD are vulnerable to chronic sleep disturbance (Germain, et al., 2013; Lamarche & De Koninck, 2007) they may be more likely to show hypoactive PSNS during sleep (Hall, 2004; Kobayashi, et al., 2014). Despite the ease of ambulatory recording, however, few studies have compared HRV across individually-determined activity periods (Agorastos, et al., 2013; Elsenbruch, Harnish, & Orr, 1999; Woodward, et al., 2009), and no study of which we are aware has investigated circadian HRV in relation to PTSD symptom severity. In the current study, we sought to determine if HRV differed across wake, rest, and sleep periods as a function of PTSD symptom severity.
Method
Participants and Procedure
Data were drawn from a larger study examining the relationship between mood, PTSD, and physical health factors in 230 adults exposed to trauma with and without current PTSD (e.g., Dennis, Watkins et al., 2014; Dennis et al., in press). In previous analyses of this sample, baseline PTSD was associated with orthostatic hypotension (Oddone et al., 2015), reduced HRV (Dennis, Watkins et al., 2014), and dyslipidemia (Dennis, Ulmer et al., 2014). Momentary PTSD symptom severity was associated with reduced minute-to-minute HRV (Green et al., 2016). The current sample includes participants from the parent study who completed both 24-hour Holter monitoring and actigraphy monitoring (N = 209), as described below.
The parent study recruited young adults (18-39 years old) via brochures and online ads (Craigslist) for a study designed to examine “the relationship between mood, trauma, PTSD, and physical health factors in young adults.” PTSD was purposively oversampled to comprise approximately half of the sample. Exclusion criteria for the parent study included: (a) organic mental disorder; (b) schizophrenia; (c) bipolar I mixed state or bipolar II; (d) lifetime PTSD without current PTSD; (e) current substance abuse/dependence; (f) current major depressive disorder without PTSD; (g) pregnancy; and (h) AIDS or HIV; and (i) uncontrolled medical condition (e.g., kidney or liver failure). Of those screened for participation in the study, 55 were excluded, as follows: older than 39 years of age (n = 1); met diagnostic criteria for lifetime PTSD but not current PTSD (n = 20); substance abuse (n = 15); major depression (n = 4); current mania (n = 4); psychotic disorder (n = 4); HIV (n = 1); more than one excluded mental health disorder (n = 1); bipolar disorder (n = 1); positive drug screen (n = 1); currently taking nitroglycerin (n = 1); and low compliance (n = 2). Medical conditions in enrolled study participants included the following: obstructive sleep apnea (mild to moderate; n = 2, neither of whom had PTSD); diabetes (n = 3, including 1 with PTSD); and hypertension (n = 4, including 1 with PTSD). The study was approved by both the Durham Veterans Affairs and Duke University Medical Center Institutional Review Boards. All patients gave written informed consent prior to participation. Data were collected between August 2008 and September 2013. Demographics of the current sample were 18-39 years old; 105 women; 95 black, 93 white, 11 multiracial, 4 Asian, 2 other, and 4 missing racial status; complete data are shown in Table 1. There were 95 participants (45.5%) who met CAPS criteria for current PTSD. Nearly half of these were military veterans. Participants with PTSD were older than those without PTSD and were more likely to smoke and smoke heavily (see Table 1).
Table 1.
Participant Characteristics by PTSD Status
| No PTSD N = 114 |
PTSD N = 95 |
||||
|---|---|---|---|---|---|
|
| |||||
| Variable | n or M | % or SD | n or M | % or SD | t or χ2 |
| Age (years) | 28.05 | 5.48 | 30.54 | 5.39 | 3.30* |
| WH ratio | 0.89 | 0.07 | 0.89 | 0.08 | 0.49 |
| DTS total | 18.64 | 25.12 | 71.48 | 30.69 | 13.69*** |
| Trauma frequency | 2.85 | 2.69 | 7.09 | 3.82 | 9.40*** |
| Years since 1st trauma | 12.68 | 10.00 | 20.68 | 8.27 | 6.22*** |
| Mean LF HRV (ms) | 44.52 | 17.00 | 37.81 | 15.07 | 2.99** |
| Mean HF HRV (ms) | 30.68 | 16.09 | 26.63 | 14.07 | 1.92 |
|
| |||||
| Females | 55 | 48.3 | 50 | 52.6 | 0.40 |
| Minority (non-white) | 56 | 50.5 | 56 | 59.6 | 1.71 |
| Veterans | 27 | 23.7 | 45 | 47.4 | 12.87*** |
| Smoking Status | 10.44* | ||||
| 0 - Non-smoker | 69 | 61.1 | 39 | 41.1 | |
| 1 - Former smoker | 19 | 16.8 | 17 | 17.9 | |
| 2 - ≤ 10 cigs/day | 12 | 10.6 | 22 | 23.2 | |
| 3 - >10 cigs/day | 13 | 11.5 | 17 | 17.9 | |
| Meds (blockers) | 4 | 3.5 | 2 | 2.1 | nsa |
Note. DTS Total = Davidson Trauma Scale total score; HF HRV = high-frequency heart-rate variability; LF HRV = low-frequency heart-rate variability;; WH Ratio = waist-hip ratio.
