Abstract
Introduction
Nifedipine is a BCS Class II drug used for treatment of hypertension and preterm labor. Large inter-patient variability in nifedipine absorption results in variable exposure among different patients.
Methods and Materials
We conducted in vitro dissolution studies to compare nifedipine dissolution from immediate release (IR) capsules with different volumes of dissolution media. Results from dissolution studies were used to design a cross-over study in healthy volunteers to evaluate the effect of co-administered water volume with nifedipine 10 mg IR capsules on nifedipine pharmacokinetics, especially absorption (Cmax, tmax, and AUC0–6).
Results and Discussion
Dissolution studies demonstrated that larger gastric fluid volumes result in enhanced nifedipine dissolution from 10 mg IR cosolvent capsules (73% vs. 17% in 200 and 100 mL simulated gastric fluid, respectively, at 30 min). The pharmacokinetic crossover study in healthy volunteers (N=6) did not show a significant effect of the water volume administered with the capsule (50 vs. 250 ml) on Cmax, tmax, or AUC0–6 of orally administered nifedipine IR capsules (10 mg). However, administration of large water volumes resulted in lower variability in nifedipine Cmax (47% vs.70% for 250 mL and 50 mL, respectively).
Conclusion
Administration of large water volumes with nifedipine 10 mg IR cosolvent capsules reduces inter-individual variability in plasma exposure. Evaluation of similar effects in other BCS Class-II drugs is recommended.
Keywords: Nifedipine, dissolution, pharmacokinetics, absorption
Introduction
Biopharmaceutics Classification System (BCS) Class II drugs have low solubility and high intestinal permeability. Due to their low solubility, the in vivo dissolution rate for these drugs can be a rate-limiting step for absorption through the gastrointestinal (GI) tract. (1) Enhanced in vivo dissolution through innovative formulations or increased GIT fluid volumes may be important to ensure adequate and consistent absorption of these drugs. Nifedipine is a BCS Class II drug used for treatment of hypertension (2) and preterm labor. (3) Nifedipine in the immediate release (IR) form is recommended by the American College of Obstetrics and Gynecology (ACOG) for the treatment of preterm labor. (4) Despite nifedipine being one of the first-line therapies for preterm labor (5–8), there is high inter-patient variability in exposure and response, resulting in lack of efficacy in some patients. (6)
Nifedipine is primarily eliminated through CYP3A metabolism (9) with an elimination half-life of 1.25–2 hours in healthy individuals. (10) Given its low solubility, nifedipine absorption is highly variable and its bioavailability ranges from 50 to 70%. (11) Absorption also depends on the dosage form used. (12) While extended release forms are commonly used for long-term treatment of hypertension, IR capsules are used for the acute treatment of preterm labor. These IR capsules are typically liquid-filled cosolvent formulations in which the drug is solubilized. Despite the drug being administered as a solution, high inter-patient variability during the absorption phase has been reported. (12) In a previous study of nifedipine pharmacokinetics in 14 pregnant women, dose-corrected Cmax (maximum observed plasma concentration) ranged from 41 to 397 µg/L. (13)
Administration of different doses of nifedipine IR capsules also results in variable absorption profiles. Administration of nifedipine IR 20 mg capsules has been shown to result in proportional increase in AUC (area under the plasma concentration time curve) but less than proportional increase in Cmax as compared to doses of 5 and 10 mg, possibly due to reduced absorption rate. (11) Thelen and colleagues attributed this to drug precipitation in the stomach. (14) However, this hypothesis has not been tested in prospective clinical trials.
Investigation of the rate and extent of in vitro drug dissolution in physiologically-relevant dissolution fluids can be used to try and understand and predict in vivo absorption profiles. Fasted-state simulated gastric fluid (FaSSGF) has been shown to provide the closest composition (sodium taurocholate, pepsin, lecithin, sodium chloride, and HCl) to human gastric contents (15–17) and dissolution studies in FaSSGF can be used to investigate factors affecting drug dissolution prior to evaluation in humans.
The objectives of our studies were to evaluate the effect of gastric fluid volume on nifedipine in vitro dissolution and in vivo pharmacokinetics. We conducted in vitro and clinical studies to test the hypothesis that small gastric fluid volumes will lead to reduced nifedipine dissolution in vitro and decreased and/or delayed absorption in humans.
Methods
Determination of Nifedipine Equilibrium Solubility
Nifedipine equilibrium solubility was determined in vitro using different fluids to evaluate the effects of fluid composition and pH on nifedipine solubility. Various fluids simulating gastric and intestinal pH were used to predict nifedipine solubility in the stomach and small intestine.
Nifedipine solubility was studied in FaSSGF (sodium taurocholate (80 µM), pepsin (0.1 mg/ml), lecithin (20 µM), sodium chloride (0.2%), HCl (to adjust pH to 1.6), and distilled water) (16), 0.01 M HCl, and 0.05 M phosphate buffer (pH 6.5). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Nifedipine equilibrium solubility was determined in each of the three fluids by adding excess drug to the media and constant shaking in a 37°C water bath with protection from light throughout the duration of the experiment. Samples were collected at 12, 24, 36, 48, and 72 hours. Collected samples were immediately centrifuged at 5000 rpm for 10 minutes and the clear supernatant was aspirated and stored at −20°C until analysis.
Nifedipine concentrations were determined by HPLC (Agilent 1100, Agilent Technologies®, Santa Clara, CA, USA) coupled with a UV detector (λ = 240 nm) and using diazepam as an internal standard. Analytes were separated on a C18 column 5 µm, 4.6 × 15 mm column (Phenomenex) with isocratic mobile phase consisting of 35% acetonitrile, 17% methanol and 48% water at a flow rate of 1.2 ml/min. The lower limit of quantification for nifedipine was 0.2 mg/L. Interday and intraday coefficients of variation were less than 15%.
Nifedipine Dissolution Profiles
Dissolution of nifedipine from IR capsules (Nifedipine Actavis®, Actavis Elizabeth LLC, NJ, USA) was studied in FaSSGF. Experiments were conducted to evaluate the effect of various nifedipine doses and simulated gastric fluid volumes on nifedipine in vitro dissolution: 10 mg IR capsule in 100 mL FaSSGF (“10/100”), 10 mg IR capsule in 200 mL FaSSGF (“10/200”), 20 mg (2×10 mg IR capsules) in 200 mL FaSSGF (“20/200”), and 20 mg (2×10 mg IR capsules) in 400 mL FaSSGF (“20/400”).
Dissolution experiments were conducted using a USP-II paddle apparatus (SR8PLUS Dissolution test station, Hanson Virtual Instruments, San Jose, CA, USA). A standardized mini-paddle apparatus using smaller paddles and 200-mL vessels was used for experiments performed with 100 and 200 mL of media. Dissolution fluid (FaSSGF) was prepared as described above and was maintained at 37±0.5°C during the experiment. The apparatus was protected from light throughout the experiment. A paddle speed of 75 rpm was used since this has been shown to provide a high similarity in drug release profiles between the standard paddle and mini-paddle apparatus for most immediate release dosage forms. (18)
Dissolution experiments were started by adding one or two 10 mg IR capsules to the dissolution vessels filled with the appropriate volume of FaSSGF. Samples (0.5 ml) were collected at 10, 15, 20, 30, 45, and 60 minutes after the start of the experiment. A sampling cannula with an attached 10 µm porous full flow filter (FIL010-HR, Quality Lab Accessories, Telford, PA, USA) was placed in each vessel and was used to collect and filter the samples without stopping the experiment. Samples were stored at −20°C until analysis (as described above). The percentage of the total dose dissolved at each time point was calculated for each of six replicates and a mean percentage dissolved was obtained for each time point.
Effects of Coadministered Water Volume on Nifedipine Absorption in Humans
The effect of water volume administered orally with nifedipine IR capsules on nifedipine pharmacokinetics was evaluated in a prospective clinical trial. The trial was designed as a pilot study to compare nifedipine pharmacokinetics, primarily absorption, after administration of IR capsules with small and large volumes of water.
The study design and all study procedures were approved by the Indiana University-Purdue University at Indianapolis (IUPUI) Institutional Review Board. Study procedures were conducted in the Indiana Clinical Research Center (ICRC) (part of the Indiana Clinical and Translational Sciences Institute) at Indiana University Hospital (Indianapolis, IN) and all participants provided written informed consent.