Fisher exact test.
p < .05.
p < .01.
p < .001.
At an initial screening session, participants completed the Clinician Administered PTSD Scale (CAPS; Blake et al, 1995) to determine current PTSD status. The CAPS is a 17-item structured interview that corresponds to the DSM-IV criteria for PTSD. In the current study, CAPs was used for diagnostic purposes only. Administration of the CAPS was modified such that the interview was discontinued as soon as a person failed to report enough symptoms in a particular symptom cluster that would rule out current PTSD diagnosis. The Davidson Trauma Scale (DTS: Davidson et al, 1997) is a 17-item self-report measure that assesses the 17 DSM-IV symptoms of PTSD rated on a 5-point frequency (0 = not at all to 4 = every day) and severity scales (0 = not at all distressing to 4 = extremely distressing). The DTS was used as a continuous measure to quantify PTSD symptom severity. Trauma exposure was measured using the Traumatic Life Events Questionnaire (TLEQ; Kubany et al., 2000). Participants reported the number of distinct types of traumas that they experienced during their lifetime as well as their age when they first experienced a traumatic event resulting in feelings of fear, helplessness, and horror. Smoking status and demographic information, including age, gender, racial minority status, pack-year history, and the use of blood-pressure medications were also collected at screening, as well as anthropometric measures (waist-hip ratio). Blood pressure medications were categorized as follows: beta blockers; calcium-channel blockers; ACE inhibitors; or other hypertension medications.
Approximately 1 week after the initial screening session, participants were instrumented with devices to assess electrical activity of the heart (electrocardiogram-ECG) and movement (actigraphy). A Del Mar Reynolds Lifecard CF, 3-channel digital Holter recorder was used for ECG in this study, and was digitized at 125 Hz. ECG sessions lasted for 20 to 24 hours, and began at approximately 2:00 PM. A wristwatch-style actigraph was used concurrently to assess activity (sleep, rest, wake) (Philips Respironics | Mini Mitter, Bend, OR). The Actiwatch-L and Actiware 5 (sensitivity set to medium) software were used to derive objective estimates of sleep, rest (quiescence) and active periods during 24-hr of monitoring. The Actiware software employs an algorithm to interpret and categorize data sequences into one of three interval types: active, rest, and sleep. Interval type is based on user-defined sensitivity and activity count information. In the current study, we utilized default settings with medium sensitivity.
Minute-by-minute changes in the amplitude of the LF and HF components of HRV were assessed by complex demodulation, a nonlinear time-domain method for assessing time-dependent changes in nonstationary oscillatory components within a predefined frequency band. Briefly, time-dependent changes in LF and HF amplitudes were extracted continuously by demodulating the frequency bands of 0.04-0.15 Hz and 0.15-0.45 Hz, respectively, and the amplitude time series of LF and HF components were averaged over every 1-minute segment (Hayano et al., 1993).
Data Analysis
Multilevel modeling (MLM) was used to determine whether the association between PTSD symptom severity and HRV differed across interval types. MLM is appropriate for analyzing repeated-measures data. Unlike repeated-measures ANOVA, MLM can accommodate imbalanced data and data missing at random (Searle, Casella, & McCulloch, 1992). Data collected for each monitoring period were broken into the 3 software-derived interval types discussed above (active, rest, sleep). Mean LF and HF amplitudes (ms) were then tabulated for each of these intervals. Two models were specified, one for LF amplitude and another for HF amplitude. Interval type and total DTS score were entered as correlates, as well as the interaction between interval type and total DTS score. Specified models included variables having established associations with cardiac function in prior studies: age; sex; waist-hip ratio; smoking status; and use of beta and/or calcium-channel blockers. The variables were entered into each model. All continuous variables were centered. There were 4 participants (n = 1 with PTSD) who were missing waist-hip or smoking status data and were thus excluded from the following adjusted analyses. Missingness was not associated with mean LF amplitude, t(207) = 1.02, p = .301 , or mean HF amplitude, t(207) = 0.79, p = .43.