Inclusion/Exclusion Criteria
Healthy adults (18–45 years) were eligible for enrollment in the study if they were not pregnant or lactating, had baseline systolic and diastolic blood pressures of at least 100 and 60 mm Hg, respectively, had normal hemoglobin (12–15 g/dl for female and 14–18 g/dl for male subjects) and hematocrit (35–49% for female and 40–54% for male subjects), had no history of allergy or hypersensitivity to nifedipine, had no significant clinical illness within a two week period preceding each study phase, had no cardiac, renal, hepatic, gastrointestinal, psychiatric or other diseases, and had no history of drug addiction and/or alcohol abuse. Administration of any medication (prescription or over the counter, except for oral contraceptives), herbal/botanical supplement, or investigational drug was prohibited within a three week period preceding each study phase.
Study Design and Procedures
Subjects were asked to avoid alcohol consumption and smoking for 24 hours before and throughout the study phases and to avoid eating or drinking grapefruit, grapefruit juice, and citrus juices for 48 hours before and throughout the study phases. Subjects were also instructed to fast for 10 hours and to avoid consumption of water for 3 hours prior to admission to ICRC.
Following baseline blood draws, subjects took a 10 mg nifedipine IR capsule (Nifedipine Actavis®, Lot No. 363G12; Actavis Elizabeth LLC, NJ, USA) orally with either 50 mL (small volume) or 250 mL (large volume) of water. The volume of water administered on the large volume phase was chosen as it is similar to commonly used water volumes in clinical trials of oral dosage forms in healthy volunteers (240–250 ml). A volume of 50 ml was chosen for the small volume phase as the minimum volume deemed by the study investigators to allow easy administration and complete swallowing of the nifedipine capsules. The order of the two phases was randomized and they were separated by a wash out period of at least 2 days.
Blood samples (2 ml) for determination of nifedipine plasma concentrations were collected in EDTA-treated blood collection tubes before and 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 2, 4, and 6 hours following administration of nifedipine. Samples were immediately centrifuged at 2000 rpm for 7 min at 4°C, and plasma transferred to polypropylene tubes for storage at −80°C until analyzed by LC/MS/MS by the Clinical Pharmacology Analytical Core Laboratory at Indiana University as described previously. (19) The assay was linear between 0.01 and 1000 ng/mL and inter- and intra-day coefficients of variation were <15%. Heart rate and blood pressure were monitored every 30 min for the first 2 hours and at 4 and 6 hours following nifedipine dose administration. Lunch (excluding food containing citrus or grapefruit) was provided 3 hrs after nifedipine administration.
Pharmacokinetic Analyses
A non-compartmental pharmacokinetic analysis (NCA) was performed using Microsoft Office Excel 2007© (Microsoft Corporation, Redmond, WA) PKSolver add-in (20) to determine each subject’s nifedipine Cmax, tmax (time for maximum plasma concentration), and AUC0–6 for each of the two phases. Cmax and tmax were observed from the individual plasma concentration time profiles and AUC0–6 was estimated by PKSolver add-in using nifedipine dose and observed concentration-time profiles.
Statistical Analyses