Results
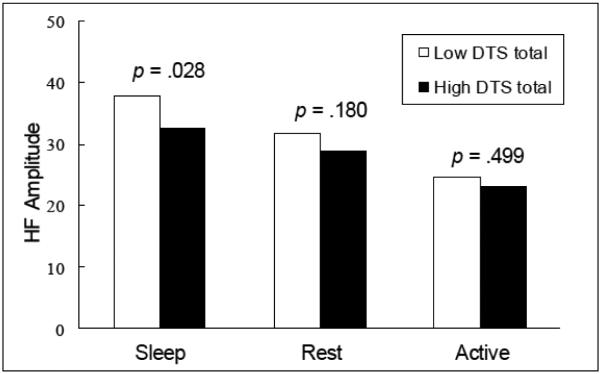
Participants were monitored for both HRV and actigraphically-derived activity for a mean of 23.76 hours (SD = 0.50), with 14.82 hours (SD = 2.15) deemed wake, 1.47 (SD = 1.23) rest, and 7.47 (SD = 1.98) sleep. Hours spent in wake, t(207) = 0.46, p = .645, and sleep, t(207) = 1.10, p = .274, did not vary by PTSD status. Participants with PTSD had lower mean LF amplitudes (see Table 1). Analysis of the null multilevel models (i.e., models without correlates) indicated that 72.3% of the variance in LF amplitude and 68.0% of the variance in HF amplitude were attributable to interindividual variability as opposed to intraindividual variability. Results for the full models with predictors are depicted in Table 2. Age and waist-hip ratio were negatively associated with both measures of HRV, and females demonstrated higher LF HRV than males. PTSD symptom severity was negatively associated with LF amplitude but did not interact with interval type in the model. The interaction between PTSD symptom severity and interval type, however, was significant for HF amplitude (p=.03)(Table 2). Post hoc analyses were conducted to decompose the interaction. Individuals with high PTSD symptom severity exhibited lower HF HRV than individuals with low PTSD symptom severity during periods of sleep, t(1083) = 2.20, p = .028, Cohen’s d = 0.12, but not during periods of activity, t(1083) = 1.34, p = .499, Cohen’s d = 0.05, or rest, t(1083) = 1.34, p = .180, Cohen’s d = 0.09, (Figure 1).
Table 2.
Multilevel models of LF and HF Amplitude
| LF Amplitude | HF Amplitude | |||
|---|---|---|---|---|
| Variable | Coeff. | SE | Coeff. | SE |
| Within-person | ||||
| Intercept | 34.69** | 1.48 | 22.01** | 1.46 |
| Sleepinga | −1.34 | 0.74 | 11.38** | 0.69 |
| Restinga | 3.59** | 0.61 | 6.43** | 0.57 |
| Between-person | ||||
| Age | −0.79** | 0.19 | −0.97** | 0.18 |
| Female | 11.35** | 2.06 | 3.53 | 2.05 |
| WH ratio | −31.84* | 13.90 | −35.94** | 13.78 |
| Smoking status | −2.19* | 0.97 | −1.33 | 0.96 |
| Meds (blockers) | −3.71 | 5.75 | 4.36 | 5.71 |
| DTS total | −0.07* | 0.03 | −0.02 | 0.03 |
| Cross-level interaction | ||||
| DTS Total × Sleepinga | −0.01 | 0.02 | −0.05** | 0.02 |
| DTS Total × Restinga | −0.02 | 0.02 | −0.02 | 0.01 |
Note. N = 205; Model coefficients and standard errors (in parentheses). Coeff=Coefficient; DTS Total = Total score on Davidson Trauma Scale; LF Amp = Low-frequency amplitude; HF Amp = High-frequency amplitude; Meds (Blockers)=Use of beta and/or calcium-channel blockers; SE=Standard Error; WH Ratio = Waist-hip ratio;
Active status used as reference value.
p < .05.
p< .01.