For determination of Nifedipine equilibrium solubility, nifedipine concentrations were compared between the three dissolution fluids at each time point using one-way ANOVA (α=0.05) with Bonferroni’s posthoc test for pairwise comparisons. For dissolution experiments, the mean area under nifedipine dissolution curve (AUCdiss) was compared between the 10/100 and 10/200 experiments and the 20/200 and 20/400 experiments using unpaired Student’s t-test (α=0.05). In addition, the effect of fluid volume on percent of nifedipine dose dissolved after 20, 30, 45, and 60 minutes was compared using repeated measures ANOVA with posthoc Bonferroni comparisons.
The sample size for the cross-over study was estimated from the variance observed in nifedipine Cmax by Yu and colleagues (21), in which the average (±SD) nifedipine Cmax after a 10 mg dose was 124 (±52) ng/ml. Based on this, we determined that six subjects were needed to detect a 60% difference in the mean nifedipine Cmax between the two phases, with an alpha of 0.05 and power of 90%. Pharmacokinetic parameters estimated from the NCA for the two phases were compared using a paired Student’s t-test for normally distributed data and Wilcoxon signed rank test for non-normally distributed data (SPSS®; Version 21.0. Armonk, NY: IBM Corp.).
Results
Determination of Nifedipine Equilibrium Solubility
Equilibrium solubility of nifedipine was achieved within 24 hours and determined to be 10.91± 2.27, 9.58±1.34 and 7.47±1.35 µg/mL in 0.01 M HCl, FaSSGF and pH 6.5 phosphate buffer, respectively. Nifedipine concentrations were similar between the dissolution fluids after 24 hours (P>0.05, one-way ANOVA). Based on these results, it was concluded that nifedipine solubility is likely to be constant across the GI tract and does not change between gastric and intestinal pH. Further dissolution experiments of nifedipine capsules were conducted in FaSSGF which most closely simulates gastric fluid.
Fluid Volume Effect on Nifedipine in Vitro Dissolution
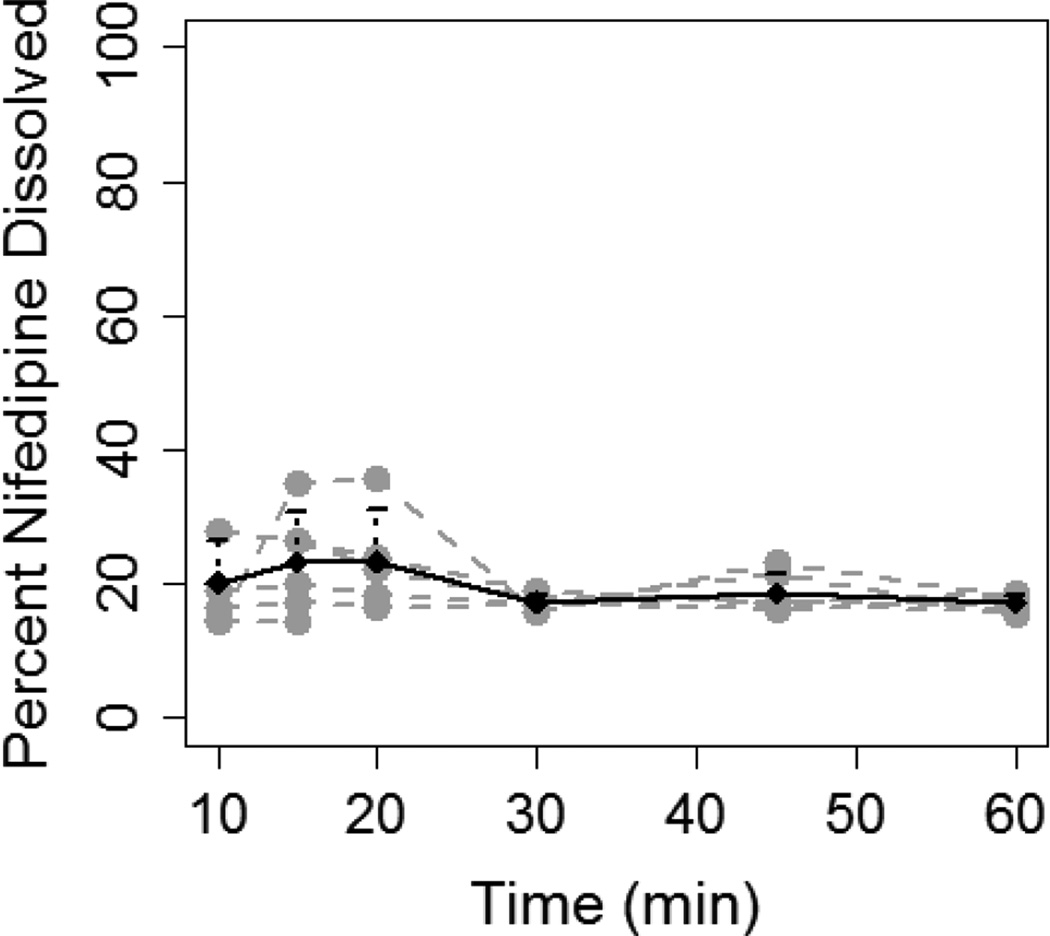
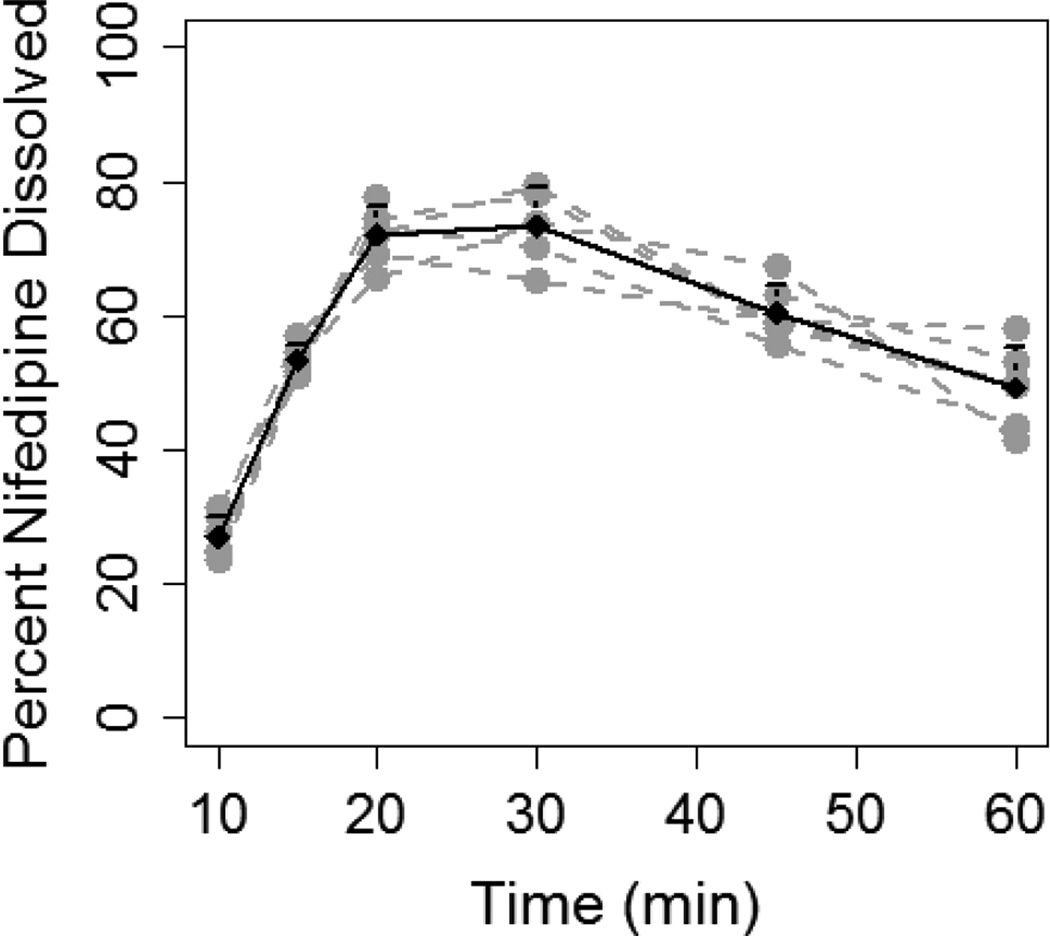
Nifedipine dissolution from IR capsules was determined using 10 and 20 mg of nifedipine in varying volumes of FaSSGF. Nifedipine 10 mg capsules dissolved to a greater extent in in 200 mL FaSSGF (10/200) compared to 100 mL (10/100) (Figure 1). The percentage of nifedipine dissolved was significantly higher in the 10/200 compared to the 10/100 experiment at all of the time points studied: 20 min (72.1±4.2% vs. 23.3±7.6%, p=0.003); 30 min (73.4±5.8% vs. 17.3±1.1%, p=0.003); 45 min (60.4±4.3% vs. 18.6±2.9%, p=0.01); and 60 min (49.3±6.1% vs. 17.2±1.2%, p=0.02). AUCdiss was more than 3-fold higher in the 10/200 experiment compared to 10/100 (51.1±1.8 vs. 15.8±2.1 ng.hr/ml, P<0.001).
Figure 1.
Dissolution of 10 mg nifedipine capsule in 100 mL (A) and 200 mL (B) of FaSSGF. Grey lines and dots represent individual dissolution profiles for each of the six experiments. Black line and dots represent the mean dissolution profile for all six experiments and the error bars represent standard deviation.
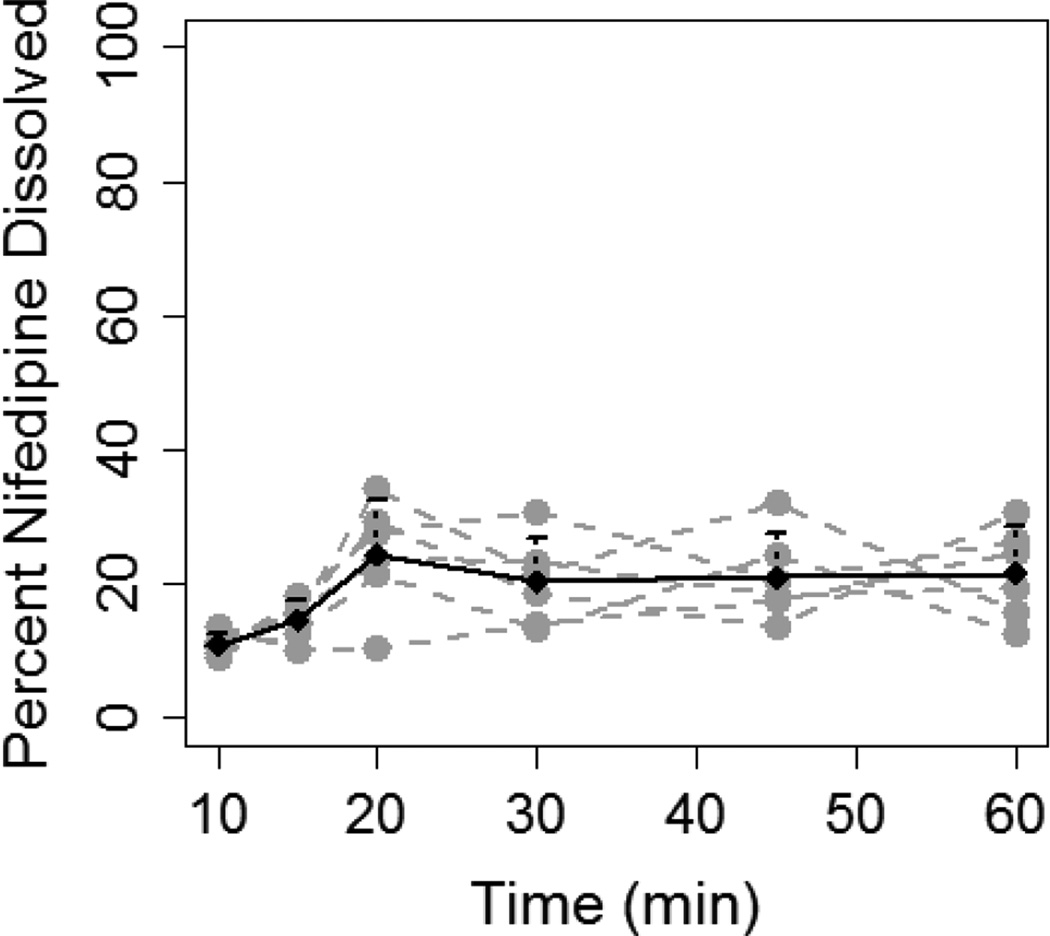
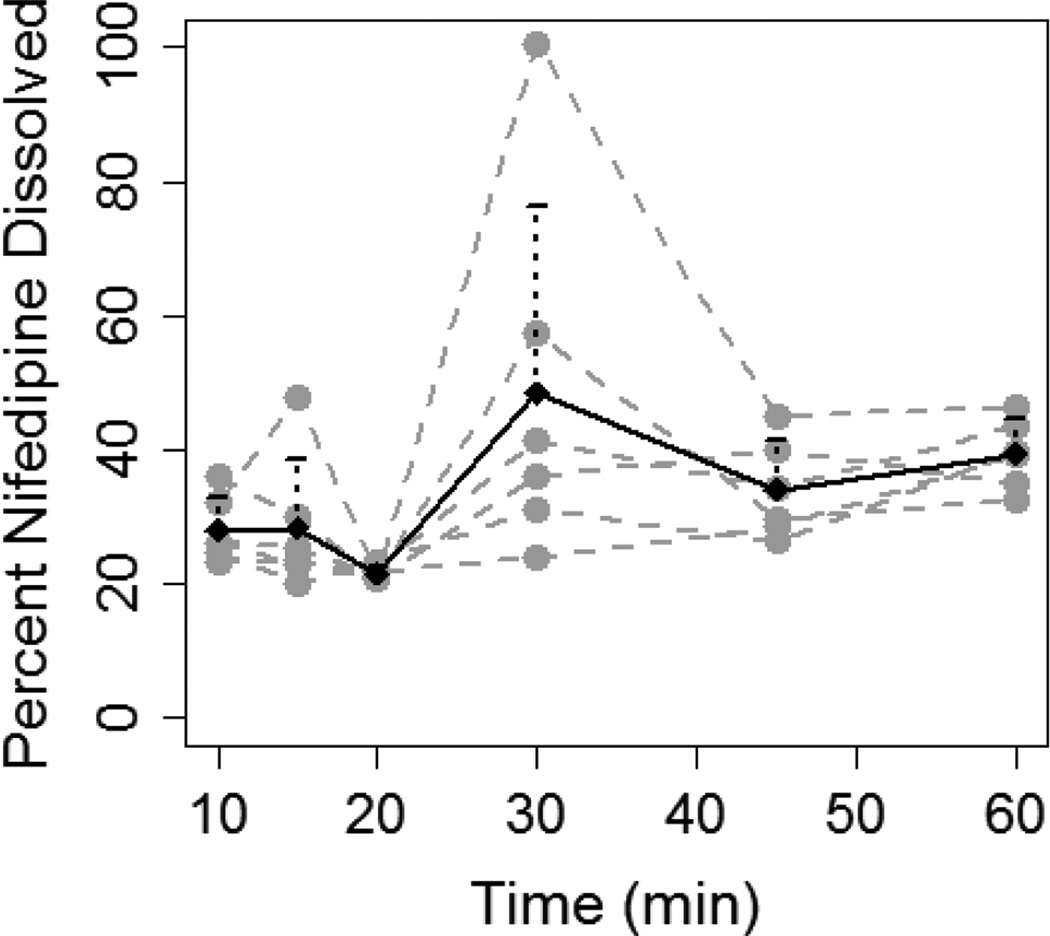
Conversely, dissolution of 20 mg of nifedipine was not consistently higher in 400 mL as compared to 200 mL of FaSSGF (Figure 2). The percentage of nifedipine dissolved in the 400 mL solution was significantly higher only at 60 min (39.3±5.1% vs. 21.6±6.9%, P=0.03). Although, AUCdiss was significantly higher in the 20/400 experiment as compared to 20/200 (30.3±7.5 vs. 17.4±2.9 ng.hr/ml, P=0.003), the magnitude of difference was less than that seen with the 10 mg capsule at different fluid volumes (1.7-fold vs. 3.2 -fold).