Figure 1. High-frequency amplitude by PTSD symptom severity (DTS total) and activity.
Low and high DTS totals calculated as 1-standard deviation offsets from the sample mean.
Discussion
PTSD symptom severity in the sample was associated with PSNS amplitude during sleep, but not during rest or wake; these results concord with previous investigations demonstrating decreased parasympathetic tone during sleep in individuals with PTSD (Agorastos, et al., 2013; Woodward, et al., 2009). Sleep physiology is globally distinguished by two stages: non-REM (NREM) and REM sleep; NREM is normally characterized by a progressive increase in parasympathetic tone from Stage 1 to Stage 3 (Versace, Mozzato, De Min Tona, Cavallero, & Stegagno, 2003). The NREM sleep of individuals with PTSD is characterized by parasympathetic modulation of HRV due, in part, to a predominance of lighter (Stage 1) versus deeper, more restorative (Stage 3) sleep (Kobayashi, Boarts, & Delahanty, 2007); similar modulation has been replicated in individuals without PTSD via experimental exposure to a presleep stressful task (Hall, et al., 2004). REM sleep disturbance in PTSD is characterized by frequent arousals and sympathetic modulation of HRV (Kobayashi, Boarts, & Delahanty, 2007). Cumulative evidence suggests the involvement of HPA axis dysfunction in the autonomic dysfunction and sleep disturbance observed in individuals with PTSD (van Liempt, et al., 2013). Our findings suggest that in individuals with high PTSD symptoms, nocturnal sleep may represent a period of increased vulnerability for decreased parasympathetic cardiac control.
In light of disagreement among researchers regarding the extent to which LF-HRV truly reflects sympathetic cardiac control (Reyes del Paso, et al, 2013), it is interesting that we found significant differences between those with and without PTSD on LF-HRV. Reyes del Paso et al (2013) assert that the LF component of HRV is more likely determined by the parasympathetic nervous system, and that baroflex control plays an important role. This viewpoint converges with our findings across 2 studies. In a sample of women, we found that lower baroreceptor sensitivity was associated with poorer sleep in women with PTSD, but not in women without PTSD (Ulmer et. al, 2009). The current findings that PTSD was associated with lower LF amplitude are consistent with previous findings from Shah and colleagues (2013) that PTSD symptom severity was related to reduced oscillations in heart rate across the LF band in US male veteran twins. Previous studies have also found that negative affect and perceived stress are temporally associated with a reduction in the LF component of HRV during experimental stress in young healthy individuals (Swenne, Bootsma and vanBolhuis, 1995) as well as during ambulatory monitoring in coronary heart disease patients (Bacon et al, 2004). This direction of change supports previous findings that the LF component of HRV is not simply a marker of sympathetic nervous system modulation but rather is under dual control by the sympathetic and parasympathetic nervous systems (Goldstein, Bentho, Park, et al, 2011; Houle et al, 1999). Moreover, findings from pharmacological blockade studies demonstrate that under most conditions, LF power is secondary to the parasympathetic nervous system and largely a function of baroreceptor-mediated adjustments to the variations in peripheral resistance and blood pressure, or Mayer waves, occurring at this frequency.
Regardless of the etiology for the autonomic dysfunction revealed in our study, a finding that PTSD symptom severity may be associated with decreased HF-HRV during sleep is not without consequence. Reduced HRV is associated with a greater risk for cardiovascular disease in both combat veterans (Wentworth, et al., 2013) and general populations (Palatini & Julius, 1997). Thus, our findings reinforce the assertion that individuals with high PTSD symptoms -- including those not meeting DSM criteria for PTSD -- may require regular medical screening for prevention and early detection of cardiovascular disease. Moreover, these results underscore the need for interventions that address sleep quality in individuals with PTSD pathology.
The current finding, that PTSD was not associated with reduced HF-HRV during resting conditions, is in contrast to earlier findings (Shah et al, 2013; Dennis et al, 2014). The failure to observe a significant association between PTSD and reduced HF HRV during resting conditions in the current study is unlikely due to less reliable HRV data due to ECG artifact because the technique of complex demodulation used in the current study provides a robust tool that is less limited by the requirement of stationary data than traditional frequency domain analyses (Hayano et al 1993).