Figure 2.
Dissolution of 20 mg nifedipine (two 10 mg capsules) in 200 mL (A) and 400 mL (B) of FaSSGF. Grey dashed lines and grey dots represent individual dissolution profiles for each of the six experiments. Black solid line and black dots represent the mean dissolution profile for all six experiments and the error bars represent standard deviation.
Effects of Coadministered Water Volume on Nifedipine Absorption in Humans
A prospective randomized two-phase crossover clinical trial was conducted to evaluate the effect of coadministered water volume on pharmacokinetics of IR nifedipine capsules. A nifedipine dose of 10 mg was used for the clinical trial based on results from in vitro dissolution studies that showed a more pronounced effect of fluid volume on 10 mg than on 20 mg.
Six healthy volunteers (4 men and 2 women) enrolled in and completed the two phases of the study. One subject was a cigarette smoker and two consumed alcohol occasionally, but all abstained from alcohol and smoking for 24 hours before and during each study phase. Table 1 lists the demographic characteristics for the six subjects at the time of enrollment.
Table 1.
Baseline demographic characteristics for study subjects (N=6).
| Demographic Characteristic | Mean±SD/Number |
|---|---|
| Male/Female | 4/2 |
| Age (yrs) | 29±10 |
| Weight (kg) | 70±5 |
| Height (cm) | 173±6 |
| Race | 4 Caucasian |
| 2 African American | |
| Baseline Heart Rate (bpm) | 73±14 |
| Baseline Systolic Blood Pressure (mm Hg) | 123±10 |
| Baseline Diastolic Blood Pressure (mm Hg) | 71±8 |
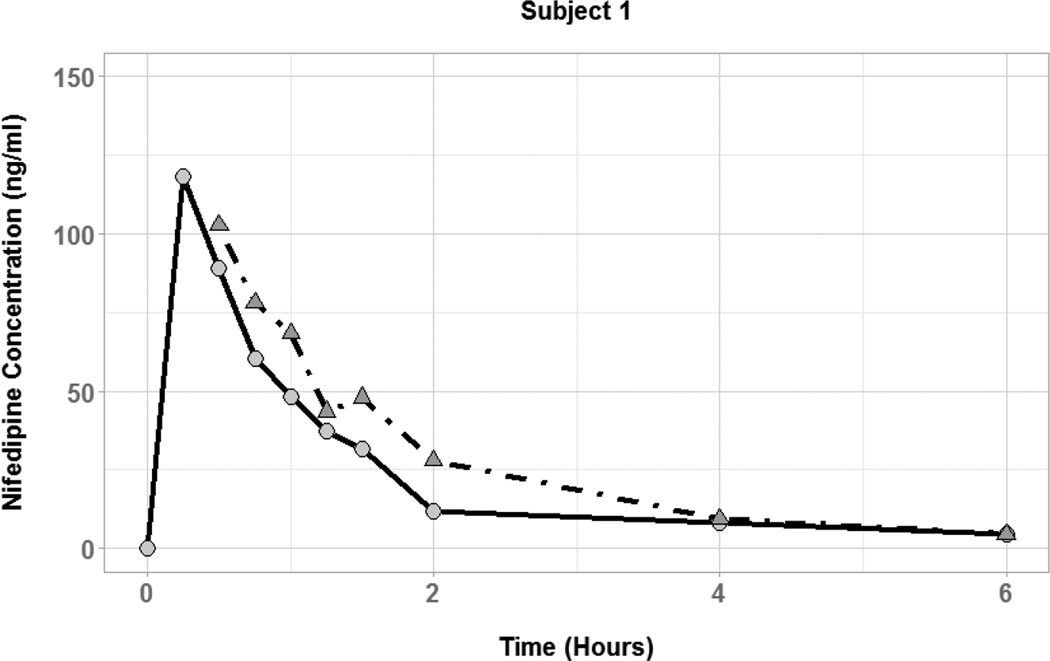
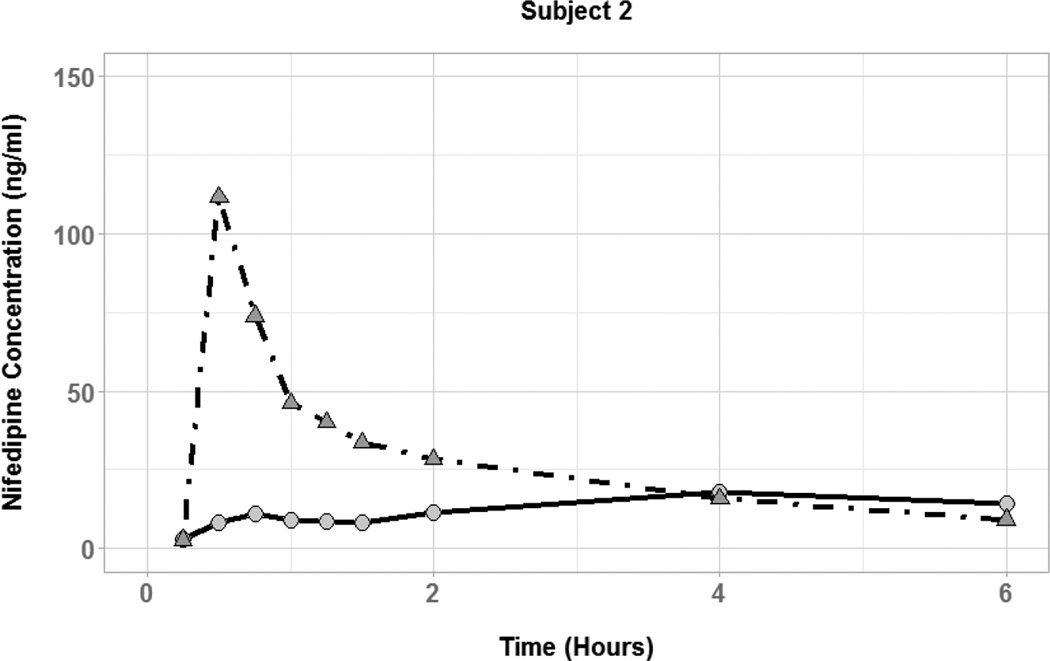
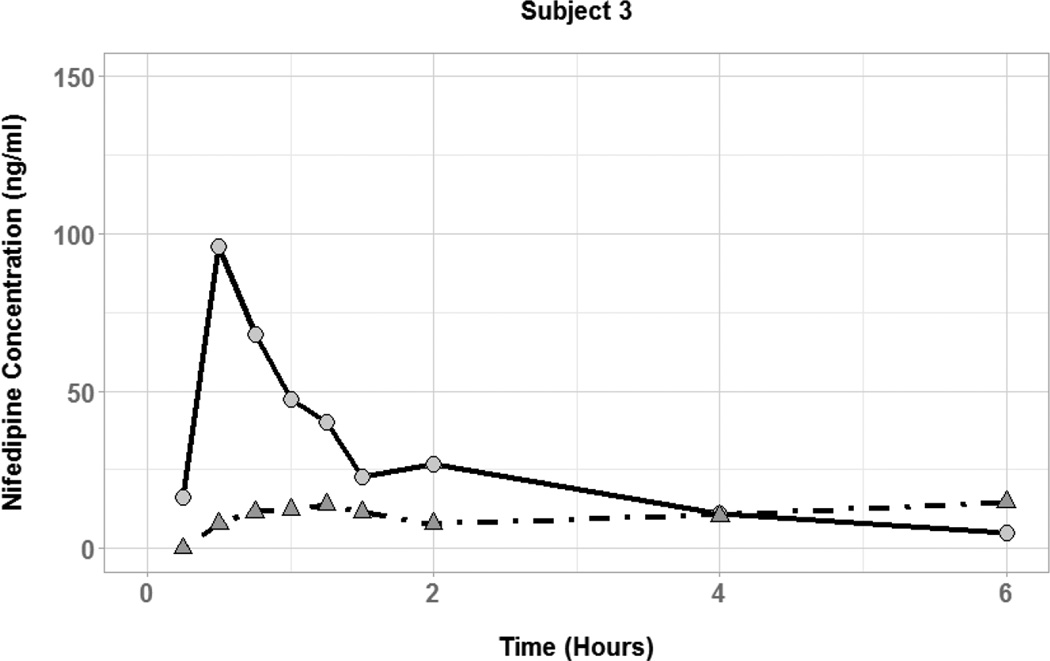
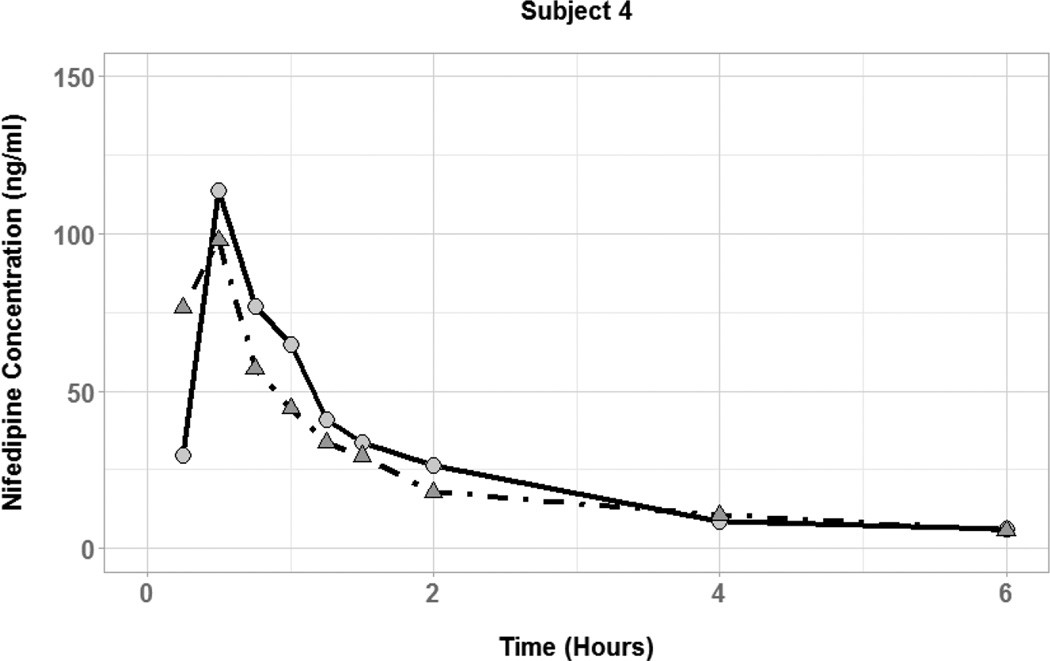
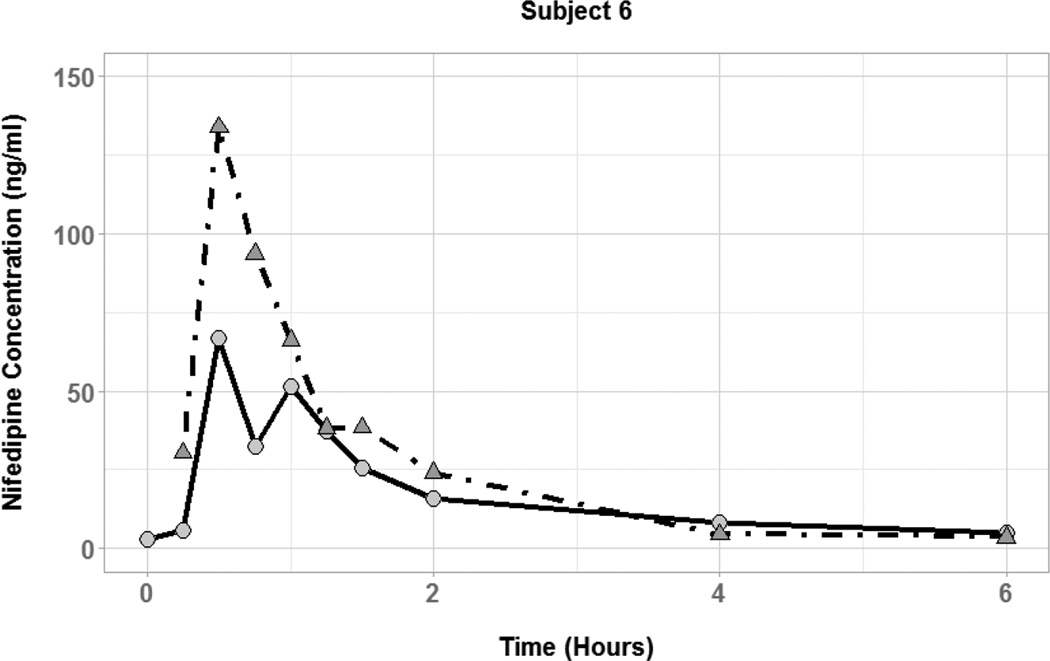
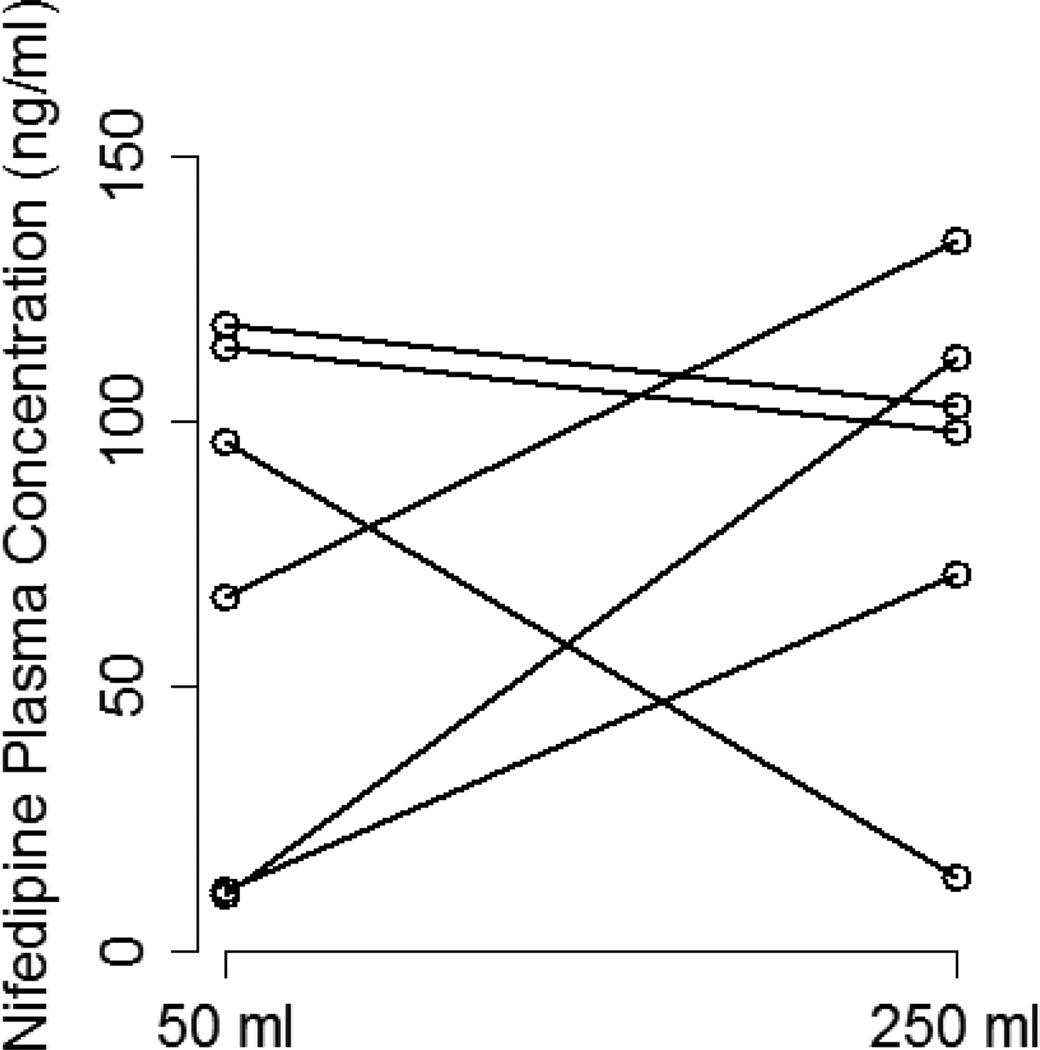
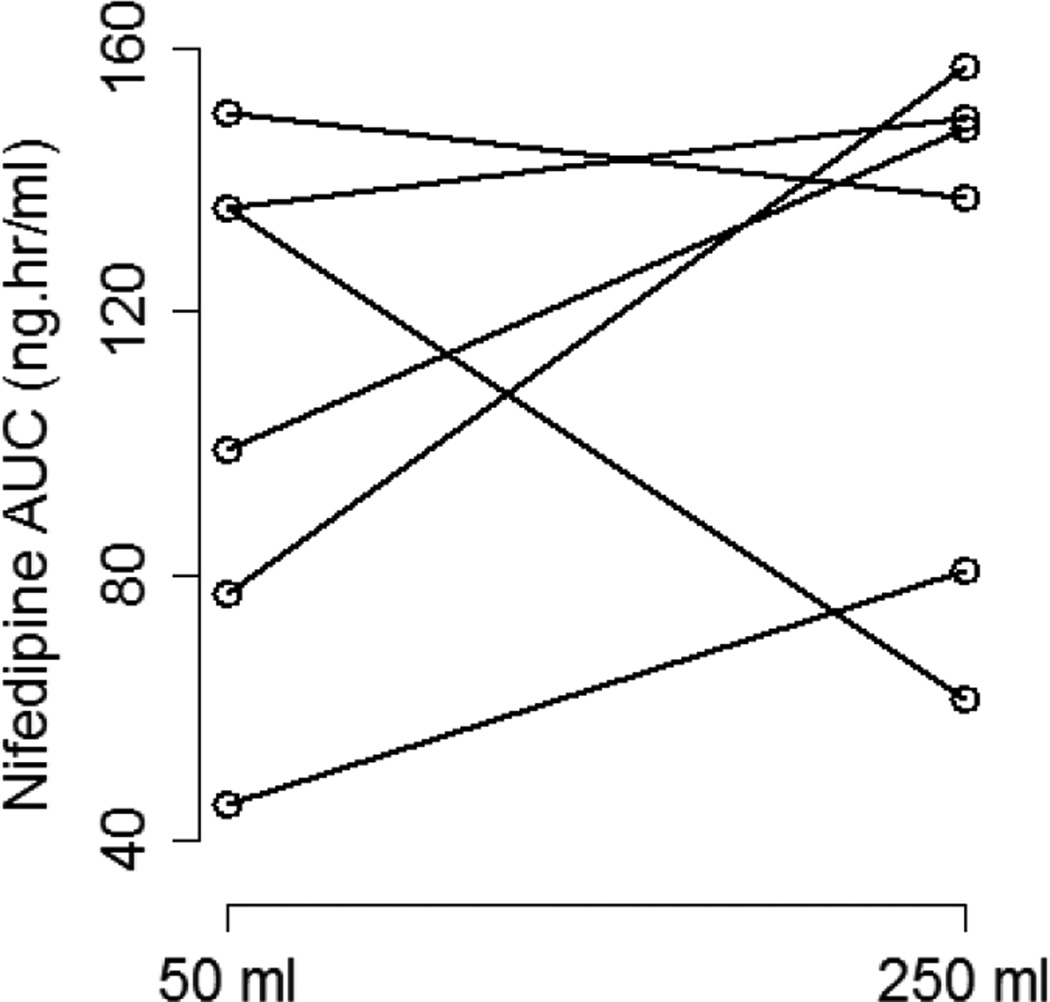
The effect of coadministered water volume on nifedipine pharmacokinetics during the absorption phase was evaluated by comparing each subject’s Cmax, tmax, and AUC0–6 between the two phases (Figure 3). There was high interindividual variability in the effect of 50 vs. 250 mL of coadministered water volume on nifedipine pharmacokinetic parameters. Plasma concentration-time profiles for three of the six subjects showed substantially higher nifedipine concentrations during the large volume phase compared to the small volume phase (Figure 4). In those subjects, the large volume phase was associated with 2-, 6.5-, and 10-fold increase in Cmax and 1.5-, 1.8-, and 2-fold increase in AUC0–6. Two of the six subjects had similar nifedipine concentrations in the two phases with minimal change in Cmax or AUC0–6. On the other hand, one subject had higher nifedipine plasma concentrations during the small volume phase with 7-and 2-fold increase in Cmax and AUC0–6, respectively. Median tmax was similar between the two phases of the study (0.5 hours) with a range of 0.25–0.75 and 0.5–0.75 for the small and large volume phases, respectively. Table 2 summarizes the nifedipine pharmacokinetic parameters for each of the six subjects. Comparison of nifedipine pharmacokinetic parameters between the two phases resulted in no significant differences between the phases in any of the parameters.
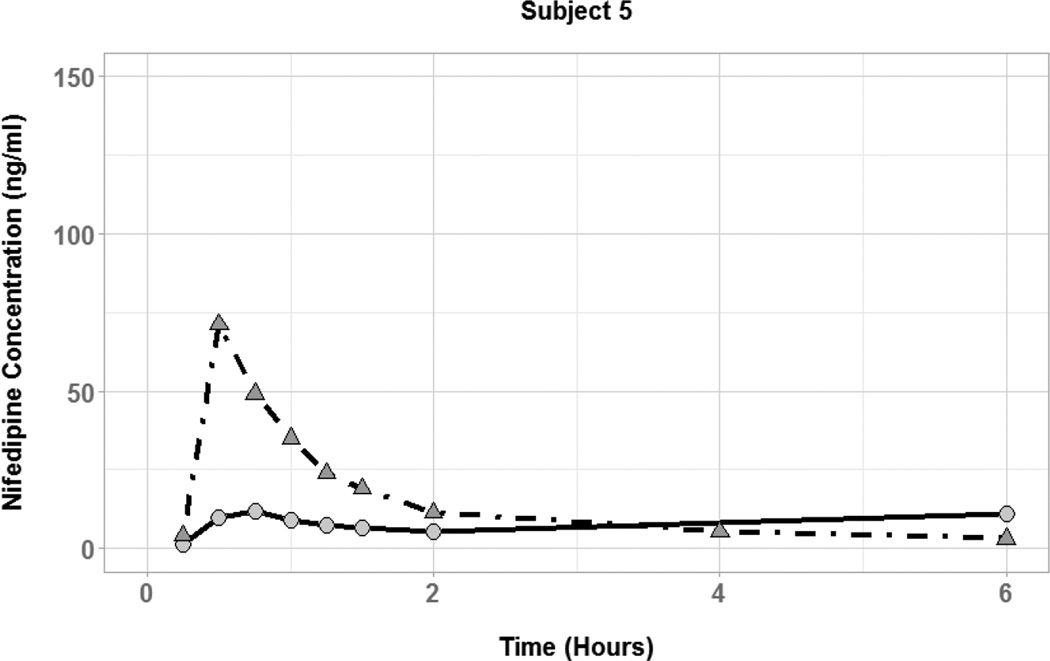
Figure 3.