Our study is not without limitations. First and foremost, the cross-sectional design of the current study prevents any identification of the temporal pattern of change between PTSD and reduced HRV. Hyper-reactivity to traumatic cues during daily life and consequent prolonged reductions in HRV have been documented in the literature and support the view that the presence of PTSD results in reduced HRV (Norte et al 2013; Keary et al, 2009). Recent findings, however, demonstrate that signs of over-reactivity to stress in the form of low HRV or elevated inflammation are present prior to the development of PTSD (Eraly, et al, 2014; Minassian et al, 2015). Secondly, in contrast with previous investigations of sleep/wake activity and HRV in PTSD, our study sample was relatively young, gender- and minority-balanced, and predominantly civilian as opposed to veteran. Not surprisingly, veterans endorsed more PTSD symptoms on average which introduced an imbalance between groups with regard to Veteran status. Third, polysomnography was not utilized in the parent study. This would have assisted both in differentiating REM and non-REM sleep, and also would have allowed detection of sleep-disordered breathing. As noted above, 2 individuals diagnosed with mild to moderate sleep apnea were included in this study, and this condition confers its own adverse impact on cardiac function (Raghurman et al., 2014). Both of these individuals, however, were part of the Non-PTSD group. As such, their inclusion is more likely to have increased Type 1 error versus Type 2 error. Nevertheless, we did not screen for occult sleep apnea as part of the parent study, so it is possible that others with sleep apnea were included in these secondary analyses.
In summary, our study finds evidence of an association between PTSD symptom severity and increased vulnerability to autonomic dysfunction during sleep, thus strengthening an association between sleep disturbance, autonomic dysfunction and increased cardiovascular morbidity in individuals with PTSD symptoms. Compromised functioning and poor health may be prevented by detecting and treating sleep disturbances, such as insomnia and nightmares, in those with elevated PTSD symptoms.
Acknowledgments
This work was supported in part by a VA Research Career Development Award #CDA 09-218 (Dr. Ulmer) from Health Services Research & Development (HSR&D) Service of the VA ORD; the National Institute of Mental Health grant #501MH062482 (Dr. Beckham); a VA Research Career Scientist Award (Dr. Beckham) from the Clinical Science Research and Development (CSR&D) Service of the VAORD; and with resources and the use of facilities at the Durham Veterans Affairs Medical Center, Durham, North Carolina. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veteran Affairs.
References
- Agorastos A, Boel JA, Heppner PS, Hager T, Moeller-Bertram T, Haji U, et al. Diminished vagal activity and blunted diurnal variation of heart rate dynamics in posttraumatic stress disorder. Stress. 2013;16:300–310. doi: 10.3109/10253890.2012.751369. doi: 10.3109/10253890.2012.751369. Epub 2013 Jan 3. [DOI] [PubMed] [Google Scholar]
- Bertram F, Jamison AL, Slightam C, Kim S, Roth HL, Roth WT. Autonomic arousal during actigraphically estimated waking and sleep in male veterans with PTSD. Journal of Traumatic Stress. 2014;27:610–617. doi: 10.1002/jts.21947. doi: 10.1002/jts.21947. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69:935–943. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Medicine Reviews. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. doi: 10.1016/j.smrv.2009.09.002. Epub 2009 Nov 30. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Miodownik H, Cassuto Y. Power spectral analysis of heart rate variability in posttraumatic stress disorder patients. Biological Psychiatry. 1997;41:627–629. doi: 10.1016/s0006-3223(96)00525-2. [DOI] [PubMed] [Google Scholar]
- Dennis PA, Ulmer CS, Calhoun PS, Sherwood A, Watkins LL, Dennis MF, Beckham JC. Behavioral health mediators of the link between posttraumatic stress disorder and dyslipidemia. Journal of Psychosomatic Research. 2014;77:45–50. doi: 10.1016/j.jpsychores.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PA, Watkins LL, Calhoun PS, Oddone A, Sherwood A, Dennis MF, Beckham JC. Posttraumatic stress, heart rate variability, and the mediating role of behavioral health risks. Psychosomatic Medicine. 2014;76:629–637. doi: 10.1097/PSY.0000000000000110. doi: 10.1016/j.jpsychores.2014.05.001. Epub 2014 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsenbruch S, Harnish MJ, Orr WC. Heart rate variability during waking and sleep in healthy males and females. Sleep. 1999;22:1067–1071. doi: 10.1093/sleep/22.8.1067. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, Baker DG. Assessment of Plasma C-Reactive Protein as a Biomarker of Posttraumatic Stress Disorder Risk. JAMA Psychiatry. 2014;71:423–431. doi: 10.1001/jamapsychiatry.2013.4374. doi:10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, James J, Insana S, Herringa RJ, Mammen O, Price J, Nofzinger E. A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Research. 2013;211:176–179. doi: 10.1016/j.pscychresns.2012.05.007. doi: 10.1016/j.pscychresns.2012.05.007. Epub 2012 Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KT, Dennis PA, Neal LC, Hobkirk A, Hicks TA, Watkins LL, Beckham JC. Exploring the relationship between posttraumatic stress disorder symptoms and momentary heart rate variability. Journal of Psychosomatic Research. 2016;82:31–34. doi: 10.1016/j.jpsychores.2016.01.003. doi: 10.1016/j.jpsychores.2016.01.003. Epub 2016 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M, Vasko R, Buysse D, Ombao H, Chen Q, Cashmere JD, et al. Acute stress affects heart rate variability during sleep. Psychosomatic Medicine. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- Hayano J, Taylor JA, Yamada A, Mukai S, Hori R, Asakawa T, Fujinami T. Continuous assessment of hemodynamic control by complex demodulation of cardiovascular variability. American Journal of Physiology-Heart and Circulatory Physiology. 1993;264:H1229–H1238. doi: 10.1152/ajpheart.1993.264.4.H1229. [DOI] [PubMed] [Google Scholar]
- Keary TA, Hughes JW, Palmieri PA. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. International Journal of Psychophysiology. 2009;73:257–264. doi: 10.1016/j.ijpsycho.2009.04.003. doi: 10.1016/j.ijpsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology. 2007;44:660–669. doi: 10.1111/j.1469-8986.2007.537.x. Epub 2007 May 22. [DOI] [PubMed] [Google Scholar]
- Kobayashi I, Lavela J, Mellman TA. Nocturnal Autonomic Balance and Sleep in PTSD and Resilience. Journal of Traumatic Stress. 2014;27:712–16. doi: 10.1002/jts.21973. doi: 10.1002/jts.21973. Epub 2014 Nov 17. [DOI] [PubMed] [Google Scholar]
- Kubany ES, Haynes SN, Leisen MB, Owens JA, Kaplan AS, Watson SB, Burns K. Development and preliminary validation of a brief broad-spectrum measure of trauma exposure: The Traumatic Life Events Questionnaire. Psychological Assessment. 2000;12:210–224. doi: 10.1037//1040-3590.12.2.210. [DOI] [PubMed] [Google Scholar]
- Lamarche LJ, De Koninck J. Sleep disturbance in adults with posttraumatic stress disorder: a review. The Journal of clinical psychiatry. 2007;68:1257–1270. doi: 10.4088/jcp.v68n0813. [DOI] [PubMed] [Google Scholar]
- Lee EA, Bissett JK, Carter MA, Cowan PA, Pyne JM, Speck PM, Tolley EA. Preliminary findings of the relationship of lower heart rate variability with military sexual trauma and presumed posttraumatic stress disorder. J Trauma Stress. 2013 Apr;26(2):249–56. doi: 10.1002/jts.21797. doi: 10.1002/jts.21797. [DOI] [PubMed] [Google Scholar]
- Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127:1–19. doi: 10.1159/000354910. doi: 10.1159/000354910. Epub 2013 Oct 24. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M. Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biological Psychiatry. 2004;55:953–956. doi: 10.1016/j.biopsych.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB. Association of Predeployment Heart Rate Variability With Risk of Postdeployment Posttraumatic Stress Disorder in Active-Duty Marines. JAMA Psychiatry. 2015;72:979–986. doi: 10.1001/jamapsychiatry.2015.0922. doi:10.1001/jamapsychiatry.2015.0922. [DOI] [PubMed] [Google Scholar]