Nifedipine plasma concentration time profiles for subjects with coadministration of small (50 mL; circles and solid lines) and large (250 mL; triangles and dot-dashed lines) volumes of water.
Figure 4.
Change in nifedipine Cmax (A) and AUC0–6 (B) between the two study phases for each of the six subjects.
Table 2.
Nifedipine individual pharmacokinetic parameters for each of the two study phases.
| Cmax (ng/ml) | AUC0–6 (ng.hr/ml) | |||
|---|---|---|---|---|
| Subject Number (Sex, Race, Age, Weight) |
Small Volume |
Large Volume |
Small Volume |
Large Volume |
| 1 (M, C, 24, 71) | 118.1 | 102.7 | 135.7 | 149.2 |
| 2 (M, AA, 23, 71) | 10.81 | 111.8 | 77.14 | 157.1 |
| 3 (M, AA, 18, 71) | 96.06 | 13.92 | 135.9 | 61.19 |
| 4 (F, C, 33, 76) | 113.7 | 97.87 | 150.3 | 137.1 |
| 5 (M, C, 45, 67) | 11.61 | 71.19 | 45.3 | 80.59 |
| 6 (F, C, 28, 62) | 66.64 | 133.9 | 99.19 | 147.8 |
| Geometric Least Squares Mean |
47.0 | 72.8 | 99.3 | 115.2 |
| Geometric Least Squares Mean Ratio (90% CI) |
0.65 (0.18–2.29) | 0.86 (0.55–1.35) | ||
CI: Confidence Interval, M: Male, F: Female, C: Caucasian, AA: African American
No subject experienced any clinical adverse event due to changes in blood pressure or heart rate; although the study was not primarily designed to assess pharmacodynamic changes in a healthy volunteer population.
Discussion
As a BCS Class II drug, nifedipine absorption is known to be highly variable and dependent on the dosage form used. (12) While the majority of nifedipine is prescribed as an extended release formulation, the obstetric community continues to employ IR nifedipine as a first-line treatment for preterm labor. (22) However, large variability in nifedipine pharmacokinetics and tocolytic effects prevents it from being effective in all cases of preterm labor. For example, observed nifedipine concentrations in 14 pregnant women treated for preterm labor showed a 10-fold range in dose-corrected Cmax. (13)
Several factors may contribute to the observed variability in nifedipine absorption. First, variability in nifedipine dissolution within the GI tract may result in different fractions of the dose being available for absorption at different times and locations within the GI tract. Second, differences in CYP3A-mediated first-pass metabolism may result in variability in nifedipine absorption. However, this is unlikely to be the major factor contributing to differences in absorption rates since differences in first-pass metabolism usually affect oral bioavailability and not the absorption rate.
We conducted a series of in vitro dissolution studies and a pilot clinical study to describe the dissolution and absorption of nifedipine. Our dissolution studies demonstrate a significant increase in the dissolution of 10 mg nifedipine IR capsule with larger dissolution media volume. Increasing the volume of FaSSGF used in dissolution experiments from 100 to 200 mL resulted in more than three-fold increase in AUCdiss and significantly higher percentages of nifedipine dissolved at all of the time points. Since the solubility in the dissolution media is lower than the concentrations achieved, a supersaturated system has been formed. Supersaturated systems undergo precipitation, which explains the observed peak followed by decline in cumulative nifedipine dissolved. A supersaturated system emerges here since the dosage form is a cosolvent-based formulation. Upon exposure to aqueous media, drug concentrations exceed equilibrium solubility in the cosolvent/ aqueous system and supersaturation is observed. The larger the volume of dissolution media, the higher the degree of supersaturation observed. (18)
A significant improvement in nifedipine dissolution with higher volumes was also observed with 20 mg doses although the magnitude of change was much smaller (1.7-fold increase in AUCdiss) than that observed with 10 mg doses. These findings suggest that the effect of fluid volume on nifedipine absorption from 20 mg doses may not be easily observed in vivo since a substantial increase in volume beyond 400 mL may be needed to induce a significant improvement in dissolution. Given the substantial improvement in dissolution of 10 mg doses with the increase in gastric volume to only 200 ml; the effect of fluid volume on nifedipine absorption in vivo was studied with this dose.
Using a randomized cross-over design, we evaluated the effect of co-administered water volume (50 and 250 ml) on nifedipine Cmax, Tmax, and AUC0–6 in healthy volunteers. Overall, nifedipine pharmacokinetic parameters did not significantly differ between the two phases of the study. However, there was wide variability between individuals in response to change in fluid volume. The a priori sample size calculation was based on a previously reported variability in nifedipine Cmax of 42%. (21) However, in our study, we observed a much higher variability (up to 70%), which may have resulted in a lack of power to detect a significant difference between the two phases.
A more detailed investigation of the effect of fluid volume in individual subjects was conducted to provide some insight into its possible role in nifedipine absorption. Plasma concentration-time profiles for three of the six subjects showed substantially higher nifedipine concentrations during the large volume phase. This is consistent with the results obtained from the in vitro dissolution studies which indicated increased dissolution in 200 mL vs. 100 mL of fluid. In these three individuals, nifedipine dissolution was increased in the presence of additional water, leading to higher availability of drug for absorption within the first six hours.
For two of the six subjects enrolled in the study, Cmax and AUC0–6 were similar between the two study phases, indicating no effect of water volume on the observed concentration-time profiles. Both subjects had concentration-time profiles and Cmax on the small volume phase similar to what would be expected for the large volume phase. This lack of effect is inconsistent with the effect seen in the in vitro dissolution studies. However, it is possible that these individuals had larger than average residual intragastric volumes in the fasted state, which may result in adequate dissolution even with small volume of coadministered water. Although the average basal gastric fluid volume in the fasted state is usually 50 mL (23), a larger volume in a specific subject is possible due to physiological inter-individual variability or inter-occasion variability within the same subject. Additionally, it is possible that these subjects did not comply with pre-study instructions and consumed extraneous fluids prior to the study visit.
Plasma concentration-time profiles observed in the sixth subject in our study showed an effect of fluid volume opposite to what would be expected based on our initial hypothesis and the results of the dissolution studies (higher concentrations during low volume phase). The unexpectedly high nifedipine exposure in the small volume phase can be explained by one or both of the reasons discussed above. However, the substantially low nifedipine exposure in the large volume phase cannot be similarly explained by variability in intragastric fluid volume. Given the relatively small number of subjects in this pilot study and the substantial variability in nifedipine absorption, larger future studies are needed to further evaluate the impact of coadministered water volume on nifedipine PK from IR capsules and assess the reproducibility of current findings in a larger sample size.
Effects of nifedipine on blood pressure in women with preeclampsia have been shown to correlate with plasma concentrations with nadirs of blood pressure occurring at the same time of Cmax. (24) Consequently, clinically significant variability in tocolytic or blood pressure reducing effects may be expected as a result of variability in nifedipine Cmax. The administration of large volume of water was associated with a smaller variability in observed Cmax (CV% = 47% versus 70% for small volume phase). Further, if we exclude the subject who had low nifedipine exposure on the large volume phase, the reduction in variability with the large water volume was even more pronounced (CV% = 22% versus 81% for small volume phase).
Previous studies have investigated the effect of coadministered water volume on the pharmacokinetics of BCS Class II drugs in humans. In 1978, Welling et al. investigated the effect of food and coadministered water volume on the bioavailability of erythromycin in healthy volunteers using erythromycin stearate film-coated tablets. The results of their study showed that higher and more uniform systemic concentrations of erythromycin were achieved when the coadministered water volume was increased from 20 to 250 ml. (25) In 1984, Bustrack et al. studied digoxin bioavailability from tablets and capsules following coadministration with 30 or 240 mL of water. Their study, however, did not show an effect of coadministered water volume on digoxin Cmax or AUC0–12. (26) Differences in bioavailability between digoxin and erythromycin may explain the different results obtained in those two studies. Digoxin bioavailability from tablets and capsules (70–80%) is higher than that for erythromycin from erythromycin stearate tablets (30%). (27, 28) Low erythromycin bioavailability can result in more pronounced effects of larger coadministered water volumes than that observed with digoxin where bioavailability is already relatively high. The lack of effect of coadministered water volume on overall nifedipine PK parameters in some subjects may be due to the relatively higher nifedipine bioavailability from IR capsules (50–70%) as compared to erythromycin. (11)
Overall, our pilot study results indicate that administration of large water volume (250 ml) with 10 mg doses of nifedipine IR cosolvent capsules may reduce the variability in observed Cmax, which, if confirmed in larger future studies, may warrant the use of large fluid volumes when nifedipine IR capsules are administered for treatment of preterm labor. Low solubility may play a role in limiting or delaying GI absorption of BCS Class II drugs and future studies with other BCS Class II drugs are needed to evaluate the applicability of the current findings to other low solubility drugs with different formulations.