- Nielsen T, Paquette T, Solomonova E, Lara-Carrasco J, Colombo R, Lanfranchi P. Changes in cardiac variability after REM sleep deprivation in recurrent nightmares. Sleep. 2010;33:113–122. doi: 10.1093/sleep/33.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte CE, Souza GGL, Vilete L, Marques-Portella C, Coutinho ESF, Figueira I, Volchan E. They know their trauma by heart: An assessment of psychophysiological failure to recover in PTSD. Journal of Affective Disorders. 2013;15:136–141. doi: 10.1016/j.jad.2012.11.039. doi: 10.1016/j.jad.2012.11.039. Epub 2012 Dec 27. [DOI] [PubMed] [Google Scholar]
- Oddone AE, Dennis PA, Calhoun PS, Watkins LL, Sherwood A, Dedert EA, Beckham JC. Orthostatic hypotension in young adults with and without posttraumatic stress disorder. Psychological Trauma: Theory, Research, Practice, and Policy. 2015;7:229–233. doi: 10.1037/a0036716. doi: 10.1037/a0036716. Epub 2014 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatini P, Julius S. Heart rate and the cardiovascular risk. Journal of Hypertension. 1997;15:3–17. doi: 10.1097/00004872-199715010-00001. [DOI] [PubMed] [Google Scholar]
- Player MS, Peterson LE. Anxiety disorders, hypertension, and cardiovascular risk: a review. International Journal of Psychiatry in Medicine. 2011;41:365–377. doi: 10.2190/PM.41.4.f. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin. 2007;133(5):725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Raghuram A, Clay R, Kumbam A, Tereshchenko LG, Khan A. A systematic review of the association between obstructive sleep apnea and ventricular arrhythmias. Journal of Clinical Sleep Medicine. 2014;10:1155–60. doi: 10.5664/jcsm.4126. doi: 10.5664/jcsm.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes del Paso GA, Langewitz W, Mulder LJM, Van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology. 2013;50:477–487. doi: 10.1111/psyp.12027. doi: 10.1111/psyp.12027. Epub 2013 Feb 27. [DOI] [PubMed] [Google Scholar]
- Searle SR, Casella G, McCulloch CE. Variance components. Wiley; New York: 1992. [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biological Psychiatry. 2013;73:1103–10. doi: 10.1016/j.biopsych.2013.01.019. doi: 10.1016/j.biopsych.2013.01.019. Epub 2013 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Vaccarino V. Heart Rate Variability in the Prediction of Risk for Posttraumatic Stress Disorder. JAMA Psychiatry. 2015;72:964–965. doi: 10.1001/jamapsychiatry.2015.1394. doi: 10.1001/jamapsychiatry.2015.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European Heart Journal. 1996;17:354–381. [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. doi: 10.1016/j.ijcard.2009.09.543. Epub 2009 Nov 11. [DOI] [PubMed] [Google Scholar]
- Ulmer CS, Calhoun PS, Edinger JD, Wagner R, Beckham JC. Sleep Disturbance and Baroreceptor Sensitivity in Women with Posttraumatic Stress Disorder. Journal of Traumatic Stress. 2009;22:643–747. doi: 10.1002/jts.20464. doi: 10.1002/jts.20464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Liempt S, Arends J, Cluitmans PJ, Westenberg HG, Kahn RS, Vermetten E. Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38:155–165. doi: 10.1016/j.psyneuen.2012.05.015. doi: 10.1016/j.psyneuen.2012.05.015. Epub 2012 Jul 7. [DOI] [PubMed] [Google Scholar]
- Versace F, Mozzato M, De Min Tona G, Cavallero C, Stegagno L. Heart rate variability during sleep as a function of the sleep cycle. Biological Psychology. 2003;63:149–162. doi: 10.1016/s0301-0511(03)00052-8. [DOI] [PubMed] [Google Scholar]
- Williamson JB, Porges EC, Lamb DG, Porges SW. Maladaptive autonomic regulation in PTSD accelerates physiological aging. Front Psychol. 2014;5:1571. doi: 10.3389/fpsyg.2014.01571. Published online 2015 January 21. doi: 10.3389/fpsyg.2014.01571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth BA, Stein MB, Redwine LS, Xue Y, Taub PR, Clopton P, et al. Post-traumatic stress disorder: a fast track to premature cardiovascular disease? Cardiology in Review. 2013;21:16–22. doi: 10.1097/CRD.0b013e318265343b. doi: 10.1097/CRD.0b013e318265343b. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Arsenault NJ, Voelker K, Nguyen T, Lynch J, Skultety K, et al. Autonomic activation during sleep in posttraumatic stress disorder and panic: a mattress actigraphic study. Biological Psychiatry. 2009;66:41–46. doi: 10.1016/j.biopsych.2009.01.005. doi: 10.1016/j.biopsych.2009.01.005. Epub 2009 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]