Acknowledgments
This study was supported, in part, by the Indiana Clinical and Translational Sciences Institute funded, in part by Grant # UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. SKQ is supported by 1K23HD071134 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
We thank Indiana Clinical Research Center nursing staff for their efforts in coordinating the conduct of the clinical study, Janelle Owens for her efforts with chromatographic analysis of nifedipine dissolution samples, and the Clinical Pharmacology Analytical Core Laboratory at Indiana University for analysis of clinical samples.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 2.Guazzi M, Olivari MT, Polese A, Fiorentini C, Magrini F, Moruzzi P. Nifedipine, a new antihypertensive with rapid action. Clin Pharmacol Ther. 1977;22(5 Pt 1):528–532. doi: 10.1002/cpt1977225part1528. [DOI] [PubMed] [Google Scholar]
- 3.Fox NS, Gelber SE, Kalish RB, Chasen ST. Contemporary practice patterns and beliefs regarding tocolysis among u.s. Maternal-fetal medicine specialists. Obstet Gynecol. 2008;112(1):42–47. doi: 10.1097/AOG.0b013e318176158e. [DOI] [PubMed] [Google Scholar]
- 4.ACOG. Practice Bulletin No. 159: Management of Preterm Labor. Obstet Gynecol. 2016;127(1):e29–e38. doi: 10.1097/AOG.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 5.Vogel JP, Oladapo OT, Manu A, Gulmezoglu AM, Bahl R. New WHO recommendations to improve the outcomes of preterm birth. Lancet Glob Health. 2015;3(10):e589–e590. doi: 10.1016/S2214-109X(15)00183-7. [DOI] [PubMed] [Google Scholar]
- 6.Nassar AH, Aoun J, Usta IM. Calcium channel blockers for the management of preterm birth: a review. Am J Perinatol. 2011;28(1):57–66. doi: 10.1055/s-0030-1262512. [DOI] [PubMed] [Google Scholar]
- 7.Haas DM, Caldwell DM, Kirkpatrick P, McIntosh JJ, Welton NJ. Tocolytic therapy for preterm delivery: systematic review and network meta-analysis. BMJ. 2012;345:e6226. doi: 10.1136/bmj.e6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King JF, Flenady VJ, Papatsonis DN, Dekker GA, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev. 2002;(2):CD002255. doi: 10.1002/14651858.CD002255. [DOI] [PubMed] [Google Scholar]
- 9.Patki KC, Von Moltke LL, Greenblatt DJ. In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes p450: role of cyp3a4 and cyp3a5. Drug Metab Dispos. 2003;31(7):938–944. doi: 10.1124/dmd.31.7.938. [DOI] [PubMed] [Google Scholar]
- 10.Kleinbloesem CH, van Harten J, Wilson JP, Danhof M, van Brummelen P, Breimer DD. Nifedipine: kinetics and hemodynamic effects in patients with liver cirrhosis after intravenous and oral administration. Clin Pharmacol Ther. 1986;40(1):21–28. doi: 10.1038/clpt.1986.134. [DOI] [PubMed] [Google Scholar]
- 11.Raemsch KD, Sommer J. Pharmacokinetics and metabolism of nifedipine. Hypertension. 1983;5(4 Pt 2):II18–II24. doi: 10.1161/01.hyp.5.4_pt_2.ii18. [DOI] [PubMed] [Google Scholar]
- 12.Toal CB. Formulation dependent pharmacokinetics--does the dosage form matter for nifedipine? J Cardiovasc Pharmacol. 2004;44(1):82–86. doi: 10.1097/00005344-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Haas DM, Quinney SK, Clay JM, Renbarger JL, Hebert MF, Clark S, et al. Nifedipine pharmacokinetics are influenced by CYP3A5 genotype when used as a preterm labor tocolytic. Am J Perinatol. 2013;30(4):275–281. doi: 10.1055/s-0032-1323590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thelen K, Jantratid E, Dressman JB, Lippert J, Willmann S. Analysis of nifedipine absorption from soft gelatin capsules using PBPK modeling and biorelevant dissolution testing. J Pharm Sci. 2010;99(6):2899–2904. doi: 10.1002/jps.22026. [DOI] [PubMed] [Google Scholar]
- 15.Jantratid E, Janssen N, Reppas C, Dressman JB. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharmaceutical Research. 2008;25(7):1663–1676. doi: 10.1007/s11095-008-9569-4. [DOI] [PubMed] [Google Scholar]
- 16.Vertzoni M, Dressman J, Butler J, Hempenstall J, Reppas C. Simulation of fasting gastric conditions and its importance for the in vivo dissolution of lipophilic compounds. Eur J Pharm Biopharm. 2005;60(3):413–417. doi: 10.1016/j.ejpb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Vertzoni M, Pastelli E, Psachoulias D, Kalantzi L, Reppas C. Estimation of intragastric solubility of drugs: in what medium? Pharmaceutical Research. 2007;24(5):909–917. doi: 10.1007/s11095-006-9209-9. [DOI] [PubMed] [Google Scholar]
- 18.Klein S, Shah VP. A standardized mini paddle apparatus as an alternative to the standard paddle. AAPS PharmSciTech. 2008;9(4):1179–1184. doi: 10.1208/s12249-008-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas DM, Quinney SK, Clay JM, Renbarger JL, Hebert MF, Clark S, et al. Nifedipine Pharmacokinetics Are Influenced by CYP3A5 Genotype When Used as a Preterm Labor Tocolytic. Am J Perinatol. 2012;8:8. doi: 10.1055/s-0032-1323590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99(3):306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Yu KS, Cho JY, Shon JH, Bae KS, Yi SY, Lim HS, et al. Ethnic differences and relationships in the oral pharmacokinetics of nifedipine and erythromycin. Clin Pharmacol Ther. 2001;70(3):228–236. doi: 10.1067/mcp.2001.117703. [DOI] [PubMed] [Google Scholar]
- 22.ACOG. ACOG practice bulletin no. 127: Management of preterm labor. Obstetrics and Gynecology. 2012;119(6):1308–1317. doi: 10.1097/AOG.0b013e31825af2f0. [DOI] [PubMed] [Google Scholar]
- 23.Schiller C, Frohlich CP, Giessmann T, Siegmund W, Monnikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment Pharmacol Ther. 2005;22(10):971–979. doi: 10.1111/j.1365-2036.2005.02683.x. [DOI] [PubMed] [Google Scholar]
- 24.Barton JR, Prevost RR, Wilson DA, Whybrew WD, Sibai BM. Nifedipine pharmacokinetics and pharmacodynamics during the immediate postpartum period in patients with preeclampsia. Am J Obstet Gynecol. 1991;165(4 Pt 1):951–954. doi: 10.1016/0002-9378(91)90446-x. [DOI] [PubMed] [Google Scholar]
- 25.Welling PG, Huang H, Hewitt PF, Lyons LL. Bioavailability of erythromycin stearate: influence of food and fluid volume. J Pharm Sci. 1978;67(6):764–766. doi: 10.1002/jps.2600670608. [DOI] [PubMed] [Google Scholar]
- 26.Bustrack JA, Katz JD, Hull JH, Foster JR, Hammond JE, Christenson RH. Bioavailability of digoxin capsules and tablets: effect of coadministered fluid volume. J Pharm Sci. 1984;73(10):1397–1400. doi: 10.1002/jps.2600731018. [DOI] [PubMed] [Google Scholar]
- 27.Beveridge T, Nüesch E, Ohnhaus EE. Absolute bioavailability of digoxin tablets. Arzneimittel-Forschung. 1978;28(4):701–703. [PubMed] [Google Scholar]
- 28.Mather LE, Austin KL, Philpot CR, McDonald PJ. Absorption and bioavailability of oral erythromycin. Br J Clin Pharmacol. 1981;12(2):131–140. doi: 10.1111/j.1365-2125.1981.tb01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]